The Effect of Plasma Treatment on the Mechanical and Biological Properties of Polyurethane Artificial Blood Vessel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material Characterization
2.3. Cell Compatibility (In Vitro)
2.4. Blood Compatibility (In Vitro)
2.5. Statistical Analysis
3. Results and Discussion
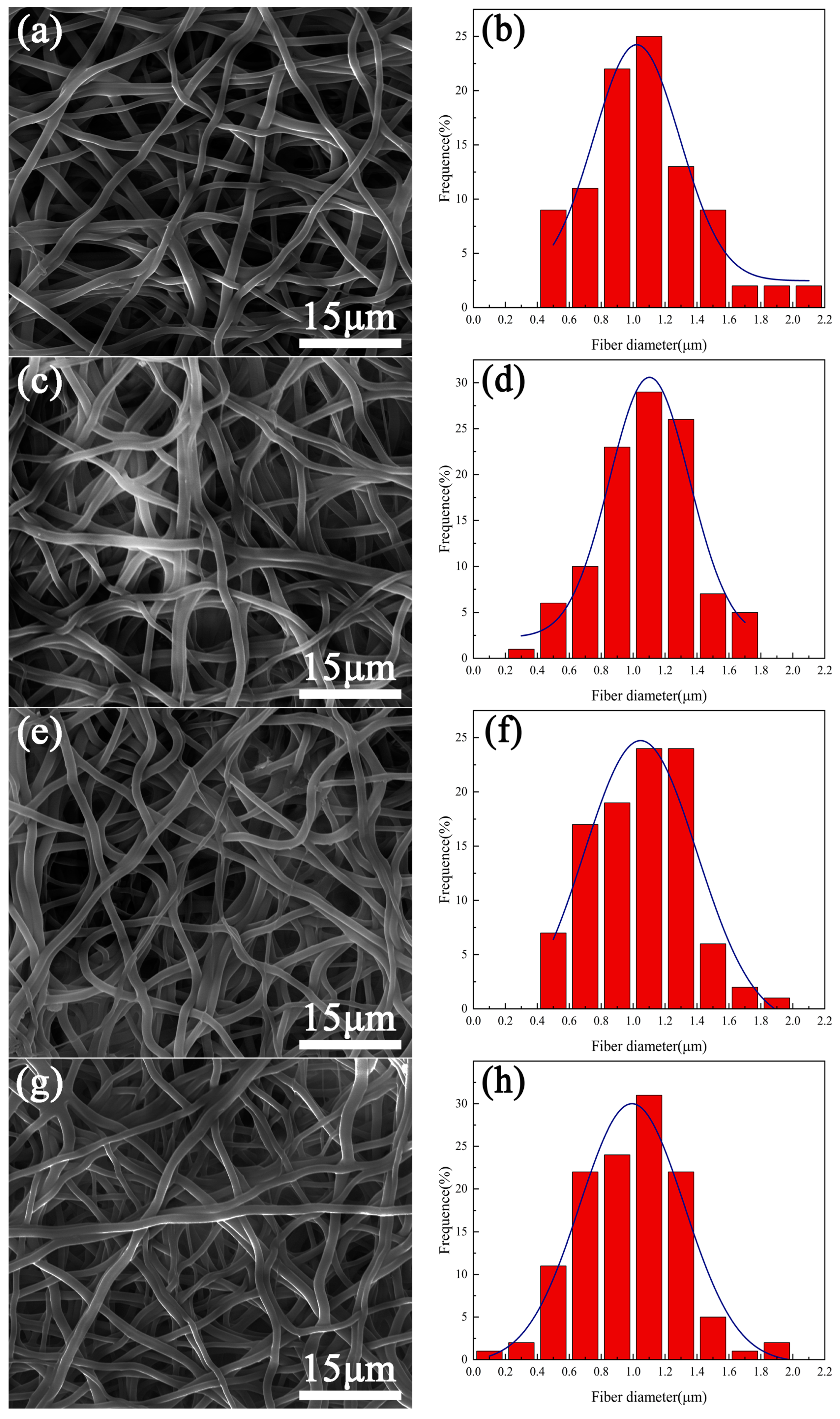
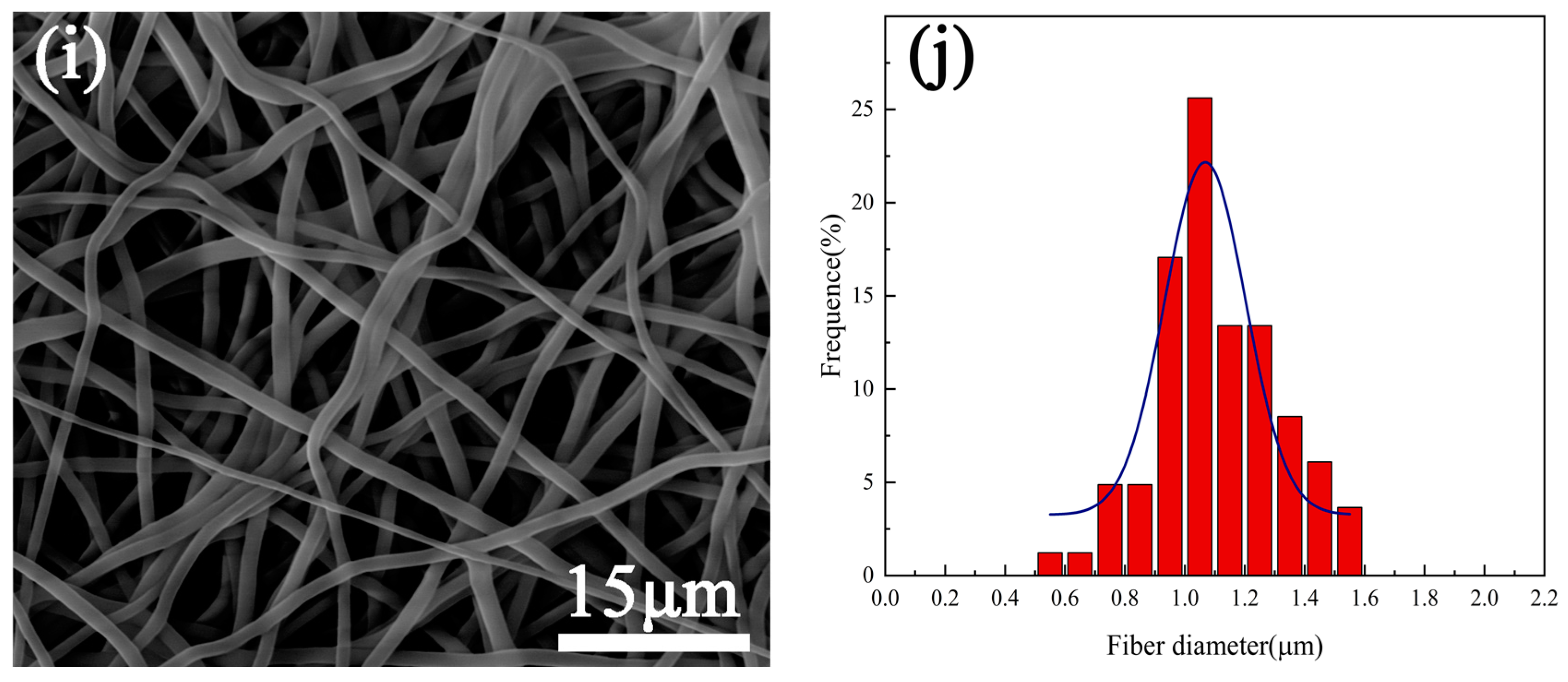
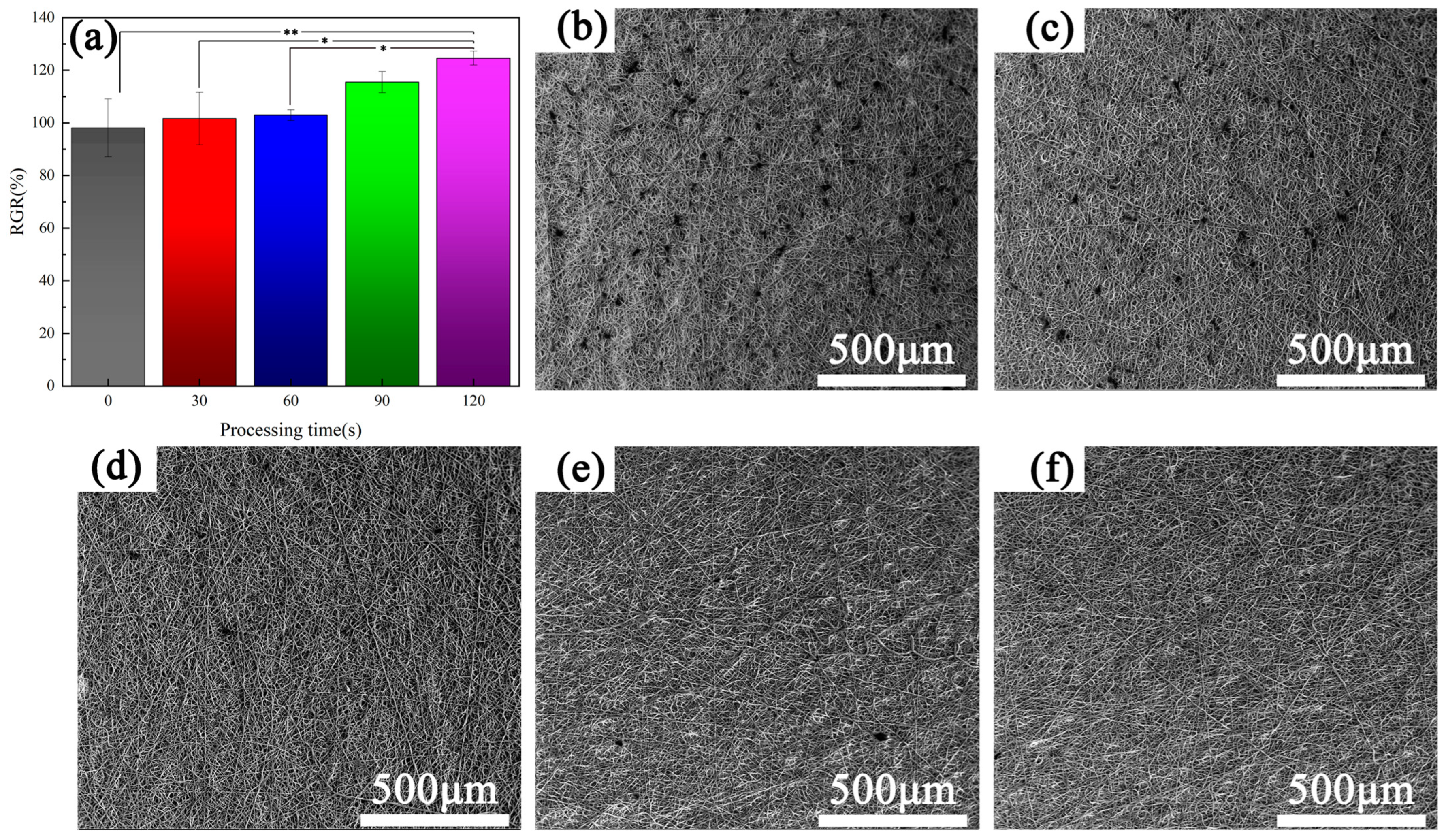
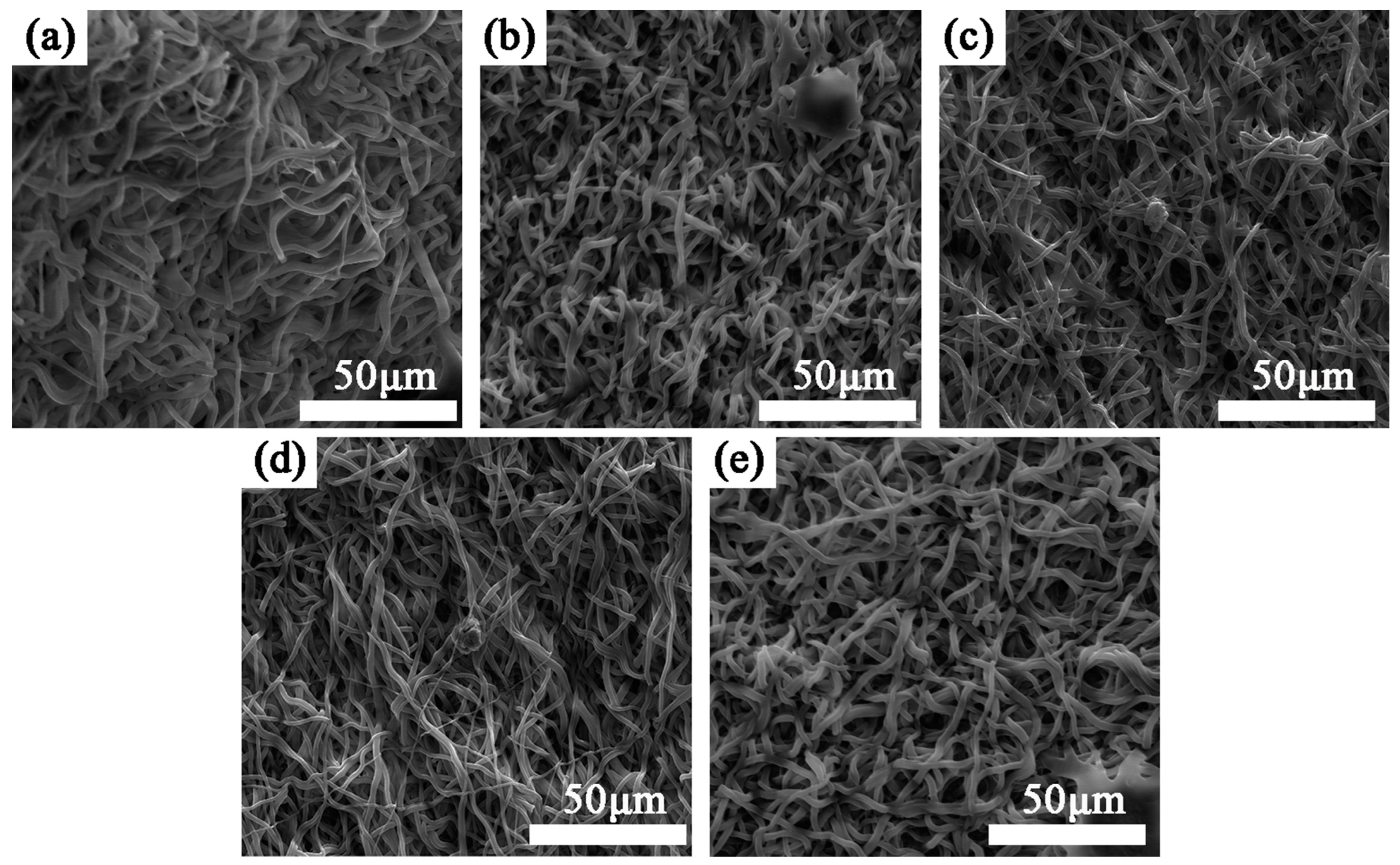
3.1. Surface Morphology
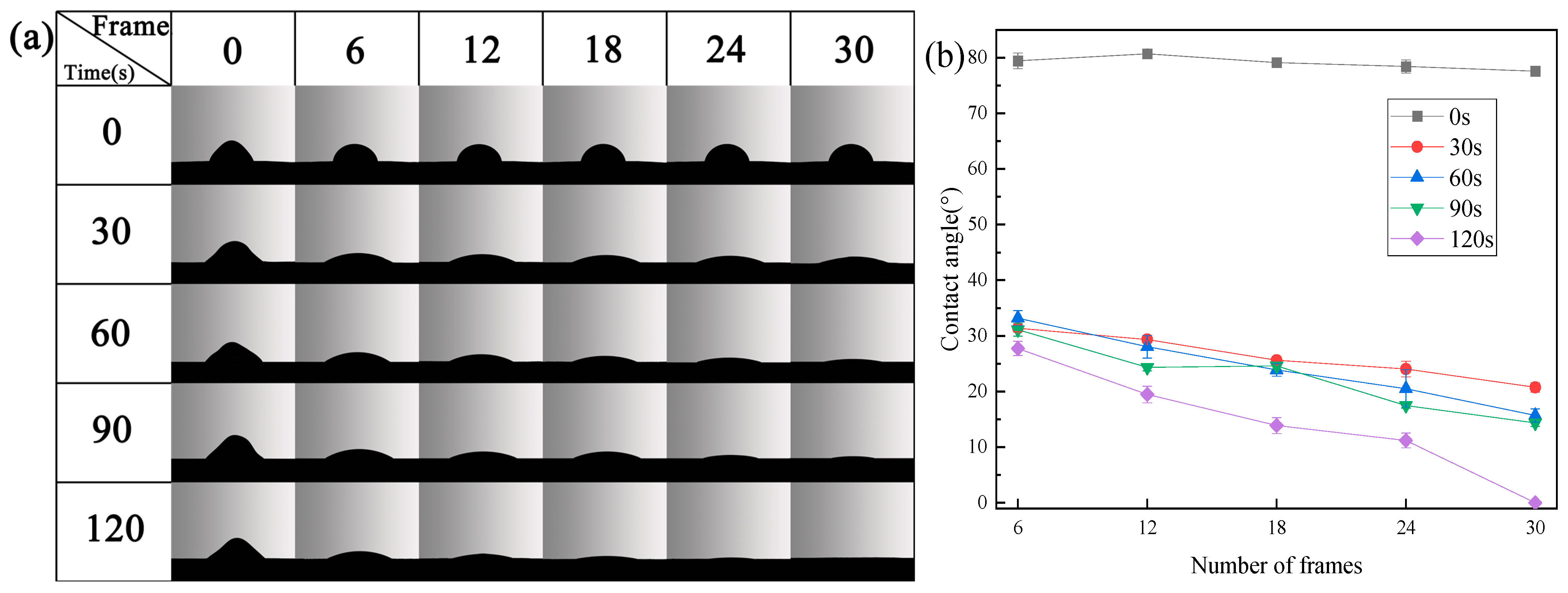
3.2. Wettability of Sample Surface
3.3. Surface Chemical Composition
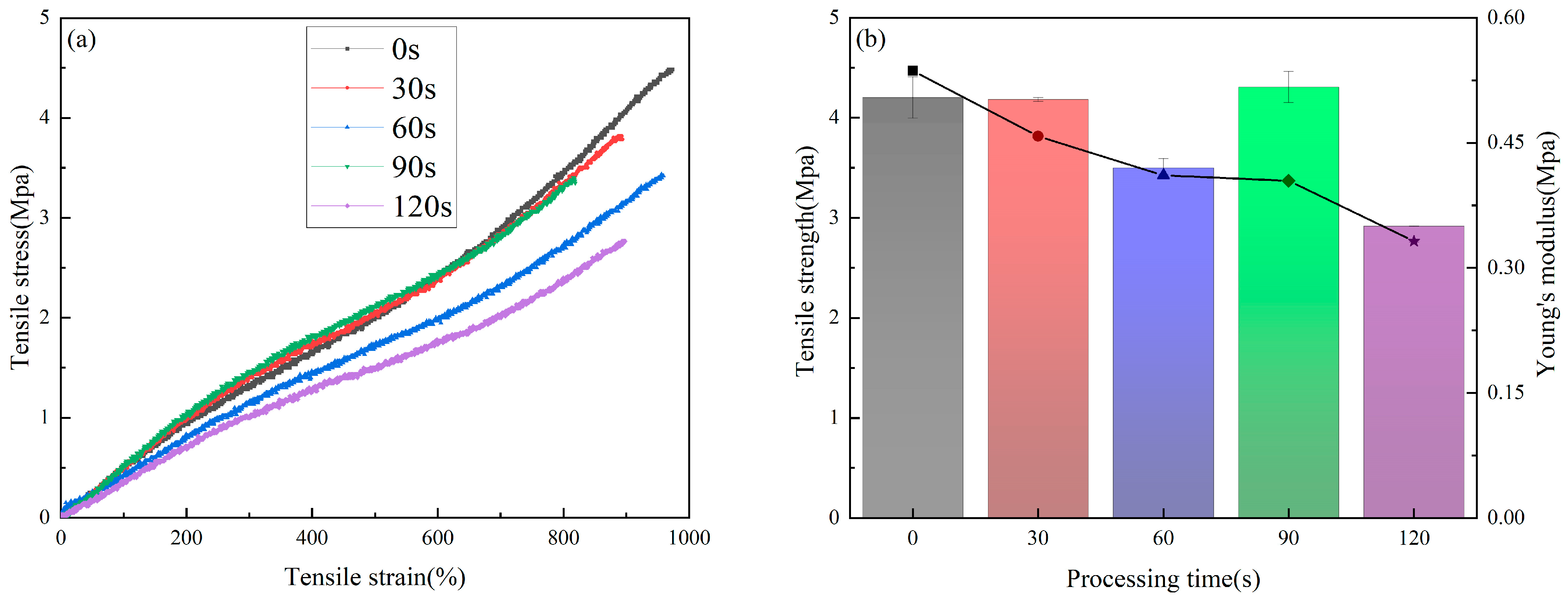
3.4. Mechanical Properties
3.5. Biocompatibility Evaluation In Vitro
3.5.1. Cell Experiment
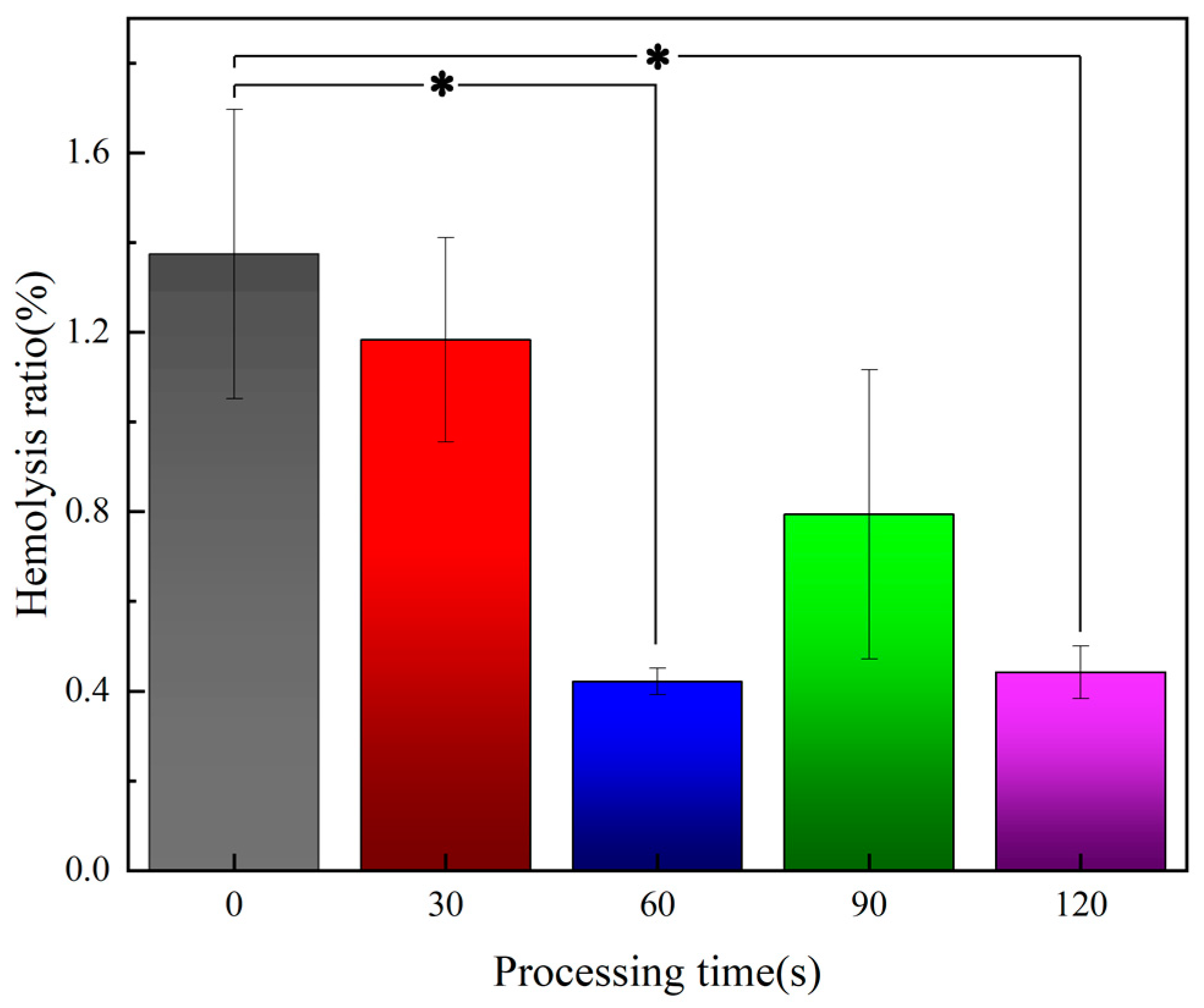
3.5.2. Blood Test Results
4. Conclusions
- Under the plasma treatment conditions selected for this experiment, plasma treatment did not cause significant changes in the morphology of polyurethane fibers.
- Plasma treatment can introduce oxygen-containing functional groups, which can significantly improve the wettability of the sample and increase the proliferation rate of endothelia cells on the sample.
- Plasma treatment can reduce the risk of hemolysis of the material, improve the anti-platelet adhesion properties. Overall, plasma treatment can improve the blood compatibility of polyurethane fiber materials.
- Due to the etching effect of plasma, the mechanical properties of the sample decreased to a certain extent; this tendency to decrease should be used as the basis to balance the biological properties and the mechanical properties during the design of PU artificial blood vessels.
