Antibiofilm Property and Biocompatibility of Siloxane-Based Polymer Coatings Applied to Biomaterials
Abstract
:1. Introduction
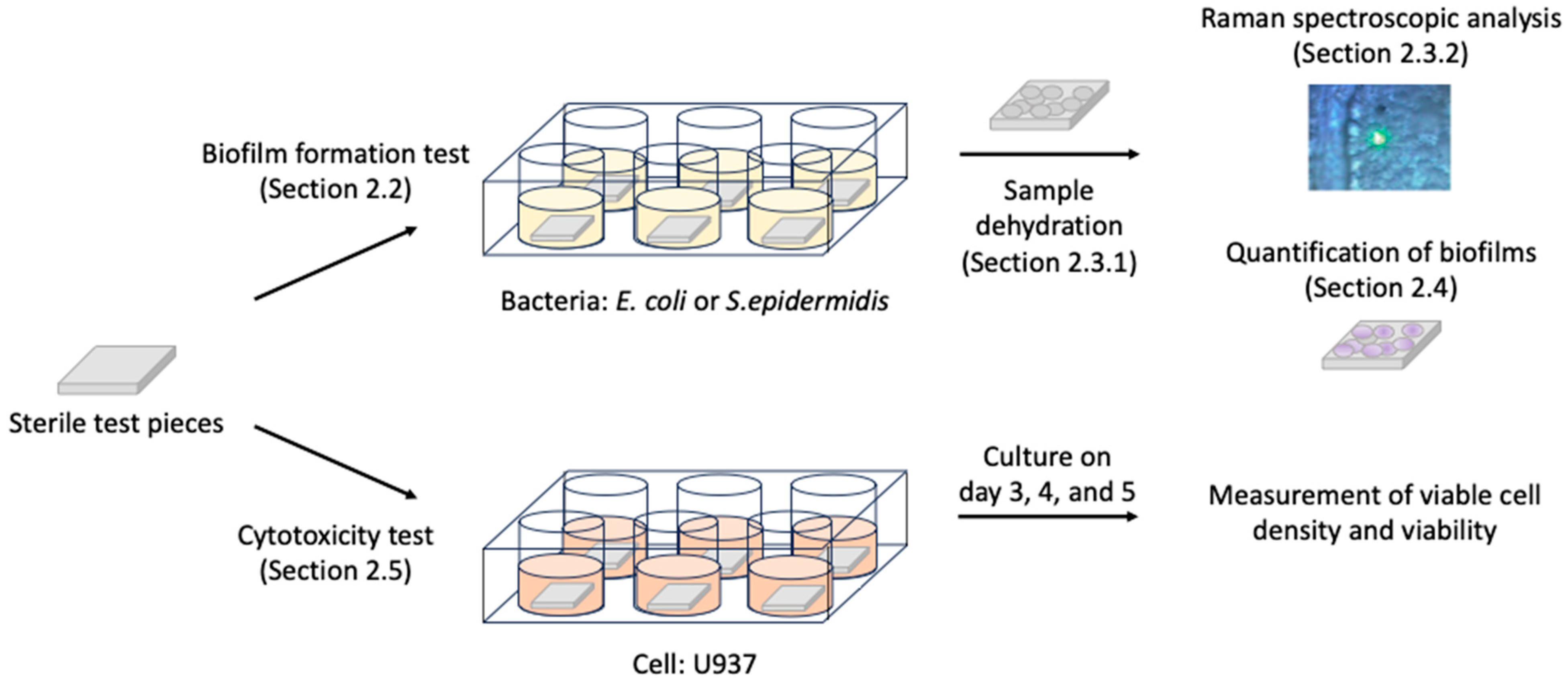
2. Materials and Methods
2.1. Specimens
2.1.1. Preparation of Siloxane-Based Polymer Coating
- An alkylalkoxysilane permeate having methyl and phenyl groups (8.0 g, D&D Co.) was mixed with N-2-(aminomethyl)-3-aminopropyltrimethoxysilane (2.0 g, KBM-603, Shin-Etsu Chemical Co., Tokyo, Japan) in a 100 mL polypropylene cup at 1000 rpm for 10 min using a stirring device (PRIMIX, Tokyo, Japan).
- The mixture was filtered with a #110 nylon mesh (AS ONE Co.).
- The filtered mixture was packed into an air-spray gun (Airtex, Osaka, Japan).
- The coating reagent (from (3)) was applied to the clean test pieces until the thickness of the coating was about 10 μm.
