Synthesis of a Stable and High-Concentration BaHfxTi1−xO3 Sol–Gel for High Electromechanical Performance of Bulk Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pure Sol Preparation
2.2. Thin Film Elaboration
2.3. Ceramic Elaboration
3. Results
3.1. Pure Sol Characterization, Stabilization, and Thin Film Elaboration
3.1.1. Sol Characterizations
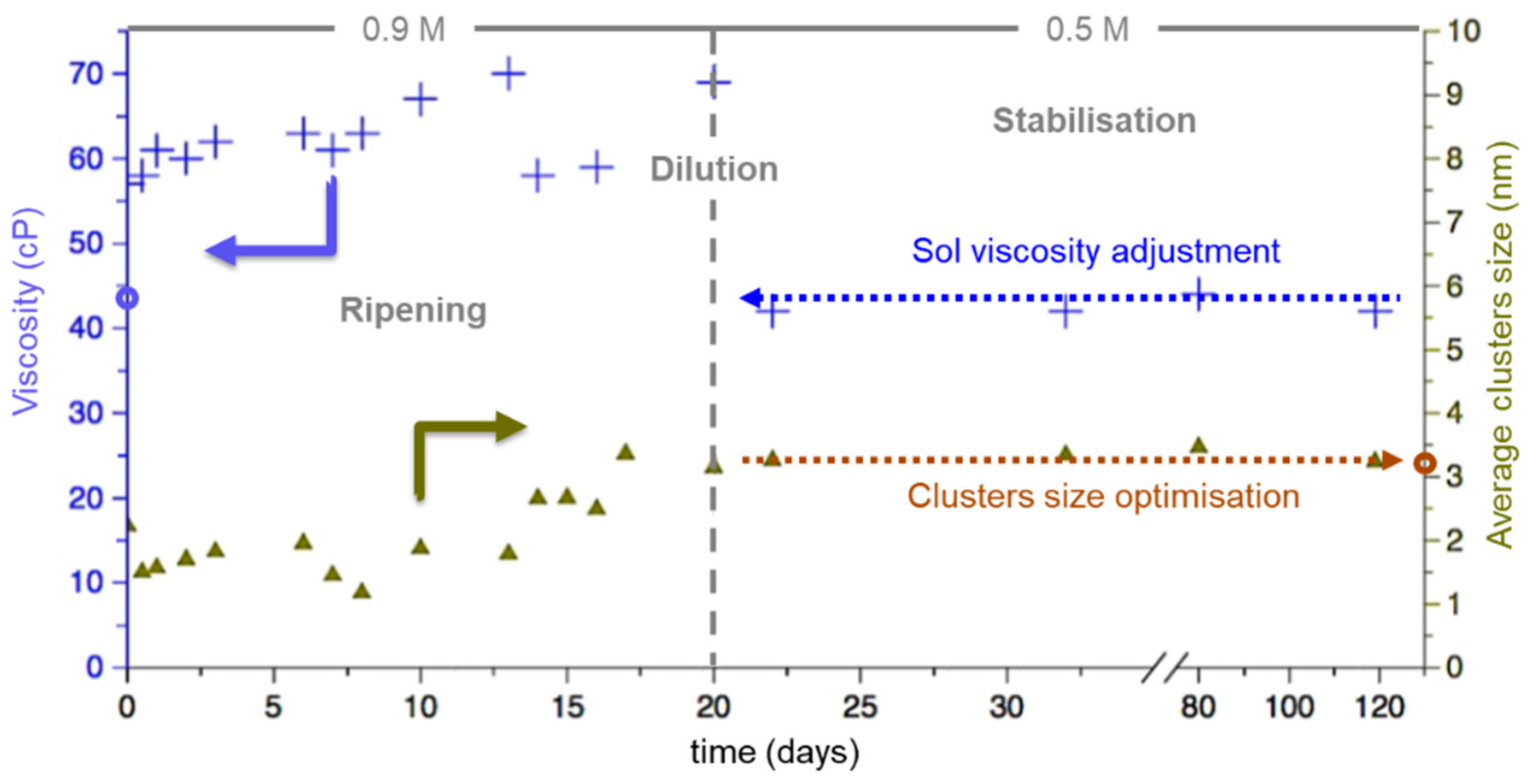
3.1.2. Ripening and Stabilization of the Sol
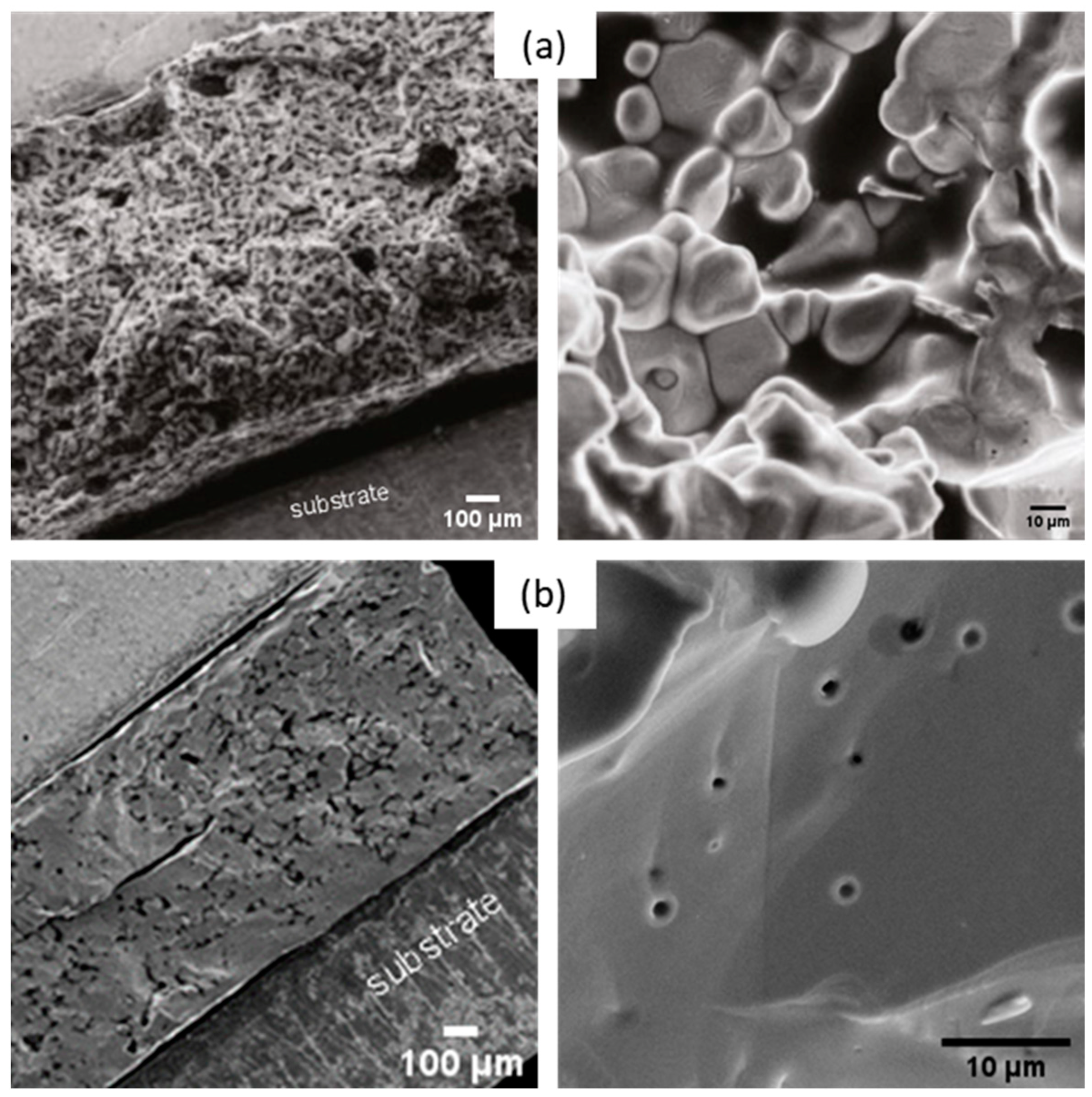
3.1.3. Thin Film Elaboration
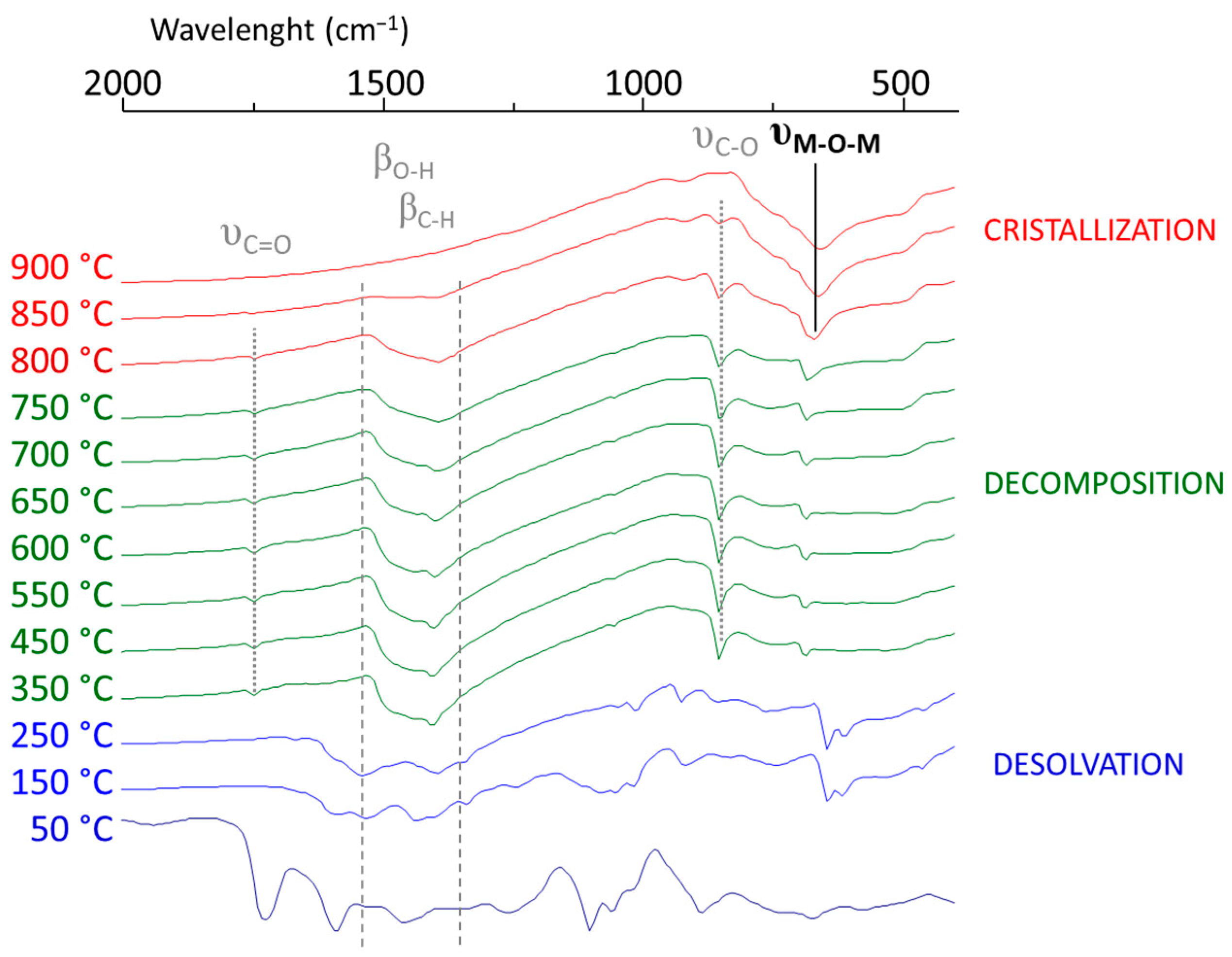
- Up to 250 °C, a desolvation step to obtain the dried sample was performed, depicted in the disappearance of the 1710, 1100, and 1000 cm−1 bands of ethylene glycol and acetic acid and the peaks at 1550 and 1400 cm−1 corresponding to the symmetric and asymmetric C-O stretching bands of carboxylic acid groups.
- The decomposition of the last organic parts occurred during 2 calcination steps (450 °C for 10 min and then 600 °C for 20 min.) The remaining acetate started to decompose, creating carbonate, which appeared at 1750 and 1410 cm−1 in the spectra.
- The multilayer film was crystallized with the complete removal of carbonates at 900 °C for 10 min. The crystallization of BHT films was confirmed by the disappearance of the characteristic IR bands of carbonate groups, along with the appearance of metal–oxide–metal (M-O-M) bands at 660 cm−1.
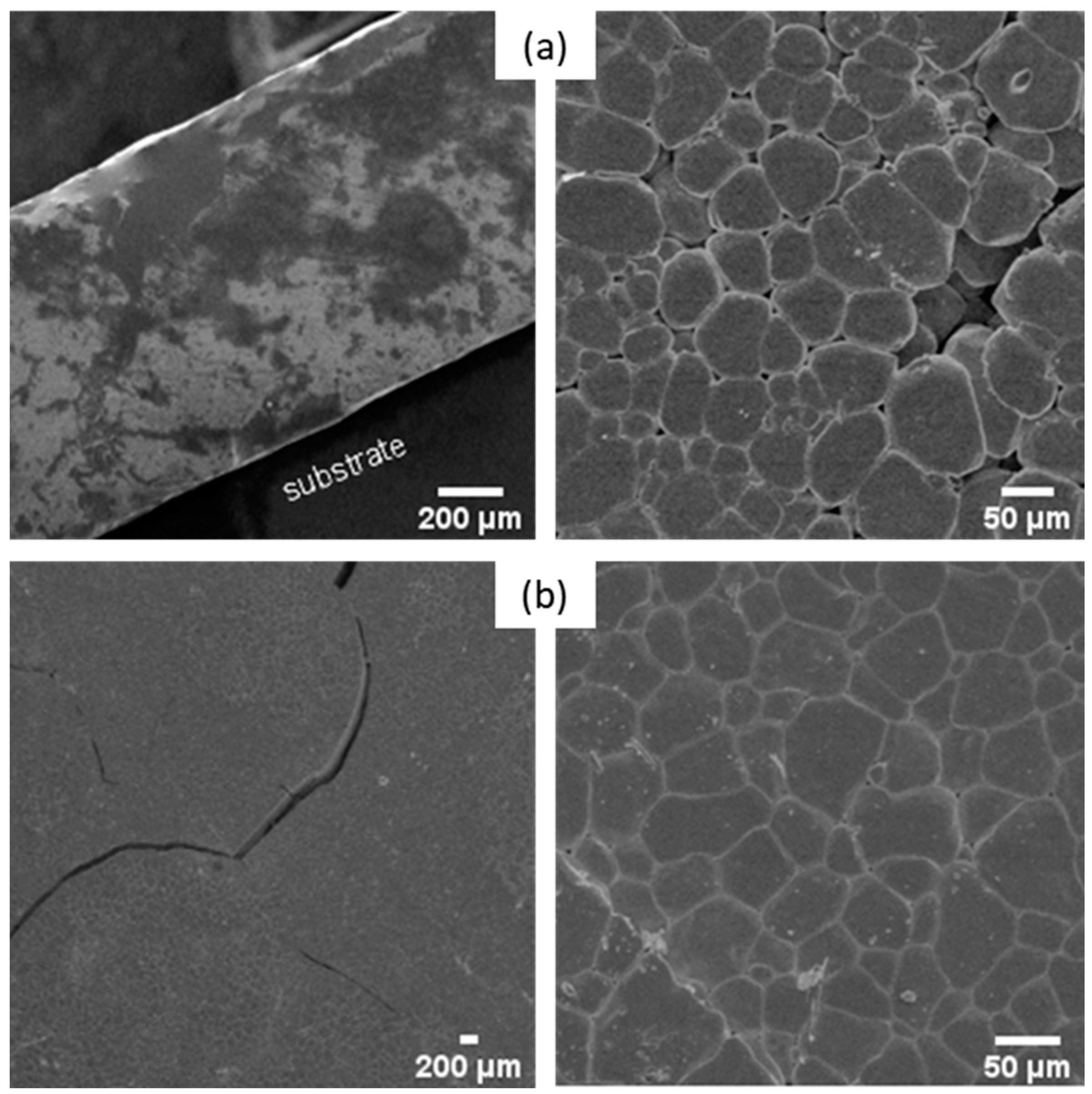
3.2. Powders and Pellet Elaborations from Pure Sol
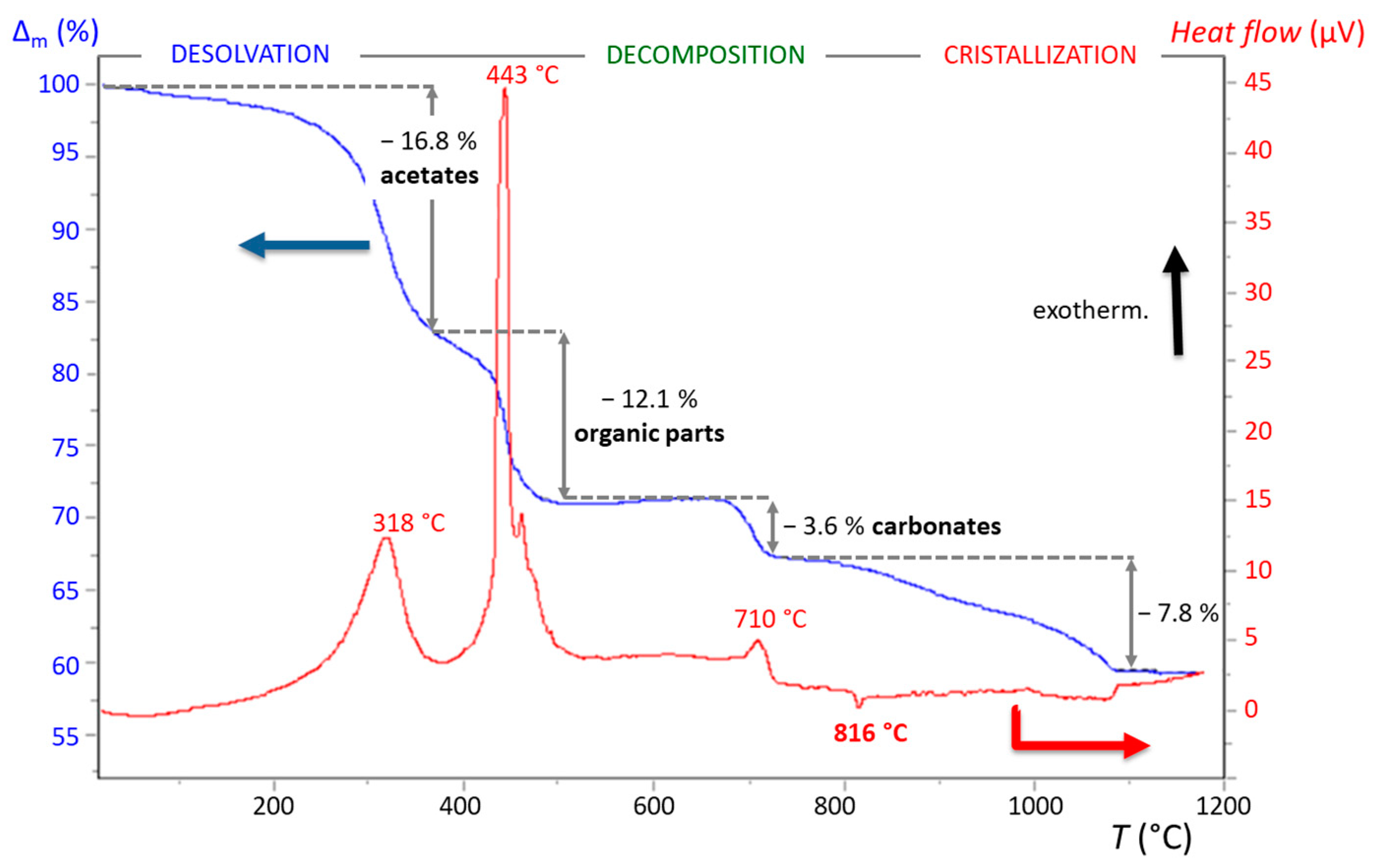
