Preparation of Steel-Slag-Based Hydrotalcite and Its Adsorption Properties on Cl− and SO42−
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Acidolysis of Steel Slag
2.3. Preparation of Hydrotalcite
2.4. Adsorption of Cl− and SO42− from Salt-Washing Wastewater by Hydrotalcite
- q: Adsorption amount of hydrotalcite (mg/g);
- C0: Initial concentration of Cl− and SO42− (mg/L);
- Cr: Ion concentration (mg/L) at the end of adsorption;
- V: Volume of solution (L);
- m: Amount of hydrotalcite added (g).
2.5. Testing Methods
3. Results
3.1. Determination of Steel Slag Acidolysis System
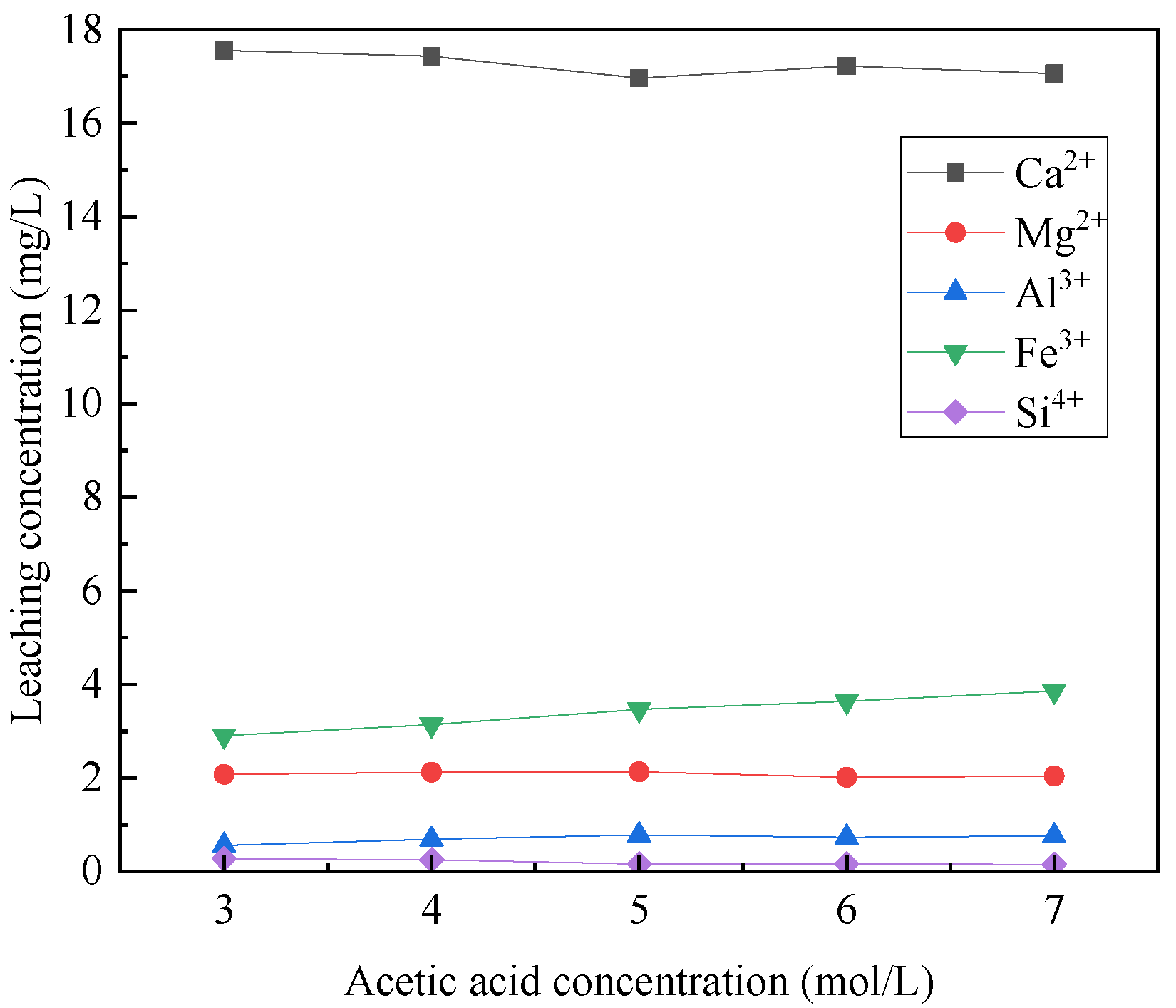
3.1.1. The Effect of Acetic Acid Concentration
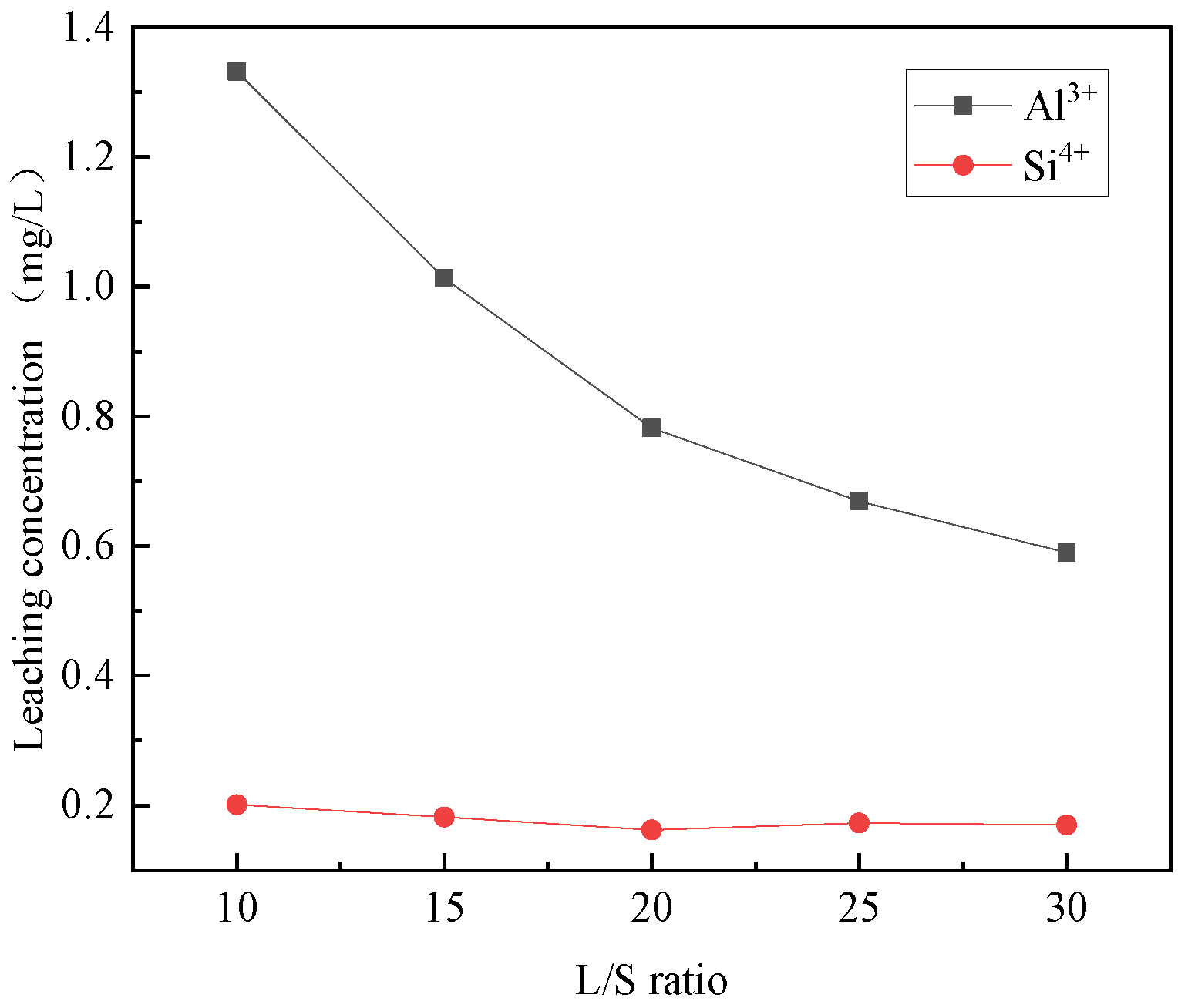
3.1.2. The Effect of Liquid–Solid Ratio
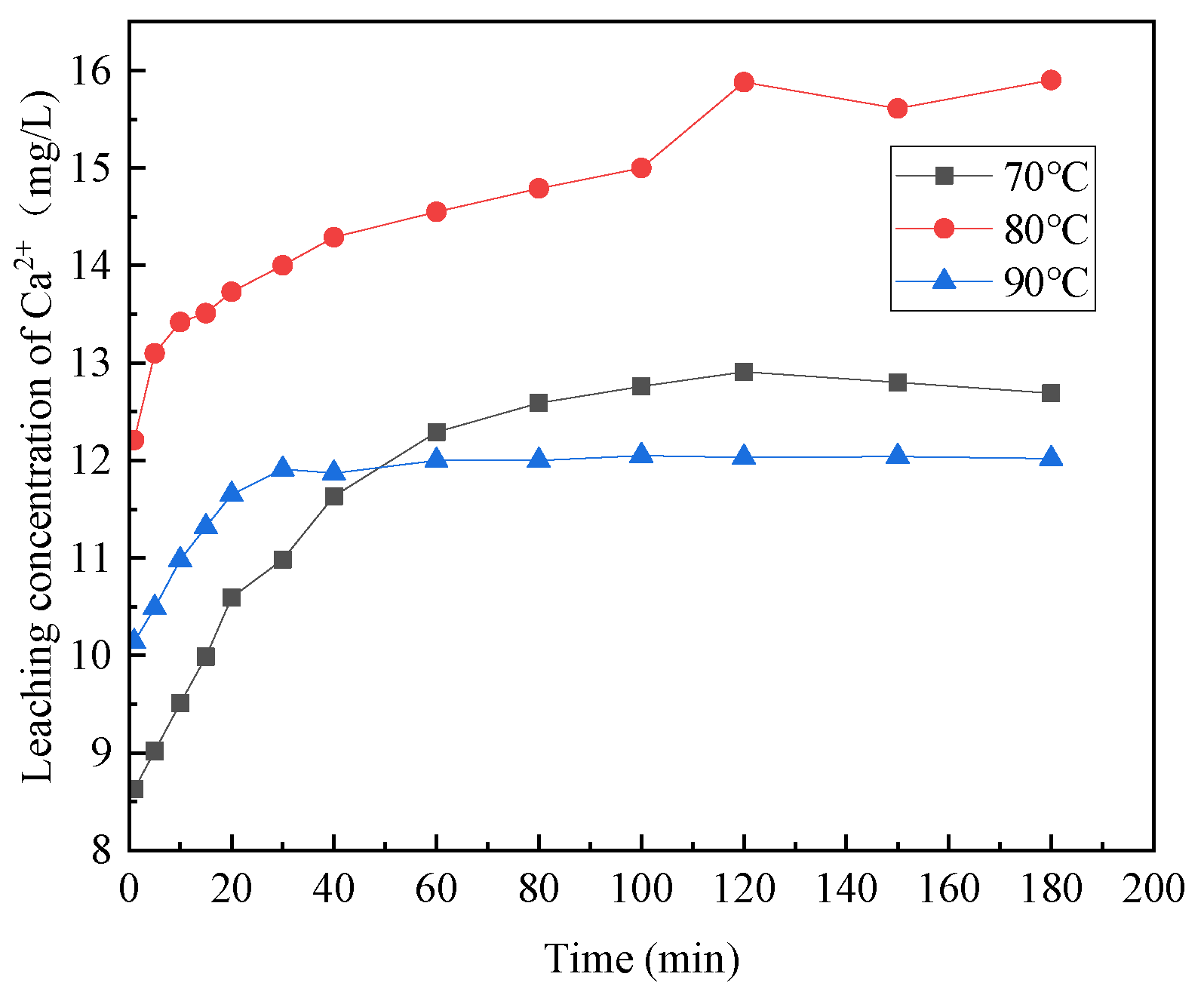
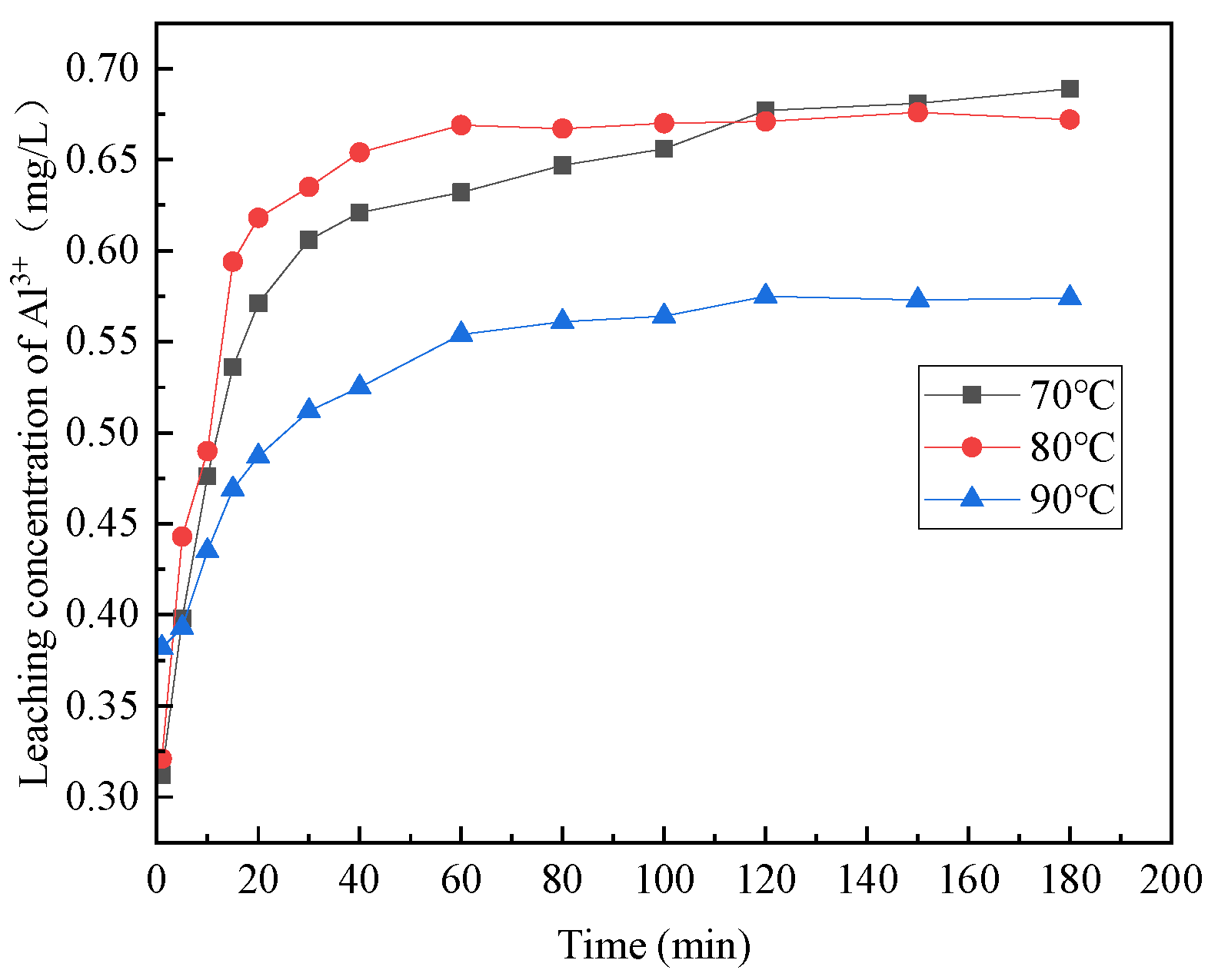
3.1.3. The Effect of Reaction Temperature
3.2. Preparation of Steel-Slag-Based Hydrotalcite
