Author Contributions
Conceptualization, R.Z.; methodology, R.Z.; software, R.Z.; validation, R.Z., M.K., J.S., M.P., and J.O.; formal analysis, R.Z.; investigation, R.Z.; resources, R.Z., M.K., J.S., M.P., and J.O.; data curation, R.Z.; writing—original draft preparation, R.Z.; writing—review and editing, R.Z.; visualization, R.Z.; supervision, R.Z.; project administration, R.Z.; funding acquisition, R.Z. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Mai Implants® showing (a) milled metal CoCr alloy + porcelain bridge (PFM); (b) milled titanium alloy + acryl (Ti-acryl).
Figure 1.
Mai Implants® showing (a) milled metal CoCr alloy + porcelain bridge (PFM); (b) milled titanium alloy + acryl (Ti-acryl).
Figure 2.
The model of a single subperiosteal implant along with numbering. Anchoring points are numbered from 1–9 as “O-shaped” whereas multi-units are numbered from 1–3 at the top of the figure.
Figure 2.
The model of a single subperiosteal implant along with numbering. Anchoring points are numbered from 1–9 as “O-shaped” whereas multi-units are numbered from 1–3 at the top of the figure.
Figure 3.
Flow chart showing the process of Finite Element Analysis (FEA).
Figure 3.
Flow chart showing the process of Finite Element Analysis (FEA).
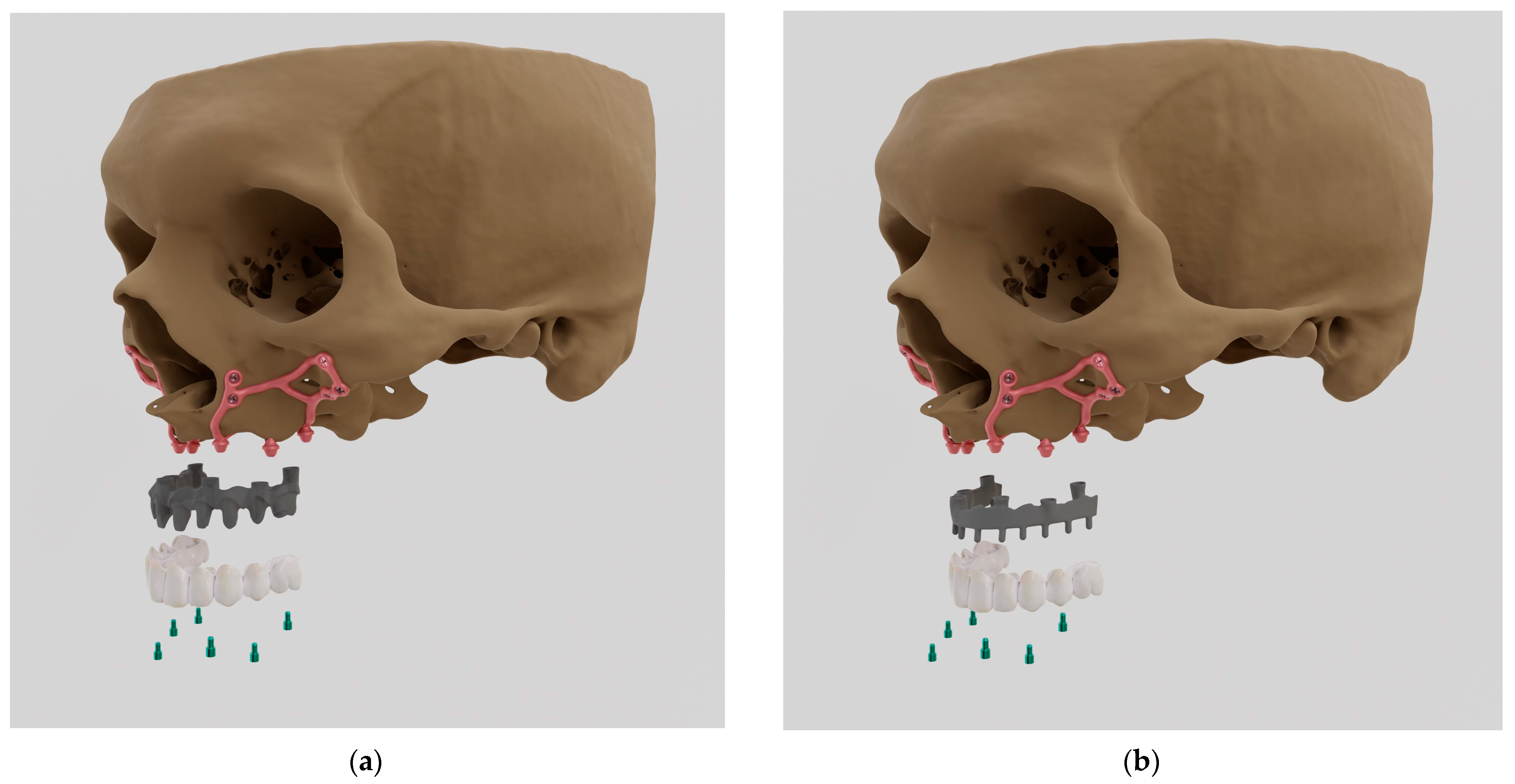
Figure 4.
