Structure Analysis and Its Correlation with Mechanical Properties of Microcellular Polyamide Composites Reinforced with Glass Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
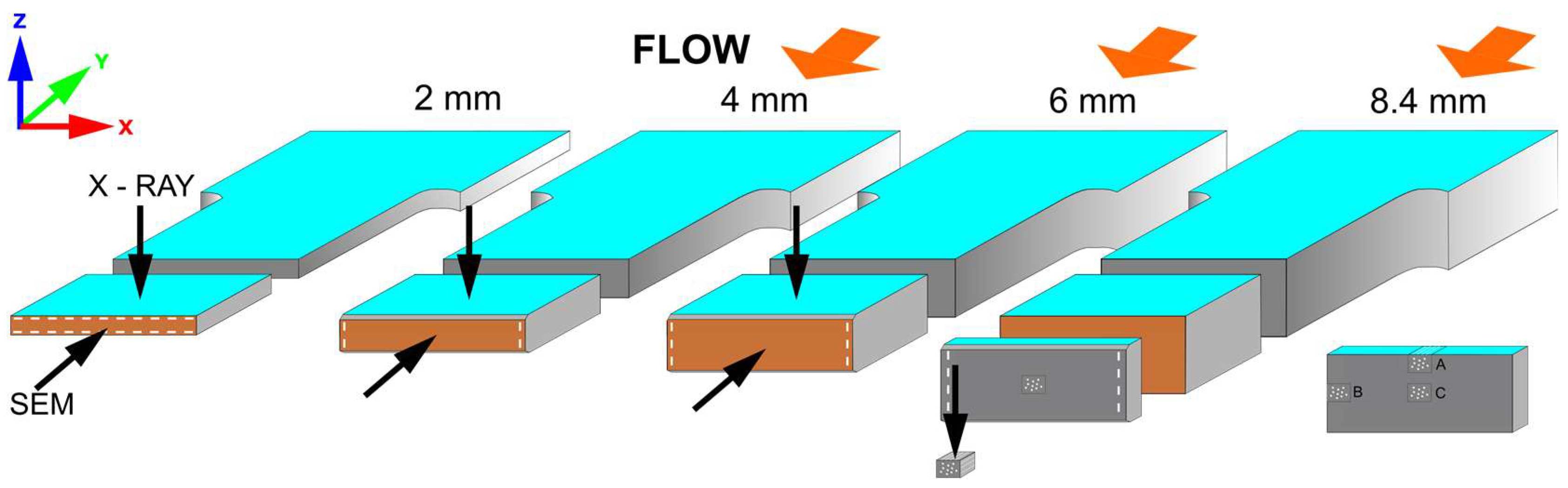
- Preparation of 2 mm thick samples
- Preparation of 4 mm thick samples