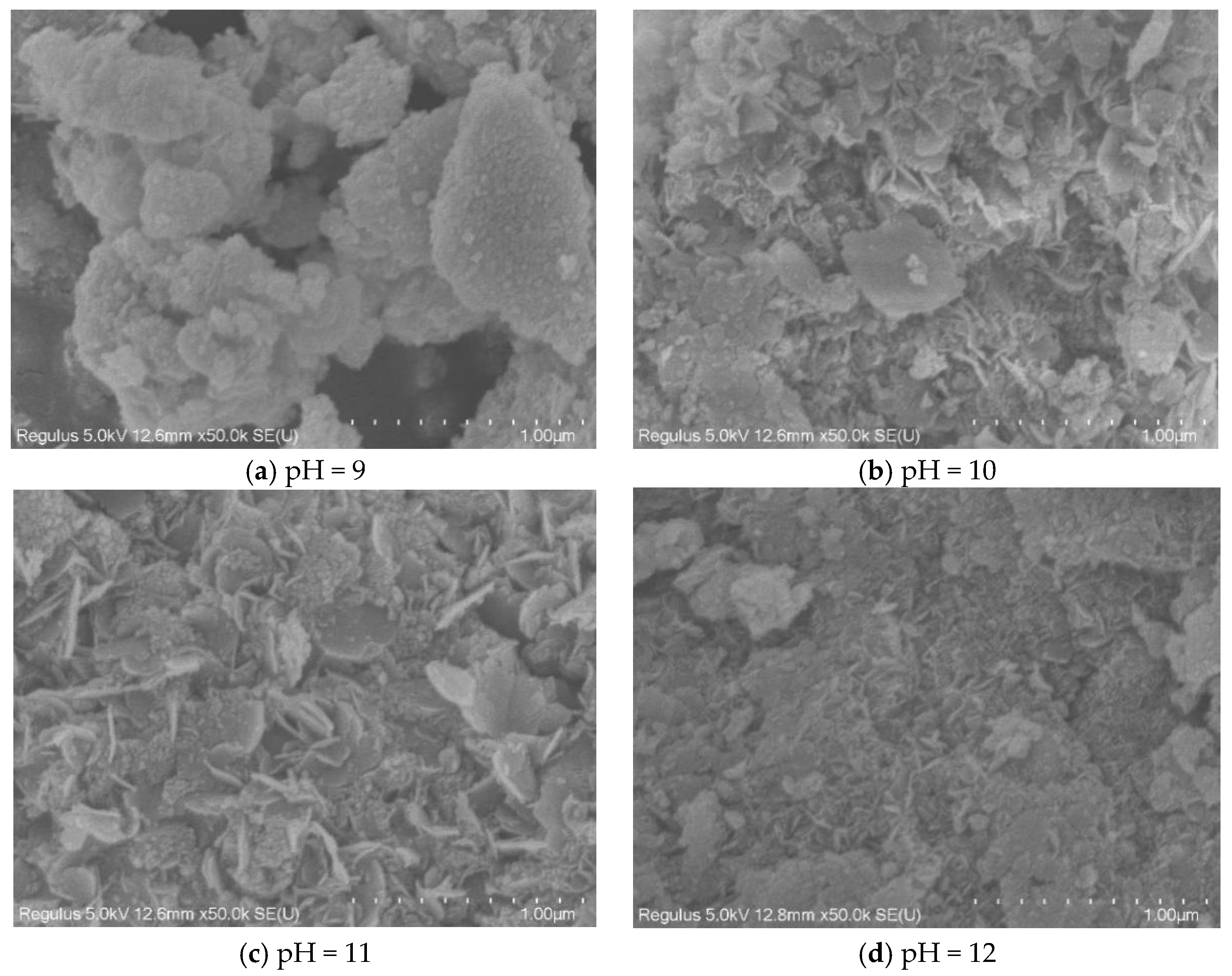
3.2.1. The Effect of pH
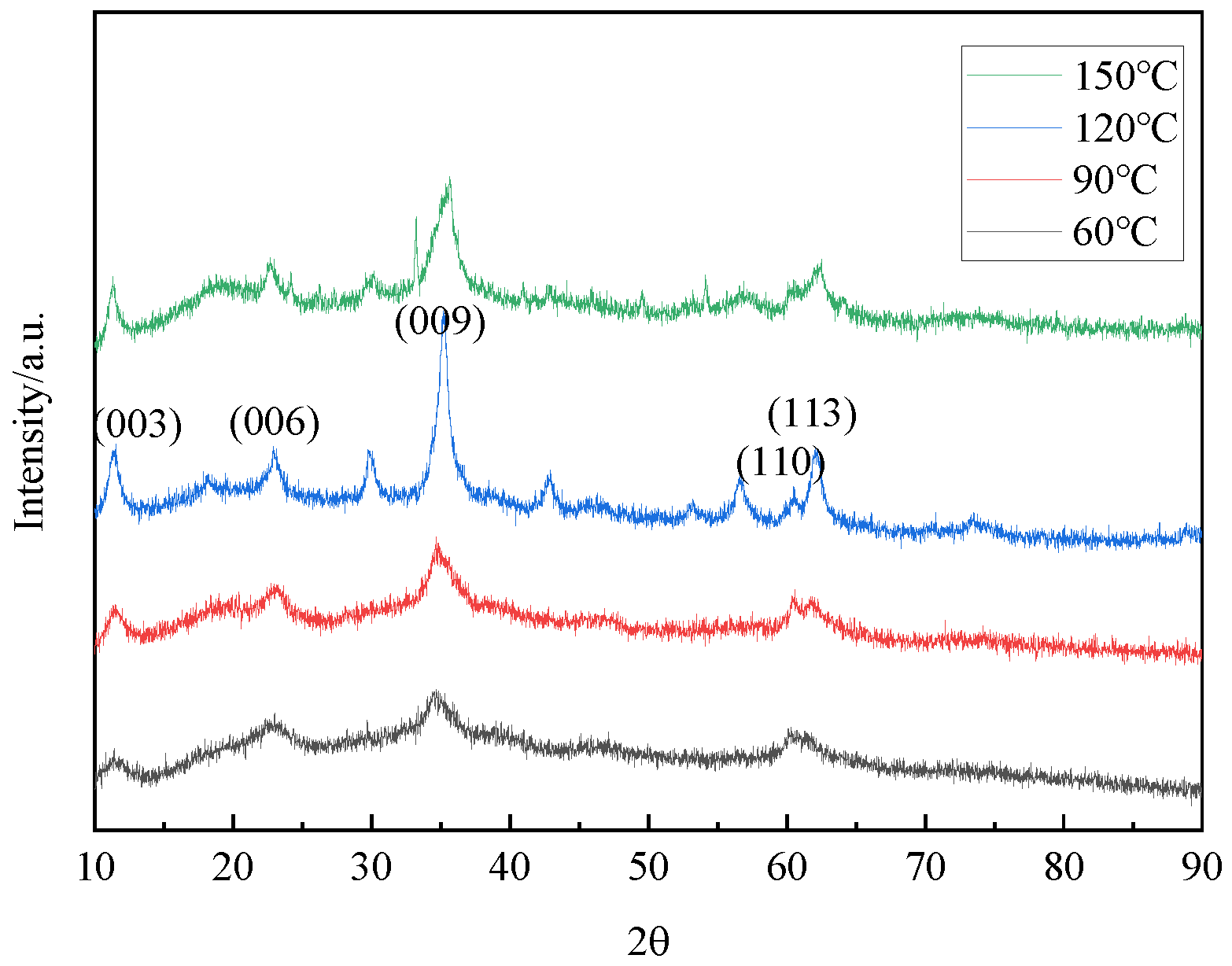
3.2.2. The Effect of Reaction Temperature
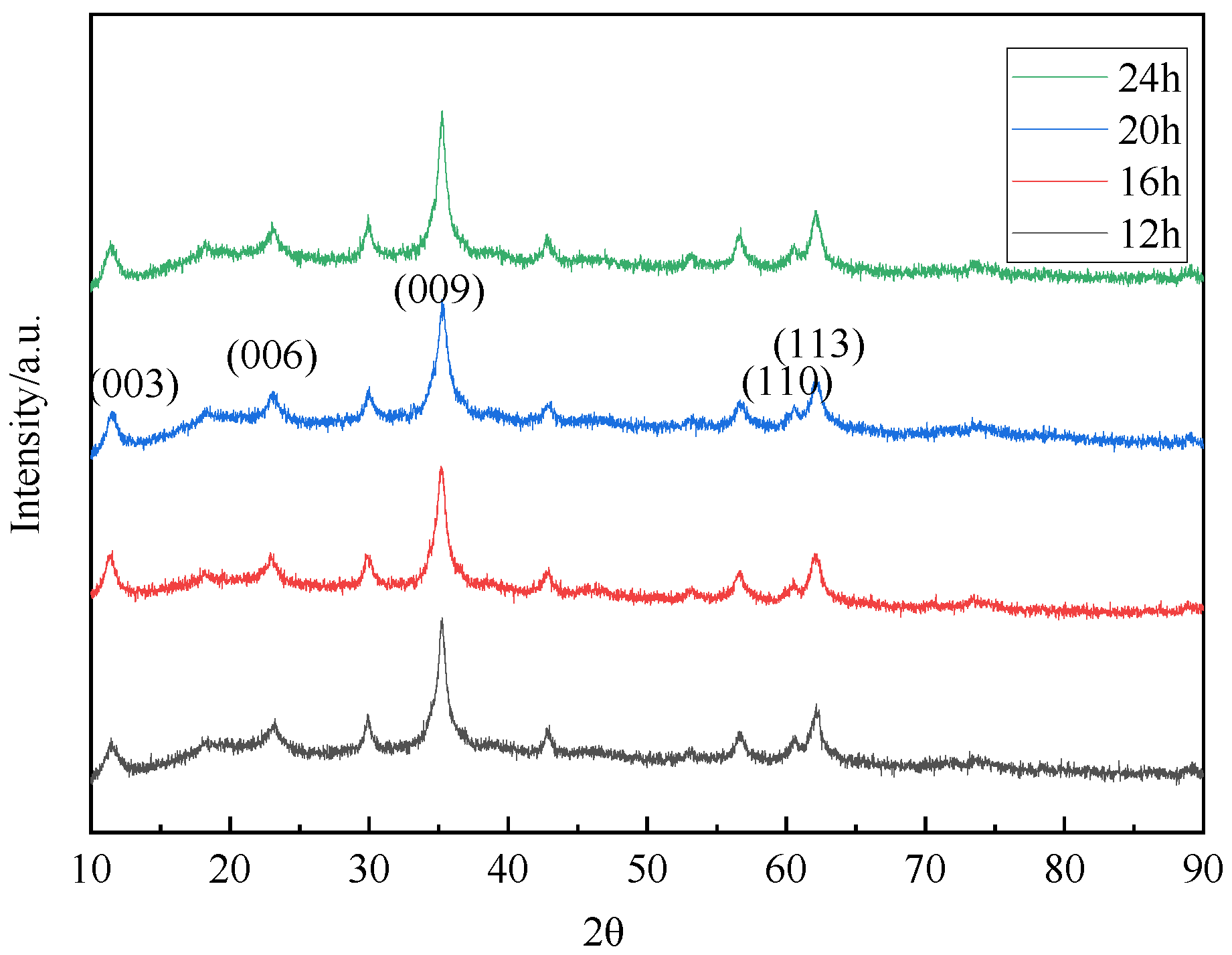
3.2.3. The Effect of Reaction Time
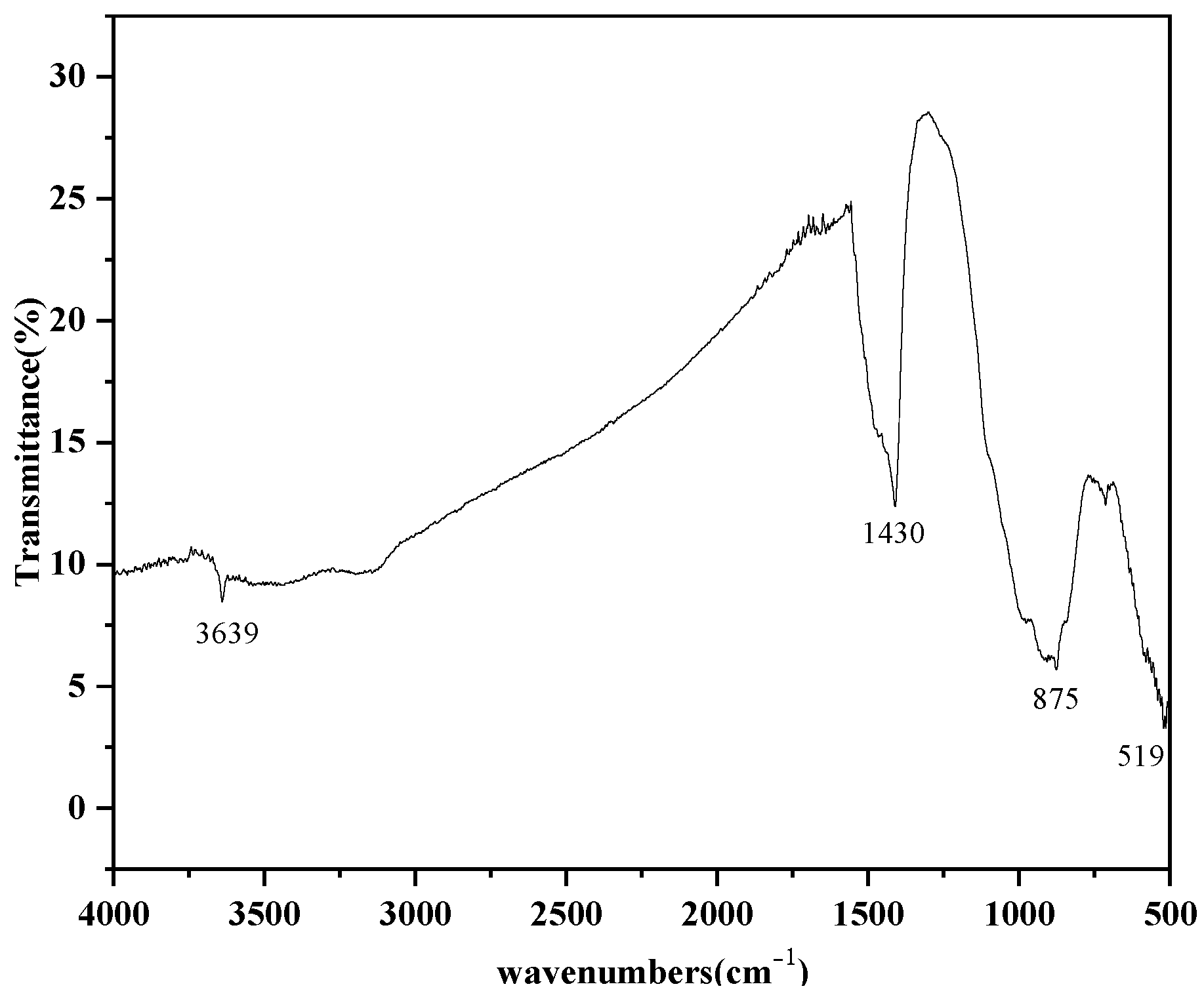
3.3. Characterization of Steel-Slag-Based Hydrotalcite
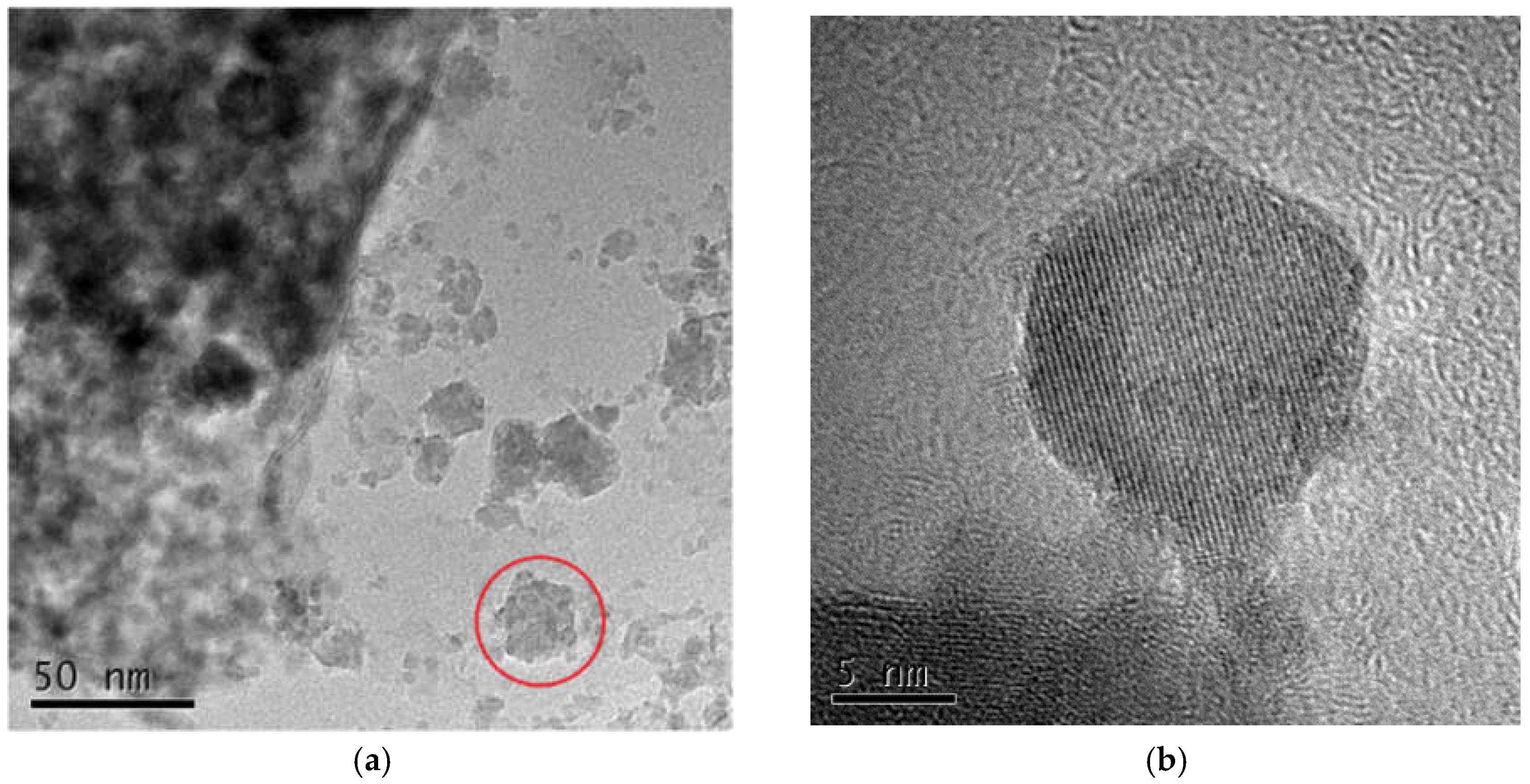
3.3.1. Morphology of Hydrotalcite
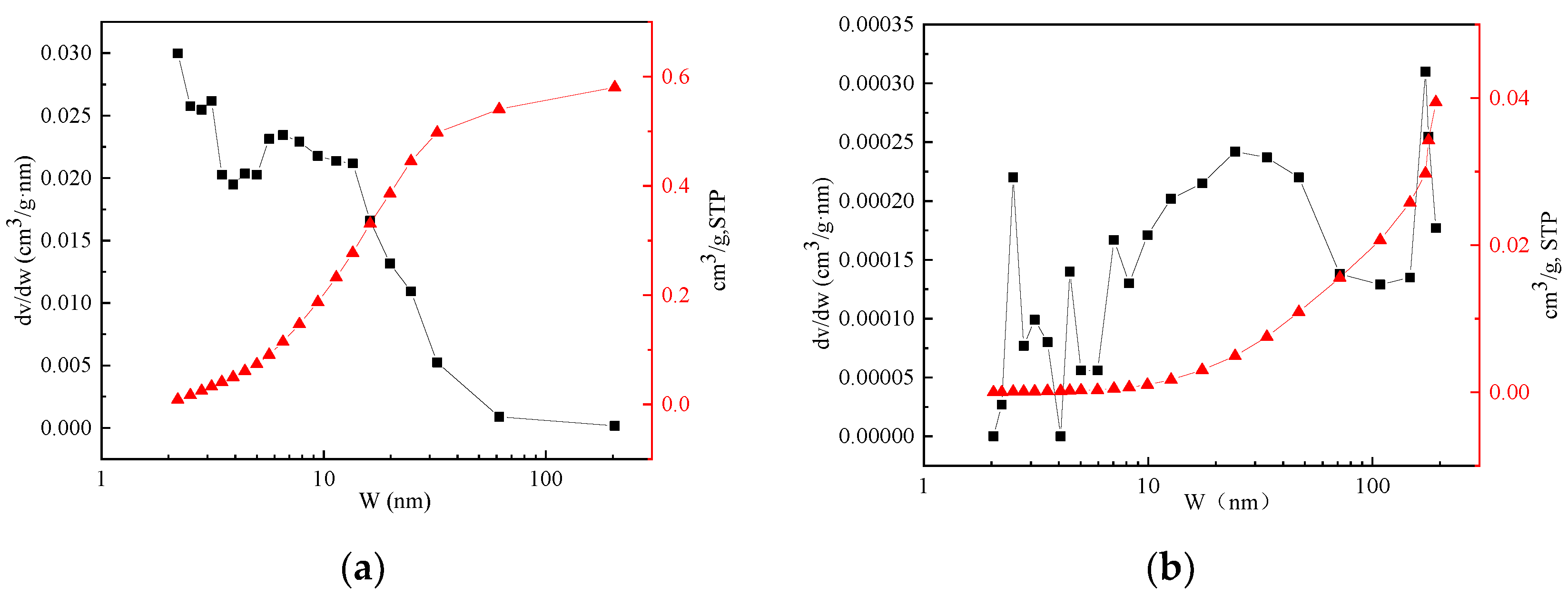