- In view of the influence of plasma treatment on mechanical properties and biocompatibility, polyurethane samples treated with plasma for 90 s have good mechanical properties and biocompatibility, and they also improve the wettability of the sample surface, which lays a foundation for the preparation of subsequent functional coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, A.; Ren, H.; Ma, Z.; Alam, N.; Jia, L.; Liu, E. Trends and characteristics of COVID-19 and cardiovascular disease related studies. Front. Pharmacol. 2023, 14, 1105459. [Google Scholar] [CrossRef]
- Yamagishi, K.; Sankai, T.; Muraki, I.; Umesawa, M.; Cui, R.; Imano, H.; Kihara, T.; Noda, H.; Ikeda, A.; Ohira, T.; et al. Trends in stroke, cardiovascular disease, and medical expenditure under a community-based long-term stroke prevention program. J. Hypertens. 2023, 41, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.R.; Malhotra, R.; Hilaire, C.S.; Aikawa, E.; Blumenthal, R.S.; Gackenbach, G.; Goyal, P.; Johnson, A.; Nigwekar, S.U.; Shanahan, C.M.; et al. Molecular Mechanisms of Vascular Health: Insights from Vascular Aging and Calcification. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Beccacece, L.; Abondio, P.; Bini, C.; Pelotti, S.; Luiselli, D. The Link between Prostanoids and Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 4193. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.J.; Montezano, A.C.; Camargo, L.L.; Touyz, R.M. Impact of Environmental Factors on Hypertension and Associated Cardiovascular Disease. Can. J. Cardiol. 2023, 39, 1229–1243. [Google Scholar] [CrossRef]
- Poznyak, A.V.V.; Sukhorukov, V.N.N.; Eremin, I.I.I.; Nadelyaeva, I.I.I.; Orekhov, A.N.N. Diagnostics of atherosclerosis: Overview of the existing methods. Front. Cardiovasc. Med. 2023, 10, 1134097. [Google Scholar] [CrossRef]