- After application, the coated test pieces were allowed to cure at 18–25 °C for seven days.
2.1.2. Sterilization of Specimens
2.2. Biofilm Formation Test
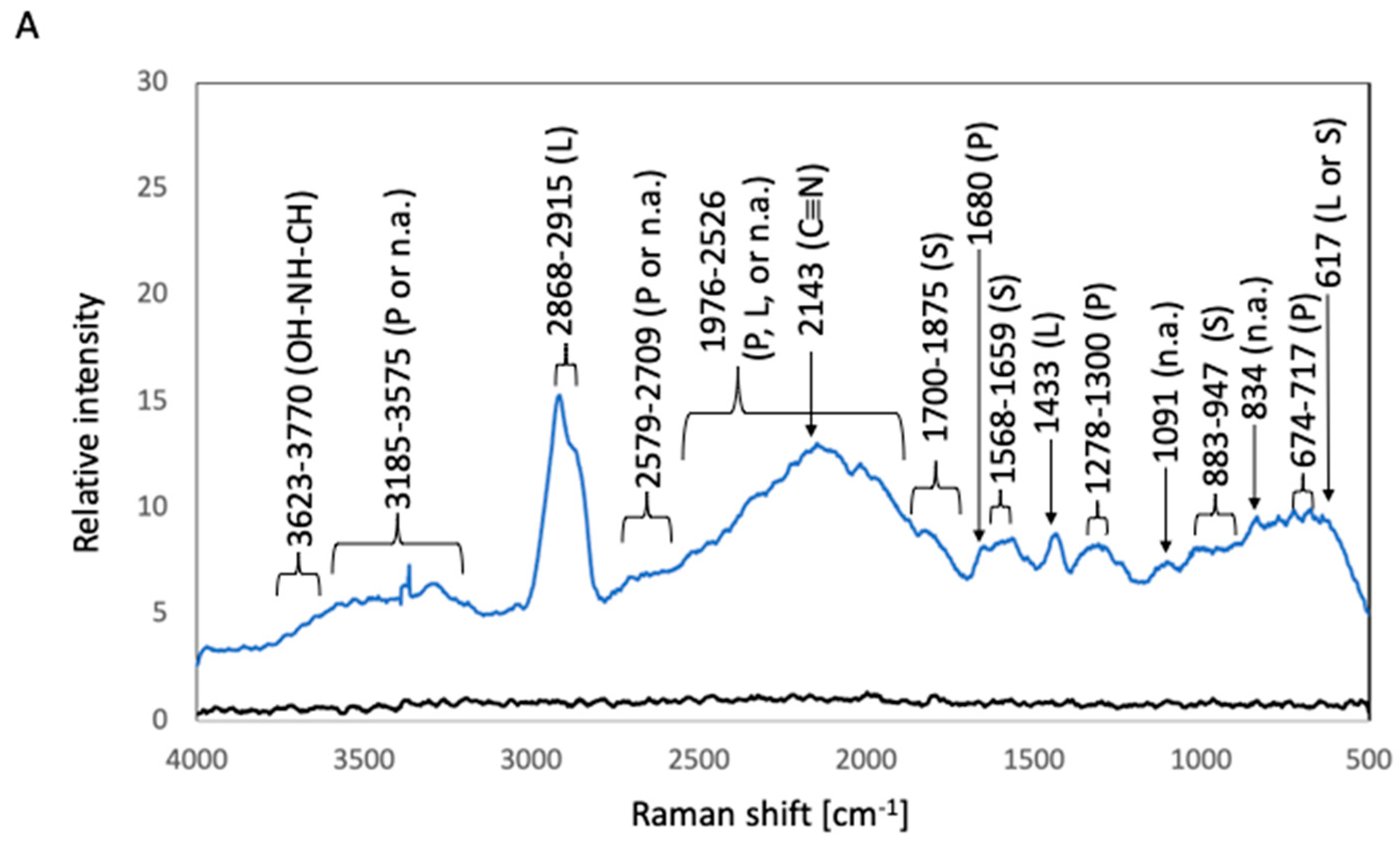
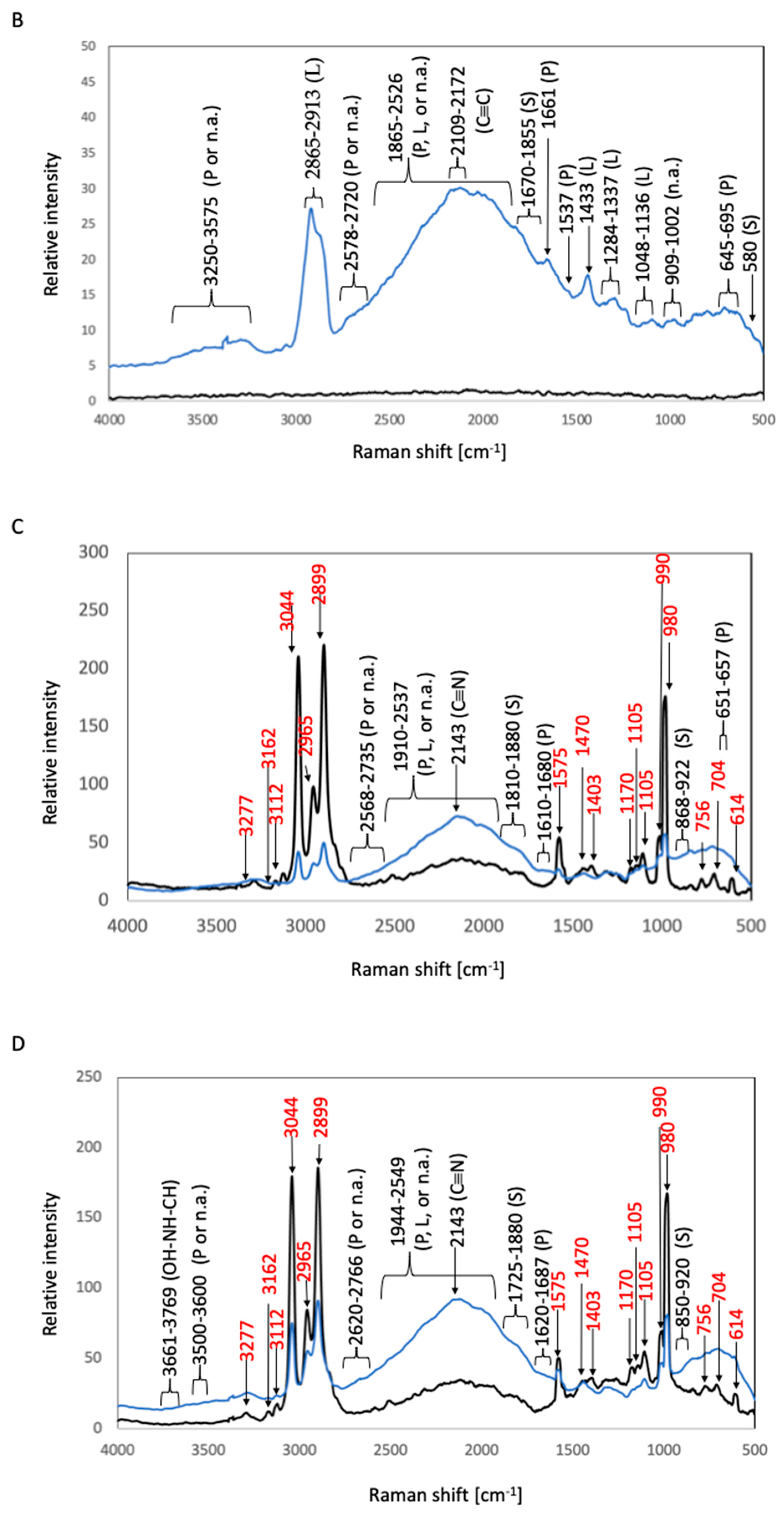
2.3. Sample Preparation for Raman Spectroscopic Analysis
2.3.1. Sample Dehydration
- One mL of 30% (v/v) ethanol was added to the sample case and left for 15 min, then aspirated out using a micropipette.
- The same procedure as in step 2 was repeated with 50% (v/v), 60% (v/v), 70% (v/v), 80% (v/v), 90% (v/v), 95% (v/v), 98% (v/v), and 99.5% (v/v) ethanol followed by 30% (v/v) t-butanol/70% (v/v) ethanol, 50% (v/v) t-butanol/50% (v/v) ethanol, 70% (v/v) t-butanol/30% (v/v) ethanol, and t-butanol, in that order.
- The samples were left in a refrigerator (5–10 °C) for 30 min.
- The sample cases were placed in a desiccator (AS ONE) with the lids open, connected to an aspirator (AS ONE), and aspirated for 60 min to dry the solvent completely.
