Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study
Abstract
:1. Introduction
1.1. Background of the Study
1.2. Rationale
1.3. Aim of the Study
2. Materials and Methods
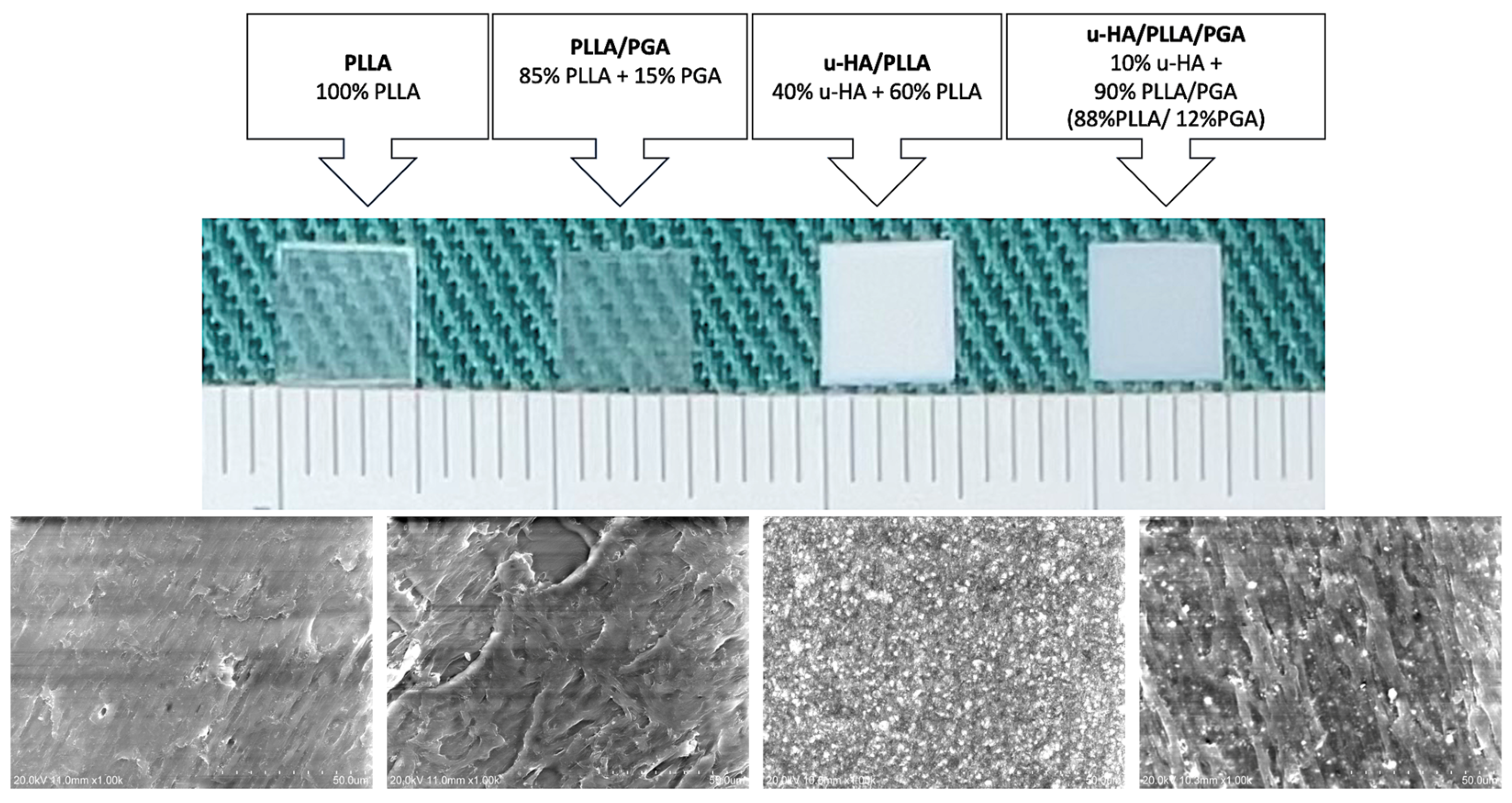
2.1. Materials Used
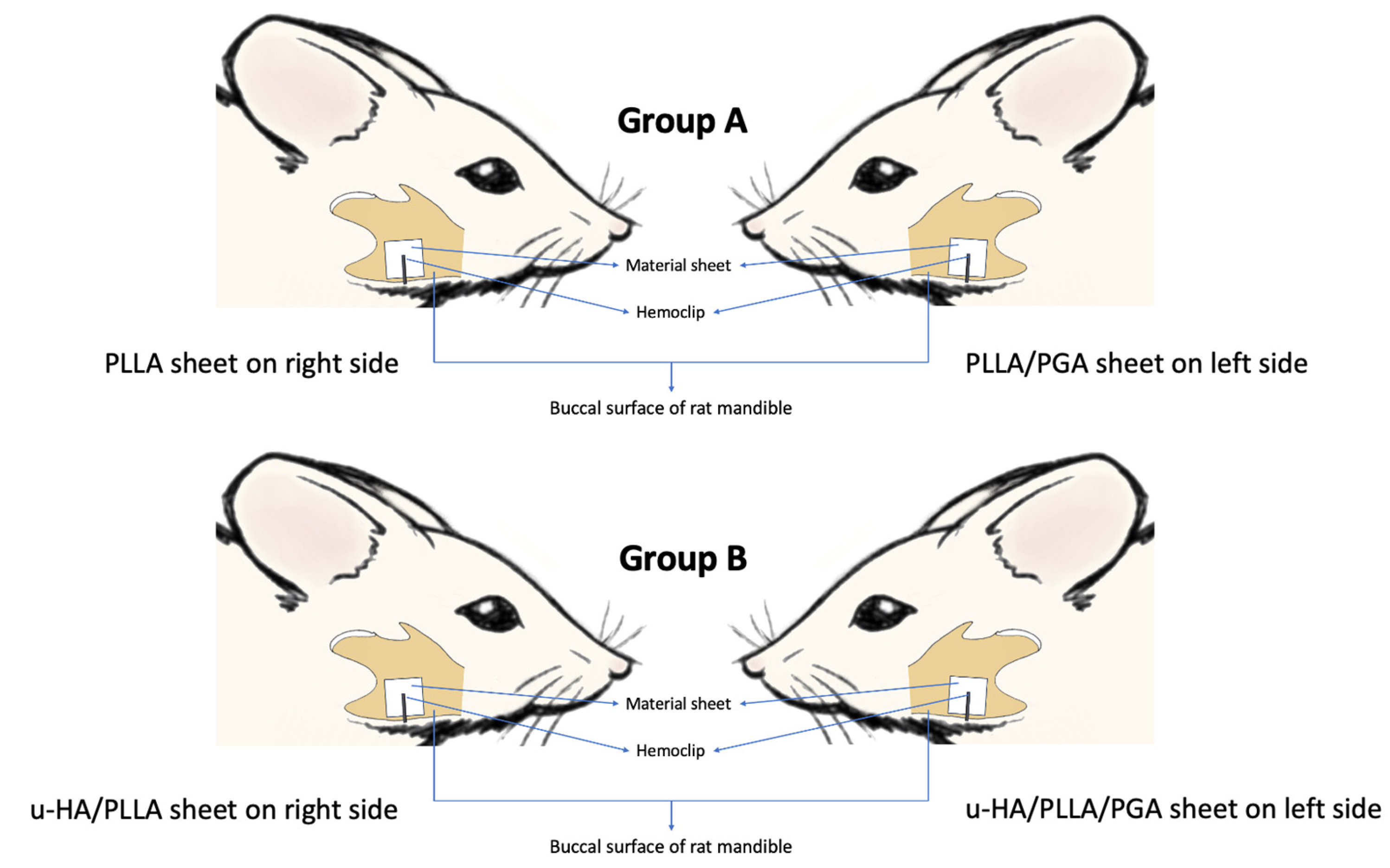
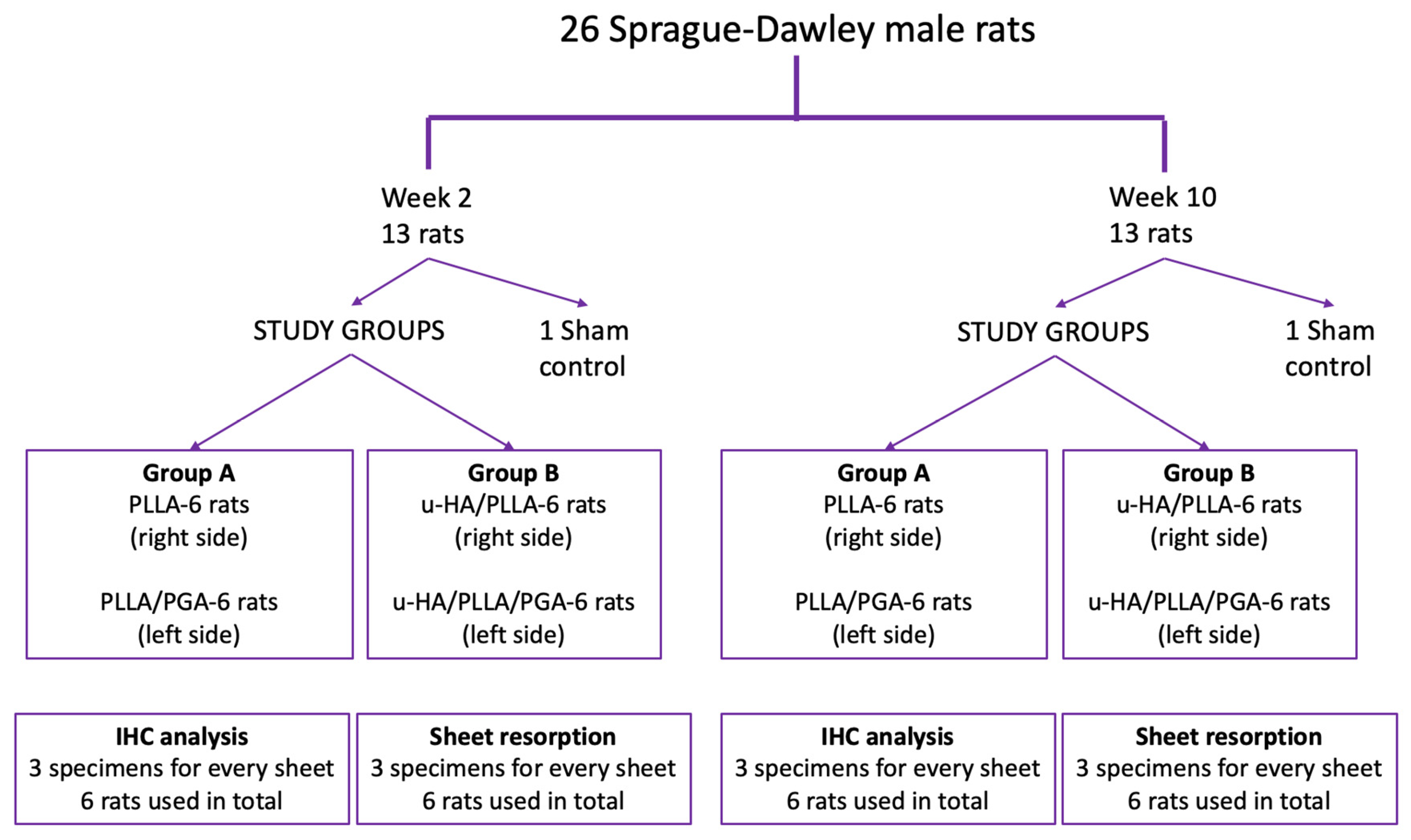
2.2. Animal Protocol: Surgical Procedure and Sacrifice
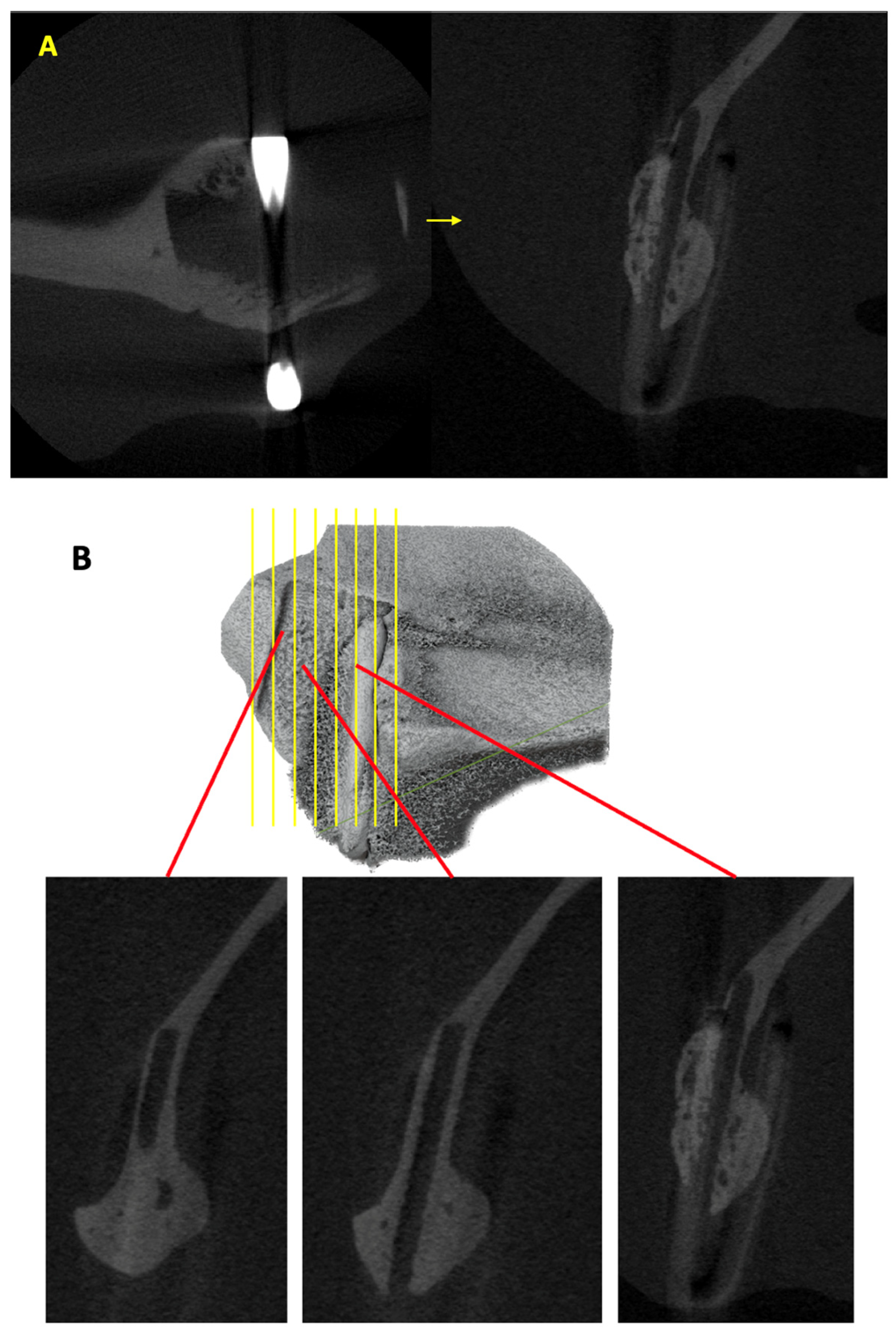
2.3. Assessment of New Bone Formation Using Micro-CT
2.4. Tissue Conditioning for IHC Evaluation
2.5. Hematoxylin-Eosin (HE) and IHC Staining
2.6. IHC Assessments
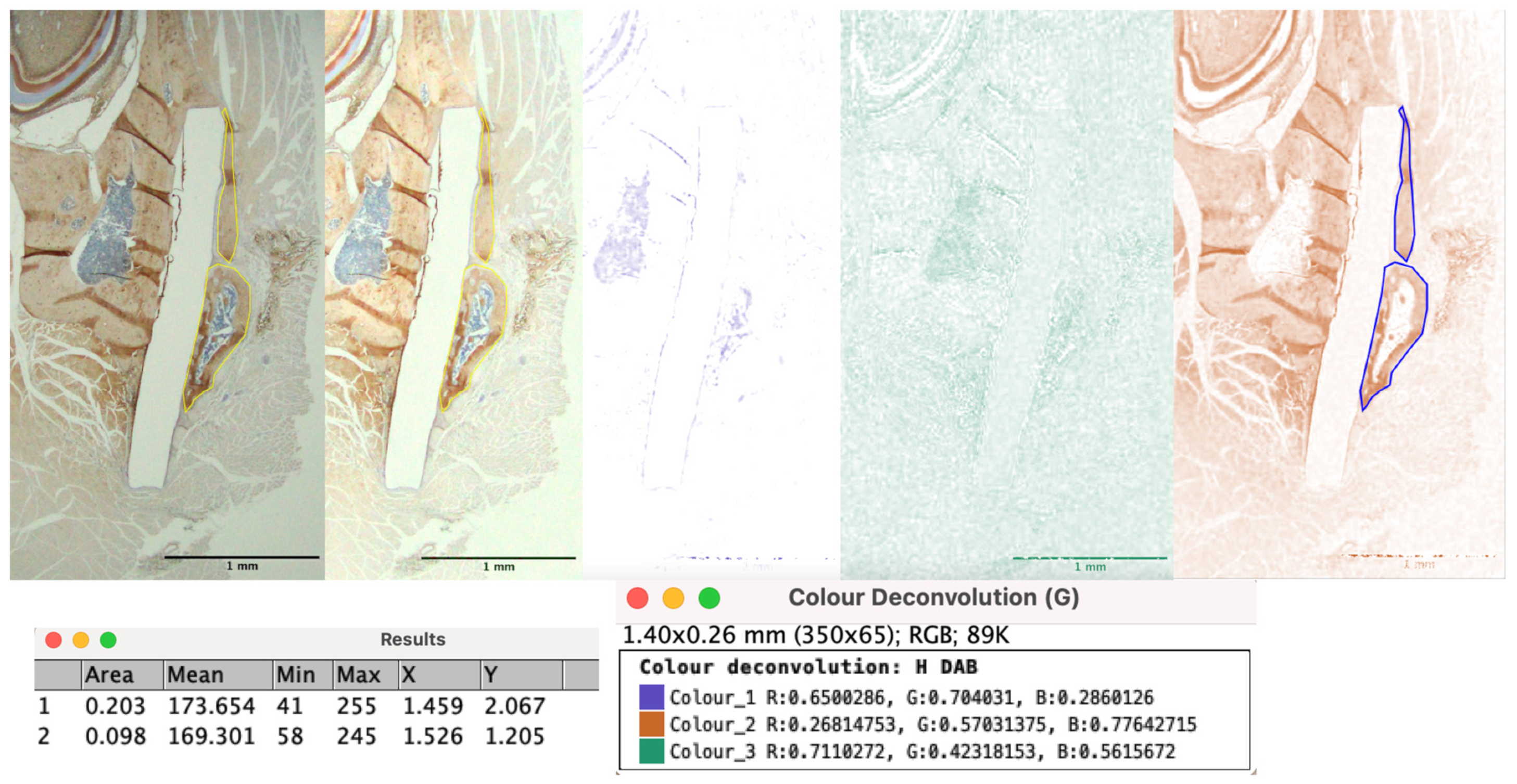
2.7. Estimation of Bioresorbable Sheet Resorption In Vivo
2.8. Statistical Analysis
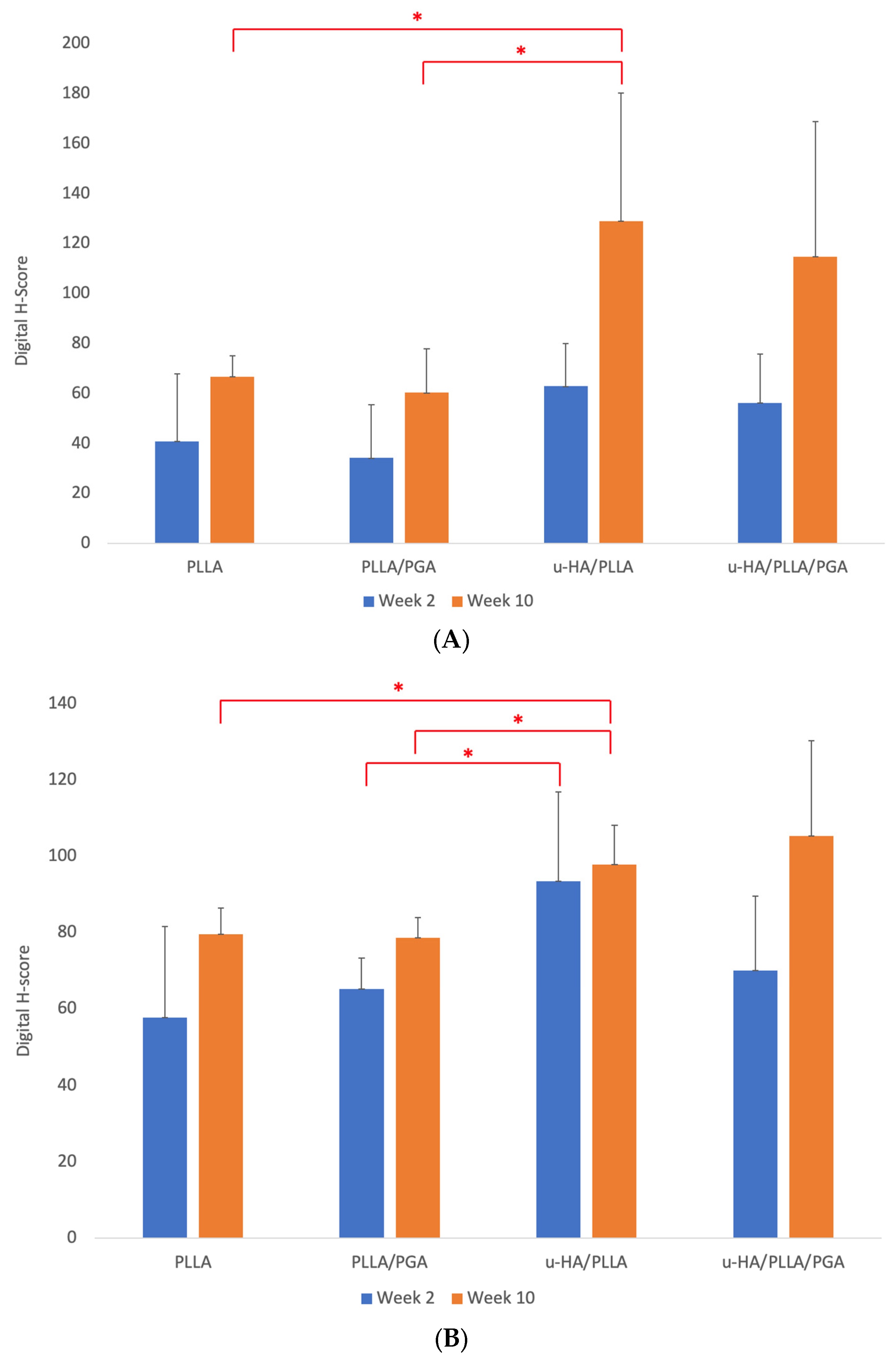
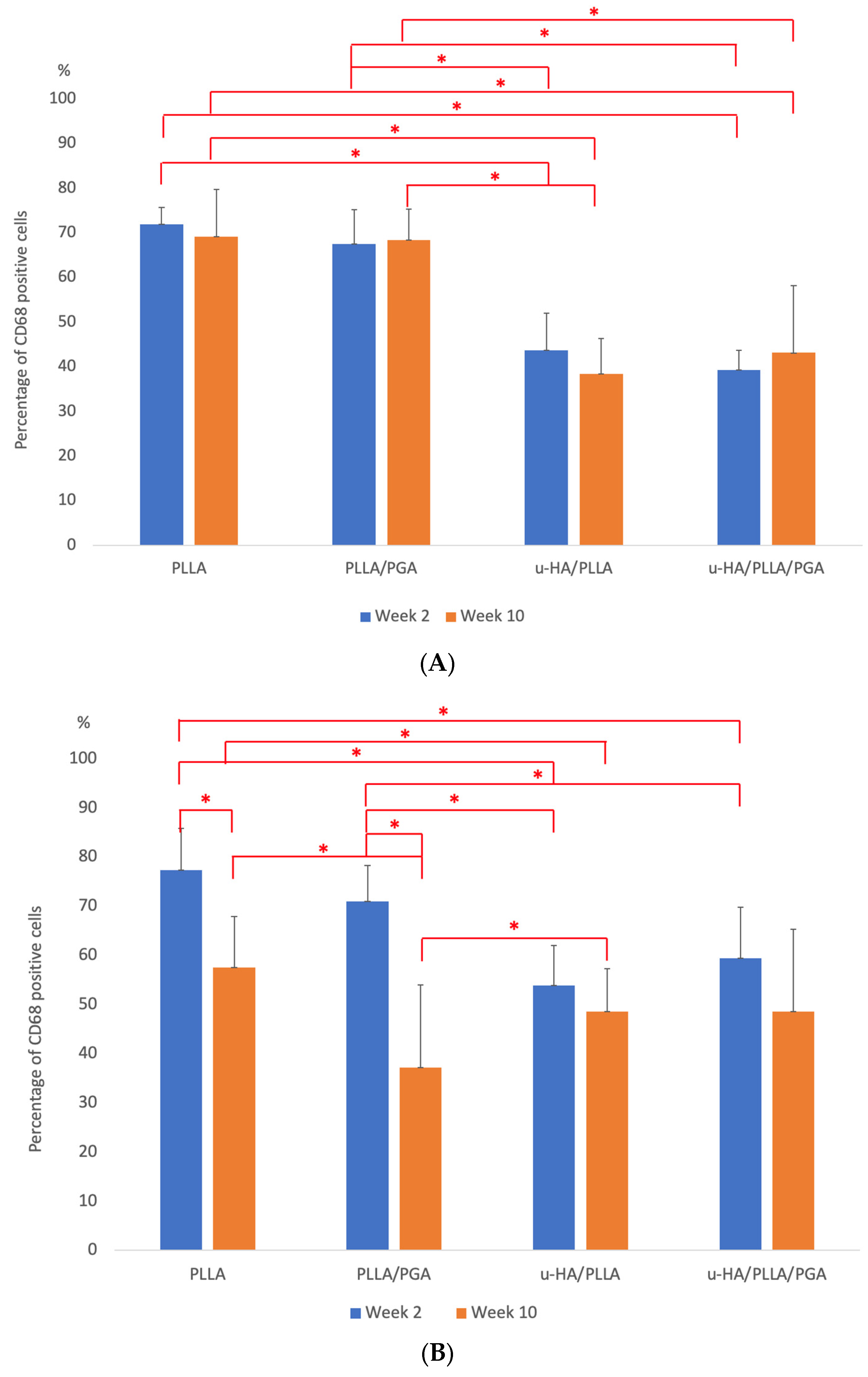
3. Results
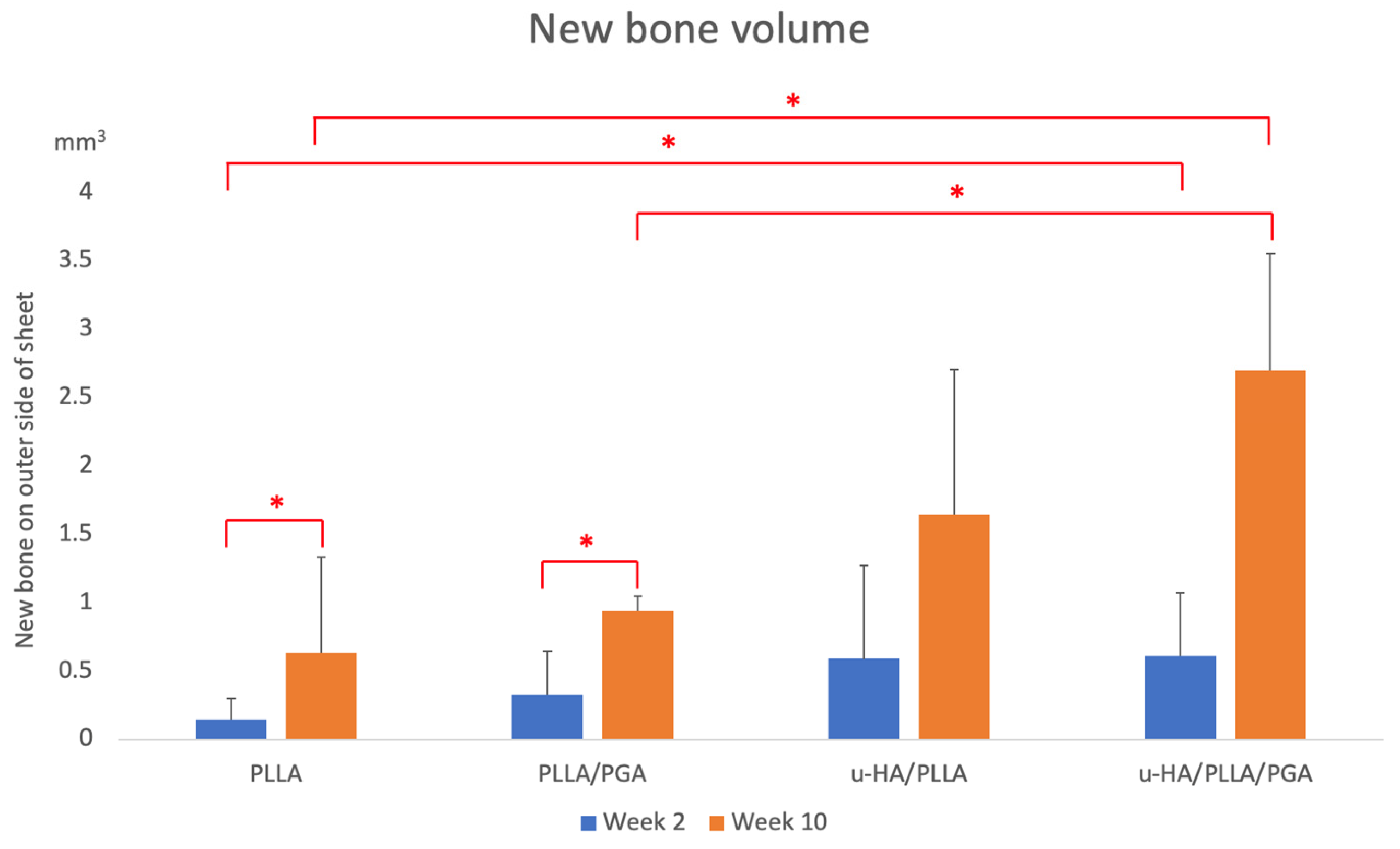
3.1. Volume of New Bone Formed Outside Sheet
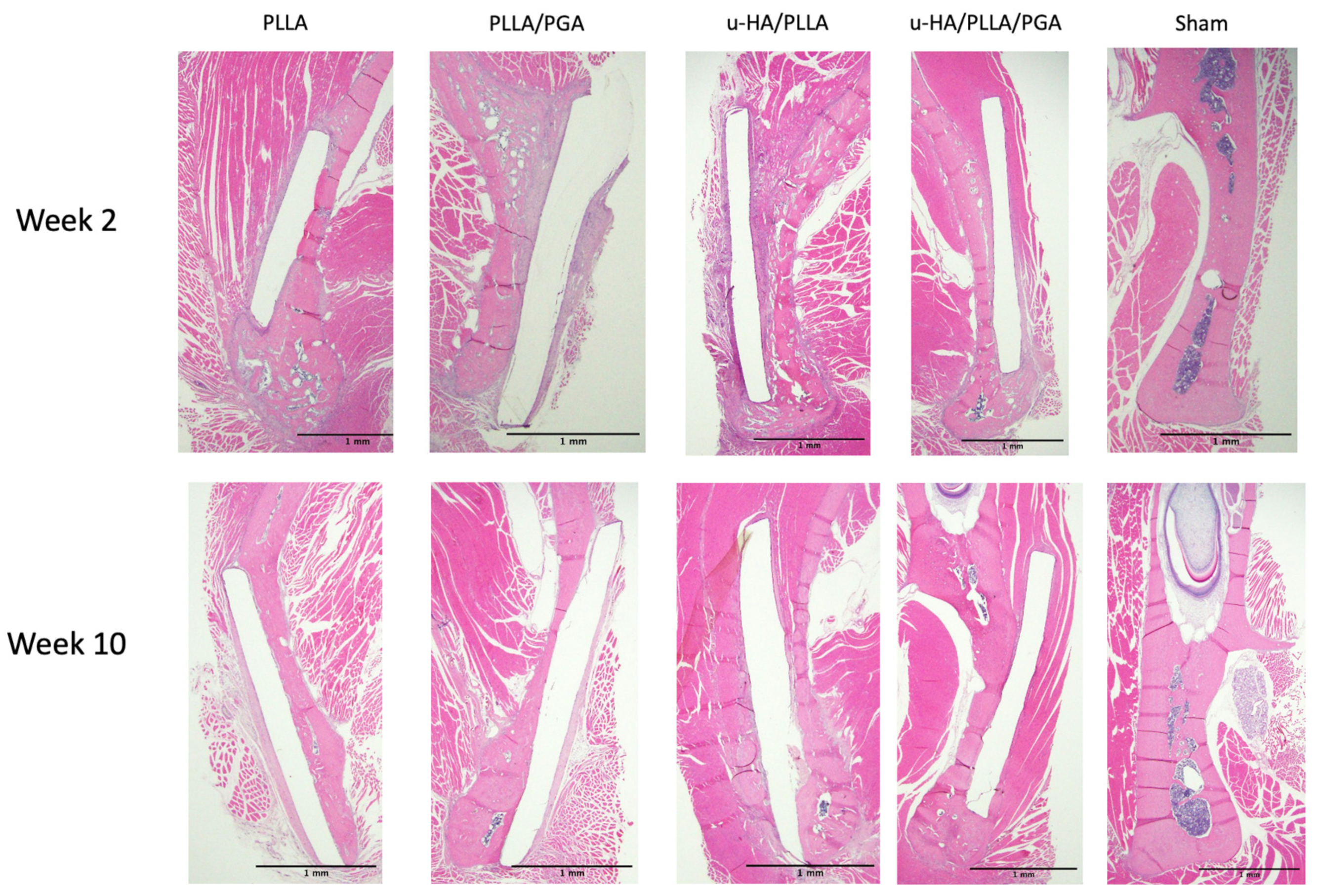
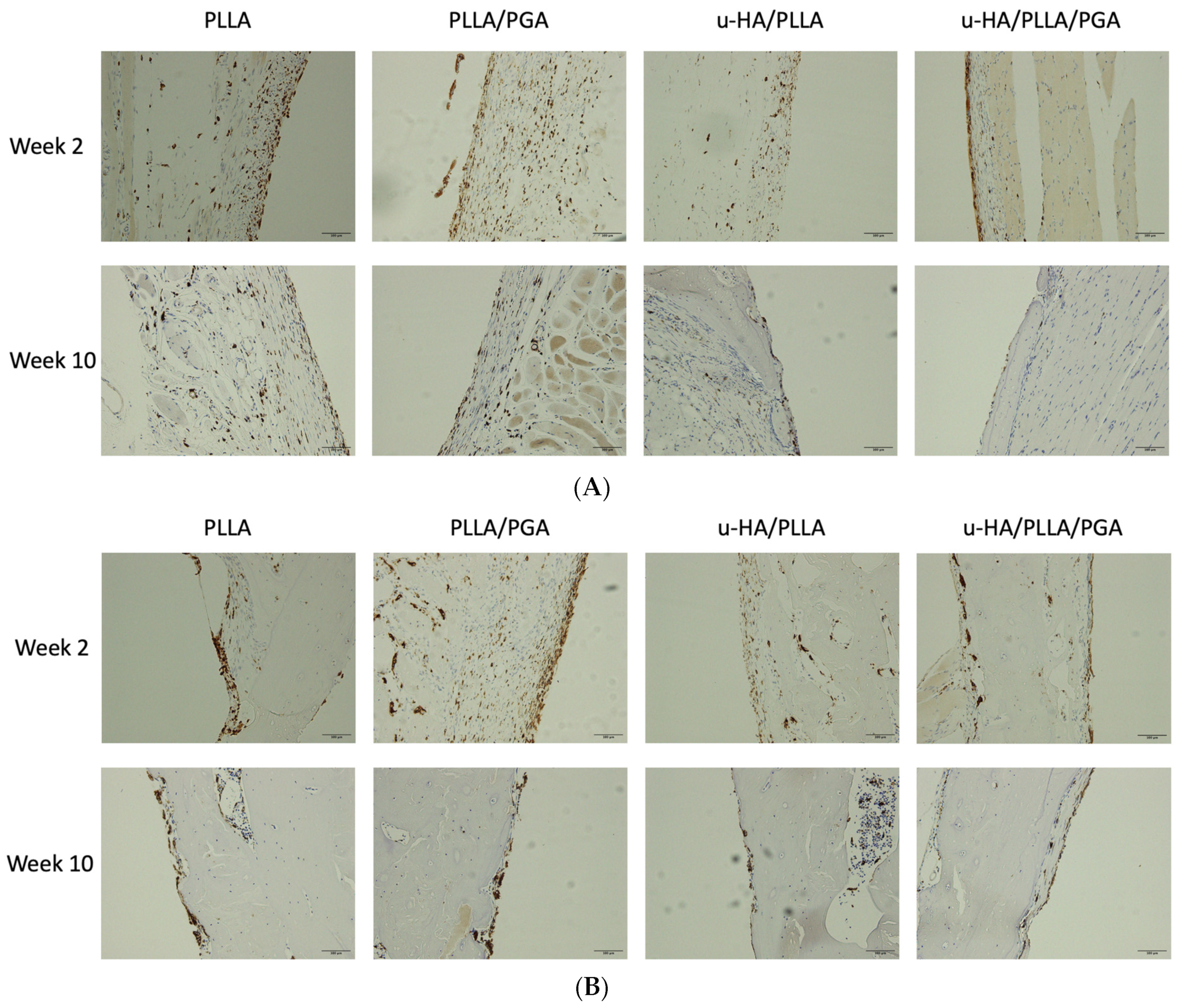
3.2. HE Staining
3.3. IHC Analysis
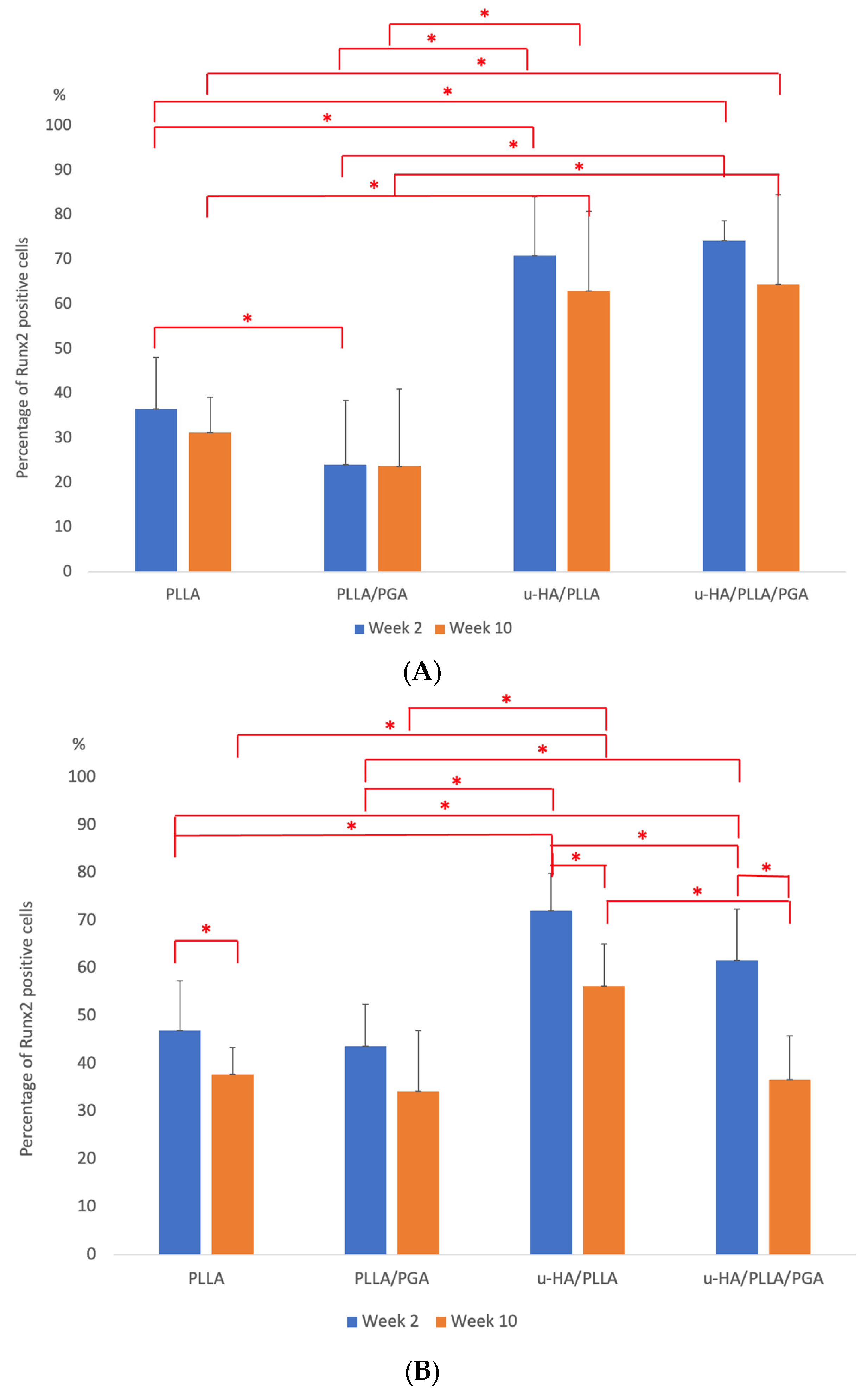
3.3.1. Runx2 Expression
3.3.2. OCN Expression
3.3.3. CD68 Expression
3.4. Bioresorbable Sheet Resorption Rate
4. Discussion
4.1. Biomaterial Induced Bone Formation
4.2. IHC Analysis—Runx2, OCN Biomarker Relevance
4.3. Inflammatory Conditions Elicited around the Biomaterials—CD68 Analysis
4.4. Bioresorbable Sheet Resorption Characteristics
4.5. Application of Each Generation Bioresorbable Material in Maxillofacial Osteosynthesis
4.5.1. Bioresorbable Osteosynthesis in Orthognathic Surgery
4.5.2. Bioresorbable Osteosynthesis in Maxillofacial Trauma
4.6. Limitations of Our Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukegawa, S.; Kanno, T.; Yamamoto, N.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H.; Furuki, Y. Biomechanical Loading Comparison between Titanium and Unsintered Hydroxyapatite/Poly-L-Lactide Plate System for Fixation of Mandibular Subcondylar Fractures. Materials 2019, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.N.; Barthélémy, I.; Bekara, F. From rigid bone plate fixation to stable dynamic osteosynthesis in mandibular and craniomaxillo-facial surgery: Historical evolution of concepts and technical developments. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; de Visscher, J.G.A.M.; Hoppenreijs, T.J.M.; Bergsma, J.E.; van Minnen, B.; Stegenga, B.; Bos, R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: A multicenter randomized controlled trial. PLoS ONE 2017, 11, e0177152. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 7, 2782. [Google Scholar] [CrossRef] [PubMed]
