Influence of HCl Concentration on Corrosion Behavior between Au or Cu Bonding Wires and the Bond Pad for Semiconductor Packaging
Abstract
:1. Introduction
2. Materials and Methods
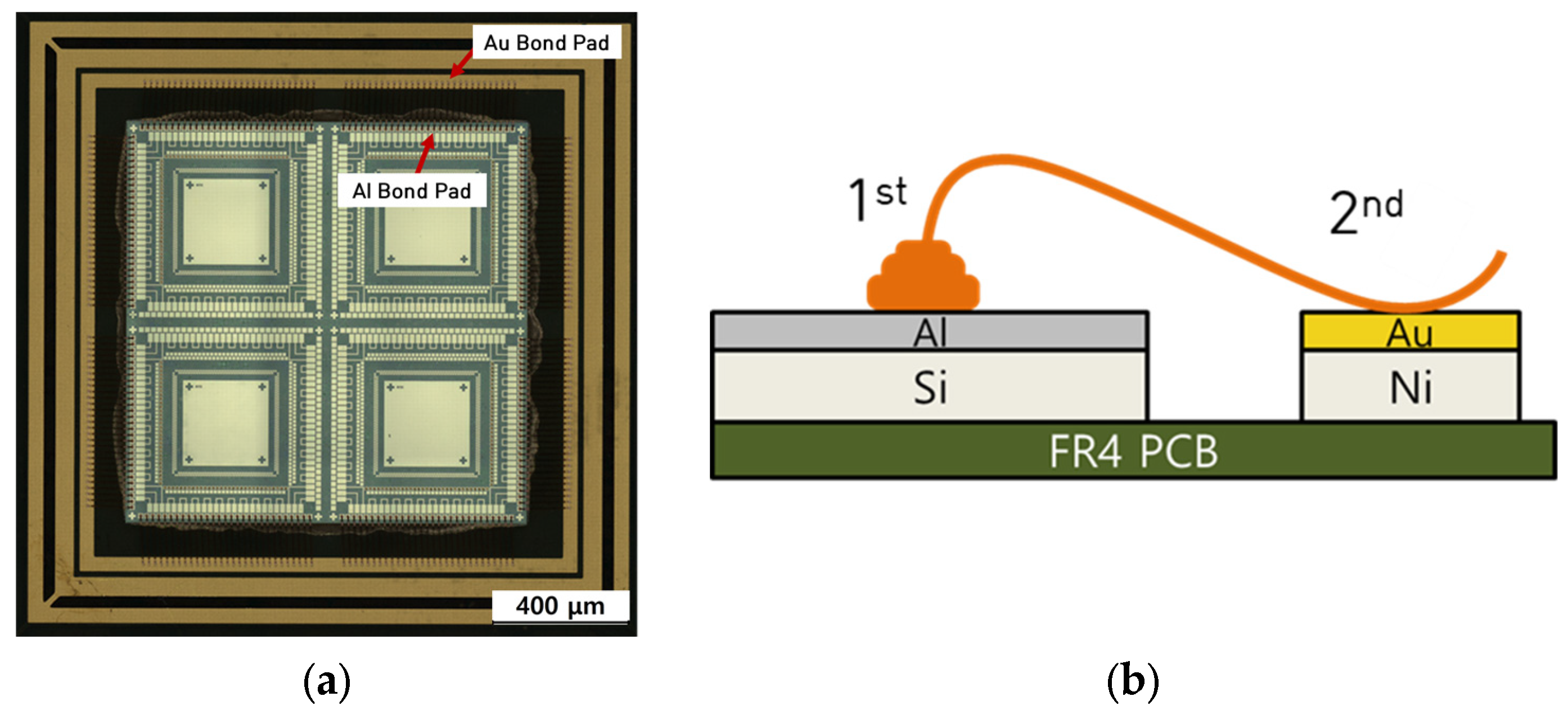
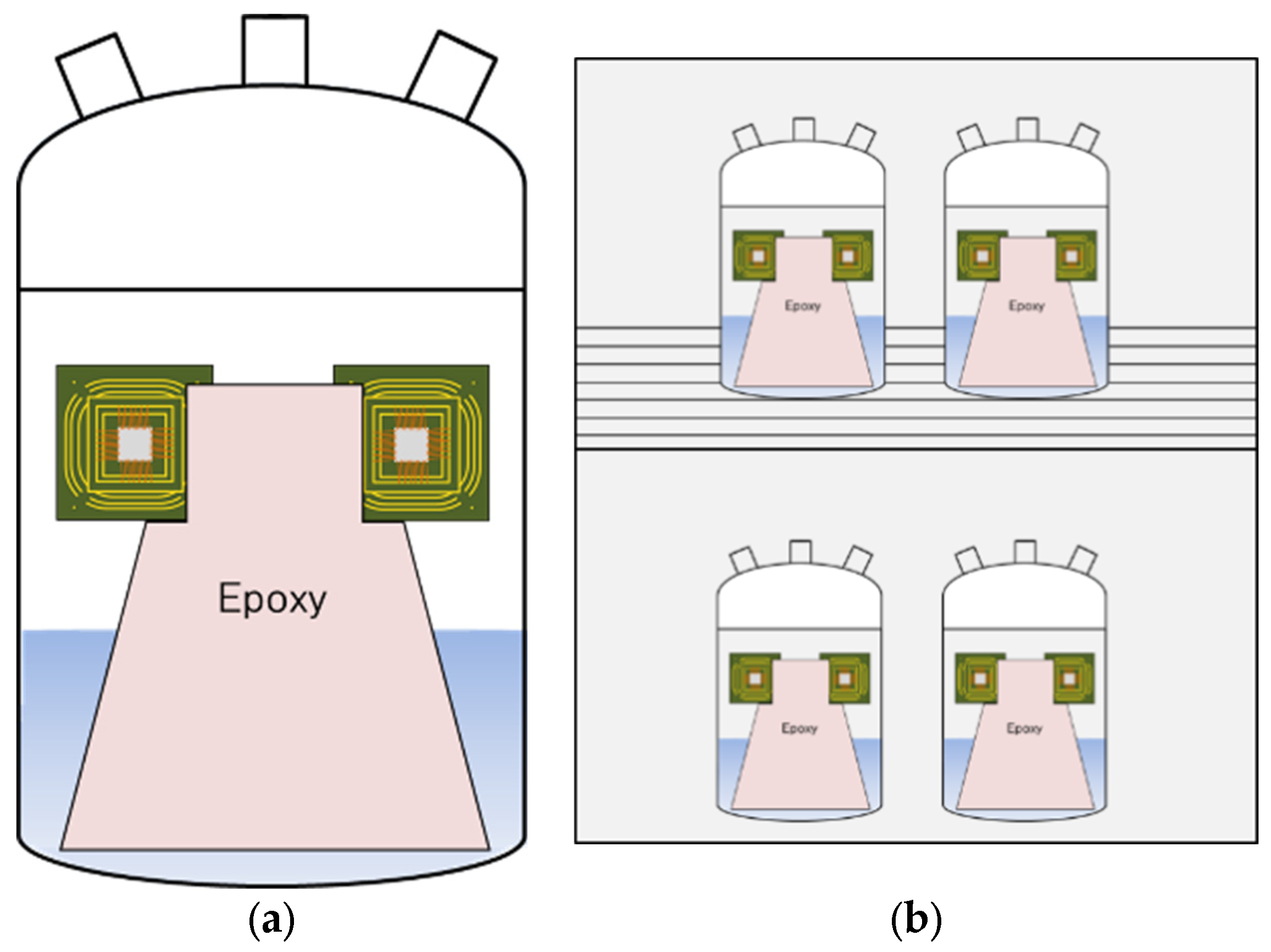
2.1. Test Specimen
2.2. Polarization Test
2.3. Electrochemical Galvanic Corrosion Test
2.4. Temperature Humidity Test (THT)
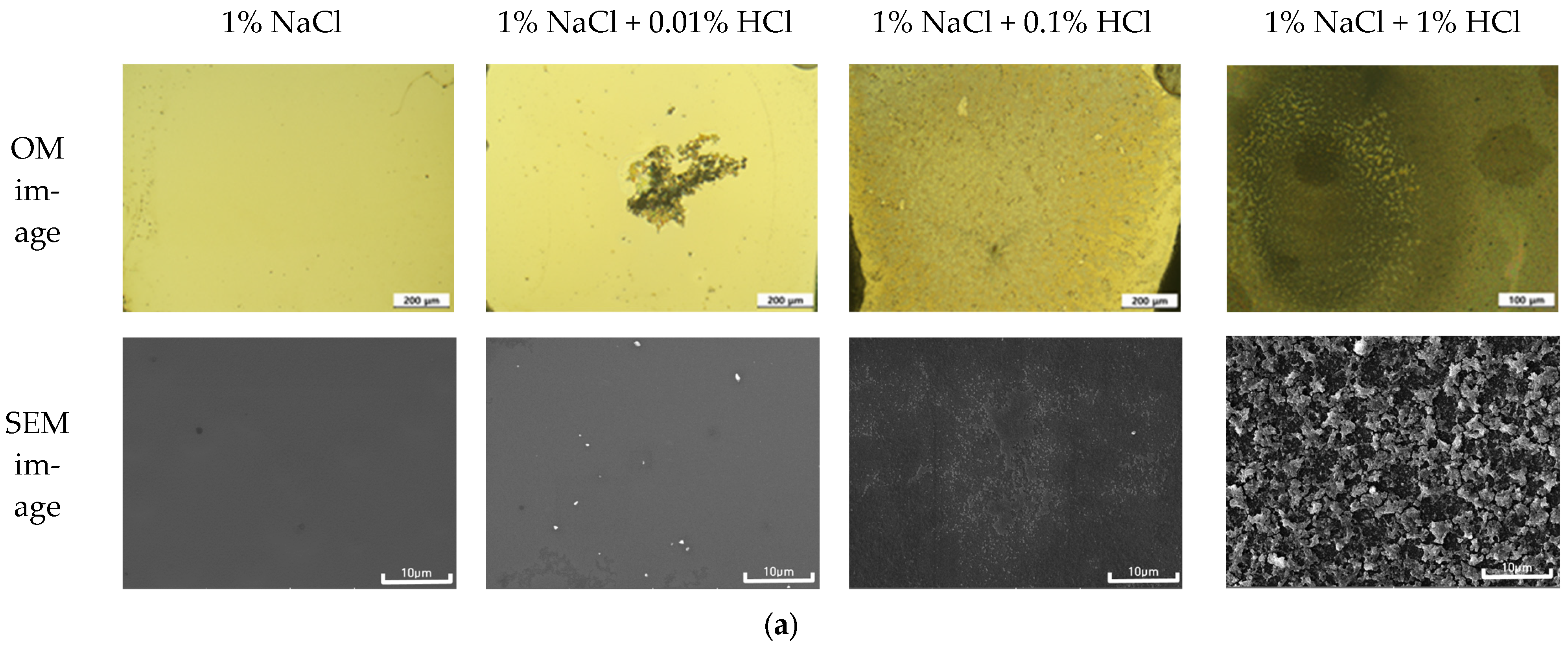
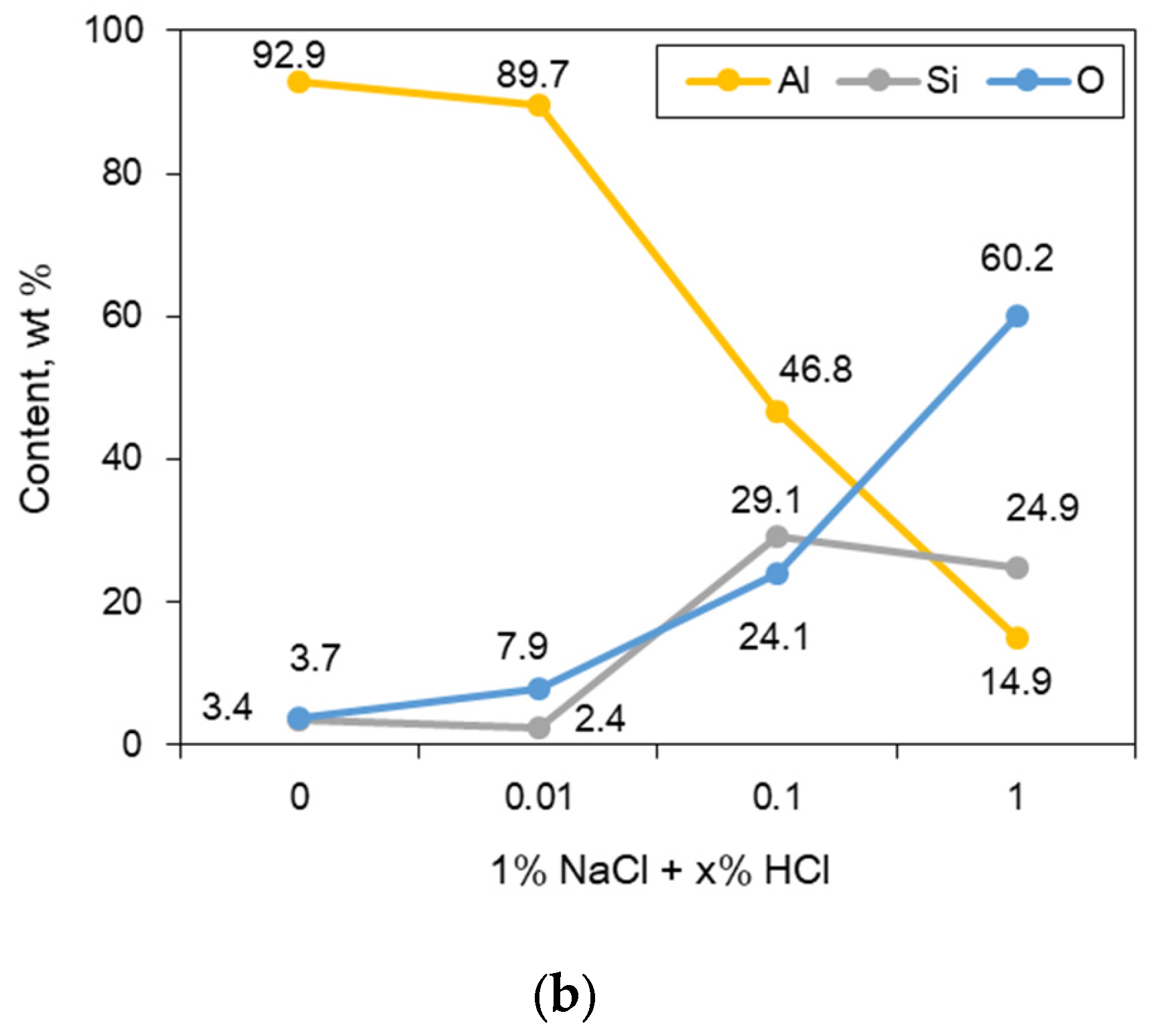
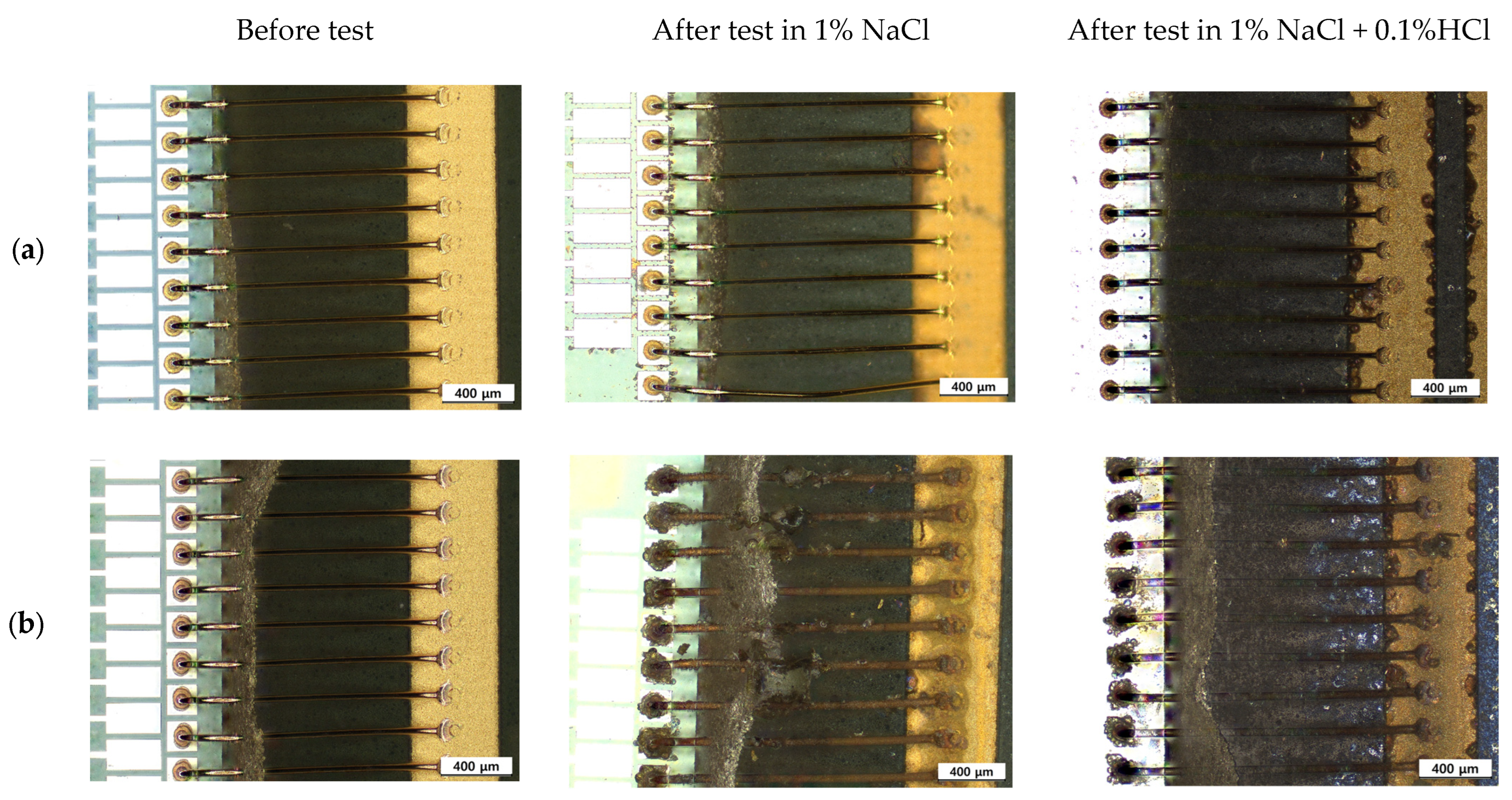
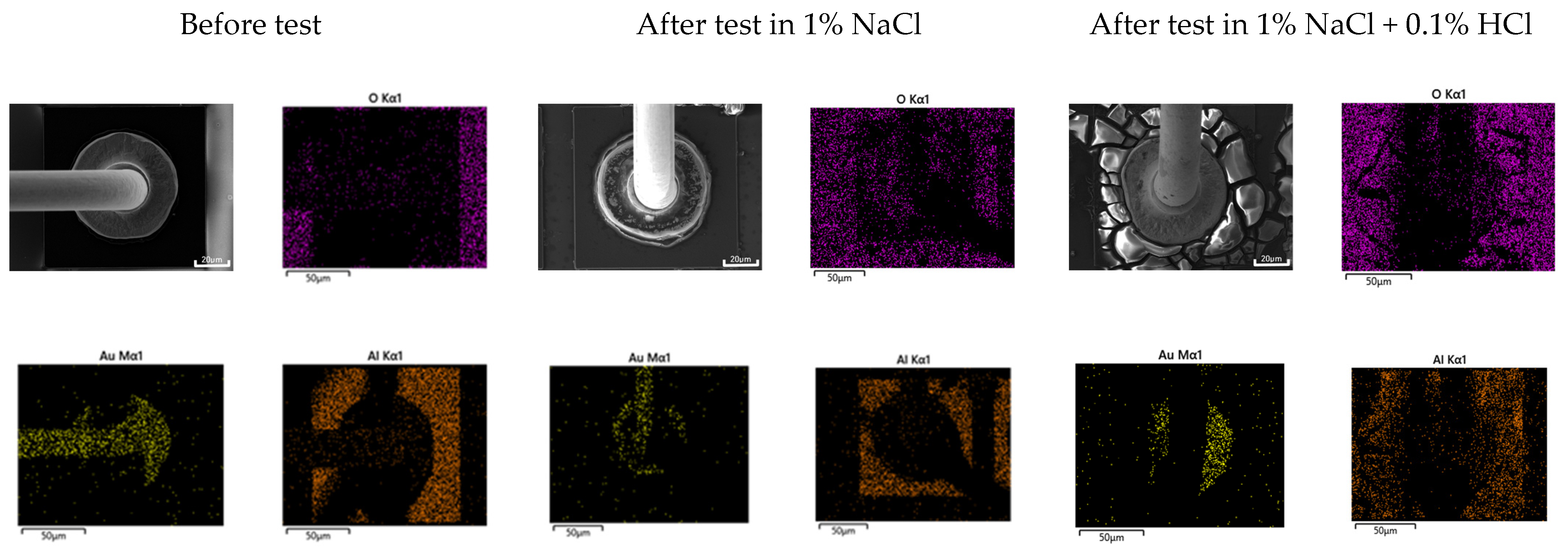
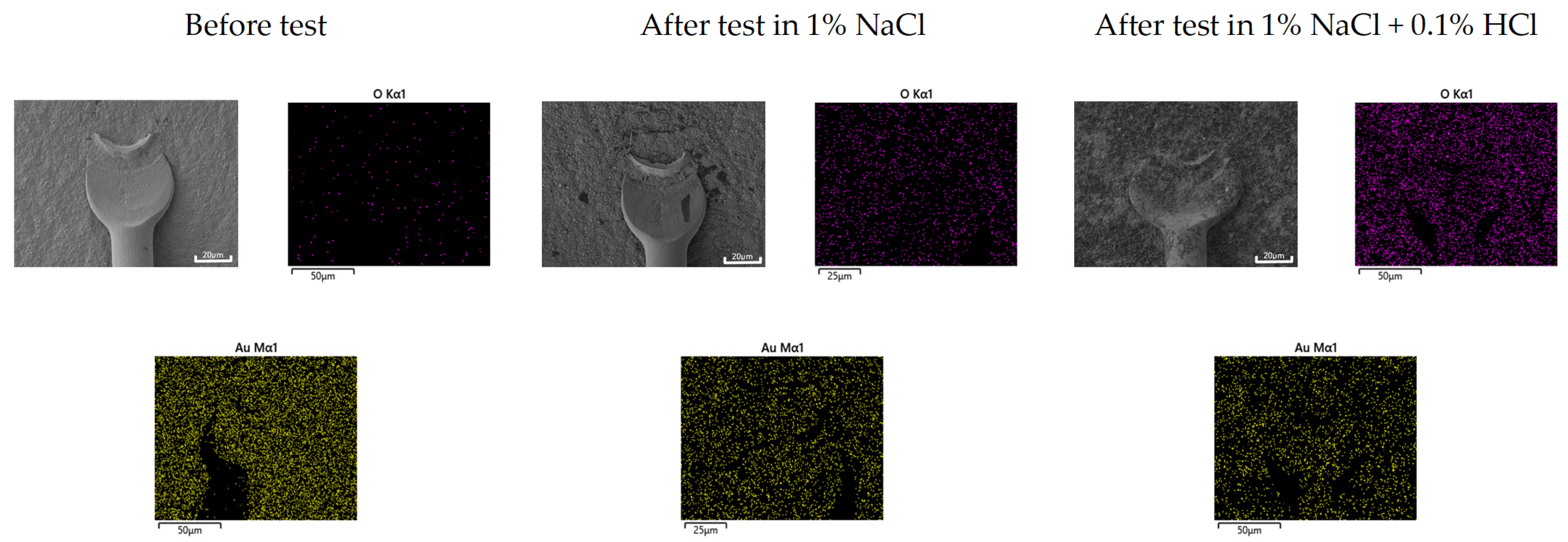
2.5. Optical and Three-Dimensional Microscopic Observation and SEM-EDS Analysis
2.6. X-ray Diffraction (XRD) Analysis
3. Results and Discussion
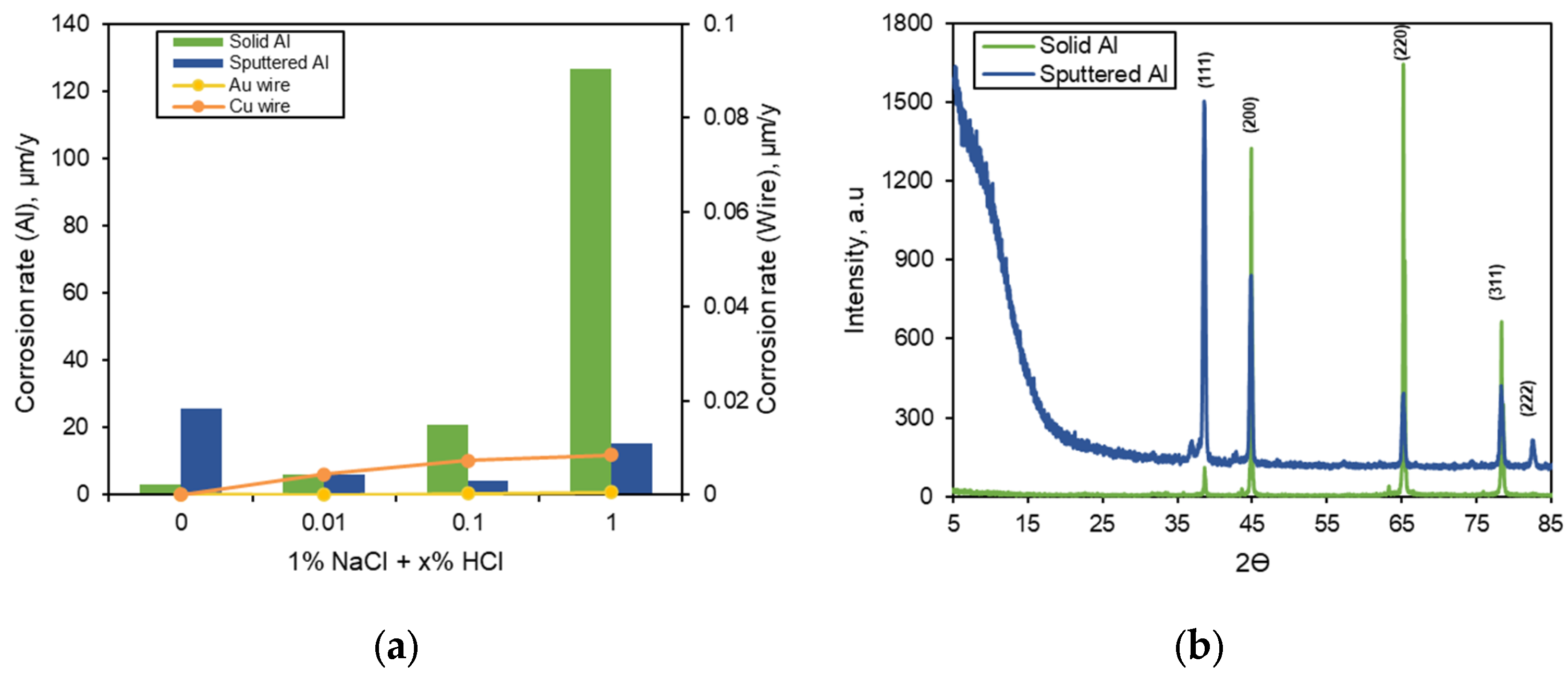
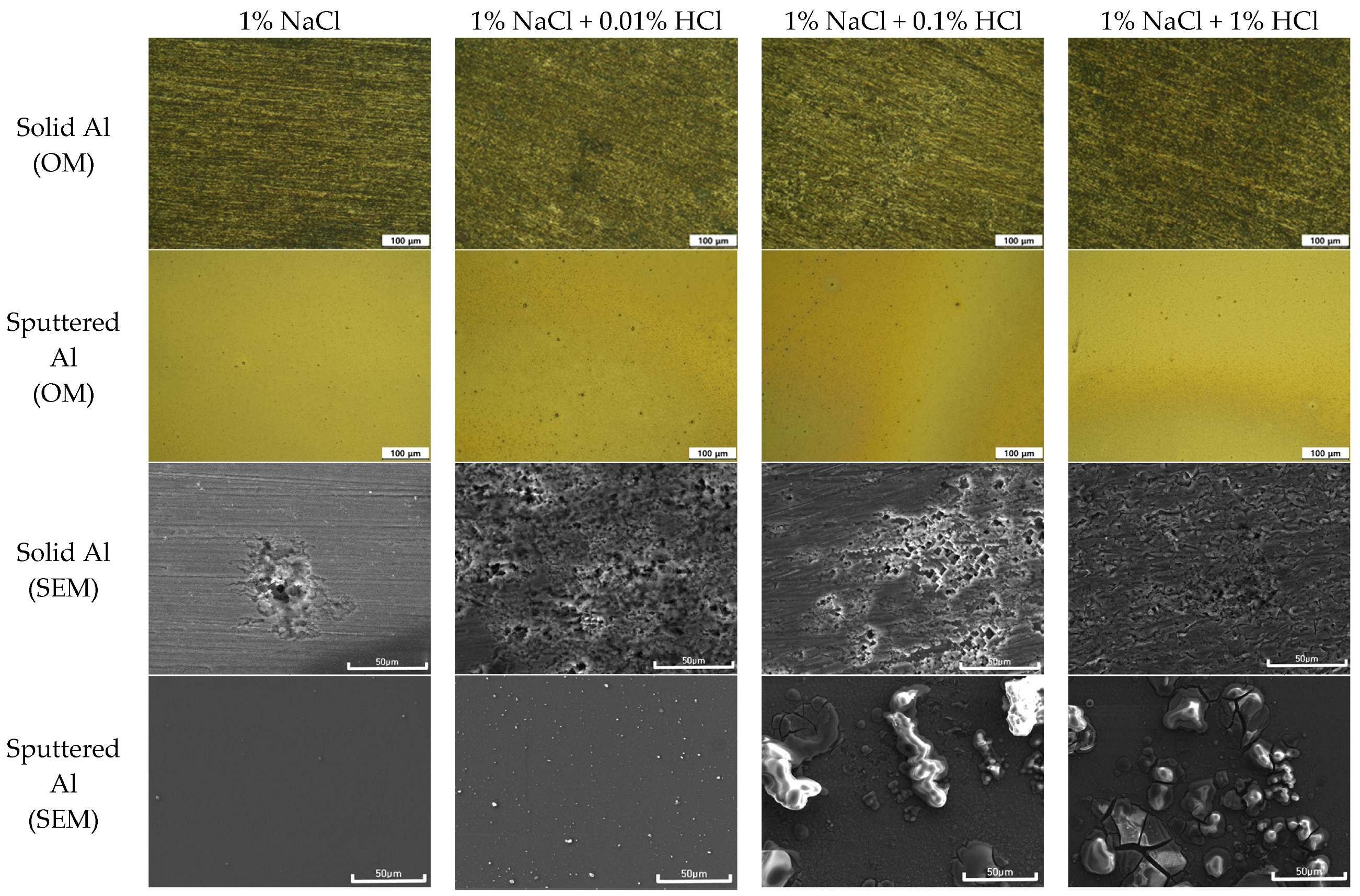
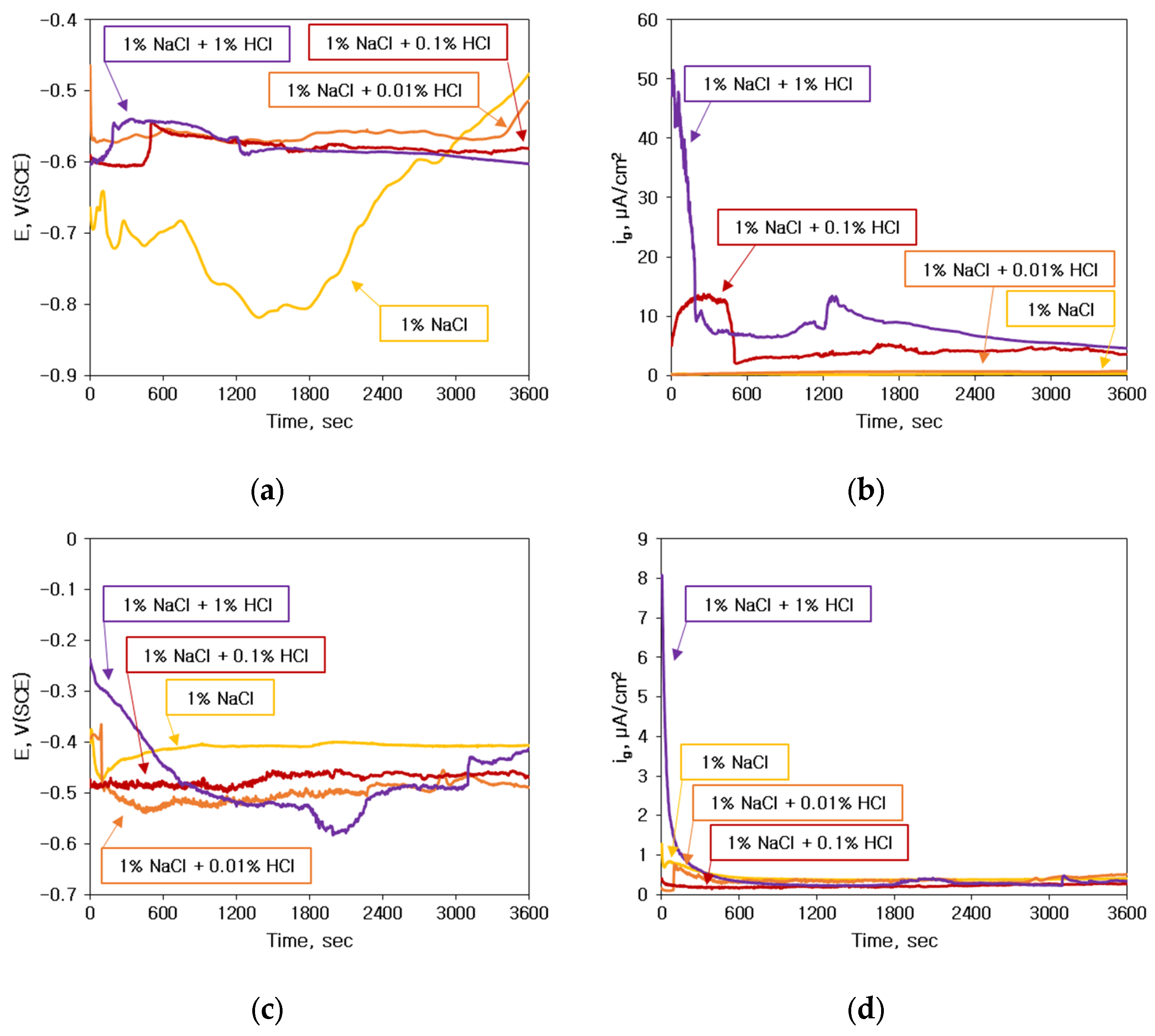
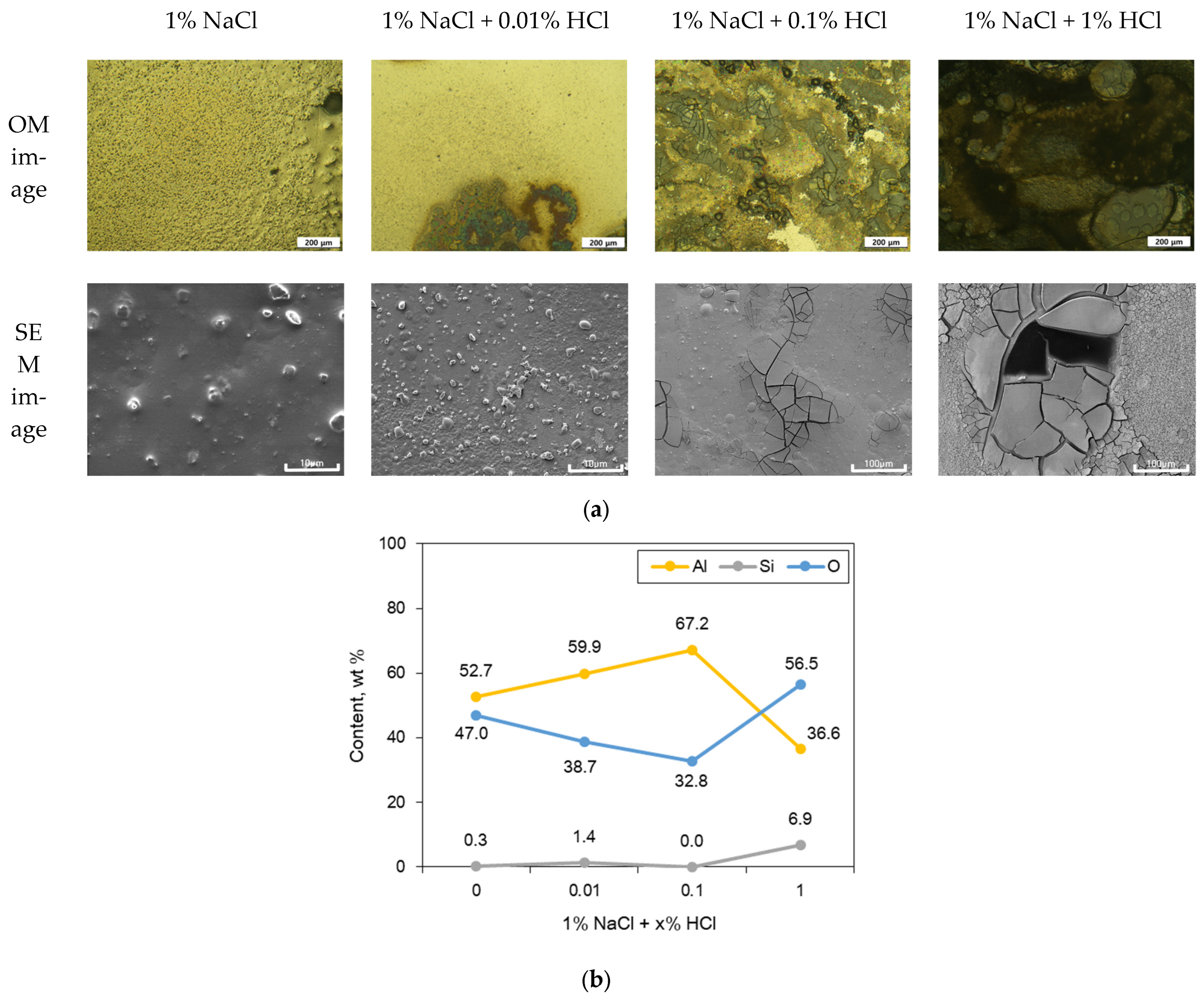
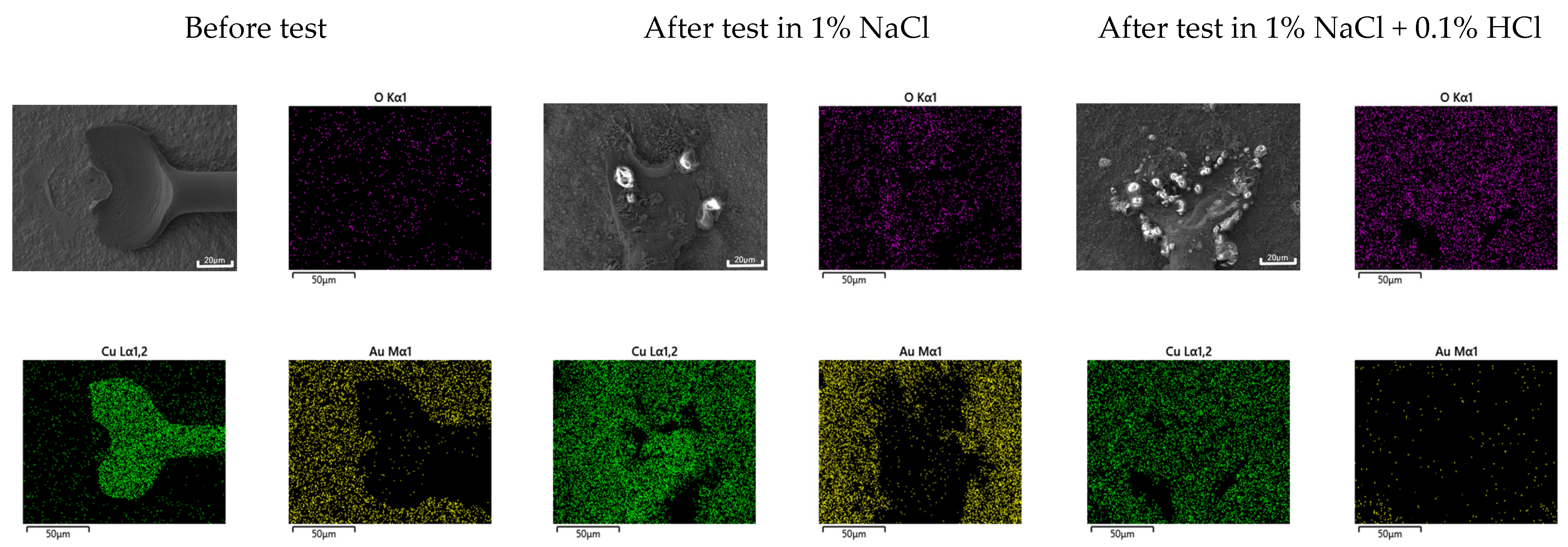
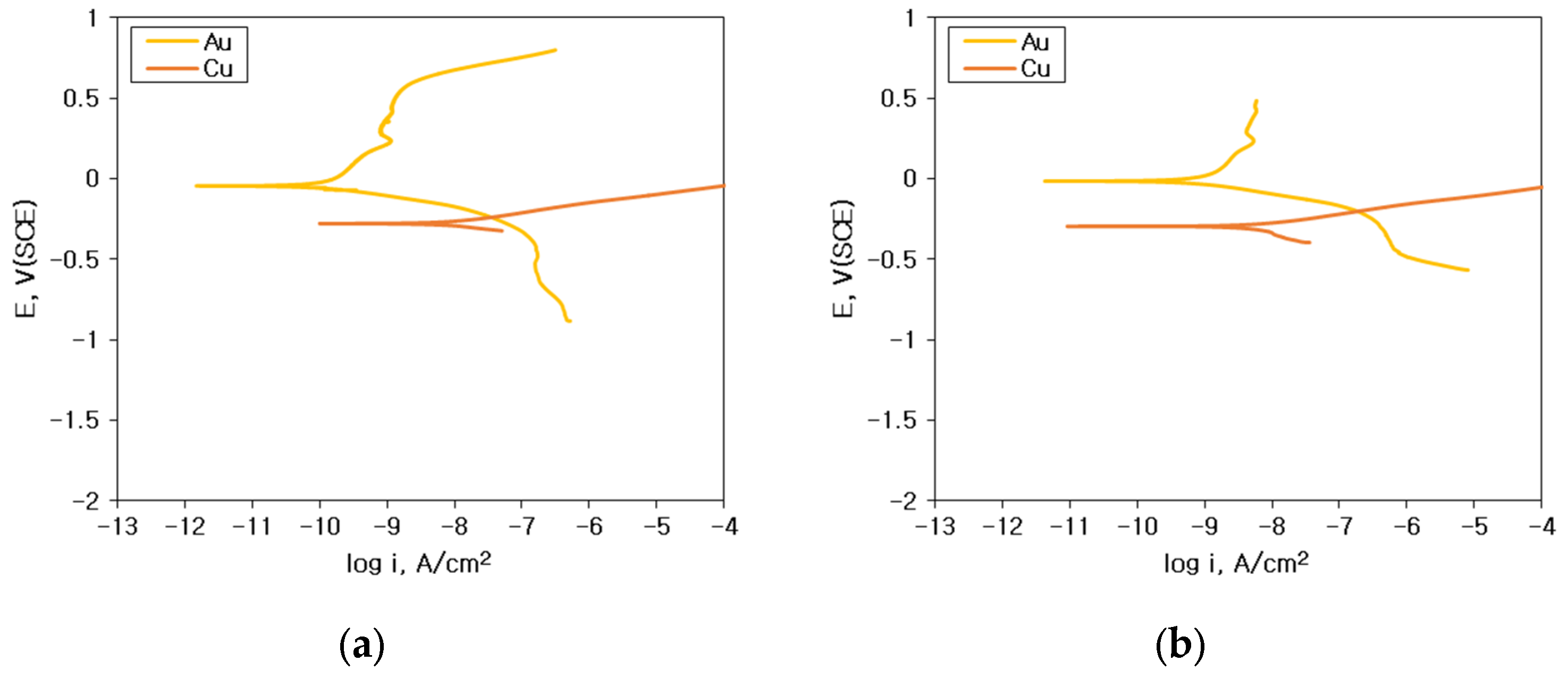
3.1. Corrosion Behavior of Single Specimen
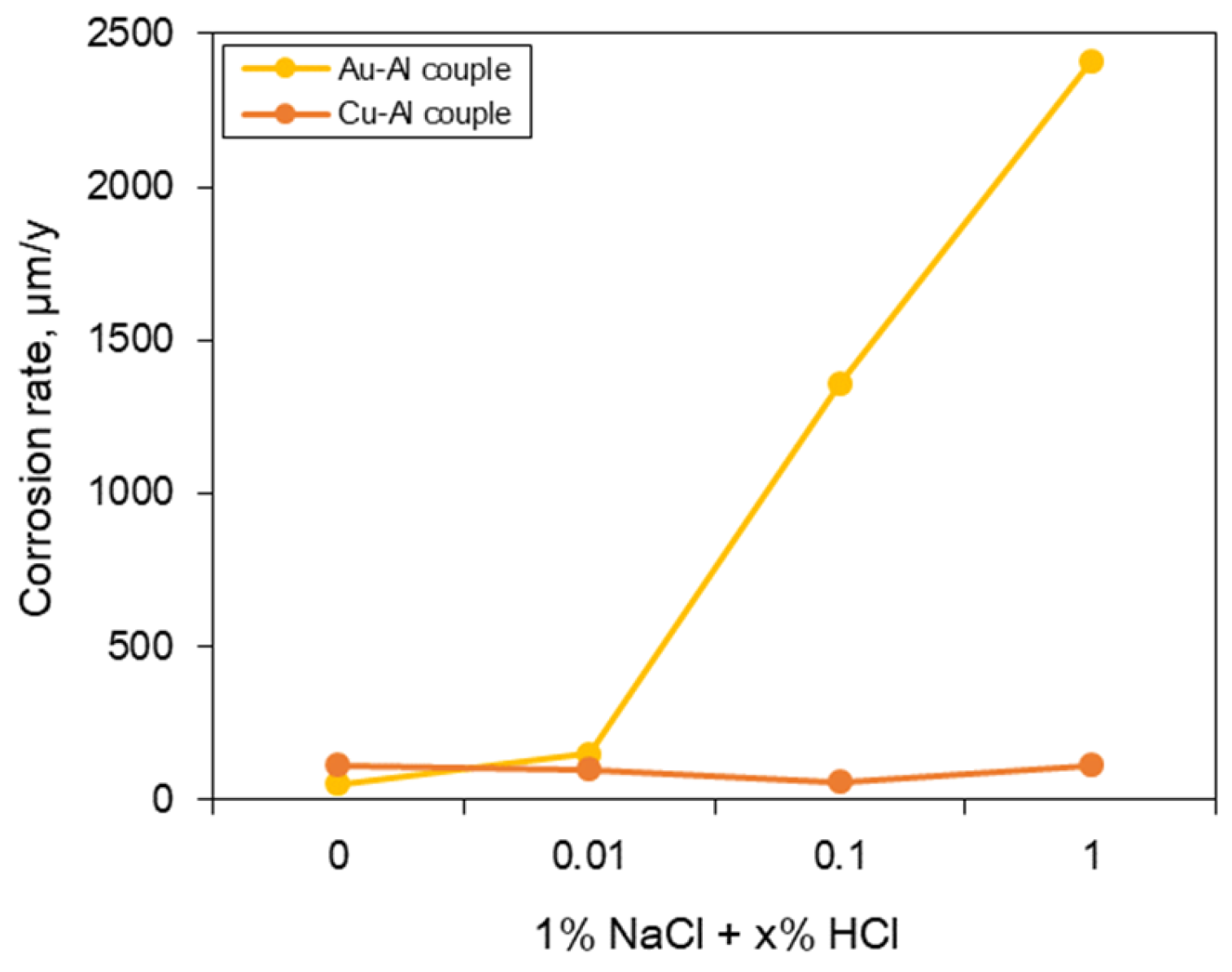
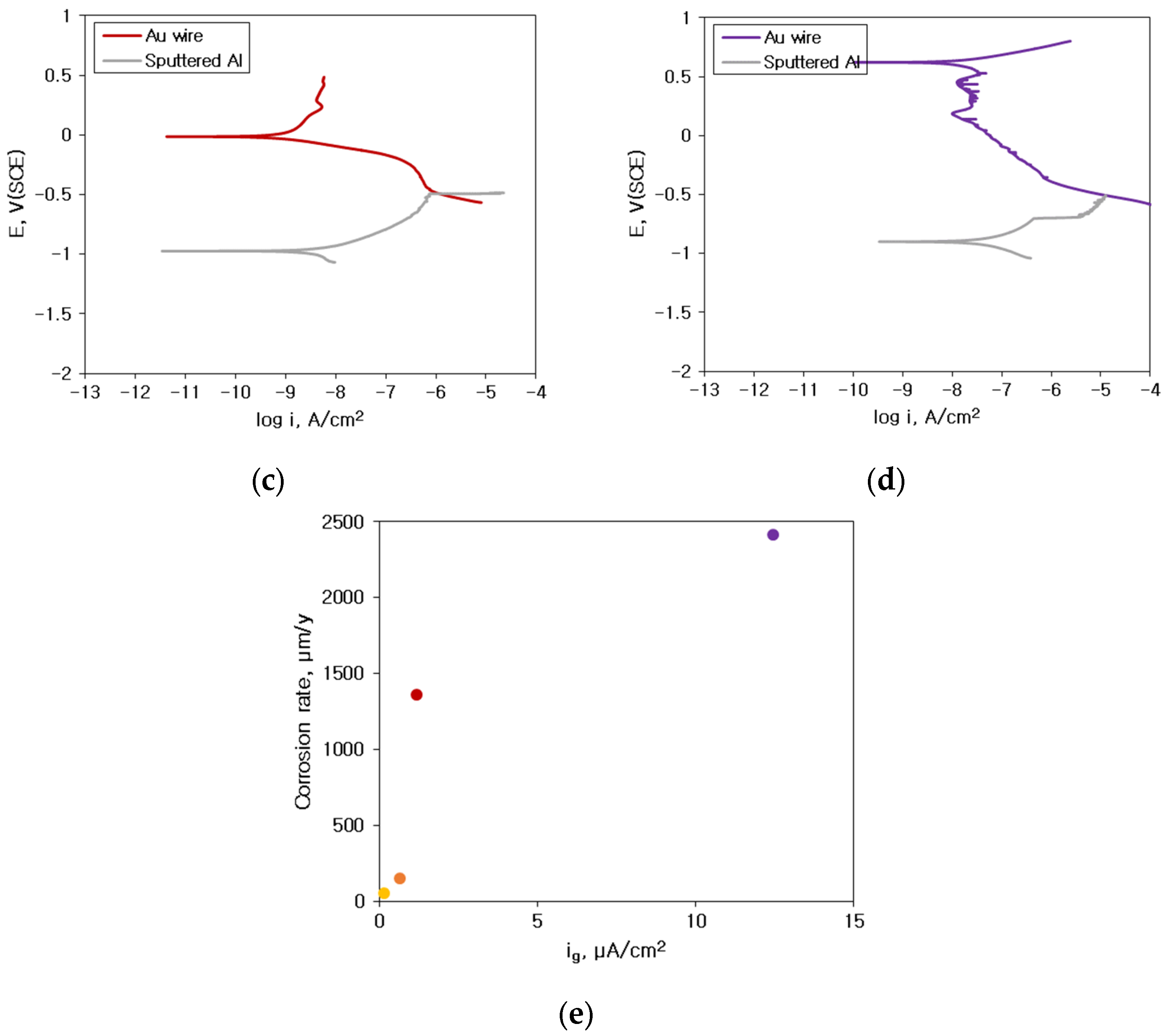
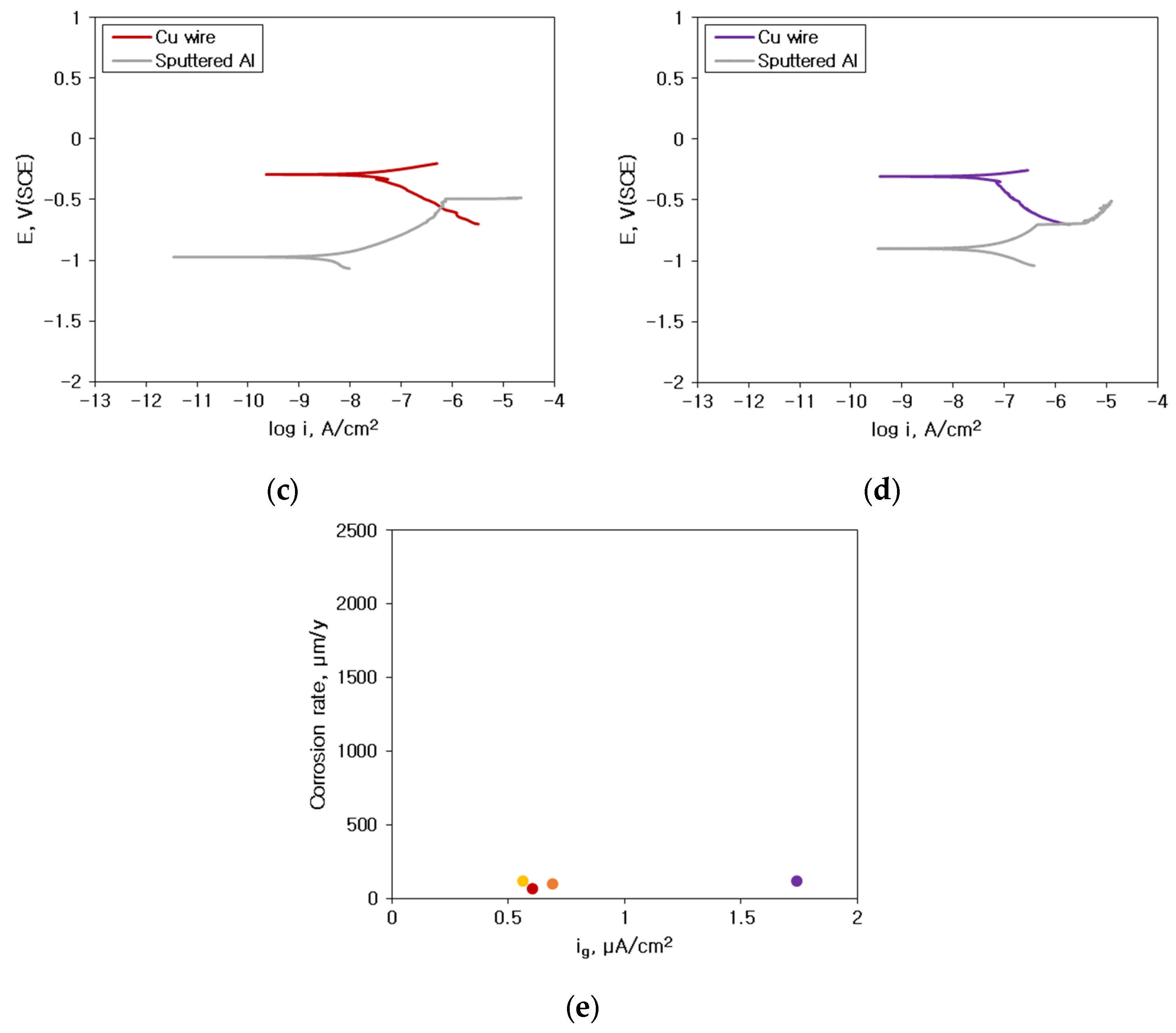
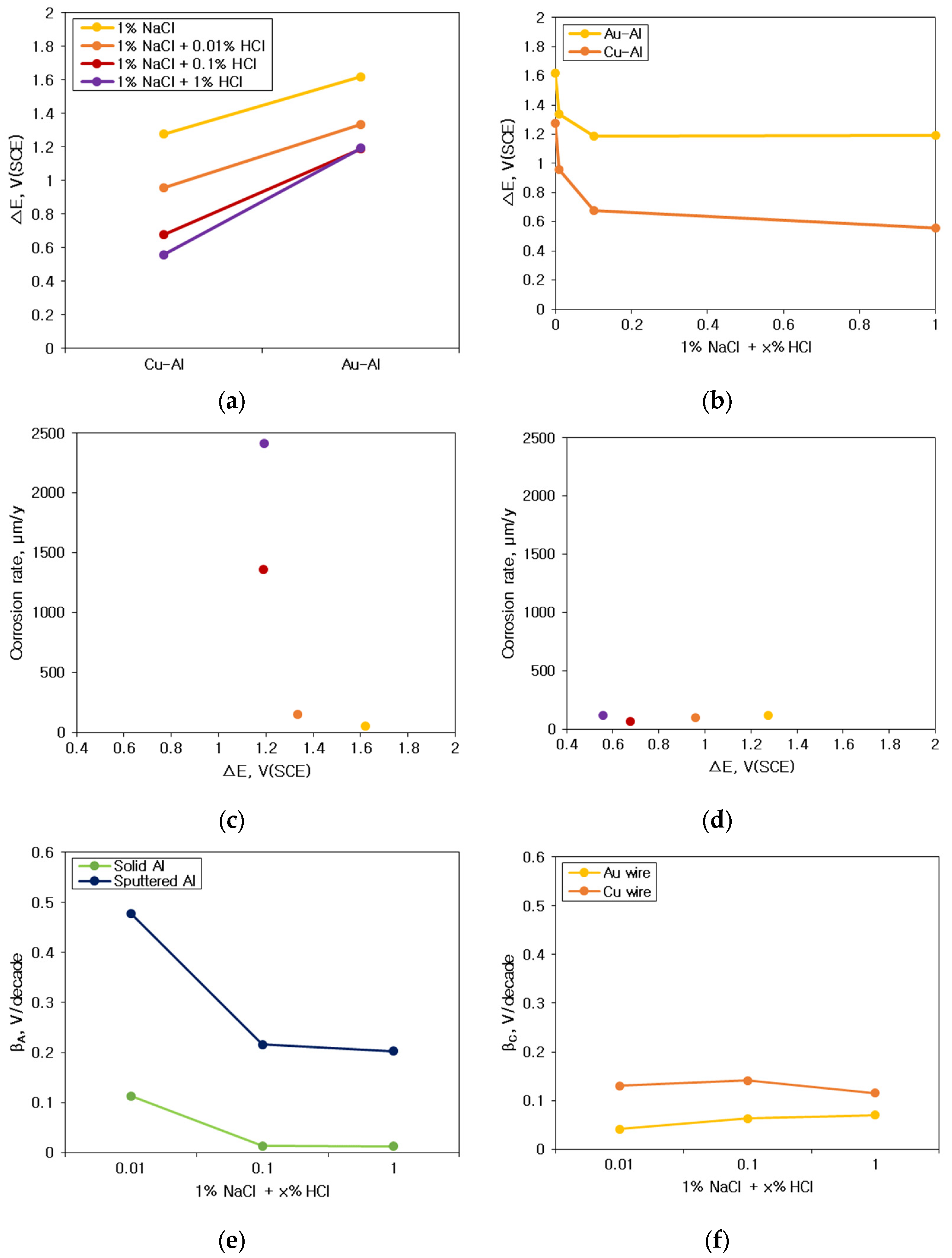
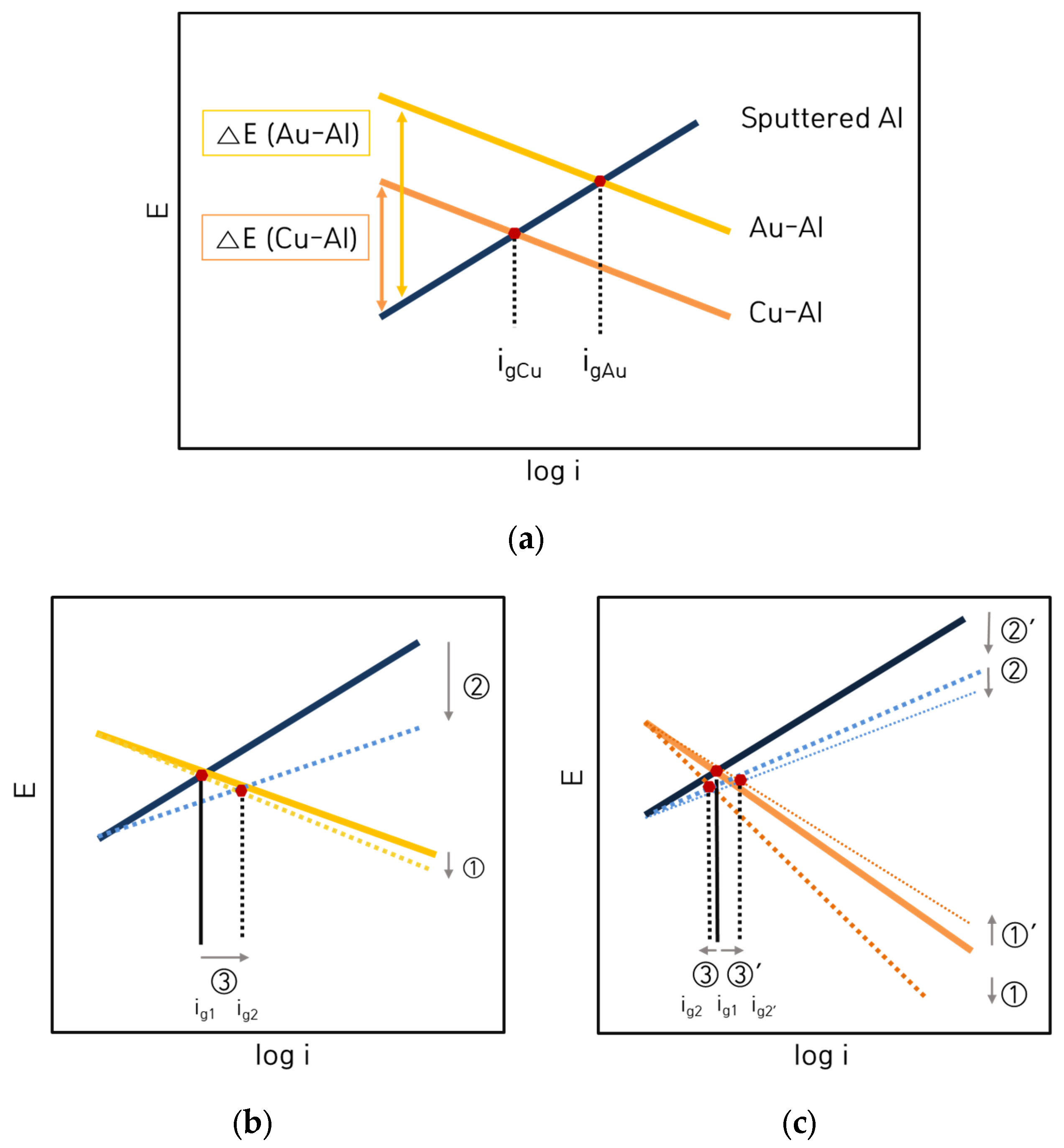
3.2. Galvanic Corrosion Behavior between Au or Cu Wires and Al Pad
3.3. Galvanic Corrosion Behavior in First and Second Bond Areas on the PCB
4. Conclusions
- The corrosion rate of the sputtered Al with HCl concentration was much lower than that of solid Al. This difference in corrosion rate is attributable to the presence of some amorphous structure in sputtered Al specimen. However, the corrosion rate of sputtered Al galvanically coupled with Au or Cu wires was greatly higher than that of single sputtered Al.