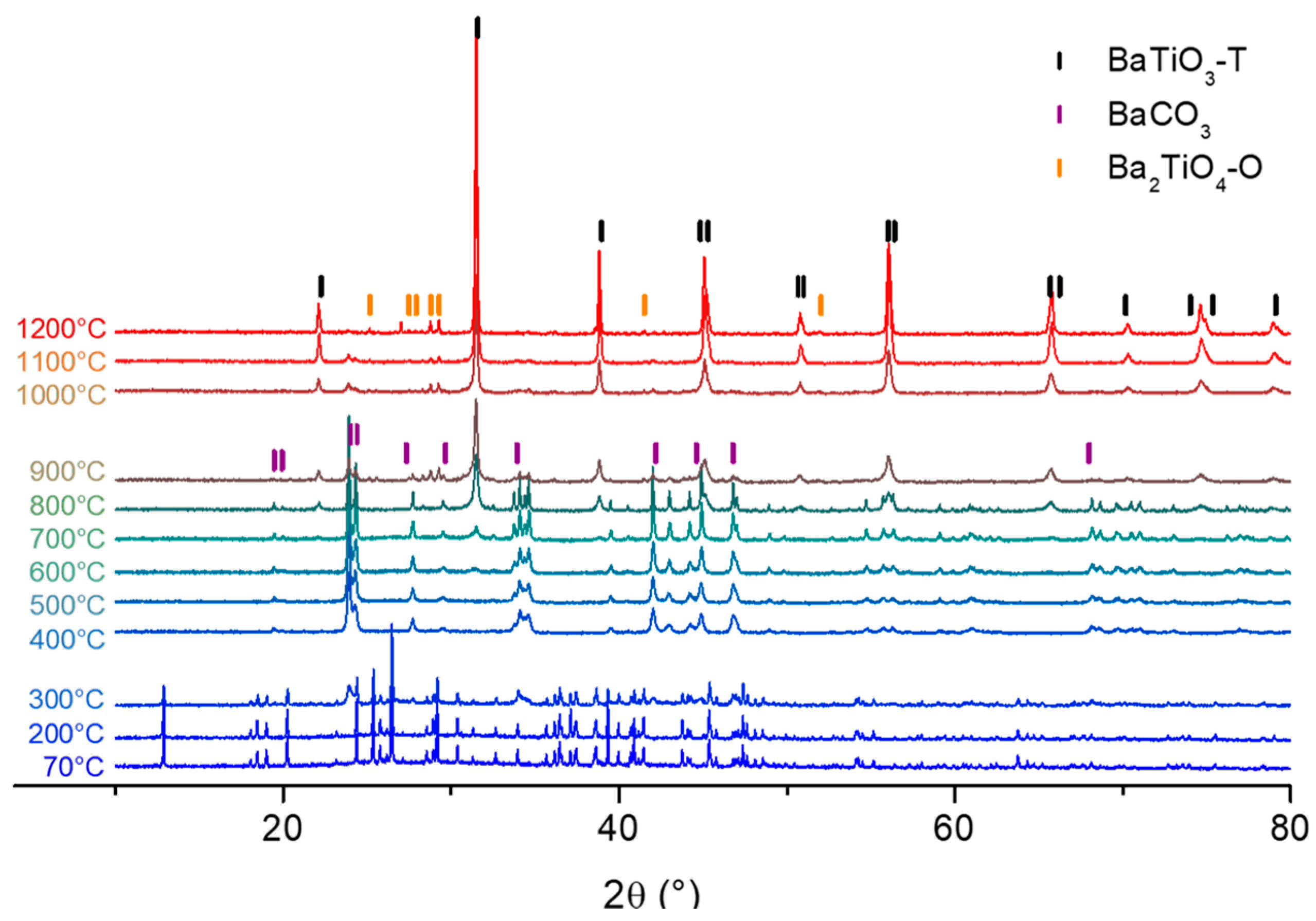
3.2.1. Xerogel Crystallization
3.2.2. Mechanical Attrition
3.2.3. Sintering Conditions of the Pellets
3.2.4. Dielectric Properties of BHT5 Ceramic
3.2.5. Electromechanical Properties of BHT Ceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, J.; Hong, X.; Ahn, C.H. Device performance of ferroelectric/correlated oxide heterostructures for non-volatile memory applications. Nanotechnology 2011, 22, 254014. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Ong, C.K. Coupled microstrip line microwave phase shifter using ferroelectric thin film varactors. J. Appl. Phys. 2012, 111, 044506. [Google Scholar] [CrossRef]
- Yin, S.; Niu, G.; Vilquin, B.; Gautier, B.; Le Rhun, G.; Defay, E.; Robach, Y. Epitaxial growth and electrical measurement of single crystalline Pb(Zr0.52Ti0.48)O3 thin film on Si(001) for micro-electromechanical systems. Thin Solid Films 2012, 520, 4572–4575. [Google Scholar] [CrossRef]
- Guillon, O.; Chang, J.; Schaab, S.; Kang, S.-J.L. Capacitance Enhancement of Doped Barium Titanate Dielectrics and Multilayer Ceramic Capacitors by a Post-Sintering Thermo-Mechanical Treatment. J. Am. Ceram. Soc. 2012, 95, 2277–2281. [Google Scholar] [CrossRef]
- Yang, X.; Su, X.; Shen, M.; Zheng, F.; Xin, Y.; Zhang, L.; Hua, M.; Chen, Y.; Harris, V.G. Enhancement of Photocurrent in Ferroelectric Films Via the Incorporation of Narrow Bandgap Nanoparticles. Adv. Mater. 2012, 24, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.F.; Chan, H.L.W.; Choy, C.L. PZT ceramic/ceramic 0–3 nanocomposite films for ultrasonic transducer applications. Thin Solid Films 2000, 375, 95–99. [Google Scholar] [CrossRef]