- Vacaras, S.; Baciut, M.; Lucaciu, O.; Dinu, C.; Baciut, G.; Crisan, L.; Hedesiu, M.; Crisan, B.; Onisor, F.; Armencea, G.; et al. Understanding the basis of medical use of poly-lactide-based resorbable polymers and composites—A review of the clinical and metabolic impact. Drug Metab. Rev. 2019, 51, 570–588. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, D.S.; Mayer, M.H.; Ellenbogen, R.G.; Centeno, J.A.; Johnson, F.B.; Mullick, F.G.; Manson, P.N. Detection of titanium in human tissues after craniofacial surgery. Plast. Reconstr. Surg. 1997, 99, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Cavuşoğlu, T.; Yavuzer, R.; Başterzi, Y.; Tuncer, S.; Latifoğlu, O. Resorbable plate-screw systems: Clinical applications. Ulus. Travma Acil Cerrahi Derg. 2005, 11, 43–48. [Google Scholar] [PubMed]
- Kanno, T.; Karino, M.; Yoshino, A.; Koike, T.; Ide, T.; Tatsumi, H.; Tsunematsu, K.; Yoshimatsu, H.; Sekine, J. Feasibility of single folded unsintered hydroxyapatite particles/poly-L-lactide composite sheet in combined orbital floor and medial wall fracture reconstruction. J. Hard Tissue Biol. 2017, 26, 237–244. [Google Scholar] [CrossRef]
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic acid for surgical implants. Arch. Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef]
- Kulkarni, R.K.; Moore, E.G.; Hegyeli, A.F.; Leonard, F. Biodegradable poly(lactic acid) polymers. J. Biomed. Mater. Res. 1971, 5, 169–181. [Google Scholar] [CrossRef]
- Pihlajamäki, H.; Böstman, O.; Hirvensalo, E.; Törmälä, P.; Rokkanen, P. Absorbable pins of self-reinforced poly-L-lactic acid for fixation of fractures and osteotomies. J. Bone Jt. Surg. Br. 1992, 74, 853–857. [Google Scholar] [CrossRef]
- Schumann, P.; Lindhorst, D.; Wagner, M.E.H.; Schramm, A.; Gellrich, L.C.; Rücker, M. Perspectives on resorbable osteosynthesis materials in craniomaxillofacial surgery. Pathobiology 2013, 80, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; de Visscher, J.G.; Hoppenreijs, T.J.; Bergsma, J.E.; Stegenga, B.; Bos, R.R. Decision-making considerations in application of biodegradable fixation systems in maxillofacial surgery—A retrospective cohort study. J. Craniomaxillofac. Surg. 2014, 42, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ferreira, J.M.F. Bioresorbable plates and screws for clinical applications: A review. J. Healthc. Eng. 2012, 3, 243–260. [Google Scholar] [CrossRef]