- The galvanic corrosion rate of the galvanic coupled Al bond pad with Au or Cu wires was closely related to the potential difference in the same corrosion environment. On the other hand, the corrosion behavior of the same galvanic couples with different corrosion environments was affected by the anodic and cathodic Tafel slopes instead of the potential difference.
- Galvanic corrosion tendency in the first bond and second bond areas of the PCB unit specimen depended upon the passive or transpassive or active states of the anodic material which encountered the cathodic materials in any corrosive environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, P.Y.; Lai, P.Y.; Ye, Z.J.; Hsieh, C.L. Effect of Pd distribution on Pd-plated Cu wire using different electronic flame off settings. J. Mater. Sci. Mater. Electron. 2017, 28, 4613–4618. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, J.W.; Koo, J.M.; Ha, S.S.; Noh, B.I.; Moon, W.C.; Moon, J.H.; Jung, S.B. Flip-chip Bonding Technology and Reliability of Electronic Packaging. J. Weld. Join. 2007, 25, 6–15. [Google Scholar] [CrossRef]
- Uno, T. Bond reliability under humid environment for coated copper wire and bare copper wire. Microelectron. Reliab. 2011, 51, 148–156. [Google Scholar] [CrossRef]
- Qin, I.; Yauw, O.; Schulze, G.; Shah, A.; Chylak, B.; Wong, N. Advances in Wire Bonding Technology for 3D Die Stacking and Fan Out Wafer Level Package. In Proceedings of the 2017 IEEE 67th Electronic Components and Technology Conference, Orlando, FL, USA, 30 May–2 June 2017. [Google Scholar] [CrossRef]
- Qin, W.; Anderson, H.; Anderson, T.; Chang, G.; Barrientos, D. Corrosion Mechanisms of Cu Wire Bonding on Al Pads. In Proceedings of the 2018 IEEE 68th Electronic Components and Technology Conference, San Diego, CA, USA, 29 May–1 June 2018. [Google Scholar] [CrossRef]