- Preparation of 6 mm and 8.4 mm thick samples
2.2. Testing Specimen and Sample Preparation
2.2.1. Foamed Specimen Preparation
- Molding of 2 mm thick samples
- Molding of 4 mm thick samples
- Molding of 6 mm and 8.4 mm thick samples
2.2.2. Sample Preparation for SEM and Micro CT Analysis
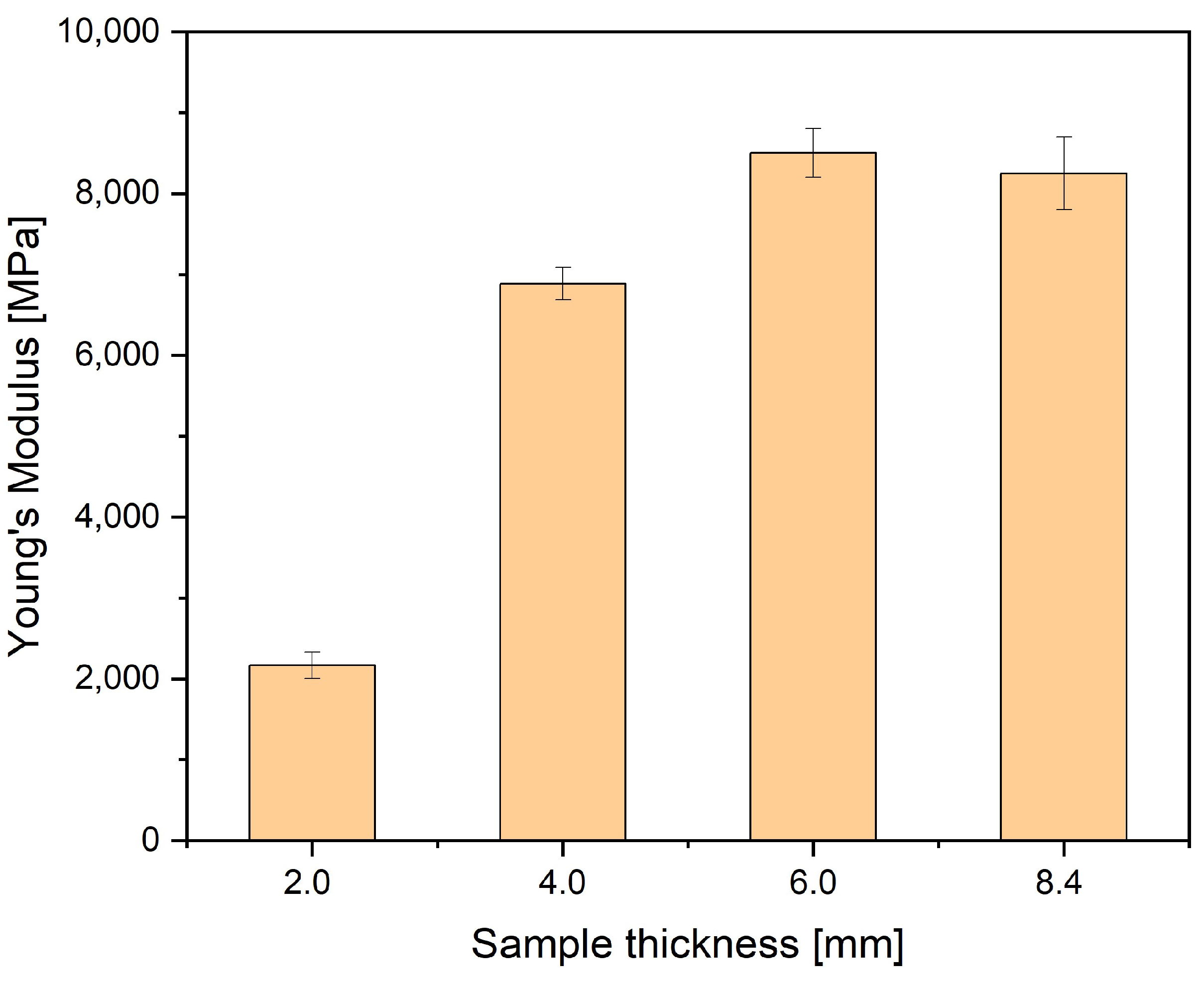