- The dehydrated samples were stored in desiccators (AS ONE) until the Raman spectroscopic observation was performed.
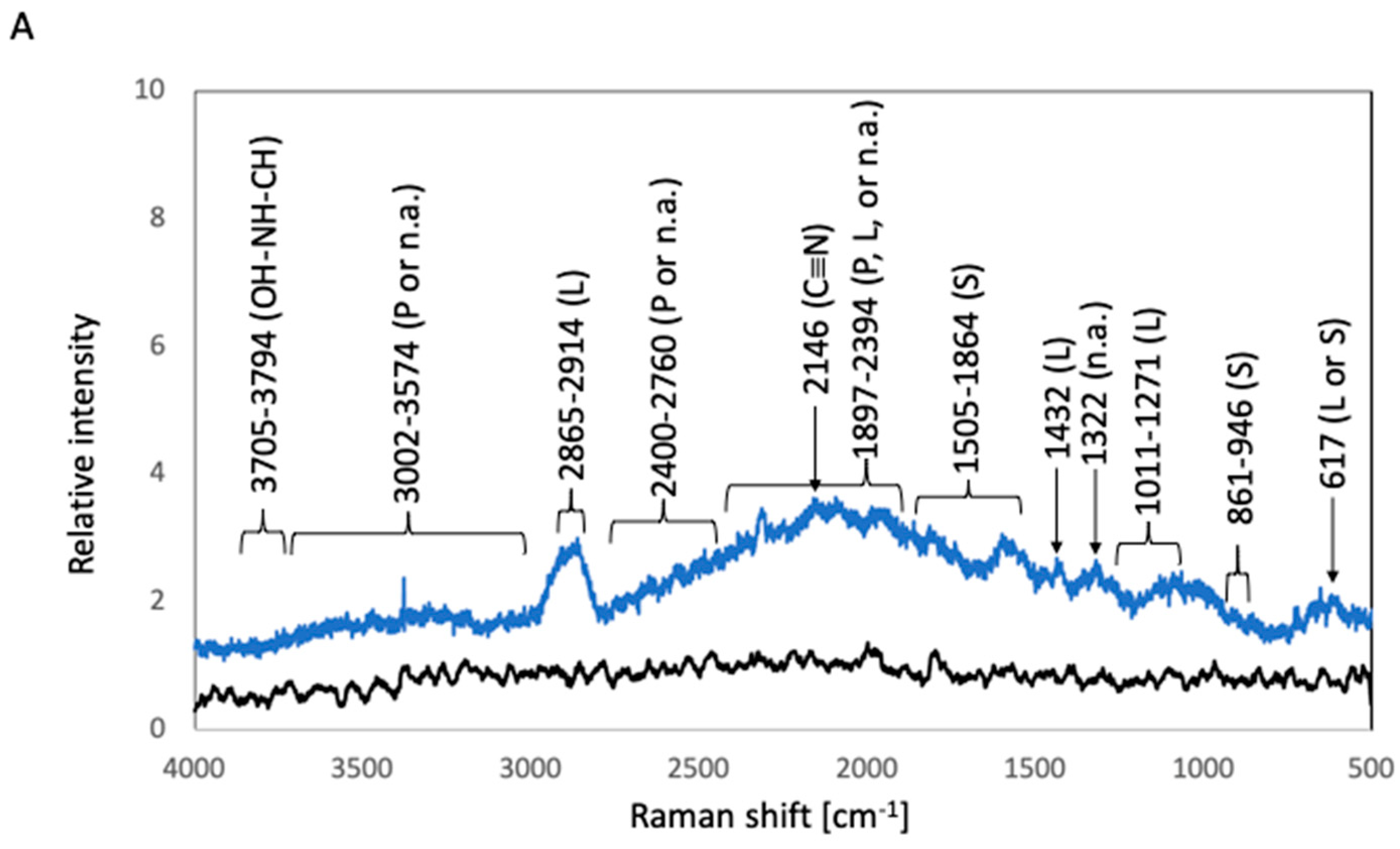
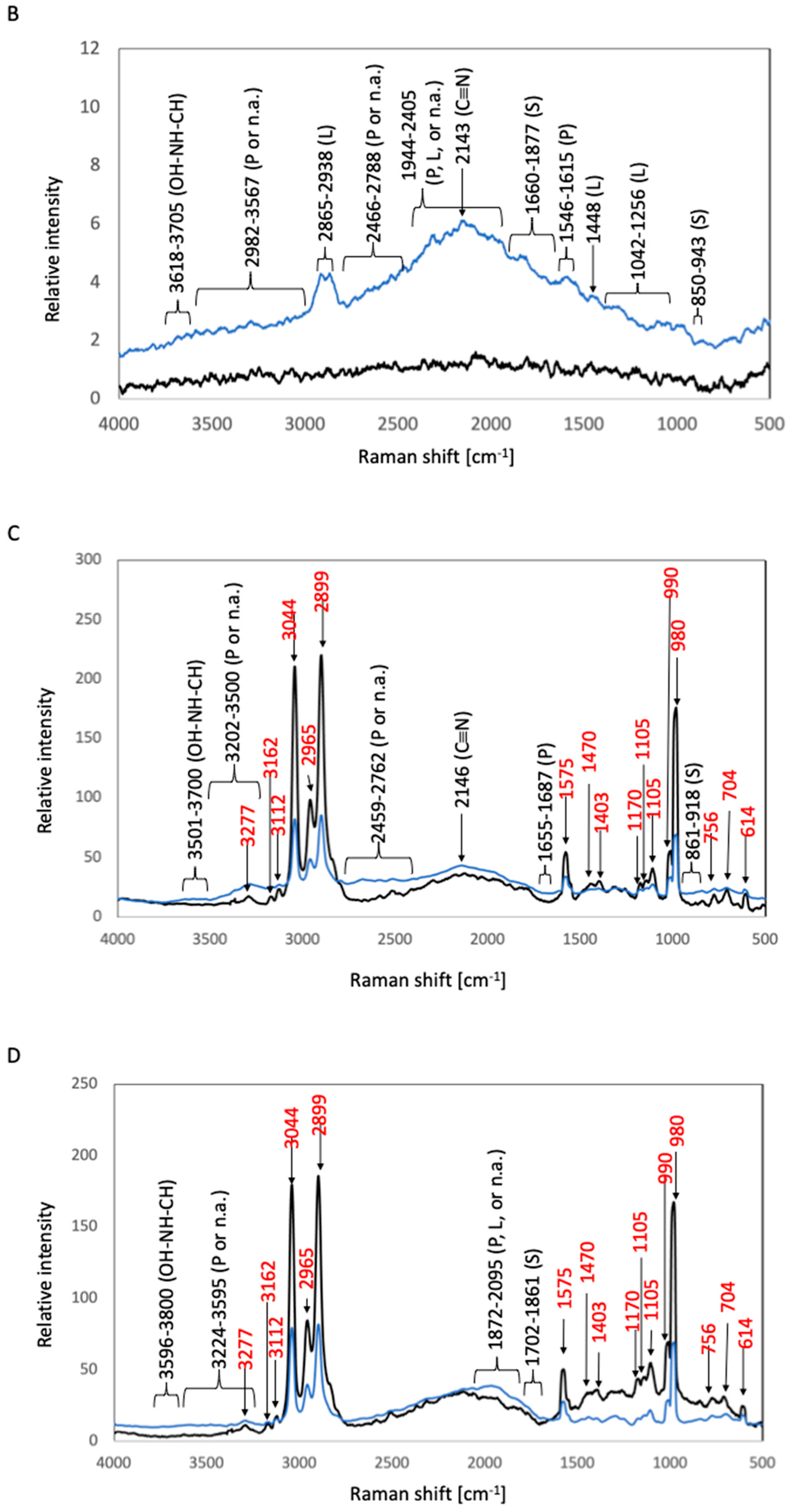
2.3.2. Raman Spectroscopic Analysis
2.4. Quantification of Biofilms
2.5. Cytotoxicity Test
2.6. Statistical Analysis
3. Results
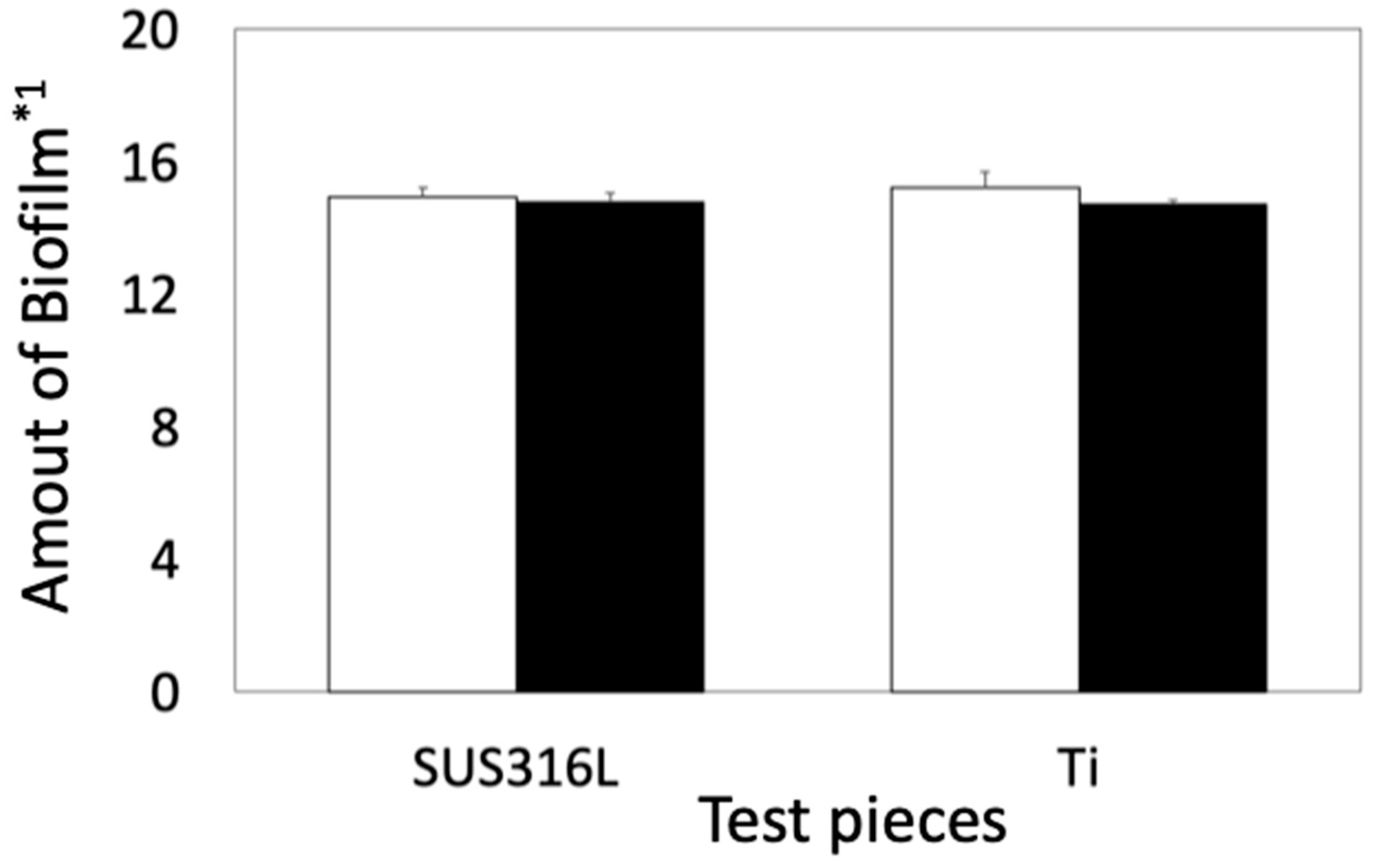
3.1. Biofilm Formation Test
3.1.1. Biofilm Formation Test in E. coli
3.1.2. Biofilm Formation Test in S. epidermidis
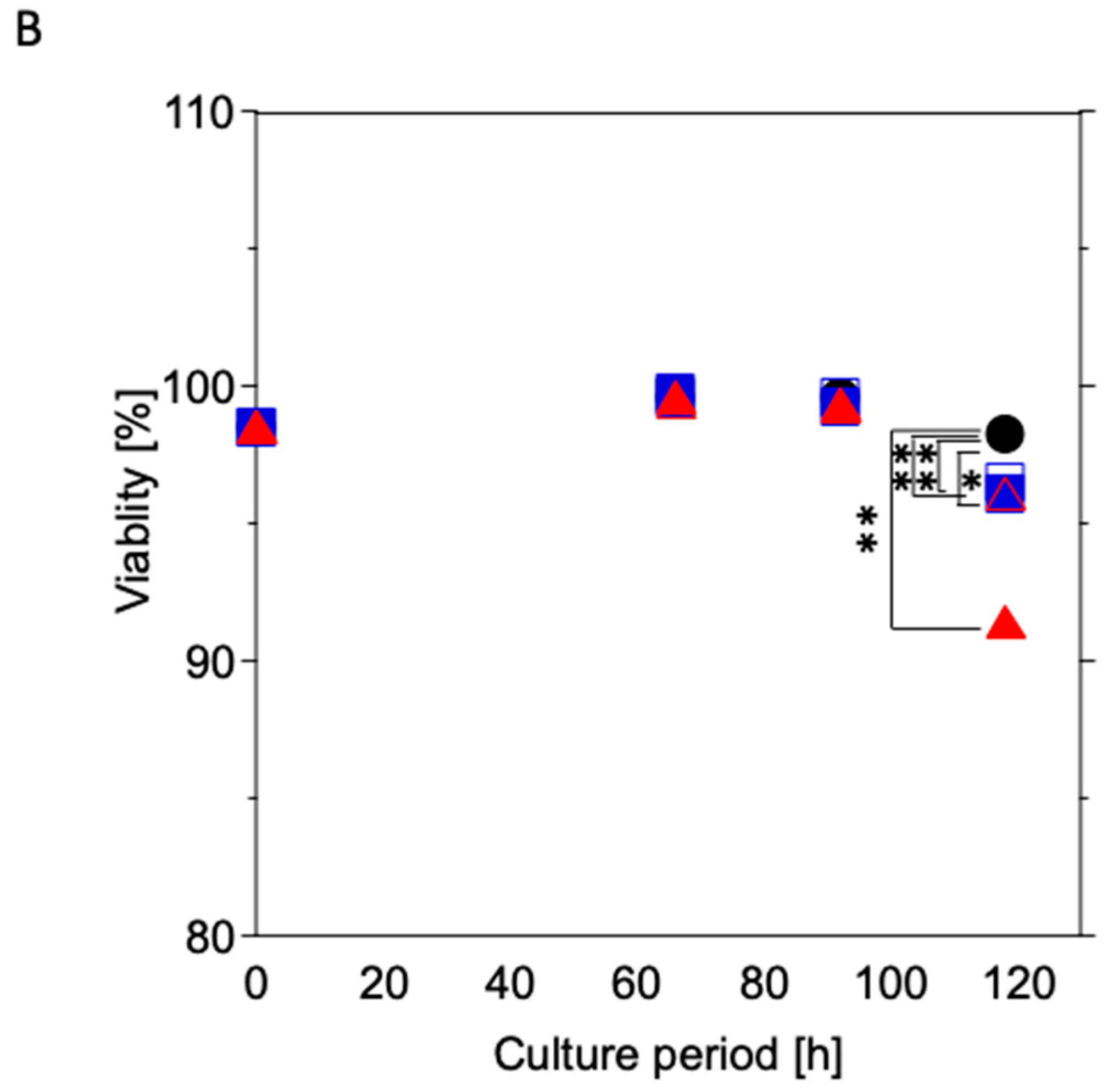
3.2. Cytotoxicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Sano, K.; Kanematsu, H.; Tanaka, T. Overview of silane-based polymer coatings and their applications. In Industrial Applications for Intelligent Polymers and Coatings; Hosseini, M., Makhlouf, H.A.S., Eds.; Springer International Publishing: Berlin, Germany, 2016; pp. 493–509. [Google Scholar] [CrossRef]
- Ogawa, A.; Kanematsu, H.; Sano, K.; Sakai, Y.; Ishida, K.; Beech, I.B.; Suzuki, O.; Tanaka, T. Effect of silver or copper nanoparticles-dispersed Silane coatings on biofilm formation in cooling water systems. Materials 2016, 9, 632. [Google Scholar] [CrossRef]