- Wu, Y.; Barton, S.C.; Lee, A. Galvanic corrosion behavior at the Cu-Al ball bond interface: Influence of Pd addition and chloride concentration. Microelectron. Reliab. 2019, 92, 79–86. [Google Scholar] [CrossRef]
- Chauhan, P.; Zhong, Z.W.; Pecht, M. Copper Wire Bonding Concerns and Best Practices. J. Electron. Mater. 2013, 42, 2415–2434. [Google Scholar] [CrossRef]
- Ross, N.; Asokan, M.; Kumar, G.I.A.; Caperton, J.; Alptekin, J.; Salunke, A.S.; Chyan, O.M. Mechanistic study of copper wire-bonding failures on packaging devices in acidic chloride environments. Microelectron. Reliab. 2020, 113, 113917. [Google Scholar] [CrossRef]
- Zhong, Z.W. Wire bonding using copper wire. Microelectron. Int. 2009, 26, 10–16. [Google Scholar] [CrossRef]
- Fu, S.W.; Lee, C.C. A corrosion study of Ag-Al intermetallic compounds in chlorine-containing epoxy molding compounds. J. Mater. Sci. Mater. Electron. 2017, 28, 15739–15747. [Google Scholar] [CrossRef]
- Gan, C.L.; Francis, C.; Chan, B.L.; Hashim, U. Extended reliability of gold and copper ball bonds in microelectronic packaging. Gold Bull. 2013, 46, 103–115. [Google Scholar] [CrossRef]
- Goh, C.S.; Chong, W.L.E.; Lee, T.K.; Breach, C. Corrosion Study and Intermetallics Formation in Gold and Copper Bonding in Microelectronics Packaging. Crystals 2013, 3, 391–404. [Google Scholar] [CrossRef]
- Ly, N.; Xu, D.E.; Song, W.H.; Mayer, M. More uniform Pd distribution in free-air balls of Pd-coated Cu bonding wire using movable flame-off electrode. Microelectron. Reliab. 2015, 55, 201–206. [Google Scholar] [CrossRef]
- Lim, A.B.Y.; Chang, A.C.K.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Ultra-fine pitch palladium-coated copper wire bonding: Effect of bonding parameters. Microelectron. Reliab. 2014, 54, 2555–2563. [Google Scholar] [CrossRef]