2.3. Measurements of Density and Mechanical Properties
2.4. SEM and Micro-CT Analysis
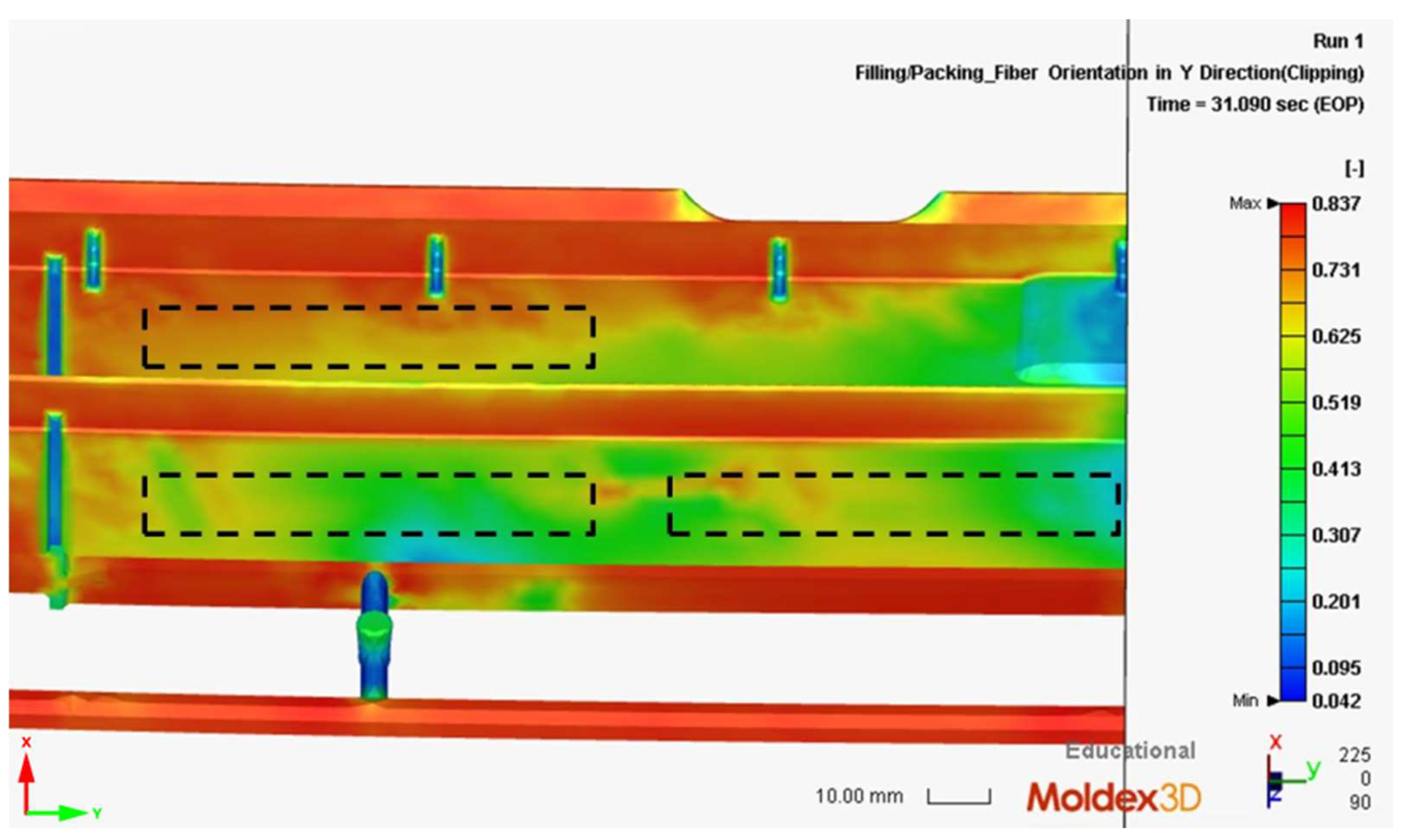
2.5. Moldex3D® Simulation of MIM Process
3. Results and Discussion
3.1. SEM Pictures Analysis
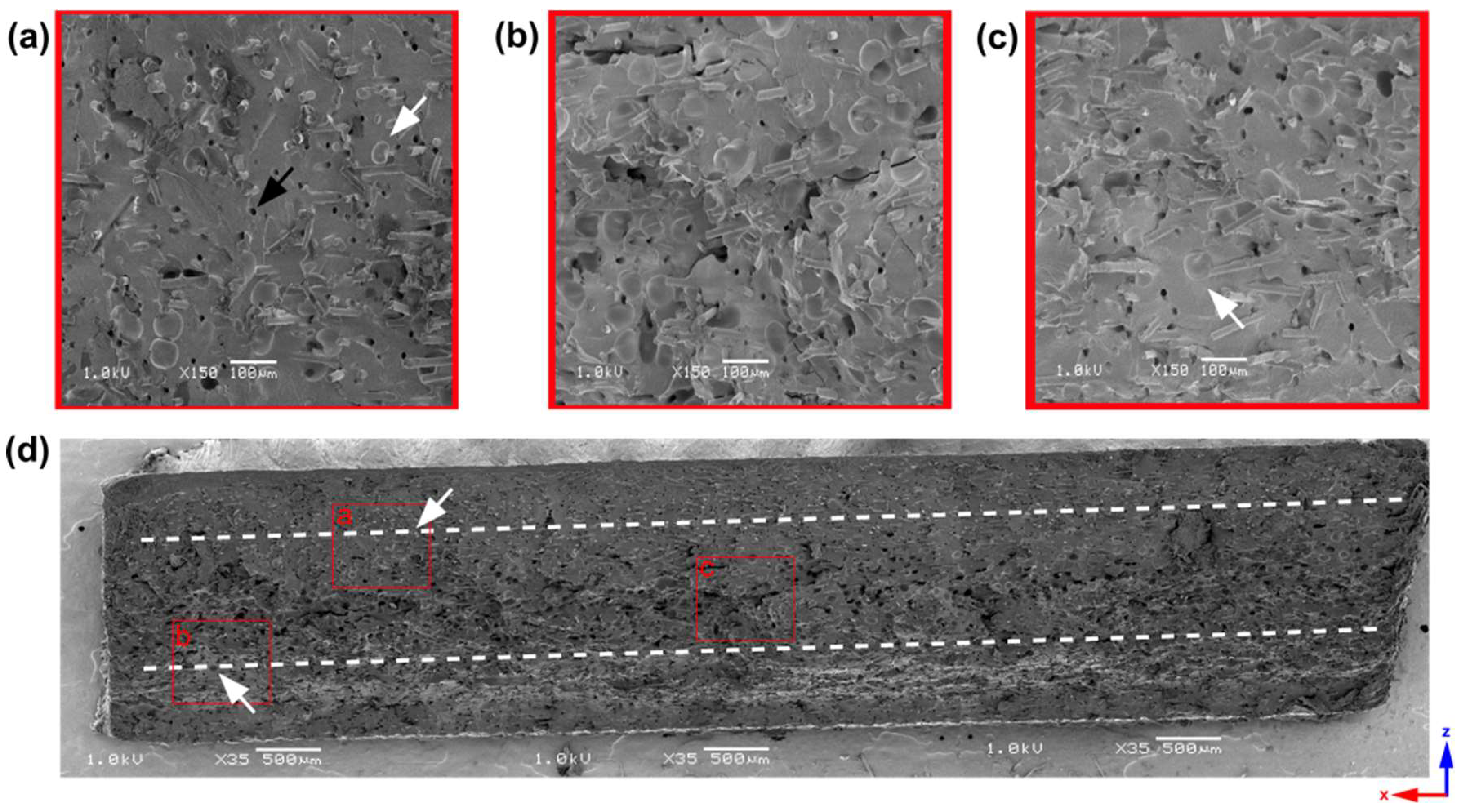
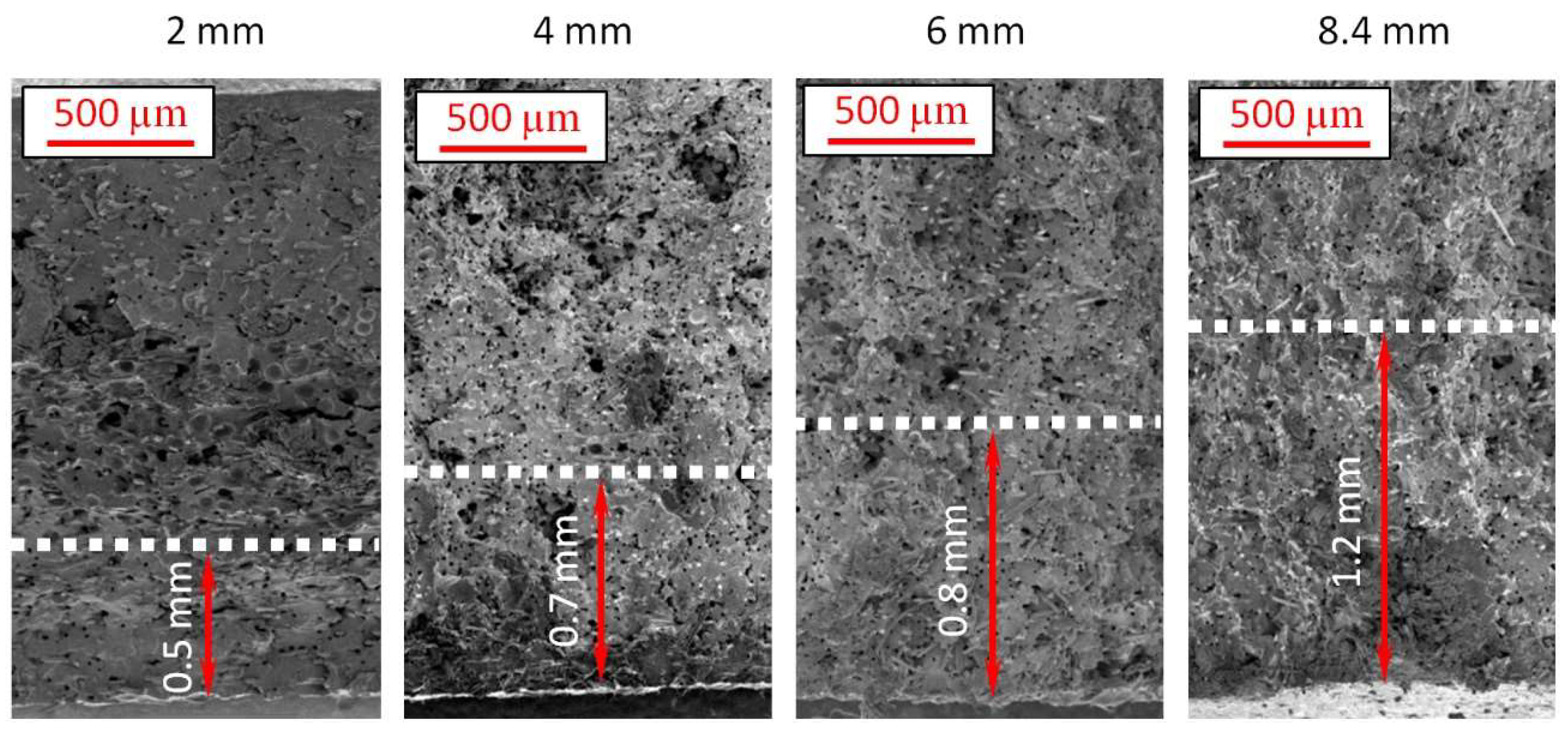