- Ridker, P.M. The Time to Initiate Anti-Inflammatory Therapy for Patients with Chronic Coronary Atherosclerosis Has Arrived. Circulation 2023, 148, 1071–1073. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yamanashi, H.; Honda, Y.; Nonaka, F.; Miyata, J.; Kawashiri, S.-Y.; Noguchi, Y.; Nakamichi, S.; Nagata, Y.; Maeda, T. Low-Density Lipoprotein Cholesterol, Structural Atherosclerosis, and Functional Atherosclerosis in Older Japanese. Nutrients 2023, 15, 183. [Google Scholar] [CrossRef]
- Niklason, L.E.; Lawson, J.H. Bioengineered human blood vessels. Science 2020, 370, 185. [Google Scholar] [CrossRef]
- Shahriari-Khalaji, M.; Shafiq, M.; Cui, H.; Cao, R.; Zhu, M. Advancements in the fabrication technologies and biomaterials for small diameter vascular grafts: A fine-tuning of physicochemical and biological properties. Appl. Mater. Today 2023, 31, 101778. [Google Scholar] [CrossRef]
- Zizhou, R.; Khoshmanesh, K.; Wang, X.; Houshyar, S. Fabrication of Nanocomposites with High Elasticity and Strength for the Load-Bearing Layer of Small-Diameter Vascular Grafts. ACS Appl. Mater. Interfaces 2023, 15, 35411–35421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Li, L.; Xie, E.Z.H.; Li, M.X.; Cao, J.H.; Yang, X.B.; Wu, D.Y. Small-diameter PCL/PU vascular graft modified with heparin-aspirin compound for preventing the occurrence of acute thrombosis. Int. J. Biol. Macromol. 2023, 249, 126058. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Jiao, Y.H.; Wang, C.; Zhang, C.C.; Wang, H.; Feng, Z.G.; Gu, Y.Q.; Wang, Z.G. Design and characterization of small-diameter tissue-engineered blood vessels constructed by electrospun polyurethane-core and gelatin-shell coaxial fiber. Bioengineered 2021, 12, 5769–5788. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.L.; Luo, R.F.; Li, J.K.; Mo, X.M.; Wang, Y.B.; Zhang, X.D. Coaxial electrospinning multicomponent functional controlled-release vascular graft: Optimization of graft properties. Colloids Surf. B–Biointerfaces 2017, 152, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, W.C.; Sun, X.M.; Lin, Z.Y.; Ren, L. Effect of Post-Treatment on Mechanical and Biological Properties of Coaxial Electrospun Core-Shell Structured Poly(lactic-co-glycolic acid)/Gelatin Methacrylamide Fibrous Scaffolds. ACS Appl. Polym. Mater. 