- Clauberg, H.; Backus, P.; Chylak, B. Nickel-palladium bond pads for copper wire bonding. Microelectron. Reliab. 2011, 51, 75–80. [Google Scholar] [CrossRef]
- Lee, T.K.; Breach, C.D.; Chong, W.L.; Goh, C.S. Oxidation and Corrosion of Au/Al and Cu/Al in Wire Bonding Assembly. In Proceedings of the 2012 13th International Conference on Electronic Packaging Technology & High Density Packaging, Guilin, China, 13–16 August 2012. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.Y.; Paik, K.W.; Koh, K.W.; Won, J.H.; Choe, S.H.; Lee, J.; Monn, J.T.; Park, Y.J. Effects of Cu/Al Intermetallic Compound (IMC) on Copper Wire and Aluminum Pad Bondability. IEEE Trans. Compon. Packag. Technol. 2003, 26, 367–374. [Google Scholar] [CrossRef]
- Chylak, B.; Clauberg, H.; Foley, J.; Qin, I.; Milton, B. Copper High Volume Manufacturing R&D to Production. In Proceedings of the 2011 IEEE 13th Electronics Packaging Technology Conference, Singapore, 7–9 December 2011. [Google Scholar] [CrossRef]
- Lall, P.; Luo, Y.; Nguyen, L. Chlorine-ion Related Corrosion in Cu-Al Wire bond Microelectronic Packages. In Proceedings of the ASME 2015 13th International Technical Conference and Exhibition and Packaging and Integration of Electronic and Photonic Microsystems, San Francisco, CA, USA, 6–9 July 2015. [Google Scholar] [CrossRef]
- Kumar, G.I.A.; Lamber, A.; Caperton, J.; Asokan, M.; Yi, W.; Chyan, O. Comparative Study of Chloride and Fluoride Induced Aluminum Pad Corrosion in Wire-Bonded Device Packaging Assembly. Corros. Mater. Degrad. 2021, 2, 447–460. [Google Scholar] [CrossRef]
- Zeng, Y.; Bai, K.; Jin, H. Thermodynamic study on the corrosion mechanism of copper wire bonding. Microelectron. Reliab. 2013, 53, 985–1001. [Google Scholar] [CrossRef]