- Analysis of 2 mm thick samples
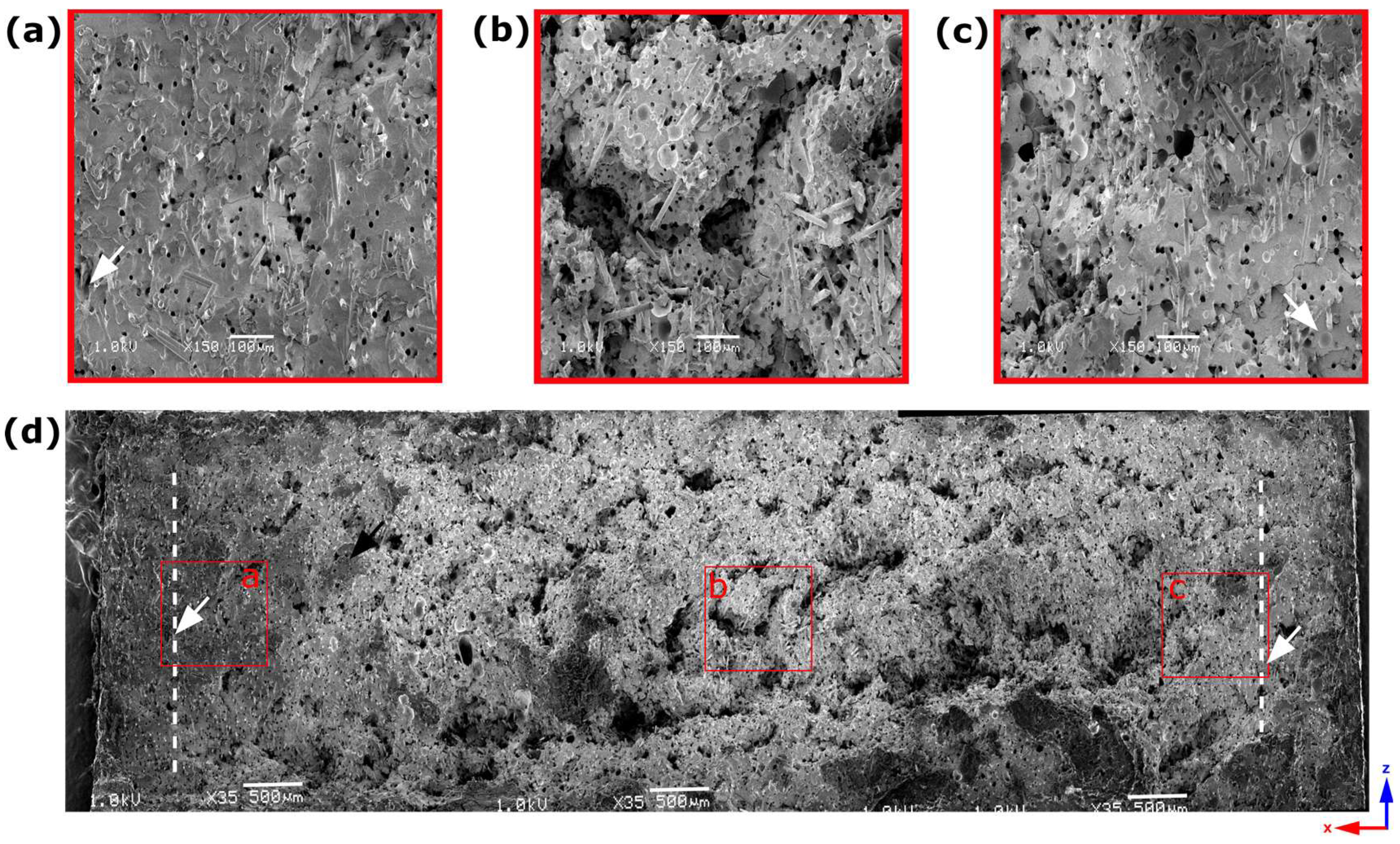
- Analysis of 4 mm thick samples
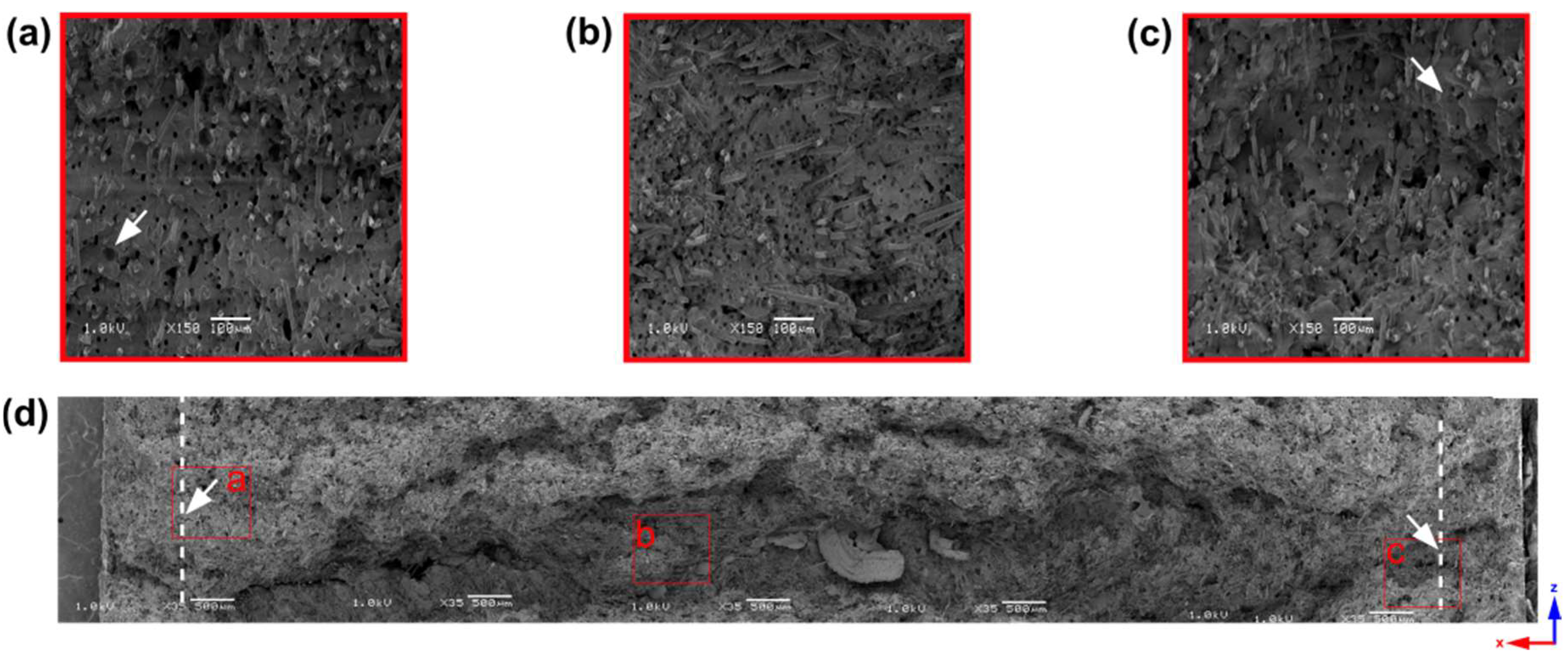
- Analysis of 6 mm thick samples
- Analysis of 8.4 mm thick samples
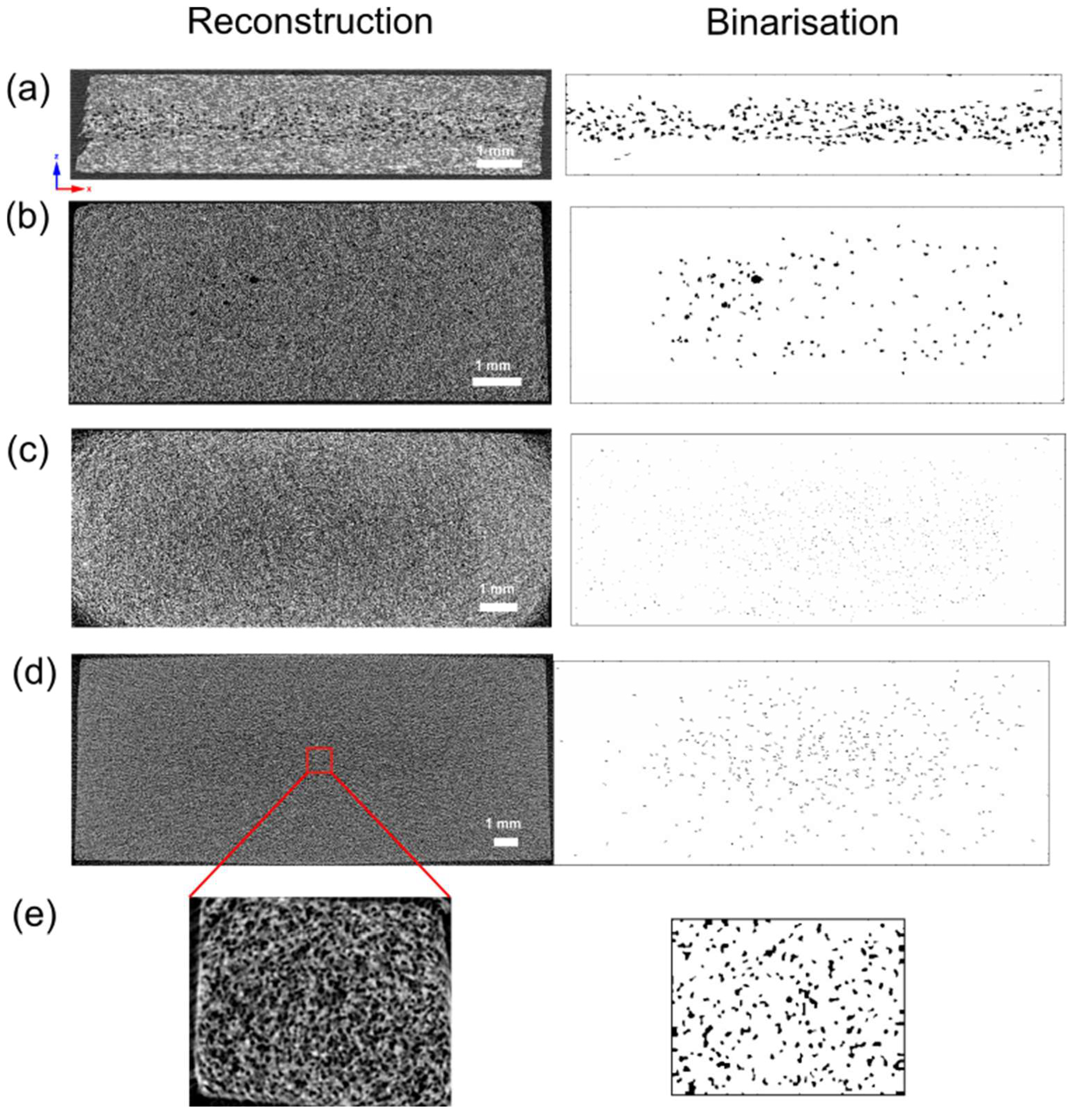
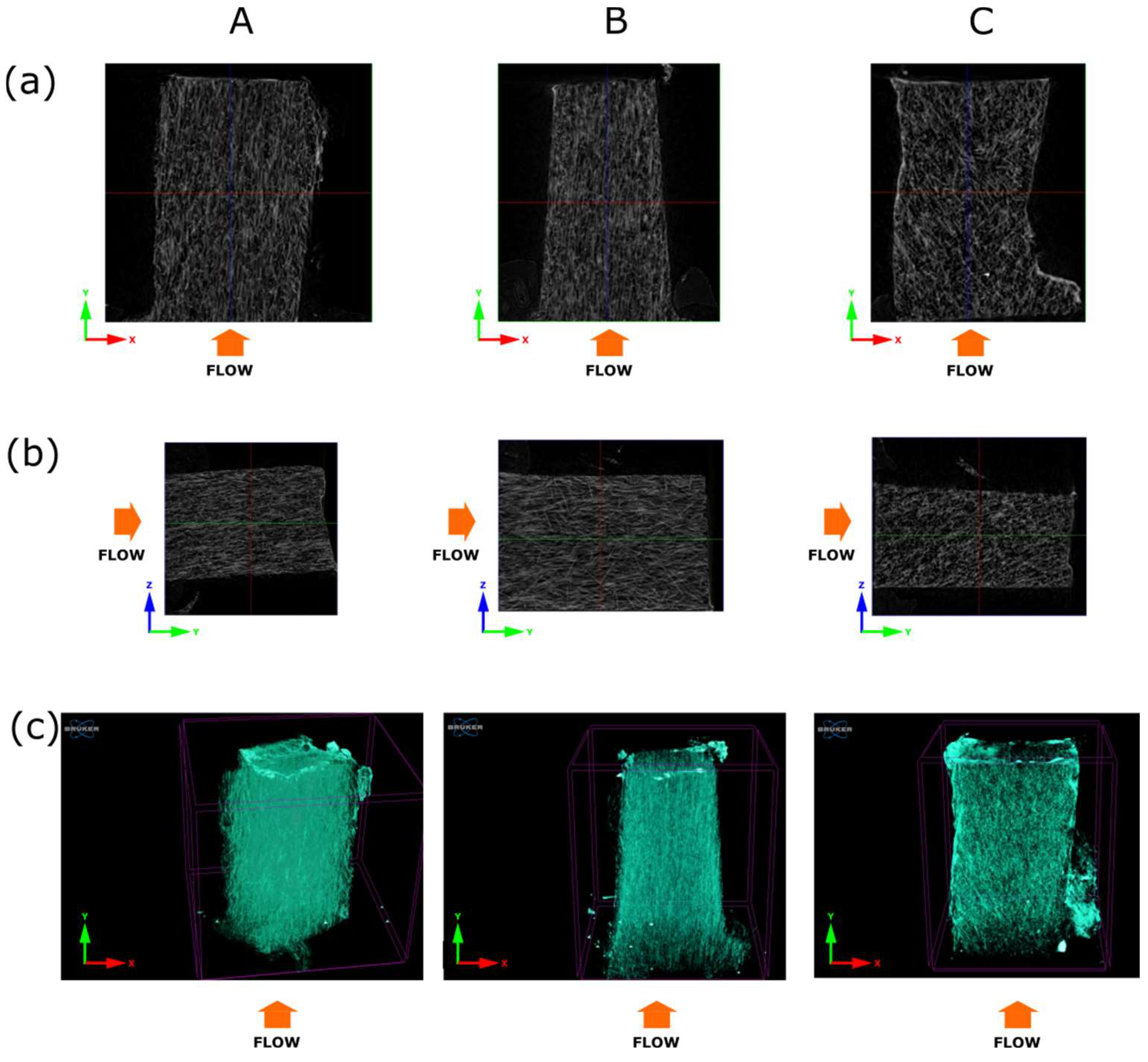
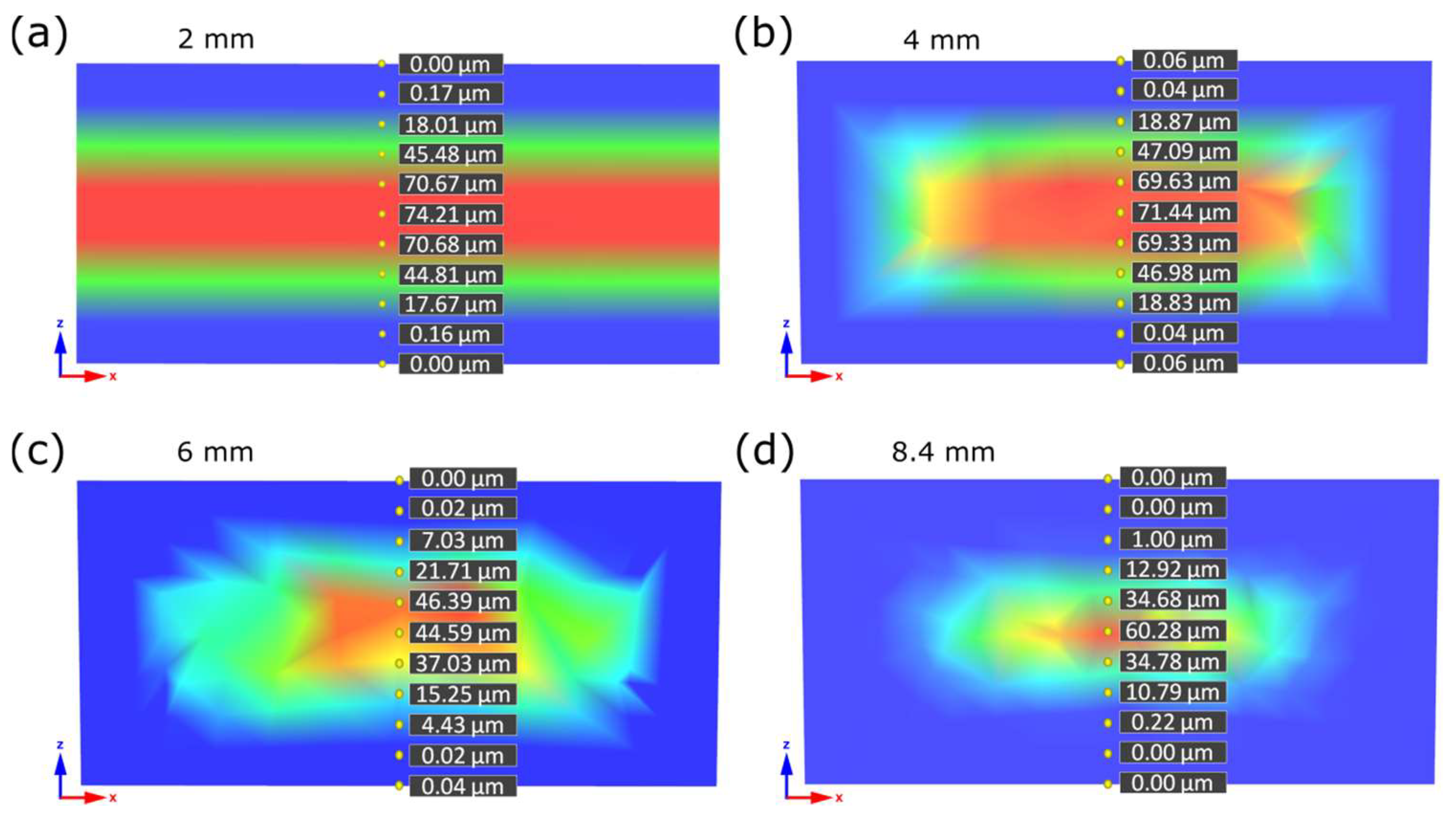