2022, 4, 987–998. [Google Scholar] [CrossRef]
- Jang, S.R.; Kim, J.I.; Park, C.H.; Kim, C.S. Development of Y-shaped small diameter artificial blood vessel with controlled topography via a modified electrospinning method. Mater. Lett. 2020, 264, 127113. [Google Scholar] [CrossRef]
- Guo, H.F.; Dai, W.W.; Qian, D.H.; Qin, Z.X.; Lei, Y.; Hou, X.Y.; Wen, C. A simply prepared small-diameter artificial blood vessel that promotes in situ endothelialization. Acta Biomater. 2017, 54, 107–116. [Google Scholar] [CrossRef]
- Guan, L.H.; Zhao, H.J.; Wang, K.; Zhang, K.Q.; Meng, K. PLCL-TPU bi-layered artificial blood vessel with compliance matching to host vessel. J. Biomater. Appl. 2023, 9, 194–202. [Google Scholar] [CrossRef]
- Gostev, A.A.; Karpenko, A.A.; Laktionov, P.P. Polyurethanes in cardiovascular prosthetics. Polymer. Bull. 2018, 75, 4311–4325. [Google Scholar] [CrossRef]
- Mo, F.; Ren, H.; Chen, S.; Ge, Z. Novel zwitterionic polyurethanes with good biocompatibility and antibacterial activity. Mater. Lett. 2015, 145, 174–176. [Google Scholar] [CrossRef]
- Marzec, M.; Kucinska-Lipka, J.; Kalaszczynska, I.; Janik, H. Development of polyurethanes for bone repair. Mater. Sci. Eng. C–Mater. Biol. Appl. 2017, 80, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, Y.S. The role of bacterial cellulose in artificial blood vessels. Mol. Cell. Toxicol. 2017, 13, 257–261. [Google Scholar] [CrossRef]