- Ekoi, E.J.; Gowen, A.; Dorrepaal, R.; Dowling, D.P. Characterisation of titanium oxide layers using Raman spectroscopy and optical profilometry: Influence of oxide properties. Results Phys. 2019, 12, 1574–1585. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hinotani, S.; Yamanaka, K. Characterization of oxide layers on SUS304L stainless steel by Raman spectroscopy. Tetsu-Hagane 1993, 79, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P. Chapter 6, IR and Raman spectra-structure correlations: Characteristic group frequencies. In Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2011; pp. 73–115. [Google Scholar] [CrossRef]
- Larkin, P. Chapter 7, General outline and strategies for IR and Raman spectral interpretation. In Infrared and Raman Spectroscopy; Larkin, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 117–133. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Benevides, J.M.; Overman, S.A.; Thomas, G.J. Raman, polarized Raman and ultraviolet resonance Raman spectroscopy of nucleic acids and their complexes. J. Raman Spectrosc. 2005, 36, 279–299. [Google Scholar] [CrossRef]
- Sano, K.; Kanematsu, H.; Kogo, T.; Hirai, N.; Tanaka, T. Corrosion and biofilm for a composite coated iron observed by FTIR-ATR and Raman spectroscopy. Trans. IMF 2016, 94, 139–145. [Google Scholar] [CrossRef]