3.3.2. Specific Surface Area Analysis of Hydrotalcite and Steel Slag
3.4. Adsorption of Cl− and SO42− from Salt-Washing Wastewater by Steel-Slag-Based Hydrotalcite
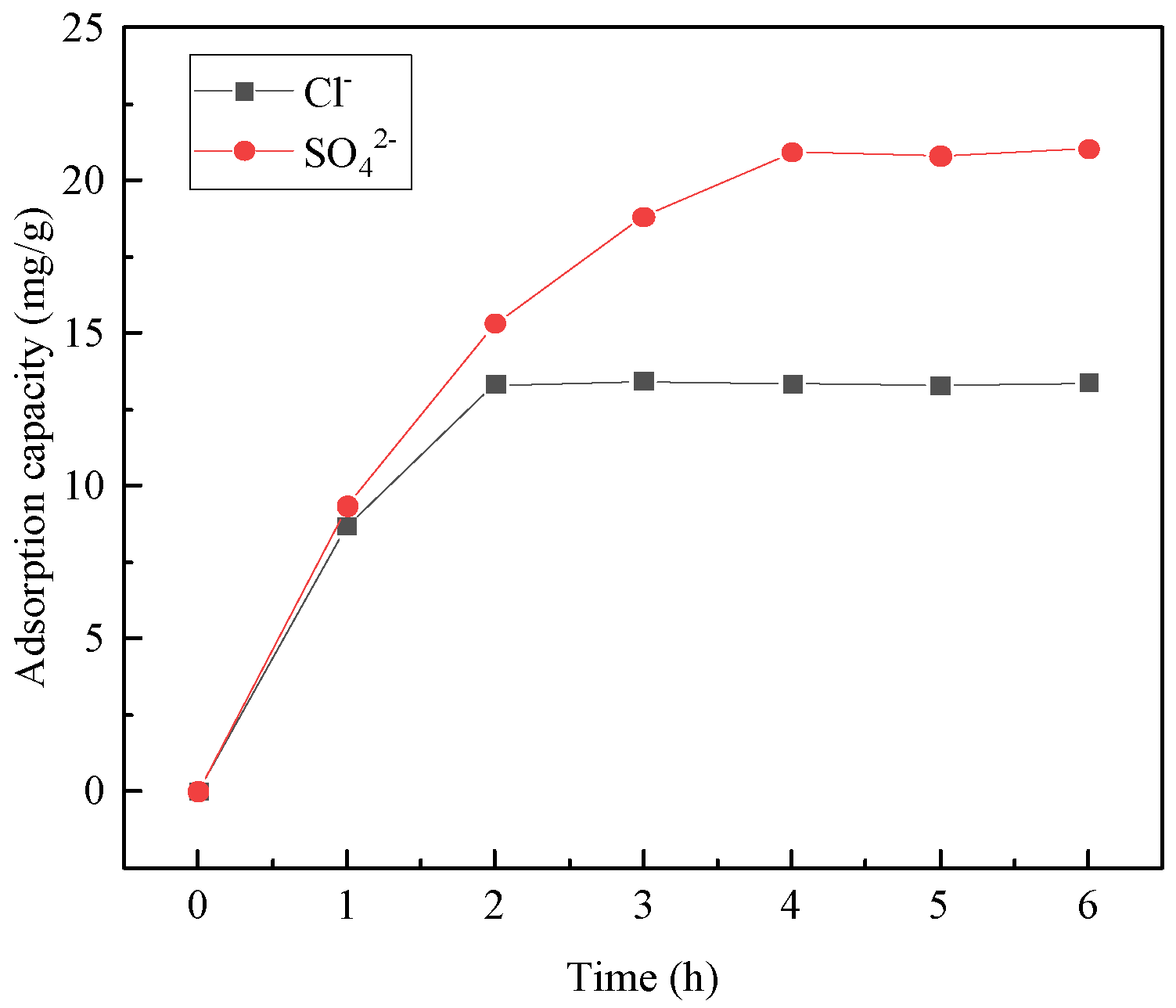
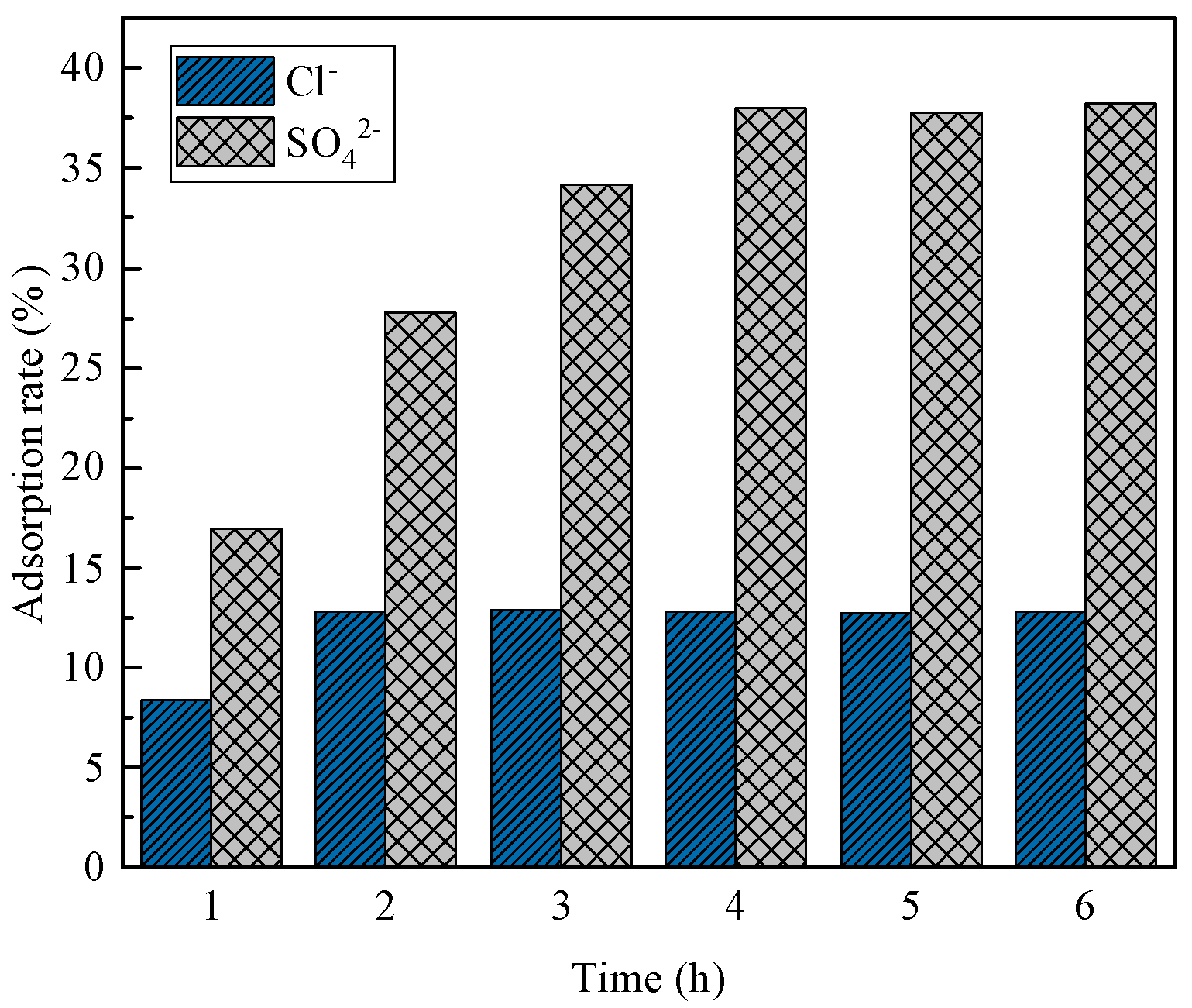
3.4.1. Effect of Adsorption Time
3.4.2. Multiple Adsorption of Steel-Slag-Based Hydrotalcite on Salt-Washing Wastewater
4. Conclusions
- The acidolysis of steel slag exhibited the highest efficiency when conducted under specific conditions: an acetic acid concentration of 5 mol/L, liquid–solid ratio of 25:1, reaction temperature of 80 °C, and reaction time of 2 h. The ratio of trivalent metal cations to the total sum of divalent and trivalent metal cations in the leaching solution was determined to be 0.167. The relatively high concentration of metal cations in the leaching solution proved advantageous for the production of a greater quantity of hydrotalcite.
- The optimum process conditions for the preparation of hydrotalcite by the hydrothermal method were pH 11, a reaction temperature of 120 °C, and reaction time of 12 h. FT-IR, FESEM, and TEM were used to analyze the hydrotalcite samples under the optimum conditions. The analytical results showed that the prepared hydrotalcite had a hexagonal lamellar structure, regular crystal structure, large specific surface area, and layered structure with ordered atomic arrangement.