- Fathi-Karkan, S.; Banimohamad-Shotorbani, B.; Saghati, S.; Rahbarghazi, R.; Davaran, S. A critical review of fibrous polyurethane-based vascular tissue engineering scaffolds. J. Biol. Eng. 2022, 16, 6. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Behl, M.; Lendlein, A.; Zhao, H.; Xiao, R.; Guo, J. Hemocompatible polyurethane/gelatin-heparin nanofibrous scaffolds formed by a bi-layer electrospinning technique as potential artificial blood vessels. Front. Chem. Sci. Eng. 2011, 5, 392–400. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Ma, S.; Wang, L.; Liu, C.; Xu, W.; Shi, J.; Qiao, W.; Yang, H. Small-diameter polyurethane vascular graft with high strength and excellent compliance. J. Mech. Behav. Biomed. Mater. 2021, 121, 104614. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Huang, Y.; Yin, T.; Wang, J.; Du, R.; Qiu, J.; Zhang, Y.; Wang, Y.; Chen, J.; Wang, G. Effects of elemene on inhibiting proliferation of vascular smooth muscle cells and promoting reendothelialization at the stent implantation site. Biomater. Sci. 2017, 5, 1144–1155. [Google Scholar] [CrossRef]
- Peng, X.; Wang, X.; Cheng, C.; Zhou, X.; Gu, Z.; Li, L.; Liu, J.; Yu, X. Bioinspired, Artificial, Small-Diameter Vascular Grafts with Selective and Rapid Endothelialization Based on an Amniotic Membrane-Derived Hydrogel. ACS Biomater. Sci. Eng. 2020, 6, 1603–1613. [Google Scholar] [CrossRef]
- Lv, Y.; Li, G.; Peng, H.; Liu, Y.; Yao, J.; Wang, G.; Sun, J.; Liu, J.; Zhang, H.; Chen, G.; et al. Development of elastic artificial vessels with a digital pulse flow system to investigate the risk of restenosis and vasospasm. Lab Chip 2020, 20, 3051–3059. [Google Scholar] [CrossRef]
- Jang, E.H.; Kim, J.-H.; Lee, J.H.; Kim, D.-H.; Youn, Y.-N. Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells. Polymers 2020, 12, 538. [Google Scholar] [CrossRef]
- Feng, L.-A.; Shi, J.; Guo, J.-Y.; Wang, S.-F. Recent strategies for improving hemocompatibility and endothelialization of cardiovascular devices and inhibition of intimal hyperplasia. J. Mater. Chem. B 2022, 10, 3781–3792. [Google Scholar] [CrossRef]
