Mechanism for Improved Curie Temperature and Magnetic Entropy Change in Sm-Doped Fe88Zr8B4 Amorphous Alloys
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
3.1. The Structure of the Fe88Zr8−xSmxB4 (x = 0, 2, 4) as-Quenched Ribbons
3.2. Thermal Properties and GFA of the Fe88Zr8−xSmxB4 (x = 0, 2, 4) AAs
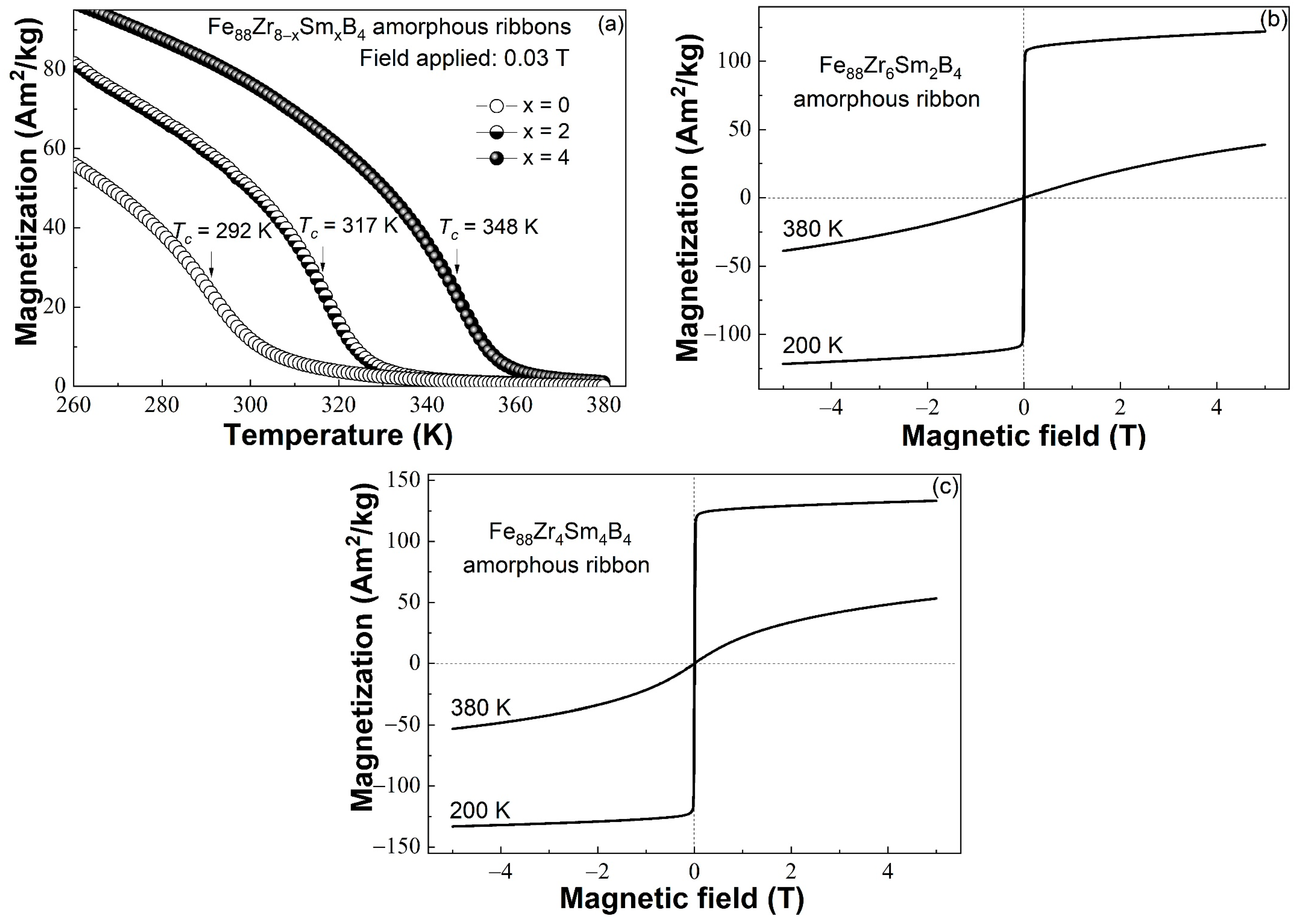
3.3. Magnetic Properties of the Fe88Zr8−xSmxB4 (x = 0, 2, 4) AAs
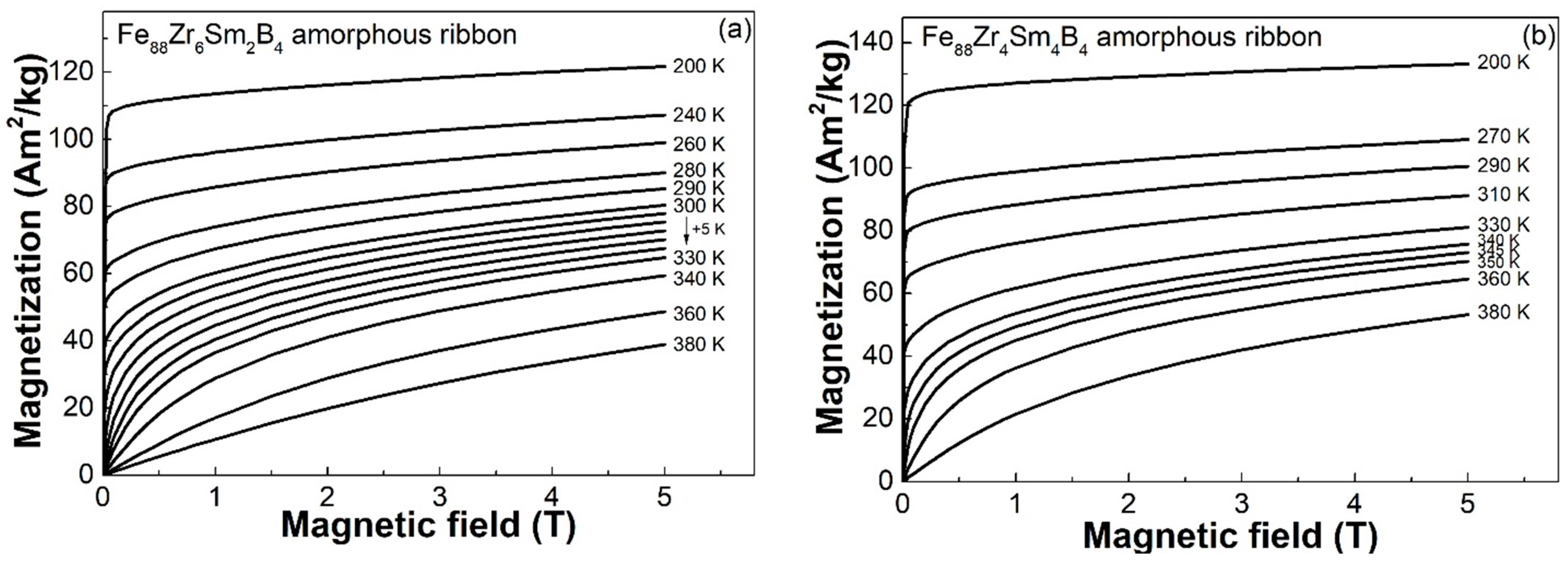
3.4. Magnetocaloric Performance of the Fe88Zr8−xSmxB4 (x = 2, 4) AAs
4. Discussion
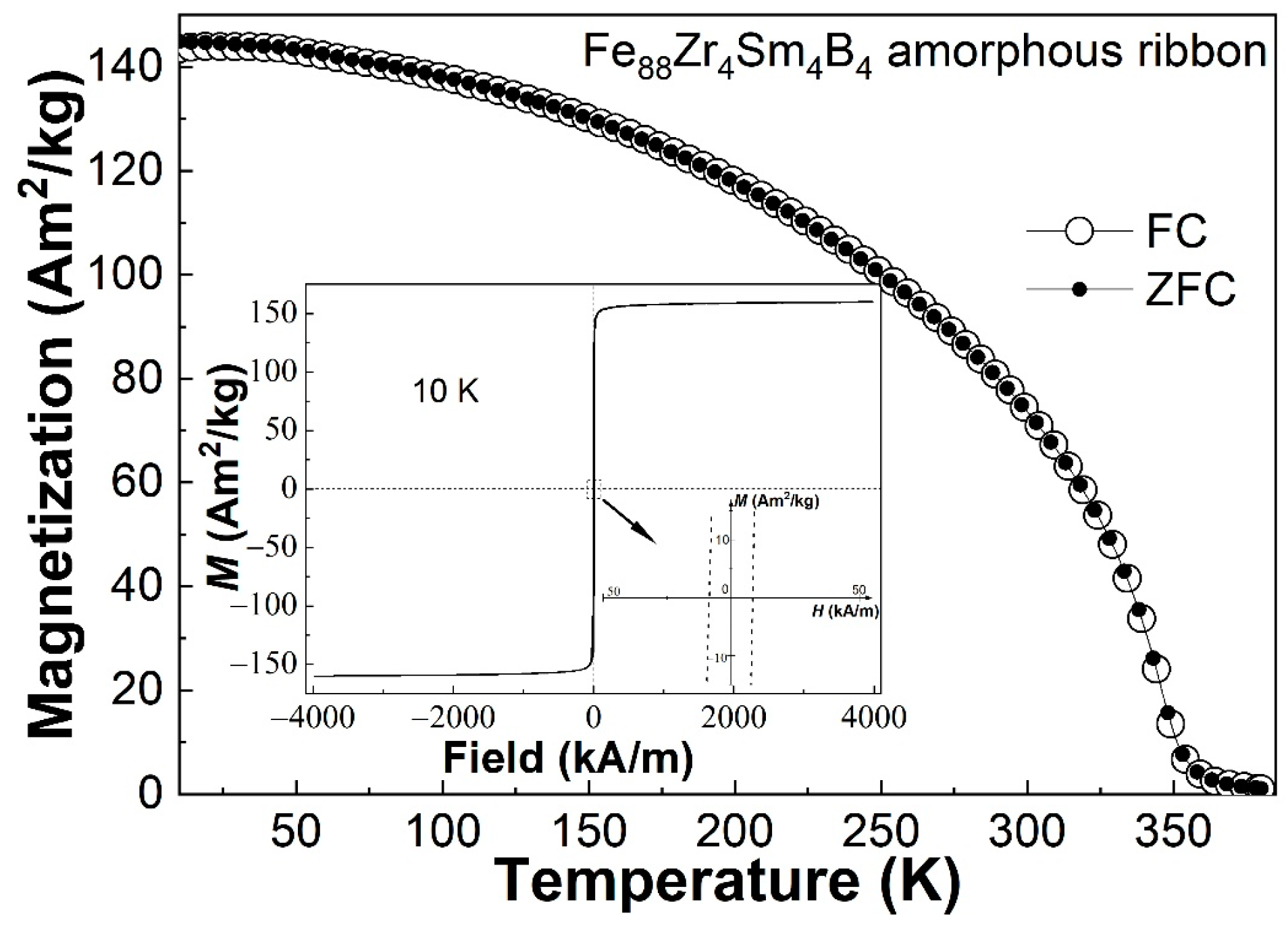