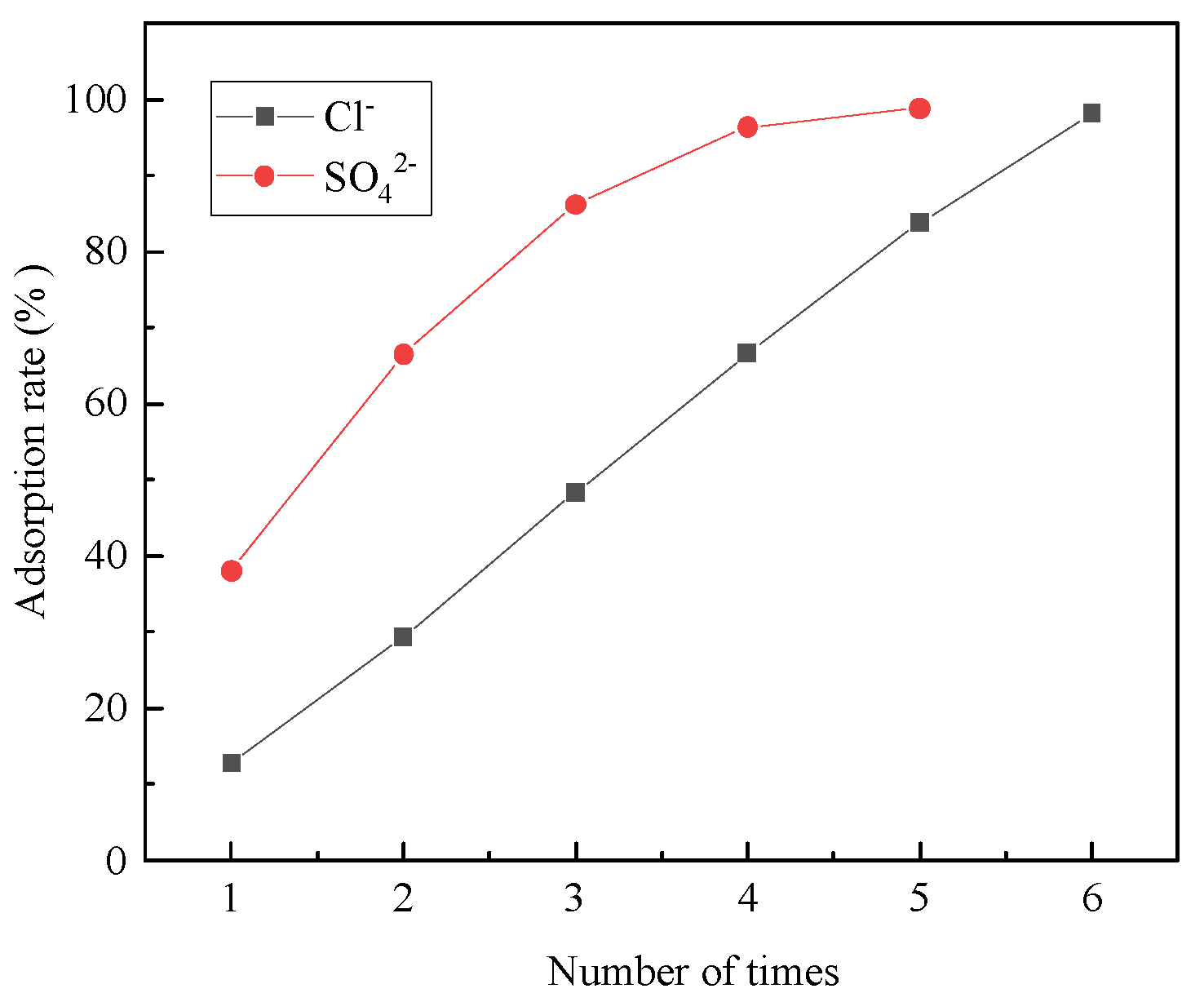
- In the adsorption experiments of steel-slag-based hydrotalcite in salt-washing wastewater, the adsorption amount of Cl− per unit mass of hydrotalcite was 13.3 mg/g, and the removal rate of Cl− in salt-washing wastewater reached 12.8%; the adsorption amount of SO42− per unit mass of hydrotalcite was 20.9 mg/g, and the removal rate of SO42− reached 38%. The adsorption of salt-washing wastewater was carried out several times, and it was found that the removal rate of SO42− reached 96.4% in the fourth adsorption, and the removal rate of Cl− reached 98% in the sixth adsorption. These findings indicated that the adsorption process effectively eliminated Cl− and SO42− from the salt-washing wastewater, essentially eliminating their presence.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapourchal, S.A.; Homaee, M. Minimizing freshwater usage for quality improvement of saline soils in semi-arid regions. Irrig. Drain. 2022, 71, 1240–1253. [Google Scholar] [CrossRef]
- Barnard, J.H.; Rensburg, L.D.; Bennie, A.T. Leaching irrigated saline sandy to sandy loam apedal soils with water of a constant salinity. Irrig. Sci. 2010, 28, 191–201. [Google Scholar] [CrossRef]
- Huang, J.; Kong, Y.; Xu, Q.; Zhu, C.; Zhu, L.; Cao, X.; Hong, X.; Zhang, J. Progresses for Characteristics and Amelioration Measures of Saline Soil. Soils 2022, 54, 18–23. [Google Scholar]
- Duan, W.; Li, G.; Wang, Z.; Wang, D.; Yu, Q.; Zhan, Y. Highly efficient production of hydrotalcite-like compounds from blast furnace slag. Appl. Clay Sci. 2022, 219, 106441. [Google Scholar] [CrossRef]
- Guo, Q.; Yin, C.; Chen, Y.; Fang, Z.; Xiao, H.; He, J.; Huang, L.; Wu, G.; Zeng, Z.; Shen, F.; et al. Periodic pH regulation controls the phosphate uptake-release behavior and structural evolution of layered double hydroxides. Chem. Eng. J. 2023, 459, 141584. [Google Scholar] [CrossRef]
- Hong, X.; Zhu, S.; Xia, M.; Du, P.; Wang, F. Investigation of the efficient adsorption performance and adsorption mechanism of 3D composite structure La nanosphere-coated Mn/Fe layered double hydrotalcite on phosphate. J. Colloid Interface Sci. 2022, 614, 478–488. [Google Scholar] [CrossRef]
- Cai, H.; Ma, K.; Zhang, Y.; Li, X.; Wang, W.; Tong, S. Carbonizing hollow metal–organic framework/layered double hydroxide (MOF/LDH) nanocomposite with excellent adsorption capacity for removal of Pb(II) and organic dyes from wastewater. Carbon Res. 2023, 2, 23. [Google Scholar] [CrossRef]
- Dai, X.; Yi, W.; Yin, C.; Li, K.; Feng, L.; Zhou, Q.; Yi, Z.; Zhang, X.; Wang, Y.; Yu, Y.; et al. 2D-3D magnetic NiFe layered double hydroxide decorated diatomite as multi-function material for anionic, cationic dyes, arsenate, and arsenite adsorption. Appl. Clay Sci. 2022, 229, 106664. [Google Scholar] [CrossRef]
- Martin, G.P.; Robinson, A.J.; Worsley, D.A. Hydrotalcite mineral stabilization of titanium dioxide photocatalysed degradation of unplasticised polyvinylchloride. Mater. Sci. Technol. 2006, 22, 375–378. [Google Scholar] [CrossRef]
- Cui, P.; Wu, S.; Xiao, Y.; Hu, R.; Yang, T. Environmental performance and functional analysis of chip seals with recycled basic oxygen furnace slag as aggregate. J. Hazard. Mater. 2021, 405, 124441. [Google Scholar] [CrossRef]
- Ma, M.; Yang, H.; Bai, B.; Xie, G. Preparation and mechanism of silicate-based sintering material from large amount of steel slag. Mater. Express 2022, 9, 125503. [Google Scholar] [CrossRef]
- Li, H.; Cui, C.; Sheng, Y.; Zhang, M.; Feng, Z.; Li, L.; Xue, H.; Zhang, Y. Application of composite steel slag as subgrade Filler: Performance evaluation and enhancement. Constr. Build. Mater. 2023, 370, 130448. [Google Scholar] [CrossRef]
- Gao, B.; Yang, C.; Zou, Y.; Wang, F.; Zhou, X.; Barbieri, D.M.; Wu, S. Compaction Procedures and Associated Environmental Impacts Analysis for Application of Steel Slag in Road Base Layer. Sustainability 2021, 13, 4396. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Y.; Dai, W.; Cang, D. Preparation Mechanism of Glass-ceramics Based on Steel Slag Using One-step Sintering Process. Bull. Chin. Ceram. Soc. 2014, 33, 3288–3294. [Google Scholar]
- Liu, L.; Liu, J.; Liu, T.; Zhang, Y.; Liang, H.; Ning, T.; Lin, X.; Bai, Z.; Lu, A. Preparation, crystallization kinetics and stabilization behavior of the heavy metal ions of all-solid waste-based glass-ceramics from steel slag and coal gangue. J. Non-Cryst. Solids 2022, 592, 121750. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Zhang, J.; Zhang, Q.; Wang, Y. Synergistic use of industrial solid wastes to prepare belite-rich sulphoaluminate cement and its feasibility use in repairing materials. Constr. Build. Mater. 2020, 264, 120201. [Google Scholar] [CrossRef]
- Adolfsson, D.; Menad, N.; Viggh, E.; Björkman, B. Steelmaking slags as raw material for sulphoaluminate belite cement. Adv. Cem. Res. 2007, 19, 147–156. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Li, J.; Wang, W.; Mao, Y.; Wu, S.; Yang, S. Heavy metals migration during the preparation and hydration of an eco-friendly steel slag-based cementitious material. J. Clean. Prod. 2021, 329, 129715. [Google Scholar] [CrossRef]