- Lukacs, M.; Sayer, M.; Foster, S. Single element high frequency (<50 MHz) PZT sol gel composite ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 148–159. [Google Scholar] [CrossRef]
- Panda, P.K.; Sahoo, B. PZT to Lead Free Piezo Ceramics: A Review. Ferroelectrics 2015, 474, 128–143. [Google Scholar] [CrossRef]
- The European Parliamant; the Concil of European Union. Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off. J. Eur. Union 2011, 174, 88. [Google Scholar] [CrossRef]
- Hoang, T.; Poulin-Vittrant, G.; Ferin, G.; Levassort, F.; Bantignies, C.; Nguyen-Dinh, C.; Bavencoffe, M. Parametric study of a thin piezoelectric cantilever for energy harvesting applications. Adv. Appl. Ceram. 2018, 117, 231–236. [Google Scholar] [CrossRef]
- Rödel, J.; Jo, W.; Seifert, K.T.P.; Anton, E.M.; Granzow, T.; Damjanovic, D. Perspective on the development of lead-free piezoceramics. J. Am. Ceram. Soc. 2009, 92, 1153–1177. [Google Scholar] [CrossRef]
- Devonshire, A.F. XCVI. Theory of barium titanate. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1949, 40, 1040–1063. [Google Scholar] [CrossRef]
- Devonshire, A.F. CIX. Theory of barium titanate—Part II. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1951, 42, 1065–1079. [Google Scholar] [CrossRef]
- von Hippel, A. Ferroelectricity, Domain Structure, and Phase Transitions of Barium Titanate. Rev. Mod. Phys. 1950, 22, 221–237. [Google Scholar] [CrossRef]