4.1. Low-Temperature Magnetic Properties and Their Influence on the Magnetocaloric Properties of Sm-Doped Fe88Zr8B4 AA Amorphous Ribbons
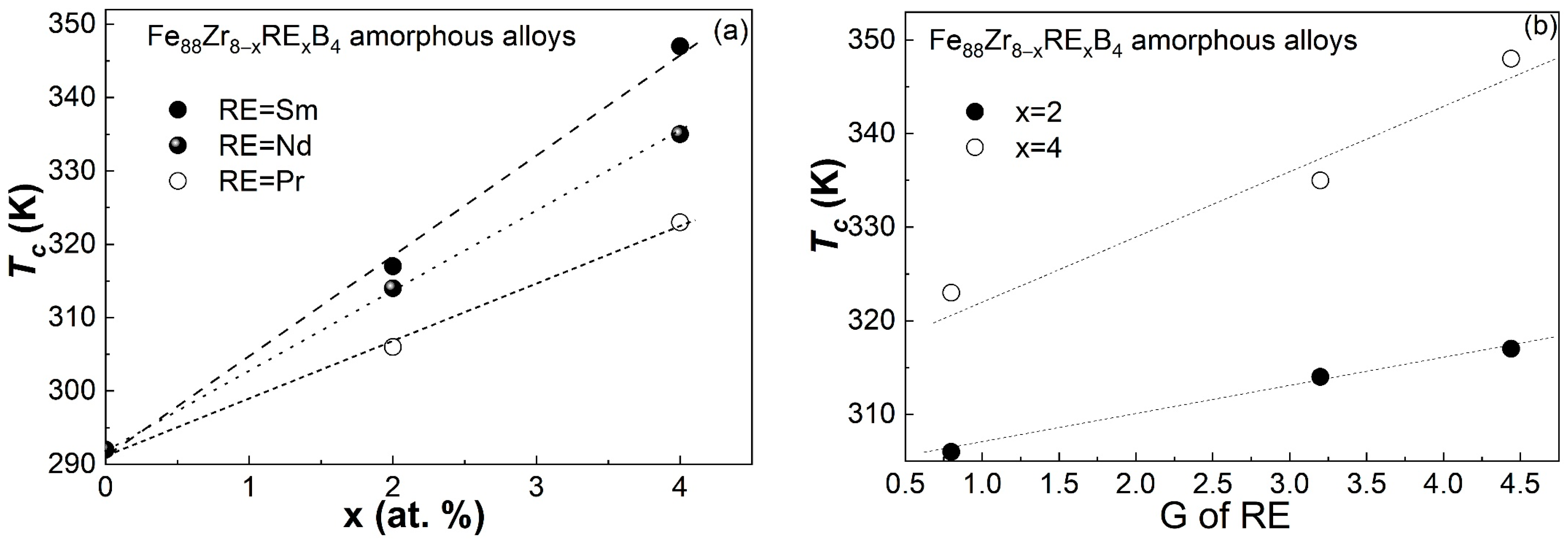
4.2. The Compositional Dependence of Tc in RE-Doped Fe88Zr8B4 AAs
4.3. Magnetocaloric Performance of RE-Doped Fe88Zr8B4 AAs
5. Conclusions
- (a)