- Shahzadi, L.; Ramzan, A.; Anjum, A.; Jabbar, F.; Khan, A.F.; Manzoor, F.; Shahzad, S.A.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. An efficient new method for electrospinning chitosan and heparin for the preparation of pro-angiogenic nanofibrous membranes for wound healing applications. J. Appl. Polym. Sci. 2022, 139, e53212. [Google Scholar] [CrossRef]
- Yao, W.C.; Gu, H.B.; Hong, T.; Wang, Y.; Chen, S.H.; Mo, X.M.; Li, W.Y.; Wang, C.S.; Zhu, T.H.; Lu, S.Y. A bi-layered tubular scaffold for effective anti-coagulant in vascular tissue engineering. Mater. Des. 2020, 194, 108943. [Google Scholar] [CrossRef]
- Vu, B.T.; Nguyen, P.D.-N.; Tran, N.M.-P.; Tang, T.-N.; Do, T.M.; Vo, T.V.; Nguyen, H.T. Fabrication of Endothelial Cell-Lining Polyurethane/Polycaprolactone Tubular Scaffold Coated with Conjugated Linoleic Acid for Small-Diameter Vascular Graft. Fibers Polym. 2023, 24, 423–432. [Google Scholar] [CrossRef]
- Morozov, I.A.; Kamenetskikh, A.S.; Beliaev, A.Y.; Izumov, R.I.; Scherban, M.G.; Lemkina, L.M.; Kiselkov, D.M. Polyurethane Treated in Ar/C2H2/Ar Plasma: Towards Deformable Coating with Improved Albumin Adsorption. Appl. Sci. 2021, 11, 9793. [Google Scholar] [CrossRef]
- Lim, H.; Chung, J.H.; Park, Y.; Baek, N.; Seo, Y.; Park, H.; Cho, Y.K.; Jung, D.; Han, D.H. Inner surface modification of ureteral stent polyurethane tubes based by plasma-enhanced chemical vapor deposition to reduce encrustation and biofilm formation. Biofouling 2022, 38, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Hesari, S.M.; Ghorbani, F.; Ghorbani, F.; Zamanian, A.; Khavandi, A. Plasma surface modification technique-induced gelatin grafting on bio-originated polyurethane porous matrix: Physicochemical and in vitro study. Polym. Polym. Compos. 2021, 29, 640–651. [Google Scholar] [CrossRef]