- Kalyani, A.K.; Brajesh, K.; Senyshyn, A.; Ranjan, R. Orthorhombic-tetragonal phase coexistence and enhanced piezo-response at room temperature in Zr, Sn, and Hf modified BaTiO3. Appl. Phys. Lett. 2014, 104, 252906. [Google Scholar] [CrossRef]
- Zhu, X.N.; Zhang, W.; Chen, X.M. Enhanced dielectric and ferroelectric characteristics in Ca-modified BaTiO3 ceramics. AIP Adv. 2013, 3, 082125. [Google Scholar] [CrossRef]
- Dixit, A.; Majumder, S.B.; Savvinov, A.; Katiyar, R.S.; Guo, R.; Bhalla, A.S. Investigations on the sol–gel-derived barium zirconium titanate thin films. Mater. Lett. 2002, 56, 933–940. [Google Scholar] [CrossRef]
- Chen, J.; Fu, C.; Cai, W.; Chen, G.; Ran, S. Microstructures, dielectric and ferroelectric properties of BaHfxTi1−xO3 ceramics. J. Alloys Compd. 2012, 544, 82–86. [Google Scholar] [CrossRef]
- Lee, S.T.F.; Lam, K.H.; Zhang, X.M.; Chan, H.L.W. High-frequency ultrasonic transducer based on lead-free BSZT piezoceramics. Ultrasonics 2011, 51, 811–814. [Google Scholar] [CrossRef]
- Yan, X.; Lam, K.H.; Li, X.; Chen, R.; Ren, W.; Ren, X.; Zhou, Q.; Shung, K.K. Correspondence: Lead-free intravascular ultrasound transducer using BZT-50BCT ceramics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1272–1276. [Google Scholar] [CrossRef]
- Sasaki, A.; Chiba, T.; Mamiya, Y.; Otsuki, E. Dielectric and Piezoelectric Properties of (Bi0.5Na0.5)TiO3–(Bi0.5K0.5)TiO3 Systems. Jpn. J. Appl. Phys. 1999, 38, 5564–5567. [Google Scholar] [CrossRef]