- Chyan, O.; Ross, N.; Asokan, M.; Lambert, A.; Berhe, S. Mechanistic Investigation and Prevention of Al Bond Pad Corrosion in Cu Wire-bonded Device Assembly. In Proceedings of the 2017 IEEE 67th Electronic Components and Technology Conference, Orlando, FL, USA, 30 May–2 June 2017. [Google Scholar] [CrossRef]
- Varughese, M.; Enakshi, W.; Sheila, F.C. Corrosion of Copper Wire Bonded Packages by Chlorine Containing Foreign Particles. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020. [Google Scholar] [CrossRef]
- Kumar, G.I.A.; Alptekin, J.; Caperton, J.; Salunke, A.; Chyan, O. Accelerated reliability testing of Cu-Al bimetallic contact by a micropattern corrosion testing platform for wire bond device application. MethodsX 2021, 8, 101320. [Google Scholar] [CrossRef]
- Soestbergen, M.V.; Mavinkurve, A.; Zaal, J.J.M.; Halloran, G.M.; Rongen, R.T.H.; Farrugia, M.L. Crevice Corrosion of Ball Bound Intermetallics of Cu and Ag Wire. In Proceedings of the 2016 IEEE 66th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 31 May–3 June 2016. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Chiang, T.H.; Chen, Y.F.; Yang, P.F.; Huang, L.; Lin, K.L. The Corrosion Performance of Cu Alloy Wire Bond on Al Pad in Molding Compounds of Various Chlorine Contents under Biased-HAST. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 27–30 May 2014. [Google Scholar] [CrossRef]
- Soestbergen, M.V.; Ernst, L.J.; Zhang, G.Q.; Rongen, R.T.H. Transport of Corrosive Constituents in Epoxy Molding Compounds. In Proceedings of the 2007 International Conference on Thermal, Mechanical and Multi-Physics Simulation Experiments in Microelectronics and Micro-Systems. EuroSime 2007, London, UK, 15–18 April 2007. [Google Scholar] [CrossRef]
- Tai, C.T.; Lim, H.Y.; Teo, C.H.; Audrey Swee, P.J. An investigation on Cu Wire Bond Corrosion and Mitigation Technique for Automotive Reliability. In Proceedings of the 2012 35th IEEE/CPMT International Electronics Manufacturing Technology Conference (IEMT), Ipoh, Malaysia, 6–8 November 2012. [Google Scholar] [CrossRef]