- Bergsma, J.E.; de Bruijn, W.C.; Rozema, F.R.; Bos, R.R.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Park, Y.W. Bioabsorbable osteofixation for orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 6. [Google Scholar] [CrossRef]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.R.; de Bruijn, W.C. Foreign body reactions to resorbable poly(L-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Katase, N.; Shibata, A.; Takahashi, Y.; Furuki, Y. Clinical evaluation of an unsintered hydroxyapatite/poly- L-lactide osteoconductive composite device for the internal fixation of maxillofacial fractures. J. Craniofac. Surg. 2016, 27, 1391–1397. [Google Scholar] [CrossRef]
- Surronen, R.; Haers, P.E.; Lindqvist, C.; Sailer, H.F. Update on bioresorbable plates in maxillofacial surgery. Facial Plast. Surg. 1999, 15, 61–72. [Google Scholar] [CrossRef]
- Edwards, R.C.; Kiely, K.D.; Eppley, B.L. The fate of resorbable poly- L-lactic/polyglycolic acid (LactoSorb) bone fixation devices in orthognathic surgery. J. Oral Maxillofac. Surg. 2001, 59, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kanno, T.; Kawai, H.; Shibata, A.; Shibata, A.; Takahashi, Y.; Nagatsuka, H.; Furuki, Y. Long-term bioresorption of bone fixation devices made from composites of unsintered hydroxyapatite particles and poly-L-lactide. J. Hard Tissue Biol. 2015, 24, 219–224. [Google Scholar] [CrossRef]
- Shikinami, Y.; Hata, K.; Okuno, M. Ultra-high-strength resorbable implants for oral and maxillofacial surgery made from composites of bioactive ceramic particles/polylactide composites. Int. J. Oral Maxillofac. Surg. 1997, 26, 37. [Google Scholar]
- Shikinami, Y.; Matsusue, Y.; Nakamura, T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly L-lactide (F-u-HA/PLLA). Biomaterials 2005, 26, 5542–5551. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, S.; Dong, Q.N.; Ngo, H.X.; Bai, Y.; Sha, J.; Toda, E.; Okui, T.; Kanno, T. Bioactive Regeneration Potential of the Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-Lactide-Co-Glycolide Biomaterial in Maxillofacial Reconstructive Surgery: An In Vivo Preliminary Study. Materials 2021, 10, 2461. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.X.; Dong, Q.N.; Bai, Y.; Sha, J.; Ishizuka, S.; Okui, T.; Sukegawa, S.; Kanno, T. Bone Regeneration Capacity of Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-l-lactide-co-glycolide Sheet in Maxillofacial Surgery: An In Vivo Study. Nanomaterials 2020, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Das, A.; Awasthi, P.; Jain, V.; Banerjee, S.S. 3D printing of maxillofacial prosthesis materials: Challenges and opportunities. Bioprinting 2023, 32, e00282. [Google Scholar] [CrossRef]
- Moiduddin, K.; Mian, S.H.; Umer, U.; Alkhalefah, H.; Ahmed, F.; Hashmi, F.H. Design, Analysis, and 3D Printing of a Patient-Specific Polyetheretherketone Implant for the Reconstruction of Zygomatic Deformities. Polymers 2023, 15, 886. [Google Scholar] [CrossRef]
- Ngo, H.X.; Bai, Y.; Sha, J.; Ishizuka, S.; Toda, E.; Osako, R.; Kato, A.; Morioka, R.; Ramanathan, M.; Tatsumi, H.; et al. A Narrative Review of u-HA/PLLA, a Bioactive Resorbable Reconstruction Material: Applications in Oral and Maxillofacial Surgery. Materials 2021, 26, 150. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Zhou, T.; Shu, J.; Mao, J.H. Quantifying chromogen intensity in immunohistochemistry via reciprocal intensity. Cancer InCytes 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Shikinami, M.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly-L-lactide (PLLA): Part I. Basic characteristics. Biomaterials 1999, 20, 859–877. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, J.; Alexander, H. Biological response of intramedullary bone to poly-L-lactic acid. J. Appl. Biomater. 1993, 4, 13–27. [Google Scholar] [CrossRef]
- Adamopoulos, I.E. Inflammation in bone physiology and pathology. Curr. Opin. Rheumatol. 2018, 30, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Moroi, A.; Ueki, K.; Okabe, K.; Marukawa, K.; Sotobori, M.; Mukozawa, A.; Miyazakia, M. Comparison between unsintered hydroxyapatite/poly-L-lactic acid mesh and titanium mesh in bone regeneration of rabbit mandible. Implant Dent. 2013, 22, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H.T.; Matsushita, T. Titania-based materials. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 485–500. ISBN 978-1-84569-422-7. [Google Scholar]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation rates of oral resorbable implants (polylactates and polyglycolates): Rate modification with changes in PLA/PGA copolymer ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Innamorati, G.; Valenti, M.T. Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Rev. Rep. 2012, 8, 891–897. [Google Scholar] [CrossRef]