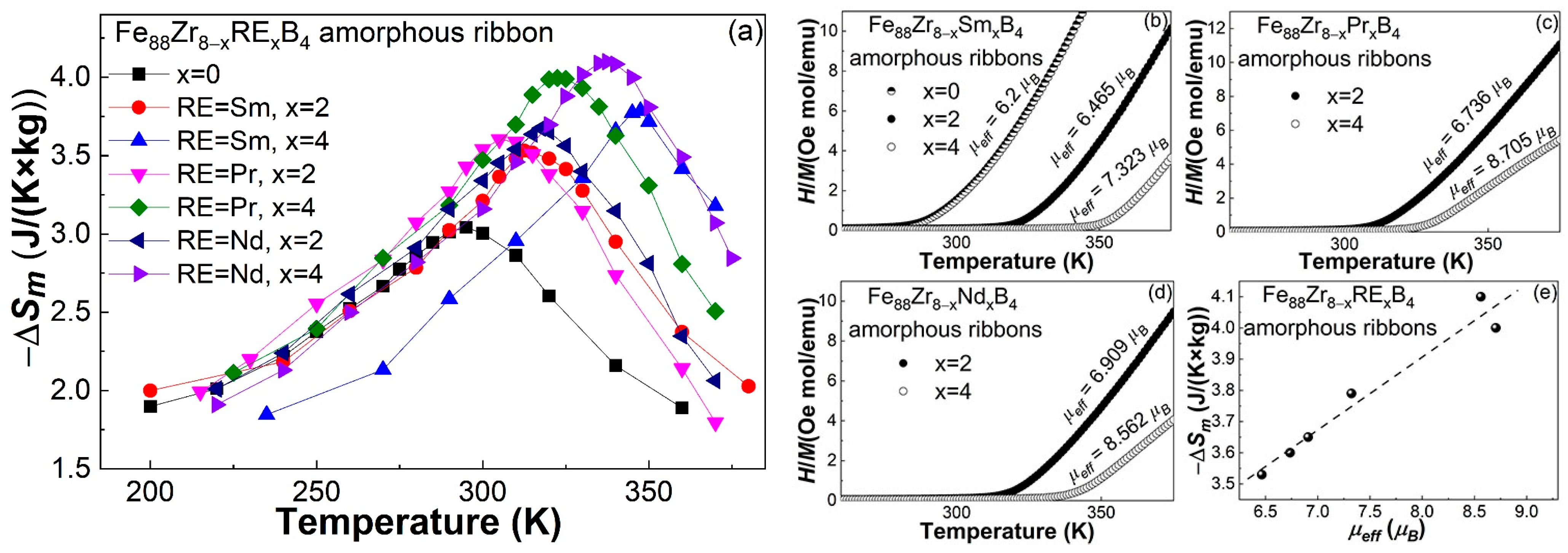
- The minor Sm-doped Fe88Zr8B4 AAs exhibited typical magnetocaloric behaviors of metallic glasses, and the invisible spin-glass-like behavior and negligible coercivity at low temperatures indicated that the minor Sm addition barely affected the magnetocaloric properties of the AAs.