- Yu, K.K.H.; Rimal, S.; Asokan, M.; Nalla, P.R.; Koskey, S.; Pillai, K.S.M.; Chyan, O.; Singh, K.J.; Suri, S. Micro-pattern Corrosion Screening on Bimetallic Corrosion for Microelectronic Application. Electrochim. Acta 2016, 210, 512–519. [Google Scholar] [CrossRef]
- Belfort, Y.; Caignard, J.M.; Keller, S.; Guerveno, J.P. Failures on DC-DC modules following a change of wire bonding material from gold to copper. Microelectron. Reliab. 2015, 55, 2003–2006. [Google Scholar] [CrossRef]
- Gan, C.L.; Ng, E.K.; Chan, B.L.; Hashim, U.; Classe, F.C. Technical Barriers and Development of Cu Wirebonding in Nanoelectronics Device Packaging. J. Nanomater. 2012, 2012, 173025. [Google Scholar] [CrossRef]
- Elisseeva, O.V.; Bruhn, A.; Cerezo, J.; Mavinkurve, A.; Rongen, R.T.H.; O’Halloran, G.M.O.; Ambat, R.; Terryn, H.; Mol, J.M.C. Novel electrochemical approach to study corrosion mechanism of Al-Au wire-bond pad interconnections. Corros. Eng. Sci. Technol. 2013, 48, 409–417. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, X.; Hua, Y. Chlorine and sulfur effects on gold-aluminum wire bond reliability. In Proceedings of the ISTFA 2022: Conference Proceedings from the 48th International Symposium for Testing and Failure Analysis, Pasadena, CA, USA, 30 October–3 November 2022. [Google Scholar] [CrossRef]
- Tian, Y.; Hang, C.; Wang, C.; Zhou, Y. Evolution of Cu/Al Intermetallic Compounds in the Copper Bump bonds during Aging Process. In Proceedings of the 2007 8th International Conference on Electronic Packaging Technology, Shanghai, China, 14–17 August 2007. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.W.; Hong, S.J.; Moon, J.T. The interface Behavior of the Cu-Al Bond System in High Humidity Conditions. In Proceedings of the 2010 12th Electronics Packaging Technology Conference, Singapore, 8–10 December 2010. [Google Scholar] [CrossRef]