- Tian, H.Y.; Wang, Y.; Miao, J.; Chan, H.L.W.; Choy, C.L. Preparation and characterization of hafnium doped barium titanate ceramics. J. Alloys Compd. 2007, 431, 197–202. [Google Scholar] [CrossRef]
- Payne, W.H.; Tennery, V.J. Dielectric and Structural Investigations of the System BaTiO3-BaHfO3. J. Am. Ceram. Soc. 1965, 48, 413–417. [Google Scholar] [CrossRef]
- Vijatović, M.M.; Bobić, J.D.; Stojanović, B.D. History and challenges of barium titanate: Part I. Sci. Sinter. 2008, 40, 155–165. [Google Scholar] [CrossRef]
- del Carmen Blanco López, M.; Fourlaris, G.; Rand, B.; Riley, F.L. Characterization of Barium Titanate Powders: Barium Carbonate Identification. J. Am. Ceram. Soc. 1999, 82, 1777–1786. [Google Scholar] [CrossRef]
- Bardaine, A.; Boy, P.; Belleville, P.; Acher, O.; Levassort, F. Influence of powder preparation process on piezoelectric properties of PZT sol-gel composite thick films. J. Solgel Sci. Technol. 2008, 48, 135–142. [Google Scholar] [CrossRef]
- Ferin, G.; Hoang, T.; Bantignies, C.; Le Khanh, H.; Flesch, E.; Nguyen-Dinh, A. Powering autonomous wireless sensors with miniaturized piezoelectric based energy harvesting devices for NDT applications. In Proceedings of the 2015 IEEE International Ultrasonics Symposium (IUS), Taipei, Taiwan, 21–24 October 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Belleville, P.; Bigarre, J.; Boy, P.; Montouillout, Y. Stable PZT sol for preparing reproducible high-permittivity perovskite-based thin films. J. Solgel Sci. Technol. 2007, 43, 213–221. [Google Scholar] [CrossRef]