- (b)
- The Tc of the Fe88Zr8−xSmxB4 AAs increased faster than those of the Fe88Zr8−xPrxB4 and Fe88Zr8−xNdxB4 AAs. The nearly linear relationship between the Tc and the de Gennes factor of Sm, Nd, and Pr when x = 2 and x = 4 demonstrated that the Tc of the Fe88Zr8−xRExB4 AAs was determined by the concentration and G value of the RE.
- (c)
- Although the −ΔSmpeak of the Fe88Zr8B4 AA was improved by the Sm substitution, the −ΔSmpeak of the Fe88Zr8−xSmxB4 was lower than that of the Fe88Zr8−xPrxB4 and Fe88Zr8−xNdxB4 amorphous ribbons when x = 2 and x = 4, respectively. The slightly lower −ΔSmpeak of the Sm-doped AAs was closely related to their lower µeff according to the roughly linear relationship between the −ΔSmpeak and the µeff of the Fe88Zr8−xRExB4 AAs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glanz, J. Making a Bigger Chill with Magnets. Science 1998, 279, 2045. [Google Scholar] [CrossRef]
- Tegus, O.; Bruck, E.; Buschow, K.H.; de Boer, F.R. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 2002, 415, 150–152. [Google Scholar] [CrossRef]
- Yu, B.F.; Gao, Q.; Zhang, B.; Meng, X.Z.; Chen, Z. Review on research of room temperature magnetic refrigeration. Int. J. Refrig. 2003, 26, 622–636. [Google Scholar] [CrossRef]
- Brück, E. Topical review—Developments in magnetocaloric refrigeration. J. Phys. D Appl. Phys. 2005, 38, R381–R391. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Ipus, J.J.; Law, J.Y.; Moreno-Ramírez, L.M.; Conde, A. Magnetocaloric effect: From materials research to refrigeration devices. Prog. Mater. Sci. 2018, 93, 112–232. [Google Scholar] [CrossRef]
- Oliveira, N.; Ranke, P. Theoretical aspects of the magnetocaloric effect. Phys. Rep. 2010, 489, 89–159. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Pecharsky, V.K.; Tsokol, A.O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005, 68, 1479–1539. [Google Scholar] [CrossRef]
- Warburg, E. Magnetische Untersuchungen. Ann. Phys. 1881, 249, 141–164. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Giant magnetocaloric effect in Gd5(Si2Ge2). Phys. Rev. Lett. 1997, 78, 4494–4497. [Google Scholar] [CrossRef]
- Shen, B.G.; Sun, J.R.; Hu, F.X.; Zhang, H.W.; Cheng, Z.H. Recent Progress in Exploring Magnetocaloric Materials. Adv. Mater. 2009, 21, 4545–4564. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Tunable magnetic regenerator alloys with a giant magnetocaloric effect for magnetic refrigeration from ~20 to ~290 K. Appl. Phys. Lett. 1997, 70, 3299–3301. [Google Scholar] [CrossRef]
- Pasquale, M.; Sasso, C.P.; Lewis, L.H.; Giudici, L.; Lograsso, T.; Schlagel, D. Magnetostructural transition and magnetocaloric effect in Ni55Mn20Ga25 single crystals. Phys. Rev. B 2005, 72, 094435. [Google Scholar] [CrossRef]
- Miao, X.F.; Wang, W.Y.; Liang, H.X.; Qian, F.J.; Cong, M.Q.; Zhang, Y.J.; Muhammad, A.; Tian, Z.J.; Xu, F. Printing (Mn,Fe)2(P,Si) magnetocaloric alloys for magnetic refrigeration applications. J. Mater. Sci. 2020, 55, 6660–6668. [Google Scholar] [CrossRef]
- Bouzidi, W.; Mliki, N.; Bessais, L. Second-order magnetic transition and low field magnetocaloric effect in nanocrystalline Pr5Co19 compound. J. Electron. Mater. 2018, 47, 2776–2781. [Google Scholar] [CrossRef]
- Hernández-González, E.L.; Watts, B.E.; Palomares-Sánchez, S.A.; Elizalde Galindo, J.T.; Mirabal-García, M. Second-order magnetic transition in La0.67Ca0.33-xSrxMnO3 (x = 0.05, 0.06, 0.07, 0.08). J. Supercond. Nov. Magn. 2016, 29, 2421–2427. [Google Scholar] [CrossRef]