- De, S.; Sharma, R.; Trigwell, S.; Laska, B.; Ali, N.; Mazumder, M.K.; Mehta, J.L. Plasma treatment of polyurethane coating for improving endothelial cell growth and adhesion. J. Biomater. Sci.–Polym. Ed. 2005, 16, 973–989. [Google Scholar] [CrossRef]
- Keshel, S.H.; Azhdadi, S.N.K.; Asefnezhad, A.; Sadraeian, M.; Montazeri, M.; Biazar, E. The relationship between cellular adhesion and surface roughness for polyurethane modified by microwave plasma radiation. Int. J. Nanomed. 2011, 6, 641–647. [Google Scholar]
- Li, P.Y.; Ma, J.; Kang, S.M.; Chen, S.S. Copper-loaded chitosan coating for improved in-vitro corrosion resistance and endothelialization of magnesium alloy stents. Mater. Chem. Phys. 2023, 305, 127931. [Google Scholar] [CrossRef]
- Abusrafa, A.E.; Habib, S.; Popelka, A. Surface Functionalization of a Polyurethane Surface via Radio-Frequency Cold Plasma Treatment Using Different Gases. Coatings 2020, 10, 1067. [Google Scholar] [CrossRef]
- Li, P.; Liu, Z.; Kang, S.; Chen, S. Effect of Plasma Treatment on Performance of Polybutylene Adipate Coating on Biomedical AZ31 Mg-alloy. Chin. J. Mater. Res. 2023, 37, 271–280. [Google Scholar]
- Zanden, C.; Voinova, M.; Gold, J.; Moersdorf, D.; Bernhardt, I.; Liu, J. Surface characterisation of oxygen plasma treated electrospun polyurethane fibres and their interaction with red blood cells. Eur. Polym. J. 2012, 48, 472–482. [Google Scholar] [CrossRef]
- García-Herrera, C.M.; Atienza, J.M.; Rojo, F.J.; Claes, E.; Guinea, G.V.; Celentano, D.J.; García-Montero, C.; Burgos, R.L. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med. Biol. Eng. Comput. 2012, 50, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Q.; Chen, S.S.; Shahzad, M.B.; Wei, Z.Y.; Leng, B. Enhanced corrosion resistance and biocompatibility of an elastic poly (butyleneadipate-co-terephthalate) composite coating for AZ31 magnesium alloy vascular stents. Prog. Org. Coat. 2022, 172, 107138. [Google Scholar] [CrossRef]