- Huang, Y.; Pei, Y.; Qian, J.; Gao, X.; Liang, J.; Duan, G.; Zhao, P.; Lu, L.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties. Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Yang, K.; Lu, D.; Yan, S.; Xu, Z. Mineral Composition and Microstructure of Portland Cement Clinker with Steel Slag in the Raw Meal. Bull. Chin. Ceram. Soc. 2013, 32, 1032–1036, 1047. [Google Scholar]
- Zhao, D.; Zhang, D.; Shen, W.; Huang, J.; Tang, X.; Yang, Y.; Deng, Y.; Wang, Y. Investigation on industrial trial production of multi-phased clinker with crude granular steel slag. J. Clean. Prod. 2022, 337, 130467. [Google Scholar] [CrossRef]
- Cao, L.; Shen, W.; Huang, J.; Yang, Y.; Zhang, D.; Huang, X.; Lv, Z.; Ji, X. Process to utilize crushed steel slag in cement industry directly: Multi-phased clinker sintering technology. J. Clean. Prod. 2019, 217, 520–529. [Google Scholar] [CrossRef]
- Chen, M.; Feng, C.; Li, D. Research on Feasibility of Using Steel Slag as Mineral Admixture in Concrete. Bull. Chin. Ceram. Soc. 2011, 30, 751–754. [Google Scholar]
- Liu, J.; Wang, D. Influence of steel slag-silica fume composite mineral admixture on the properties of concrete. Powder Technol. 2017, 320, 230–238. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Z. Influence of Steel Slag-Superfine Blast Furnace Slag Composite Mineral Admixture on the Properties of Mortar and Concrete. Adv. Civ. Eng. 2021, 2021, 9983019. [Google Scholar] [CrossRef]
- Roslan, N.H.; Ismail, M.; Abdul-Majid, Z.; Ghoreishiamiri, S.; Muhammad, B. Performance of steel slag and steel sludge in concrete. Constr. Build. Mater. 2016, 104, 16–24. [Google Scholar] [CrossRef]
- Ma, S.; Song, C.; Zhang, Z.; Zhang, Y. Preparation of hydrotalcite flame retardant from steel slag and its application in EVA foam composites. J. Mater. Eng. 2021, 49, 151–157. [Google Scholar]
- Liu, G.; Yang, J.; Xu, X. Synthesis of hydrotalcite-type mixed oxide catalysts from waste steel slag for transesterification of glycerol and dimethyl carbonate. Sci. Rep. 2020, 10, 10273. [Google Scholar] [CrossRef]
- Song, Z.; Gao, H.; Liao, G.; Zhang, W.; Wang, D. A novel slag-based Ce/TiO2@LDH catalyst for visible light driven degradation of tetracycline: Performance and mechanism. J. Alloys Compd. 2022, 901, 163525. [Google Scholar] [CrossRef]
- Qin, W.; Li, W.; Lan, Z.; Qiu, G. Simulated small-scale pilot plant heap leaching of low-grade oxide zinc ore with integrated selective extraction of zinc. Miner. Eng. 2007, 20, 694–700. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Shang, X.; Hou, X.; Li, H.; Guo, Z. Facile synthesis of Ca/Mg/Al/Fe layered double hydroxides using steelmaking slag as raw material. Mater. Lett. 2016, 173, 115–118. [Google Scholar] [CrossRef]
- Eiby, S.H.J.; Tobler, D.J.; Nedel, S.; Bischoff, A.; Christiansen, B.C.; Hansen, A.S.; Kjaergaard, H.G.; Stipp, S.L.S. Competition between chloride and sulphate during the reformation of calcined hydrotalcite. Appl. Clay Sci. 2016, 132–133, 650–659. [Google Scholar] [CrossRef]










| Component | LOI | SiO2 | CaO | MgO | Al2O3 | Fe2O3 | SO3 |
|---|---|---|---|---|---|---|---|
| wt% | 2.81 | 16.86 | 39.11 | 8.45 | 6.38 | 19.73 | 0.48 |
| Sample | (003) | (006) | (009) | ||||
|---|---|---|---|---|---|---|---|
| 2θ(003)/° | d(003)/nm | β(003)/rad | 2θ(006)/° | d(006)/nm | 2θ(009)/° | d(009)/nm | |
| pH = 10 | 11.58 | 0.7635 | 0.0142 | 23.42 | 0.3795 | 35.46 | 0.2529 |
| pH = 11 | 11.48 | 0.7704 | 0.0119 | 23.24 | 0.3824 | 35.18 | 0.2549 |
| pH = 12 | 11.24 | 0.7867 | 0.0094 | 22.78 | 0.3900 | 35.36 | 0.2537 |
| Sample | (003) | (006) | (009) | ||||
|---|---|---|---|---|---|---|---|
| 2θ(003)/° | d(003)/nm | β(003)/rad | 2θ(006)/° | d(006)/nm | 2θ(009)/° | d(009)/nm | |
| 60 °C | 11.41 | 0.7751 | 0.0084 | 22.99 | 0.3865 | 34.40 | 0.2605 |
| 90 °C | 11.32 | 0.7810 | 0.0124 | 22.72 | 0.3910 | 34.59 | 0.2591 |
| 120 °C | 11.48 | 0.7704 | 0.0119 | 23.24 | 0.3824 | 35.18 | 0.2549 |
| 150 °C | 11.30 | 0.7824 | 0.0102 | 22.66 | 0.3921 | 35.64 | 0.2517 |
| Sample | (003) | (006) | (009) | ||||
|---|---|---|---|---|---|---|---|
| 2θ(003)/° | d(003)/nm | β(003)/rad | 2θ(006)/° | d(006)/nm | 2θ(009)/° | d(009)/nm | |
| 12 h | 11.44 | 0.7727 | 0.0101 | 23.22 | 0.3827 | 35.26 | 0.2543 |
| 16 h | 11.48 | 0.7704 | 0.0119 | 23.24 | 0.3824 | 35.18 | 0.2549 |
| 20 h | 11.50 | 0.7688 | 0.0111 | 23.06 | 0.3854 | 35.24 | 0.2545 |
| 24 h | 11.40 | 0.7756 | 0.0116 | 23.08 | 0.3850 | 35.24 | 0.2545 |
| Samples | Specific Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| hydrotalcite | 207.27 | 10.89 | 0.56 |
| steel slag | 3.88 | 40.68 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Huang, B.; Zhang, T.; Liu, N.; Mao, Z. Preparation of Steel-Slag-Based Hydrotalcite and Its Adsorption Properties on Cl− and SO42−. Materials 2023, 16, 7402. https://doi.org/10.3390/ma16237402
Dong Z, Huang B, Zhang T, Liu N, Mao Z. Preparation of Steel-Slag-Based Hydrotalcite and Its Adsorption Properties on Cl− and SO42−. Materials. 2023; 16(23):7402. https://doi.org/10.3390/ma16237402
Chicago/Turabian StyleDong, Zebo, Bei Huang, Tao Zhang, Na Liu, and Zhongyang Mao. 2023. "Preparation of Steel-Slag-Based Hydrotalcite and Its Adsorption Properties on Cl− and SO42−" Materials 16, no. 23: 7402. https://doi.org/10.3390/ma16237402
APA StyleDong, Z., Huang, B., Zhang, T., Liu, N., & Mao, Z. (2023). Preparation of Steel-Slag-Based Hydrotalcite and Its Adsorption Properties on Cl− and SO42−. Materials, 16(23), 7402. https://doi.org/10.3390/ma16237402