- Thomas, G.J. Raman spectroscopy of protein and nucleic acid assemblies. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 1–27. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Yuen, S.-N.; Choi, S.-M.; Phillips, D.L.; Ma, C.-Y. Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Kalampounias, A.G. IR and Raman spectroscopic studies of sol–gel derived alkaline-earth silicate glasses. Bull. Mater. Sci. 2011, 34, 299–303. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, T. Surface-enhanced Raman scattering (SERS) revealing chemical variation during biofilm formation: From initial attachment to mature biofilm. Anal. Bioanal. Chem. 2012, 404, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-h.; Du, C.-m.; Yang, X. Recovery of phosphorus from steelmaking slag and phosphate tailings by a collaborative processing method. Sep. Purif. Technol. 2023, 313, 123499. [Google Scholar] [CrossRef]
- Adams, H.; Teertstra, W.; Koster, M.; Tommassen, J. PspE (phage-shock protein E) of Escherichia coli is a rhodanese. FEBS Lett. 2002, 518, 173–176. [Google Scholar] [CrossRef]
- Hänzelmann, P.; Dahl, J.U.; Kuper, J.; Urban, A.; Müller-Theissen, U.; Leimkühler, S.; Schindelin, H. Crystal structure of YnjE from Escherichia coli, a sulfurtransferase with three rhodanese domains. Protein Sci. 2009, 18, 2480–2491. [Google Scholar] [CrossRef]
- Lei, L.; Su, J.; Chen, J.; Chen, W.; Chen, X.; Peng, C. The role of lysophosphatidic acid in the physiology and pathology of the skin. Life Sci. 2019, 220, 194–200. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Hirota, M.; Sugita, Y.; Ishijima, M.; Ikeda, T.; Saruta, J.; Maeda, H.; Ogawa, T. UV photocatalytic activity of titanium dioxide (TiO2) surface contaminated with bacterial biofilm: Implications for photo-restoration of osteoconductivity. Mater. Today Adv. 2021, 12, 100182. [Google Scholar] [CrossRef]
- Sano, K.; Kanematsu, H.; Hirai, N.; Ogawa, A.; Kougo, T.; Tanaka, T. The development of the anti-biofouling coating agent using metal nanoparticles and analysis by Raman spectroscopy and FIB system. Surf. Coat. Technol. 2017, 325, 715–721. [Google Scholar] [CrossRef]