- Bardaine, A.; Boy, P.; Belleville, P.; Acher, O.; Levassort, F. Improvement of composite sol–gel process for manufacturing 40 μm piezoelectric thick films. J. Eur. Ceram. Soc. 2008, 28, 1649–1655. [Google Scholar] [CrossRef]
- Krimholtz, R.; Leedom, D.A.; Matthaei, G.L. New equivalent circuits for elementary piezoelectric transducers. Electron. Lett. 1970, 6, 398–399. [Google Scholar] [CrossRef]
- Lethiecq, M.; Patat, F.; Pourcelot, L.; Tran-Huu-Hue, L.P. Measurement of losses in five piezoelectric ceramics between 2 and 50 MHz. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1993, 40, 232–237. [Google Scholar] [CrossRef]
- van Kervel, S.J.H.; Thijssen, J.M. A calculation scheme for the optimum design of ultrasonic transducers. Ultrasonics 1983, 21, 134–140. [Google Scholar] [CrossRef]
- Doeuff, S.; Henry, M.; Sanchez, C.; Livage, J. Hydrolysis of titanium alkoxides: Modification of the molecular precursor by acetic acid. J. Non Cryst. Solids 1987, 89, 206–216. [Google Scholar] [CrossRef]
- Guglielmi, M.; Carturan, G. Precursors for sol-gel preparations. J. Non Cryst. Solids 1988, 100, 16–30. [Google Scholar] [CrossRef]
- Hwang, U.Y.; Park, H.S.; Koo, K.K. Behavior of Barium Acetate and Titanium Isopropoxide during the Formation of Crystalline Barium Titanate. Ind. Eng. Chem. Res. 2004, 43, 728–734. [Google Scholar] [CrossRef]