- Álvarez, P.; Sánchez Llamazares, J.L.; Gorria, P.; Blanco, J.A. Enhanced refrigerant capacity and magnetic entropy flattening using a two-amorphous FeZrB(Cu) composite. Appl. Phys. Lett. 2011, 99, 232501. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, W.H. Magnetocaloric effect in rare earth-based bulk metallic glasses. J. Alloys Compd. 2010, 495, 209–216. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kuzuhara, T.; Sahashi, M.; Inomata, K.; Tomokiyo, A.; Yayama, H. New application of complex magnetic materials to the magnetic refrigerant in an Ericsson magnetic refrigerator. J. Appl. Phys. 1987, 62, 3873. [Google Scholar] [CrossRef]
- Bingham, N.S.; Wang, H.; Qin, F.; Peng, H.X.; Sun, J.F.; Franco, V.; Srikanth, H.; Phan, M.H. Excellent magnetocaloric properties of melt-extracted Gd-based amorphous microwires. Appl. Phys. Lett. 2012, 101, 102407. [Google Scholar] [CrossRef]
- Inoue, A. Bulk amorphous and nanocrystalline alloys with high functional properties. Mater. Sci. Eng. A 2001, 304–306, 1–10. [Google Scholar] [CrossRef]
- Greer, A.L.; Rutherford, K.L.; Hutchings, I.M. Wear resistance of amorphous alloys and related materials. Int. Mater. Rev. 2002, 47, 87–111. [Google Scholar] [CrossRef]
- Liu, X.Y.; Barclay, J.A.; Gopal, R.B.; Földeàki, M.; Chahine, R.; Bose, T.K.; Schurer, P.J.; LaCombe, J.L. Thermomagnetic properties of amorphous rare-earth alloys with Fe, Ni, or Co. J. Appl. Phys. 1996, 79, 1630–1641. [Google Scholar] [CrossRef]
- Xue, L.; Shao, L.L.; Luo, Q.; Shen, B.L. Gd25RE25Co25Al25 (RE = Tb, Dy and Ho) high-entropy glassy alloys with distinct spin-glass behavior and good magnetocaloric effect. J. Alloys Compd. 2019, 790, 633–639. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Magnetocaloric effect in Gd-based bulk metallic glasses. Appl. Phys. Lett. 2006, 89, 81914. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Q.; Shen, B.L. The effect of Fe/Al ratio on the thermal stability and magnetocaloric effect of Gd55FexAl45-x (x=15–35) glassy ribbons. J. Appl. Phys. 2012, 111, 07A937. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Zhong, X.C.; Su, K.P.; Yu, H.Y.; Liu, Z.W.; Zeng, D.C. Magnetic properties and large magnetocaloric effects in amorphous Gd-Al-Fe alloys for magnetic refrigeration. Sci. China Phys. Mech. Astron. 2011, 54, 1267–1270. [Google Scholar] [CrossRef]
- Liu, G.L.; Zhao, D.Q.; Bai, H.Y.; Wang, W.H.; Pan, M.X. Room temperature table-like magnetocaloric effect in amorphous Gd50Co45Fe5 ribbon. J. Phys. D Appl. Phys. 2016, 49, 055004. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, J.Z.; Xia, L. Effect of boron on the magneto-caloric effect in Fe91-xZr9Bx (x = 3, 4, 5) amorphous alloys. J. Mater. Sci. 2017, 52, 13948–13955. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Bi, X.F. The role of Zr and B in room temperature magnetic entropy change of FeZrB amorphous alloys. Appl. Phys. Lett. 2009, 95, 262501. [Google Scholar] [CrossRef]
- Álvarez, P.; Gorria, P.; Sánchez, M.; Barquín, L.F.; Blanco, J.A. The role of boron on the magneto-caloric effect of FeZrB metallic glasses. Intermetallics 2010, 18, 2464–2467. [Google Scholar] [CrossRef]