The designed arch along with the division into left and right implants.
Figure 4.
The designed arch along with the division into left and right implants.
Figure 5.
(a) Anchoring holes; (b) surface adjustment for multi-units.
Figure 5.
(a) Anchoring holes; (b) surface adjustment for multi-units.
Figure 6.
Place and direction of vertical loads for a single model. A, B, C are numbered multi-units.
Figure 6.
Place and direction of vertical loads for a single model. A, B, C are numbered multi-units.
Figure 7.
The loading scheme of the implant according to ISO norm 14801.
Figure 7.
The loading scheme of the implant according to ISO norm 14801.
Figure 8.
Measurement location in the multi-units and the mounting hole marked by means of red arrows. Two graphs below illustrate in magnification above graph.
Figure 8.
Measurement location in the multi-units and the mounting hole marked by means of red arrows. Two graphs below illustrate in magnification above graph.
Figure 9.
Measurement location in the bar marked by means of red arrows.
Figure 9.
Measurement location in the bar marked by means of red arrows.
Figure 10.
Displacement state for a single implant: (a) 100 N, (b) 800 N.
Figure 10.
Displacement state for a single implant: (a) 100 N, (b) 800 N.
Figure 11.
Displacements on the external side of the multi-units of a single implant.
Figure 11.
Displacements on the external side of the multi-units of a single implant.
Figure 12.
Displacements on the internal side of the multi-units of a single implant.
Figure 12.
Displacements on the internal side of the multi-units of a single implant.
Figure 13.
Displacement state for a single implant under an 800 N force at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 13.
Displacement state for a single implant under an 800 N force at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 14.
Displacements on the external side of the multi-units for a single implant.
Figure 14.
Displacements on the external side of the multi-units for a single implant.
Figure 15.
Displacements on the internal side of the multi-units for a single implant.
Figure 15.
Displacements on the internal side of the multi-units for a single implant.
Figure 16.
Displacement state for the arch under load: (a) 100 N, (b) 800 N.
Figure 16.
Displacement state for the arch under load: (a) 100 N, (b) 800 N.
Figure 17.
Displacements on the external side of the multi-units for the arch.
Figure 17.
Displacements on the external side of the multi-units for the arch.
Figure 18.
Displacements on the internal side of the multi-units for the arch.
Figure 18.
Displacements on the internal side of the multi-units for the arch.
Figure 19.
Displacement state for the arch under a load of 800 N at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 19.
Displacement state for the arch under a load of 800 N at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 20.
The state of strain for a single implant under forces of (a) 100 N and(b) 800 N.
Figure 20.
The state of strain for a single implant under forces of (a) 100 N and(b) 800 N.
Figure 21.
Deformations on the external side of multi-units for a single implant.
Figure 21.
Deformations on the external side of multi-units for a single implant.
Figure 22.
Deformations on the internal side of multi-units for a single implant.
Figure 22.
Deformations on the internal side of multi-units for a single implant.
Figure 23.
The state of strain for a single implant under a force of 800 N at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 23.
The state of strain for a single implant under a force of 800 N at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 24.
Stress state for a single implant under the forces of (a) 100 N and (b) 800 N.
Figure 24.
Stress state for a single implant under the forces of (a) 100 N and (b) 800 N.
Figure 25.
Stresses on the external side of the multi-units for a single implant.
Figure 25.
Stresses on the external side of the multi-units for a single implant.
Figure 26.
Stresses on the internal side of the multi-units for a single implant. Deformation was measured with 2 different forces—100 N and 800 N.