- Lotnyk, A.; Senz, S.; Hesse, D. Formation of BaTiO3 thin films from (110) TiO2 rutile single crystals and BaCO3 by solid state reactions. Solid State Ion. 2006, 177, 429–436. [Google Scholar] [CrossRef]
- Yin, H.M.; Xu, W.J.; Zhou, H.W.; Zhao, X.Y.; Huang, Y.N. Effects of phase composition and grain size on the piezoelectric properties of HfO2-doped barium titanate ceramics. J. Mater. Sci. 2019, 54, 12392–12400. [Google Scholar] [CrossRef]
- Anwar, S.; Sagdeo, P.R.R.; Lalla, N.P.P. Ferroelectric relaxor behavior in hafnium doped barium-titanate ceramic. Solid State Commun. 2006, 138, 331–336. [Google Scholar] [CrossRef]
- Anwar, S.; Sagdeo, P.R.; Lalla, N.P. Crossover from classical to relaxor ferroelectrics in BaTi1−xHfxO3 ceramics. J. Phys. Condens. Matter 2006, 18, 3455–3468. [Google Scholar] [CrossRef]
- von Hippel, A.R.; Morgan, S.O. Dielectrics and Waves. J. Electrochem. Soc. 1955, 102, 68C. [Google Scholar] [CrossRef]
- Kittel, C.; Masi, J.F. Introduction to Solid State Physics. Phys. Today 1954, 7, 18–19. [Google Scholar] [CrossRef]
- Lethiecq, M.; Levassort, F.; Tran-Huu-Hue, L.-P.; Alguero, M.; Pardo, L.; Bove, T.; Ringgaard, E.; Wolny, W. New low acoustic impedance piezoelectric material for broadband transducer applications. In Proceedings of the IEEE Ultrasonics Symposium, 2004, Montréal, QC, Canada, 23–27 August 2004; pp. 1153–1156. [Google Scholar] [CrossRef]
- CTS Ferroperm Piezoceramics. Available online: https://www.ferropermpiezoceramics.com/ (accessed on 1 September 2023).






| Sample | Theoretical Composition | BHT Thin Film (RBS) | BHT Sol (ICPMS) |
|---|---|---|---|