- Wu, Y.B.; Wang, Q.; Tang, B.Z.; Pan, L.L.; Ding, D.; Xia, L. Outstanding glass formability and magneto-caloric effect of a Fe85Co3Zr5B4Nb3 metallic glass. J. Non-Cryst. Solids 2021, 566, 120885. [Google Scholar] [CrossRef]
- Zheng, X.N.; Wang, Q.; Yue, C.Y.; Li, A.L.; Ding, D.; Xia, L. Achieving higher magnetic entropy change peak at lower temperature by minor Ti substitution for Zr in the Fe88Zr8B4 metallic glass. Mod. Phys. Lett. B 2023, 37, 2350032. [Google Scholar] [CrossRef]
- Li, A.L.; Wang, Q.; Tang, B.Z.; Yu, P.; Ding, D.; Xia, L. Magnetocaloric effect of the Fe87M8B5 (M = Zr, Ce) amorphous alloys. Mater. Sci. Eng. B Adv. 2022, 286, 116033. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, D.; Tang, B.Z.; Yu, P.; Chan, K.C.; Xia, L. Excellent magnetocaloric performance of a Fe88Zr4Pr4B4 amorphous alloy and its amorphous hybrids. Intermetallics 2023, 161, 107982. [Google Scholar] [CrossRef]
- Wang, P.J.; Wang, Q.; Zheng, S.H.; Zhu, L.Z.; Ding, D.; Tang, B.Z.; Yu, P.; Yao, J.L.; Xia, L. Improvement of Curie temperature and magnetic entropy change of a Fe88Zr8B4 metallic glass by minor Nd substitution. J. Non-Cryst. Solids 2023, 611, 122347. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 45, 279–306. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A.; Masumoto, T. Amorphous Zr–Al–TM (TM=Co, Ni, Cu) Alloys with Significant Supercooled Liquid Region of Over 100 K. Mater. Trans. JIM 1991, 32, 1005–1010. [Google Scholar] [CrossRef]
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. Glass formation criterion for various glass-forming systems. Phys. Rev. Lett. 2003, 91, 115505. [Google Scholar] [CrossRef]
- Minouei, H.; Rizi, M.S.; Akbari, G.H.; Hong, S.I. Effect of residual nanocrystals on thermal stability and mechanical properties of metalloid-containing amorphous alloys. Mater. Charact. 2021, 173, 110914. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, Q.; Tang, B.Z.; Zhou, X.; Ding, D.; Xia, L. Achieve good magneto-caloric response near the ambient temperature in a Fe86La7B5Ce2 amorphous ribbon. J. Magn. Magn. Mater. 2022, 547, 168954. [Google Scholar] [CrossRef]
- Hashimoto, T.; Numasawa, T.; Shino, M.; Okada, T. Magnetic refrigeration in the temperature range from 10 K to room temperature: The ferromagnetic refrigerants. Cryogenics 1981, 21, 647–653. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Conde, A. Field dependence of the magnetocaloric effect in materials with a second order phase transition: A master curve for the magnetic entropy change. Appl. Phys. Lett. 2006, 88, 222512. [Google Scholar] [CrossRef]
- Du, J.; Zheng, Q.; Brück, E.; Buschow, K.H.J.; Cui, W.B.; Feng, W.J.; Zhang, Z.D. Spin-glass behavior and magnetocaloric effect in Tb-based bulk metallic glass. J. Magn. Magn. Mater. 2009, 321, 413–417. [Google Scholar] [CrossRef]
- Luo, Q.; Schwarz, B.; Mattern, N.; Eckert, J. Irreversible and reversible magnetic entropy change in a Dy-based bulk metallic glass. Intermetallics 2012, 30, 76–79. [Google Scholar] [CrossRef]
- Luo, Q.; Schwarz, B.; Mattern, N.; Eckert, J. Giant irreversible positive to large reversible negative magnetic entropy change evolution in Tb-based bulk metallic glass. Phys. Rev. B 2010, 82, 024204. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Tan, J.; Zhang, X.; Yan, J.Z.; Shi, H.; Zhu, Y.; Cheng, W.Z.; Li, H.L.; Li, W.H.; Xia, A.L. Correlation between Magnetocaloric Properties and Magnetic Exchange Interaction in Gd54Fe36B10-xSix Amorphous Alloys. Materials 2023, 16, 3629. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Li, R.; Zhang, L.L.; Zhang, T. Tunable magnetic and magnetocaloric properties in heavy rare-earth based metallic glasses through the substitution of similar elements. J. Appl. Phys. 2014, 115, 133903. [Google Scholar] [CrossRef]