- Lian, J.B.; Javed, A.; Zaidi, S.K.; Lengner, C.; Montecino, M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S. Regulatory controls for osteoblast growth and differentiation: Role of Runx/Cbfa/AML factors. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 1–41. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014, 23, 269–286. [Google Scholar] [CrossRef]
- Jonason, J.H.; Xiao, G.; Zhang, M.; Xing, L.; Chen, D. Post-translational Regulation of Runx2 in Bone and Cartilage. J. Dent. Res. 2009, 88, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Dradjat, R.S.; Sananta, P.; Rosandi, R.D.; Siahaan, L.D. Osteocalcin biomarker level evaluation on fracture healing with bone defect after stromal vascular fraction application in murine model. Ann. Med. Surg. 2021, 2, 103020. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin-A Versatile Bone-Derived Hormone. Front. Endocrinol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Klinge, U.; Dievernich, A.; Tolba, R.; Klosterhalfen, B.; Davies, L. CD68+ macrophages as crucial components of the foreign body reaction demonstrate an unconventional pattern of functional markers quantified by analysis with double fluorescence staining. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3134–3146. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 15, 450–462. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Böstman, O.; Pihlajamäki, H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: A review. Biomaterials 2000, 21, 2615–2621. [Google Scholar] [CrossRef]
- Chandorkar, Y.K.R.; Basu, B. The Foreign Body Response Demystified. ACS Biomater. Sci. Eng. 2019, 14, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Eseonu, O.I.; De Bari, C. Homing of mesenchymal stem cells: Mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology 2015, 54, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, Q.; Gu, B.; Yin, C.; Shen, K.; Tang, H.; Xia, H.; Zhang, X.; Zhao, Y.; Yang, X.; et al. Minimally invasive implantation and decreased inflammation reduce osteoinduction of biomaterial. Theranostics 2020, 18, 3533–3545, Erratum in Theranostics 2022, 21, 1734–1735. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.B.; Kang, D.H.; Gu, J.H.; Oh, S.A. Delayed Foreign Body Reaction Caused by Bioabsorbable Plates Used for Maxillofacial Fractures. Arch. Plast. Surg. 2016, 43, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Karino, M.; Okui, T.; Kanno, T. Complications of Poly-l-Lactic Acid and Polyglycolic Acid (PLLA/PGA) Osteosynthesis Systems for Maxillofacial Surgery: A Retrospective Clinical Investigation. Polymers 2021, 14, 889. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Morales, L.; Wood, R.; Pensler, J.; Goldstein, J.; Havlik, R.J.; Habal, M.; Losken, A.; Williams, J.K.; Burstein, F.; et al. Resorbable PLLA-PGA plate and screw fixation in pediatric craniofacial surgery: Clinical experience in 1883 patients. Plast. Reconstr. Surg. 2004, 15, 850–857. [Google Scholar] [CrossRef]
- Furukawa, T.; Matsusue, Y.; Yasunaga, T.; Shikinami, Y.; Okuno, M.; Nakamura, T. Biodegradation behavior of ultra-high-strength hydroxyapatite/poly (L-lactide) composite rods for internal fixation of bone fractures. Biomaterials 2000, 21, 889–898. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Manabe, Y.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Furuki, Y. Biomechanical Loading Evaluation of Unsintered Hydroxyapatite/poly-l-lactide Plate System in Bilateral Sagittal Split Ramus Osteotomy. Materials 2017, 7, 764. [Google Scholar] [CrossRef]
- Shikinami, Y.; Okuno, M. Bioresorbable devices made of forged composites of hydroxyapatite (HA) particles and poly L-lactide (PLLA). Part II: Practical properties of miniscrews and miniplates. Biomaterials 2001, 22, 3197–3211. [Google Scholar] [CrossRef]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Dewey, M.J.; Harley, B.A.C. Biomaterial design strategies to address obstacles in craniomaxillofacial bone repair. RSC Adv. 2021, 11, 17809–17827. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Atala, A. Tissue Engineering: Toward a New Era of Medicine. Annu. Rev. Med. 2017, 14, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Schakenraad, J.M.; Hardonk, M.J.; Feijen, J.; Molenaar, I.; Nieuwenhuis, P. Enzymatic activity toward poly(L-lactic acid) implants. J. Biomed. Mater. Res. 1990, 24, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Kouno, N.; Wang, H.; Tsuji, H. Crystal Structure of Poly(lactic acid) Stereocomplex: Random Packing Model of PDLA and PLLA Chains As Studied by X-ray Diffraction Analysis. Macromolecules 2017, 50, 8048–8065. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Schultze-Mosgau, S.; Schrell, U.; Wenzel, D.; Kessler, P. Biodegradable miniplates (LactoSorb): Long-term results in infant minipigs and clinical results. J. Craniofac. Surg. 2000, 11, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Quereshy, F.A.; Goldstein, J.A.; Goldberg, J.S.; Beg, Z. The efficacy of bioresorbable fixation in the repair of mandibular fractures: An animal study. J. Oral Maxillofac. Surg. 2000, 58, 1263–1269. [Google Scholar] [CrossRef]
- Turvey, T.A.; Bell, R.B.; Tejera, T.J.; Proffit, W.R. The use of self-reinforced biodegradable bone plates and screws in orthognathic surgery. J. Oral Maxillofac. Surg. 2002, 60, 59–65. [Google Scholar] [CrossRef]
- Ueki, K.; Marukawa, K.; Shimada, M.; Nakagawa, K.; Alam, S.; Yamamoto, E. Maxillary stability following Le Fort I osteotomy in combination with sagittal split ramus osteotomy and intraoral vertical ramus osteotomy: A comparative study between titanium miniplate and poly-L-lactic acid plate. J. Oral Maxillofac. Surg. 2006, 64, 74–80. [Google Scholar] [CrossRef]
- Norholt, S.E.; Pedersen, T.K.; Jensen, J. Le Fort I miniplate osteosynthesis: A randomized, prospective study comparing resorbable PLLA/PGA with titanium. Int. J. Oral Maxillofac. Surg. 2004, 33, 245–252. [Google Scholar] [CrossRef]
- Ko, E.W.; Huang, C.S.; Lo, L.J.; Chen, Y.R. Alteration of mastica- tory electromyographic activity and stability of orthognathic surgery in patients with skeletal class III malocclusion. J. Oral Maxillofac. Surg. 2013, 71, 1249–1260. [Google Scholar] [CrossRef]
- Yerit, K.C.; Enislidis, G.; Schopper, C.; Turhani, D.; Wanschitz, F.; Wagner, A.; Watzinger, F.; Ewers, R. Fixation of mandibular fractures with biodegradable plates and screws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 94, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.S.; Khambay, B.S.; Ayoub, A.F.; Koppel, D.; Wood, G. Preliminary assessment of skeletal stability after sagittal split mandibular advancement using a bioresorbable fixation system. Br. J. Oral Maxillofac. Surg. 2003, 41, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Cutright, D.E.; Hunsuck, E.E.; Beasley, J.D. Fracture reduction using a biodegradable material, polylactic acid. J. Oral Surg. 1971, 29, 393–397. [Google Scholar] [PubMed]