- Ho, H.M.; Lam, W.; Stoukatch, S.; Ratchev, P.; Vath, C.J.; Beyne, E. Direct gold and copper wires bonding on copper. Microelectron. Reliab. 2003, 43, 913–923. [Google Scholar] [CrossRef]
- Boettcher, T.; Rother, M.; Liedtke, S.; Ullrich, M.; Bollmann, M.; Pinkernelle, A.; Gruber, D.; Funke, H.J.; Kaiser, M.; Lee, K.; et al. On the Intermetallic Corrosion of Cu-Al wire bonds. In Proceedings of the 2010 12th Electronics Packaging Technology Conference, Singapore, 8–10 December 2010. [Google Scholar] [CrossRef]
- Fu, C.H.; Hung, L.Y.; Jiang, D.S.; Chang, C.C.; Wang, Y.P.; Hsiao, C.S. Evaluation of new substrate surface finish: Electroless nickel/electroless palladium/immersion gold (ENEPIG). In Proceedings of the 2008 58th Electronic Components and Technology Conference, Lake Buena Vista, FL, USA, 27–30 May 2008. [Google Scholar] [CrossRef]
- Hur, S.Y.; Kim, K.T.; Yoo, Y.R.; Kim, Y.S. Effects of NaCl Concentration and Solution Temperature on the Galvanic Corrosion between CFRP and AA7075T6. Corros. Sci. Technol. 2020, 19, 75–81. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kumagai, N.; Yoshioka, H.; Kim, J.H.; Akiyama, E.; Habazaki, H.; Mrowec, S.; Kawashima, A.; Asami, K. Corrosion-Resistant Amorphous Surface Alloy. Corros. Sci. 1993, 35, 363–370. [Google Scholar] [CrossRef]
- Akiyama, E.; Habazaki, H.; Kawashima, A.; Asami, K.; Hashimoto, K. Corrosion-resistant amorphous aluminum alloys and structure of passive films. Mater. Sci. Eng. 1997, A226–A228, 920–924. [Google Scholar] [CrossRef]




| Bond FORCE, grams | Bond Time, ms | Electrical Flame-Off (EFO) Current, mA | EFO Time, ms |