- Matsumoto, N.; Shimosaka, T. Validation of a quantitative analytical method based on the effective magnetic moment and the Curie–Weiss law. Accred. Qual. Assur. 2015, 20, 115–124. [Google Scholar] [CrossRef]


| Composition | Tgonset (K) | Txonset (K) | Tl (K) | ∆Tx (K) | Trg | γ | Zc (mm) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Fe88Zr6Sm2B4 | 710 | 861 | 1566 | 151 | 0.453 | 0.378 | 1.96 | Present work |
| Fe88Zr4Sm4B4 | 716 | 863 | 1498 | 147 | 0.478 | 0.390 | 3.24 | |
| Fe88Zr8B4 | 787 | 840 | 1611 | 53 | 0.489 | 0.35 | 0.61 | [31] |
| Fe87Co1Zr8B4 | 776 | 834 | 1580 | 58 | 0.491 | 0.354 | 0.72 | |
| Fe86Co2Zr8B4 | 780 | 835 | 1574 | 55 | 0.496 | 0.355 | 0.75 | |
| Fe87Zr7B4Co2 | 754 | 808 | 1570 | 54 | 0.48 | 0.348 | 0.56 | |
| Fe85Co3Zr5B4Nb3 | 701 | 813 | 1495 | 112 | 0.469 | 0.370 | 1.41 | |
| Fe87Zr8B4Sm1 | 828 | 859 | 1582 | 31 | 0.52 | 0.356 | 0.78 | |
| Fe86Zr8B4Sm2 | 818 | 870 | 1580 | 52 | 0.52 | 0.363 | 1.05 | |
| Fe85Zr8B4Sm3 | 828 | 883 | 1578 | 55 | 0.53 | 0.367 | 1.24 | |
| Fe88Zr6B4Ti2 | 752 | 812 | 1539 | 60 | 0.489 | 0.354 | 0.72 | [32] |
| Fe88Zr6Pr2B4 | 757 | 866 | 1509 | 109 | 0.482 | 0.377 | 1.88 | [34] |
| Fe88Zr4Pr4B4 | 768 | 865 | 1527 | 97 | 0.503 | 0.372 | 1.53 |
| Composition | −ΔSmpeak (J/(kg × K)) | Tc (K) | Refs. | |||||
|---|---|---|---|---|---|---|---|---|
| 1 T | 1.5 T | 2 T | 3 T | 4 T | 5 T | |||
| Fe88Zr6Sm2B4 a | 1.07 | 1.45 | 1.79 | 2.42 | 3.00 | 3.53 | 317 | Present work |
| Fe88Zr4Sm4B4 a | 1.14 | 1.54 | 1.92 | 2.59 | 3.21 | 3.79 | 348 | |
| Fe88Zr8B4 | 0.87 | 1.20 | 1.50 | 2.06 | 2.57 | 3.04 | 292 | [32] |
| Fe88Zr6Nd2B4 | 1.09 | 1.49 | 1.84 | 2.50 | 3.09 | 3.65 | 314 | [35] |
| Fe88Zr4Nd4B4 | 1.20 | 1.65 | 2.05 | 2.79 | 3.47 | 4.10 | 335 | |
| Fe88Zr6Pr2B4 | 1.07 | 1.47 | 1.82 | 2.46 | 3.05 | 3.60 | 306 | [34] |
| Fe88Zr4Pr4B4 | 1.20 | 1.63 | 2.02 | 2.74 | 3.39 | 4.00 | 323 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-R.; Liu, X.-J.; Zhang, H.-T.; Wang, Q.; Ding, D.; Tang, B.-Z.; Yu, P.; Yao, J.-L.; Xia, L. Mechanism for Improved Curie Temperature and Magnetic Entropy Change in Sm-Doped Fe88Zr8B4 Amorphous Alloys. Materials 2023, 16, 7274. https://doi.org/10.3390/ma16237274
Zhang Z-R, Liu X-J, Zhang H-T, Wang Q, Ding D, Tang B-Z, Yu P, Yao J-L, Xia L. Mechanism for Improved Curie Temperature and Magnetic Entropy Change in Sm-Doped Fe88Zr8B4 Amorphous Alloys. Materials. 2023; 16(23):7274. https://doi.org/10.3390/ma16237274
Chicago/Turabian StyleZhang, Zhe-Rui, Xiang-Jie Liu, He-Teng Zhang, Qiang Wang, Ding Ding, Ben-Zhen Tang, Peng Yu, Jin-Lei Yao, and Lei Xia. 2023. "Mechanism for Improved Curie Temperature and Magnetic Entropy Change in Sm-Doped Fe88Zr8B4 Amorphous Alloys" Materials 16, no. 23: 7274. https://doi.org/10.3390/ma16237274
APA StyleZhang, Z.-R., Liu, X.-J., Zhang, H.-T., Wang, Q., Ding, D., Tang, B.-Z., Yu, P., Yao, J.-L., & Xia, L. (2023). Mechanism for Improved Curie Temperature and Magnetic Entropy Change in Sm-Doped Fe88Zr8B4 Amorphous Alloys. Materials, 16(23), 7274. https://doi.org/10.3390/ma16237274