Figure 26.
Stresses on the internal side of the multi-units for a single implant. Deformation was measured with 2 different forces—100 N and 800 N.
Figure 27.
Stress state for a single implant under a load of 800 N at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 27.
Stress state for a single implant under a load of 800 N at angles of (a) 30 degrees, (b) 60 degrees, and (c) 90 degrees.
Figure 28.
Stresses on the external side of the multi-units for a single implant. Deformation was measured with 3 different angles—30°, 60°, 90°.
Figure 28.
Stresses on the external side of the multi-units for a single implant. Deformation was measured with 3 different angles—30°, 60°, 90°.
Figure 29.
Stresses on the internal side of the multi-units for a single implant.
Figure 29.
Stresses on the internal side of the multi-units for a single implant.
Figure 30.
Displacements: (a) with multi-unit fixation, (b) without fixation.
Figure 30.
Displacements: (a) with multi-unit fixation, (b) without fixation.
Figure 31.
Deformations: (a) with fixation under multi-units, (b) without fixation.
Figure 31.
Deformations: (a) with fixation under multi-units, (b) without fixation.
Table 1.
Compilation of materials, load values, and angles used in the literature [
10,
11,
12,
13,
14,
15,
16,
17].
Table 1.
Compilation of materials, load values, and angles used in the literature [
10,
11,
12,
13,
14,
15,
16,
17].
| Authors | Material | Force [N] | Angle [°] |
|---|
| Bahrami et al. | Ti-6Al-4V | 200 | 90 |
| Burlibasa et al. | Ti-6Al-4V | 50 | 90, 60, 45, 30, 0 |
| Gultekin et al. | Ti-6Al-4V | 100, 150 | 90 |
| Kayumi et al. | - | 40, 100, 200, 400, 800 | 90 |
| Liu et al. | Ti-6Al-4V | 100 | 90 |
| Materac et al. | Ti-6Al-4V | 100, 200, 500 | 90 |
| Mommaerts | Ti-6Al-4V | 100 | 90 |
| PN-EN ISO 14801 | - | 800 | 30 |
Table 2.
Compilation of displacement results for varying force values.
Table 2.
Compilation of displacement results for varying force values.
| Element | Implant | Element | Side | Single Implant (mm) | Arch (mm) |
|---|
| 100 N | 800 N | 100 N | 800 N |
|---|
| Multi-unit | Left | 1 | outer | 3.50 × 10−5 | 2.89 × 10−4 | 5.40 × 10−5 | 4.75 × 10−4 |
| inner | 1.30 × 10−4 | 1.04 × 10−5 | 8.81 × 10−5 | 7.04 × 10−4 |
| 2 | outer | 7.89 × 10−5 | 6.45 × 10−4 | 6.76 × 10−5 | 5.42 × 10−4 |
| inner | 7.55 × 10−5 | 6.05 × 10−4 | 6.99 × 10−5 | 5.61 × 10−4 |
| 3 | outer | 7.78 × 10−5 | 6.24 × 10−4 | 9.65 × 10−5 | 7.78 × 10−4 |
| inner | 5.48 × 10−5 | 4.39 × 10−4 | 2.09 × 10−5 | 1.64 × 10−4 |
| Right | 3 | outer | - | - | 9.91 × 10−5 | 7.94 × 10−4 |
| inner | - | - | 2.22 × 10−5 | 1.70 × 10−4 |
| 2 | outer | - | - | 8.07 × 10−5 | 6.41 × 10−4 |
| inner | - | - | 6.85 × 10−5 | 5.46 × 10−4 |
| 1 | outer | - | - | 5.65 × 10−5 | 4.50 × 10−4 |
| inner | - | - | 9.07 × 10−5 | 7.28 × 10−4 |
| Mounting holes | Left | 8 | - | 2.76 × 10−6 | 2.38 × 10−5 | 5.69 × 10−6 | 4.35 × 10−5 |
| Right | 8 | - | - | - | 2.52 × 10−6 | 1.41 × 10−5 |
| Bar | Left | near 3 | - | - | - | 6.89 × 10−4 | 5.48 × 10−3 |
| - | middle | - | - | - | 2.27 × 10−3 | 1.82 × 10−2 |
| Left | near 3 | - | - | - | 6.77 × 10−4 | 5.41 × 10−3 |
Table 3.
Compilation of displacement results for varying angle values.
Table 3.