- Modic, M.; Junkar, I.; Stana-Kleinschek, K.; Kostanjsek, R.; Mozetic, M. Morphology Transformations of Platelets on Plasma Activated Surfaces. Plasma Process. Polym. 2014, 11, 596–605. [Google Scholar] [CrossRef]
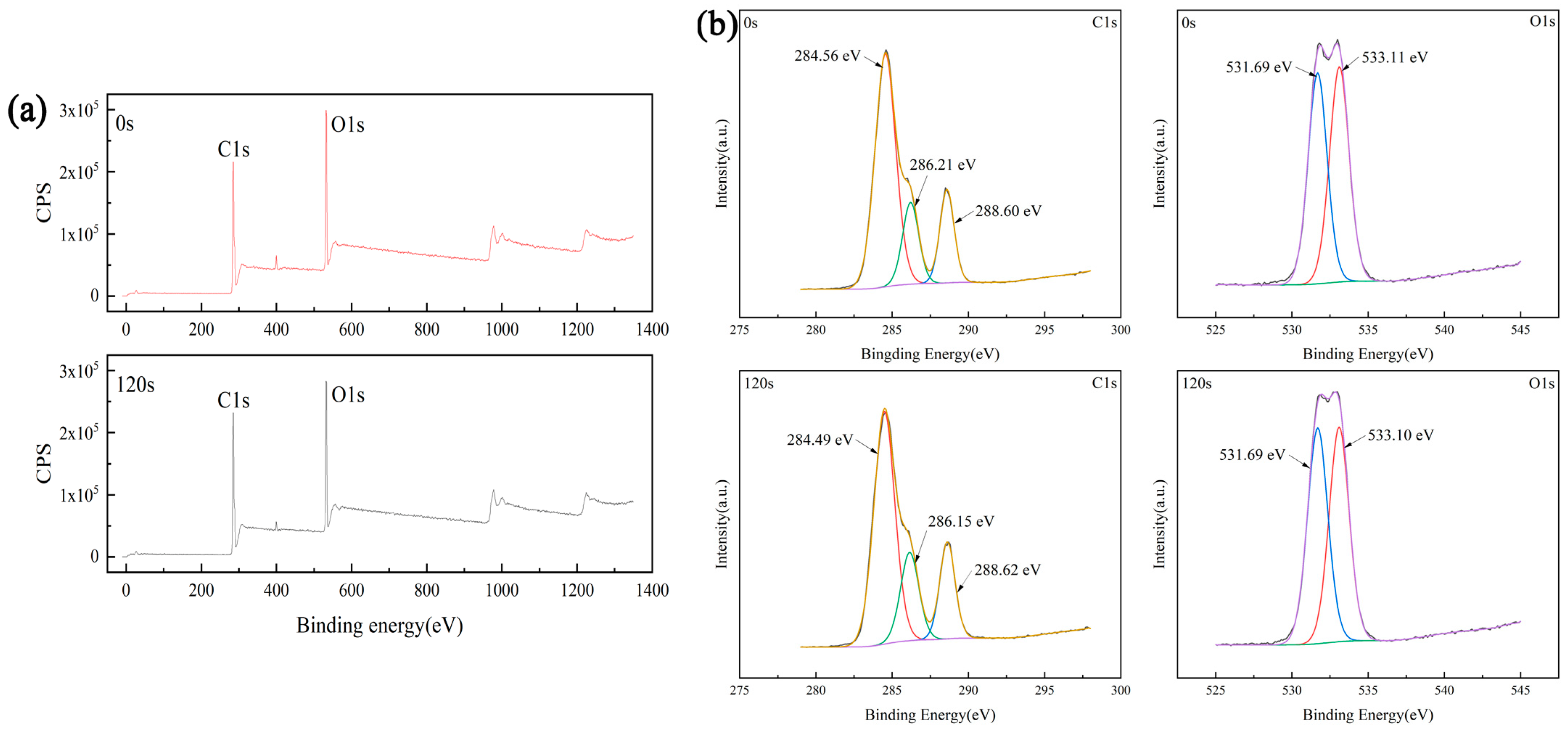
| Processing Time (s) | Element | Integral Area | Proportion of Each Element (%) |
|---|---|---|---|
| 0 | C1s | 882,842.61 | 72.40 |
| O1s | 858,638.52 | 27.60 | |
| 120 | C1s | 861,275.60 | 70.98 |
| O1s | 898,559.74 | 29.02 |
| Processing Time (s) | C–O Integral Area | Proportion of C–O (%) | C=O Integral Area | Proportion of C=O (%) |
|---|---|---|---|---|
| 0 | 126,101.08 | 51.21 | 120234.91 | 48.79 |
| 120 | 128,696.42 | 49.44 | 131748.05 | 50.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Ma, J.; Teng, Y.; Chen, S. The Effect of Plasma Treatment on the Mechanical and Biological Properties of Polyurethane Artificial Blood Vessel. Materials 2023, 16, 7231. https://doi.org/10.3390/ma16227231
Ding C, Ma J, Teng Y, Chen S. The Effect of Plasma Treatment on the Mechanical and Biological Properties of Polyurethane Artificial Blood Vessel. Materials. 2023; 16(22):7231. https://doi.org/10.3390/ma16227231
Chicago/Turabian StyleDing, Cheng, Jing Ma, Yingxue Teng, and Shanshan Chen. 2023. "The Effect of Plasma Treatment on the Mechanical and Biological Properties of Polyurethane Artificial Blood Vessel" Materials 16, no. 22: 7231. https://doi.org/10.3390/ma16227231
APA StyleDing, C., Ma, J., Teng, Y., & Chen, S. (2023). The Effect of Plasma Treatment on the Mechanical and Biological Properties of Polyurethane Artificial Blood Vessel. Materials, 16(22), 7231. https://doi.org/10.3390/ma16227231