- Ogawa, A.; Sano, K. Effect of metallic nanoparticle-dispersed silane-based polymer coatings on anti-biofilm formation. In Monitoring Artificial Materials and Microbes in Marine Ecosystems: Interactions and Assessment Methods; Takahashi, T., Ed.; Bentham Science Publisher: Singapore, 2020; pp. 188–201. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, A.; Tahori, A.; Yano, M.; Hirobe, S.; Terada, S.; Kanematsu, H. Antibiofilm Property and Biocompatibility of Siloxane-Based Polymer Coatings Applied to Biomaterials. Materials 2023, 16, 7399. https://doi.org/10.3390/ma16237399
Ogawa A, Tahori A, Yano M, Hirobe S, Terada S, Kanematsu H. Antibiofilm Property and Biocompatibility of Siloxane-Based Polymer Coatings Applied to Biomaterials. Materials. 2023; 16(23):7399. https://doi.org/10.3390/ma16237399
Chicago/Turabian StyleOgawa, Akiko, Akane Tahori, Mayumi Yano, Shunma Hirobe, Satoshi Terada, and Hideyuki Kanematsu. 2023. "Antibiofilm Property and Biocompatibility of Siloxane-Based Polymer Coatings Applied to Biomaterials" Materials 16, no. 23: 7399. https://doi.org/10.3390/ma16237399
APA StyleOgawa, A., Tahori, A., Yano, M., Hirobe, S., Terada, S., & Kanematsu, H. (2023). Antibiofilm Property and Biocompatibility of Siloxane-Based Polymer Coatings Applied to Biomaterials. Materials, 16(23), 7399. https://doi.org/10.3390/ma16237399