Compilation of displacement results for varying angle values.
| Element | Implant | Position | Side | Single Implant (mm) | Arch (mm) |
|---|
| 90° | 60° | 30° | 90° | 60° | 30° |
|---|
| Multi-unit | Left | 1 | outer | 2.89 × 10−4 | 2.43 × 10−3 | 4.41 × 10−3 | 4.75 × 10−4 | 2.74 × 10−3 | 5.10 × 10−3 |
| inner | 1.04 × 10−3 | 3.47 × 10−3 | 5.01 × 10−3 | 7.04 × 10−4 | 3.24 × 10−3 | 4.94 × 10−3 |
| 2 | outer | 6.45 × 10−4 | 1.82 × 10−3 | 3.79 × 10−3 | 5.42 × 10−4 | 2.48 × 10−3 | 4.96 × 10−3 |
| inner | 6.05 × 10−4 | 2.81 × 10−3 | 4.25 × 10−3 | 5.61 × 10−4 | 3.62 × 10−3 | 5.71 ×10−3 |
| 3 | outer | 6.24 × 10−4 | 8.88 × 10−4 | 2.33 × 10−3 | 7.78 × 10−4 | 8.02 × 10−4 | 2.19 × 10−3 |
| inner | 4.39 × 10−4 | 2.66 × 10−3 | 4.32 × 10−3 | 1.64 × 10−4 | 2.49 × 10−3 | 4.41 × 10−3 |
| Right | 3 | outer | - | - | - | 7.94 × 10−4 | 8.58 × 10−4 | 1.98 × 10−3 |
| inner | - | - | - | 1.70 × 10−4 | 2.77 × 10−3 | 4.87 × 10−3 |
| 2 | outer | - | - | - | 6.41 × 10−4 | 3.02 × 10−3 | 5.96 × 10−3 |
| inner | - | - | - | 5.46 × 10−4 | 3.62 × 10−3 | 5.73 × 10−3 |
| 1 | outer | - | - | - | 4.50 × 10−4 | 2.75 × 10−3 | 5.12 ×10−3 |
| inner | - | - | - | 7.28 × 10−4 | 3.34 × 10−3 | 5.03 × 10−3 |
| Mounting holes | Left | 8 | - | 2.38 × 10−5 | 1.15 × 10−4 | 1.74 × 10−4 | 4.35 × 10−5 | 2.77 × 10−4 | 4.55 × 10−4 |
| Right | 8 | - | - | - | - | 1.41 × 10−5 | 1.45 × 10−4 | 2.40 × 10−4 |
| Bar | Left | near 3 | - | - | - | - | 5.48 × 10−3 | 2.24 × 10−2 | 3.94 × 10−2 |
| - | middle | - | - | - | - | 1.82 × 10−2 | 1.00 × 10−1 | 1.60 × 10−1 |
| Left | near 3 | - | - | - | - | 5.41 × 10−3 | 2.28 × 10−2 | 3.95 × 10−2 |
Table 4.
Compilation of deformation results for a variable force value.
Table 4.
Compilation of deformation results for a variable force value.
| Element | Implant | Position | Side | Single Implant (%) | Arch (%) |
|---|
| 100 N | 800 N | 100 N | 800 N |
|---|
| Multi-unit | Left | 1 | outer | 2.92 × 10−5 | 2.32 × 10−4 | 5.39 × 10−5 | 4.30 × 10−4 |
| inner | 7.90 × 10−5 | 6.34 × 10−4 | 7.29 × 10−5 | 5.88 × 10−4 |
| 2 | outer | 5.24 × 10−5 | 4.65 × 10−4 | 6.34 × 10−5 | 5.06 × 10−4 |
| inner | 6.84 × 10−5 | 5.55 × 10−4 | 6.12 × 10−5 | 4.89 × 10−4 |
| 3 | outer | 5.13 × 10−5 | 4.07 × 10−4 | 8.43 × 10−5 | 6.69 × 10−4 |
| inner | 4.85 × 10−5 | 3.81 × 10−4 | 1.60 × 10−5 | 1.23 × 10−4 |
| Right | 3 | outer | - | - | 7.26 × 10−5 | 5.80 × 10−4 |
| inner | - | - | 1.64 × 10−5 | 1.25 × 10−4 |
| 2 | outer | - | - | 4.82 × 10−5 | 3.67 × 10−4 |
| inner | - | - | 6.53 × 10−5 | 5.20 × 10−4 |
| 1 | outer | - | - | 5.02 × 10−5 | 4.05 × 10−4 |
| inner | - | - | 7.09 × 10−5 | 5.73 × 10−4 |
| Mounting holes | Left | 8 | - | 1.00 × 10−5 | 7.77 × 10−5 | 1.30 × 10−5 | 9.66 × 10−5 |
| Right | 8 | - | - | - | 6.54 × 10−5 | 8.46 × 10−5 |
| Bar | Left | Near 3 | - | - | - | 6.57 × 10−5 | 5.46 × 10−4 |
| - | Middle | - | - | - | 2.76 × 10−7 | 1.56 × 10−6 |
| Right | Near 3 | - | - | - | 6.97 × 10−5 | 5.60 × 10−4 |
Table 5.