- Bos, R.R.; Boering, G.; Rozema, F.R.; Leenslag, J.W. Resorbable poly(L-lactide) plates and screws for the fixation of zygomatic fractures. J. Oral Maxillofac. Surg. 1987, 45, 751–753. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Jia, P.; Zhang, Y.; Gong, X.; Han, X.; He, Y. Application of biodegradable plates for treating pediatric mandibular fractures. J. Cranio-Maxillofac. Surg. 2015, 43, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Dorri, M.; Nasser, M.; Oliver, R. Resorbable versus titanium plates for facial fractures. Cochrane Database Syst. Rev. 2009, CD007158. [Google Scholar] [CrossRef] [PubMed]
- Bali, R.K.; Sharma, P.; Jindal, S.; Gaba, S. To evaluate the efficacy of biodegradable plating system for fixation of maxillofacial fractures: A prospective study. Natl. J. Maxillofac. Surg. 2013, 4, 167–172. [Google Scholar] [CrossRef]
- Gareb, B.; Roossien, C.C.; van Bakelen, N.B.; Verkerke, G.J.; Vissink, A.; Bos, R.R.M.; van Minnen, B. Comparison of the mechanical properties of biodegradable and titanium osteosynthesis systems used in oral and maxillofacial surgery. Sci. Rep. 2020, 23, 18143. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayasaka, K.; Ramanathan, M.; Huy, N.X.; Shijirbold, A.; Okui, T.; Tatsumi, H.; Kotani, T.; Shimamura, Y.; Morioka, R.; Kanno, T. Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study. Materials 2023, 16, 7379. https://doi.org/10.3390/ma16237379
Ayasaka K, Ramanathan M, Huy NX, Shijirbold A, Okui T, Tatsumi H, Kotani T, Shimamura Y, Morioka R, Kanno T. Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study. Materials. 2023; 16(23):7379. https://doi.org/10.3390/ma16237379
Chicago/Turabian StyleAyasaka, Kentaro, Mrunalini Ramanathan, Ngo Xuan Huy, Ankhtsetseg Shijirbold, Tatsuo Okui, Hiroto Tatsumi, Tatsuhito Kotani, Yukiho Shimamura, Reon Morioka, and Takahiro Kanno. 2023. "Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study" Materials 16, no. 23: 7379. https://doi.org/10.3390/ma16237379
APA StyleAyasaka, K., Ramanathan, M., Huy, N. X., Shijirbold, A., Okui, T., Tatsumi, H., Kotani, T., Shimamura, Y., Morioka, R., & Kanno, T. (2023). Evaluation of Hard and Soft Tissue Responses to Four Different Generation Bioresorbable Materials-Poly-l-Lactic Acid (PLLA), Poly-l-Lactic Acid/Polyglycolic Acid (PLLA/PGA), Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid (u-HA/PLLA) and Uncalcined/Unsintered Hydroxyapatite/Poly-l-Lactic Acid/Polyglycolic Acid (u-HA/PLLA/PGA) in Maxillofacial Surgery: An In-Vivo Animal Study. Materials, 16(23), 7379. https://doi.org/10.3390/ma16237379