|---|---|---|---|
| 30~60 | 15~10 | 30~40 | 700~730 |
| Specimen | 1% NaCl | 1% NaCl + 0.01% HCl | 1% NaCl + 0.1% HCl | 1% NaCl + 1% HCl | ||||
|---|---|---|---|---|---|---|---|---|
| icorr, nA/cm2 | Ecorr, mV(SCE) | icorr, nA/cm2 | Ecorr, mV(SCE) | icorr, nA/cm2 | Ecorr, mV(SCE) | icorr, nA/cm2 | Ecorr, mV(SCE) | |
| Solid Al | 10.67 | −911 | 20.93 | −791 | 76.30 | −722 | 543.00 | −754 |
| Sputtered Al | 182.00 | −1590 | 69.60 | −1240 | 15.80 | −973 | 72.70 | −901 |
| Au wire | 0.072 | 24 | 0.072 | 81 | 0.068 | 217 | 0.028 | 299 |
| Cu wire | 2.85 | −278 | 5.31 | −293 | 5.34 | −295 | 8.05 | −341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.-R.; Kim, G.; Jeon, S.-M.; Park, H.-J.; Seo, W.-W.; Moon, J.-T.; Kim, Y.-S. Influence of HCl Concentration on Corrosion Behavior between Au or Cu Bonding Wires and the Bond Pad for Semiconductor Packaging. Materials 2023, 16, 7275. https://doi.org/10.3390/ma16237275
Yoo Y-R, Kim G, Jeon S-M, Park H-J, Seo W-W, Moon J-T, Kim Y-S. Influence of HCl Concentration on Corrosion Behavior between Au or Cu Bonding Wires and the Bond Pad for Semiconductor Packaging. Materials. 2023; 16(23):7275. https://doi.org/10.3390/ma16237275
Chicago/Turabian StyleYoo, Young-Ran, Gyubinn Kim, Sung-Min Jeon, Hyun-Jun Park, Won-Wook Seo, Jeong-Tak Moon, and Young-Sik Kim. 2023. "Influence of HCl Concentration on Corrosion Behavior between Au or Cu Bonding Wires and the Bond Pad for Semiconductor Packaging" Materials 16, no. 23: 7275. https://doi.org/10.3390/ma16237275
APA StyleYoo, Y.-R., Kim, G., Jeon, S.-M., Park, H.-J., Seo, W.-W., Moon, J.-T., & Kim, Y.-S. (2023). Influence of HCl Concentration on Corrosion Behavior between Au or Cu Bonding Wires and the Bond Pad for Semiconductor Packaging. Materials, 16(23), 7275. https://doi.org/10.3390/ma16237275