Compilation of deformation results for a variable angle value.
Table 5.
Compilation of deformation results for a variable angle value.
| Element | Implant | Position | Side | Single Implant (%) | Arch (%) |
|---|
| 90° | 60° | 30° | 90° | 60° | 30° |
|---|
| Multi-unit | Left | 1 | outer | 2.32 ×10−4 | 1.38 ×10−3 | 2.65 ×10−3 | 4.30 ×10−4 | 2.24 × 10−3 | 4.33 × 10−3 |
| inner | 6.34 × 10−4 | 2.22 × 10−3 | 3.26 × 10−3 | 5.88 × 10−4 | 2.89 × 10−3 | 4.55 × 10−3 |
| 2 | outer | 4.65 × 10−4 | 1.18 × 10−3 | 2.59 × 10−3 | 5.06 × 10−4 | 2.29 × 10−3 | 4.49 × 10−3 |
| inner | 5.55 × 10−4 | 2.46 × 10−3 | 3.78 × 10−3 | 4.89 × 10−4 | 3.18 × 10−3 | 4.92 × 10−3 |
| 3 | outer | 4.07 × 10−4 | 7.17 × 10−4 | 1.83 × 10−3 | 6.69 × 10−4 | 8.37 × 10−4 | 2.17 × 10−3 |
| inner | 3.81 × 10−4 | 2.19 × 10−3 | 3.39 × 10−3 | 1.23 × 10−4 | 2.66 × 10−3 | 4.58 × 10−3 |
| Right | 3 | outer | - | - | - | 5.80 × 10−4 | 6.55 × 10−4 | 1.76 × 10−3 |
| inner | - | - | - | 1.25 × 10−4 | 2.56 × 10−3 | 4.39 × 10−3 |
| 2 | outer | - | - | - | 3.67 × 10−4 | 1.82 × 10−3 | 3.66 × 10−3 |
| inner | - | - | - | 5.20 × 10−4 | 3.94 × 10−3 | 5.51 × 10−3 |
| 1 | outer | - | - | - | 4.05 × 10−4 | 2.22 × 10−3 | 4.27 × 10−3 |
| inner | - | - | - | 5.73 × 10−4 | 2.89 × 10−3 | 4.40 × 10−3 |
| Mounting holes | Left | 8 | - | 7.77 × 10−5 | 3.76 × 10−4 | 5.71E−04 | 9.66 × 10−5 | 6.75 × 10−4 | 1.03 × 10−3 |
| Right | 8 | - | - | - | - | 6.54 × 10−5 | 4.51 × 10−4 | 7.24 × 10−4 |
| Bar | Left | near 3 | - | - | - | - | 5.46 × 10−4 | 8.10 × 10−4 | 1.13 × 10−3 |
| - | middle | - | - | - | - | 1.56 × 10−6 | 5.96 × 10−5 | 1.39 × 10−4 |
| Right | near 3 | - | - | - | - | 5.60 × 10−4 | 8.80 × 10−4 | 1.22 × 10−3 |
Table 6.
Compilation of stress results for a variable load value.
Table 6.