| BHT5 | Ba1Ti0.950Hf0.050O3 | Ba0.990Ti0.969Hf0.038O3.003 | Ba1.100Ti0.888Hf0.045O2.967 |
| BHT7.5 | Ba1Ti0.925Hf0.075O3 | Ba1.002Ti0.917Hf0.081O2.999 | Ba1.166Ti0.832Hf0.056O2.945 |
| BHT10 | Ba1Ti0.900Hf0.100O3 | Ba1.040Ti0.867Hf0.106O2.987 | Ba1.032Ti0.883Hf0.095O2.989 |
| Sintering Conditions | (%) | (GPa) | (%) | (C/m2) | (%) | ||
|---|---|---|---|---|---|---|---|
| T (°C)/Times (h) | Powder | ||||||
| 1500/4 | N.A. | 3800/60 | 42 | 570 | 17 | 5 | 34 |
| 1400/4 | A. | 4620/74 | 79 | 660 | 20 | 7 | 31 |
| 1500/4 | A. | 4420/70 | 111 | 695 | 11 | 12 | 45 |
| 1500/6 | A. | 4520/73 | 101 | 700 | 4 | 12 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brault, D.; Richardot, T.; Boy, P.; Belleville, P.; Levassort, F.; Bavencoffe, M. Synthesis of a Stable and High-Concentration BaHfxTi1−xO3 Sol–Gel for High Electromechanical Performance of Bulk Ceramics. Materials 2023, 16, 7452. https://doi.org/10.3390/ma16237452
Brault D, Richardot T, Boy P, Belleville P, Levassort F, Bavencoffe M. Synthesis of a Stable and High-Concentration BaHfxTi1−xO3 Sol–Gel for High Electromechanical Performance of Bulk Ceramics. Materials. 2023; 16(23):7452. https://doi.org/10.3390/ma16237452
Chicago/Turabian StyleBrault, Damien, Thomas Richardot, Philippe Boy, Philippe Belleville, Franck Levassort, and Maxime Bavencoffe. 2023. "Synthesis of a Stable and High-Concentration BaHfxTi1−xO3 Sol–Gel for High Electromechanical Performance of Bulk Ceramics" Materials 16, no. 23: 7452. https://doi.org/10.3390/ma16237452