Compilation of stress results for a variable load value.
| Element | Implant | Position | Side | Single Implant MPa | Arch (MPa) |
|---|
| 100 N | 800 N | 100 N | 800 N |
|---|
| Multi-unit | Left | 1 | outer | 3.0 | 23.7 | 5.5 | 44.6 |
| inner | 7.1 | 57.5 | 6.9 | 56.3 |
| 2 | outer | 5.4 | 48.6 | 6.5 | 51.7 |
| inner | 7.0 | 55.4 | 5.8 | 48.3 |
| 3 | outer | 5.3 | 42.4 | 8.9 | 70.6 |
| inner | 5.0 | 39.5 | 1.7 | 13.0 |
| Right | 3 | outer | - | - | 7.7 | 60.9 |
| inner | - | - | 1.7 | 13.0 |
| 2 | outer | - | - | 5.0 | 37.9 |
| inner | - | - | 6.7 | 54.7 |
| 1 | outer | - | - | 5.2 | 41.4 |
| inner | - | - | 6.8 | 55.9 |
| Mounting holes | Left | 8 | - | 1.0 | 7.8 | 1.3 | 9.7 |
| Right | 8 | - | - | - | 0.7 | 4.9 |
| Bar | Left | near 3 | - | - | - | 6.7 | 55.9 |
| - | middle | - | - | - | 2.4 × 10−2 | 0.1 |
| Right | near 3 | - | - | - | 7.1 | 56.2 |
Table 7.
Compilation of stress results for a variable angle value.
Table 7.
Compilation of stress results for a variable angle value.
| Implant | Position | Side | Single Implant, MPa | | Arch, MPa | |
|---|
| 90° | 60° | 30° | 90° | 60° | 30° |
|---|
| Left | 1 | Outer | 23.7 | 134.7 | 261.5 | 44.6 | 227.5 | 440.5 |
| inner | 57.5 | 189.4 | 283.5 | 56.3 | 274.8 | 416.5 |
| 2 | outer | 48.6 | 123.0 | 268.5 | 51.7 | 233.8 | 462.6 |
| inner | 55.4 | 243.9 | 371.7 | 48.3 | 303.0 | 481.0 |
| 3 | outer | 42.4 | 73.4 | 188.2 | 70.6 | 88.5 | 228.5 |
| inner | 39.5 | 223.5 | 344.9 | 13.0 | 277.5 | 481.0 |
| Right | 3 | outer | - | - | - | 60.9 | 68.7 | 176.6 |
| inner | - | - | - | 13.0 | 269.0 | 460.0 |
| 2 | outer | - | - | - | 37.9 | 181.0 | 370.6 |
| inner | - | - | - | 54.7 | 371.0 | 567.0 |
| 1 | outer | - | - | - | 41.4 | 227.3 | 436.7 |
| inner | - | - | - | 55.9 | 270.0 | 417.0 |
| Left | 8 | - | 7.8 | 37.7 | 57.1 | 9.7 | 67.1 | 103.0 |
| Right | 8 | - | - | - | - | 4.9 | 40.0 | 70.2 |
| Left | near 3 | - | - | - | - | 55.9 | 81.3 | 112.0 |
| - | middle | - | - | - | - | 0.1 | 5.1 | 15.0 |
| Right | near 3 | - | - | - | - | 56.2 | 86.0 | 119.0 |
Table 8.
Displacement results for a single implant.
Table 8.
Displacement results for a single implant.
| Element | Position | Side | Anchorage under Multi-Units, mm |
|---|
| Yes | No |
|---|
| Multi-unit | 1 | outer | 2.4 × 10−3 | 0.5 |
| inner | 3.5 × 10−3 | 0.4 |
| 2 | outer | 1.8 × 10−3 | 2.8 × 10−2 |
| inner | 2.8 × 10−3 | 8.7 × 10−2 |
| 3 | outer | 8.9 × 10−4 | 0.4 |
| inner | 2.7 × 10−3 | 0.4 |
| Mounting hole | 8 | - | 1.1 × 10−4 | - |
| 9 | - | - | 1.9 × 10−3 |
Table 9.
Deformation results for a single implant.
Table 9.
Deformation results for a single implant.
| Element | Position | Side | Anchorage under Multi-Units,
% |
|---|
| Yes | No |
|---|
| Multi-unit | 1 | outer | 1.4 × 10−3 | 4.1 × 10−3 |
| inner | 2.2 × 10−3 | 1.1 × 10−2 |
| 2 | outer | 1.2 × 10−3 | 5.9 × 10−4 |
| inner | 2.5 × 10−3 | 1.1 × 10−2 |
| 3 | outer | 7.2 × 10−4 | 1.1 × 10−2 |
| inner | 2.2 × 10−3 | 1.1 × 10−2 |
| Mounting hole | 8 | - | 3.8 × 10−4 | - |
| 9 | - | - | 6.1 × 10−3 |