3.2. X-ray Microtomography Analysis
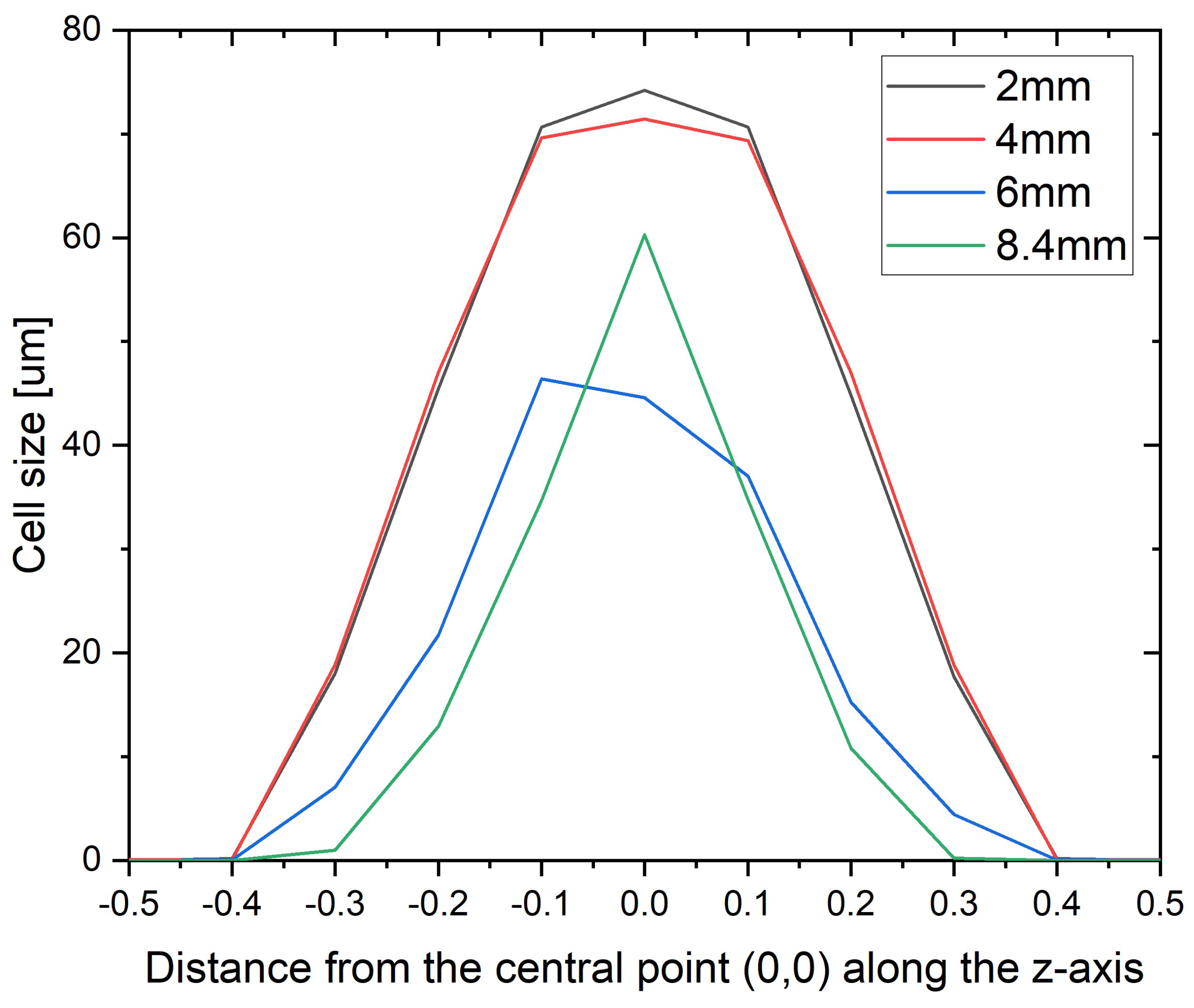
3.3. Moldex3D® Simulation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Plastics–The Facts 2022; Plastics Europe AISBL: Brussels, Belgium, 2022. [Google Scholar]
- Bielinski, M.; Muzyczuk, P. Studies on the mechanical properties in compression of porous polypropylene. Polimery 2011, 56, 309–316. [Google Scholar] [CrossRef]
- Li, M.; Huang, T.; Qin, Y.; Shen, C.; Gao, S. Preparation Process Orthogonal Optimization and Mechanical Properties of Microcellular Foam Polypropylene. Macromol. Mater. Eng. 2021, 306, 2100350. [Google Scholar] [CrossRef]
- Gómez-Monterde, J.; Hain, J.; Sánchez-Soto, M.; Maspoch, M.L. Microcellular injection moulding: A comparison between MuCell process and the novel micro-foaming technology IQ Foam. J. Mater. Process. Technol. 2019, 268, 162–170. [Google Scholar] [CrossRef]
- Simon, S.A.; Hain, J.; Osswald, T. Effect of gas pressure on the microstructure of parts foamed with the novel microcellular injection molding technology Ku-Fizz™. SPE Polym. 2021, 311–324. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Faruk, O.; Kirschling, H.; Kühn, J.; Jaszkiewicz, A. Microcellular polymer and composites–part II. Properties of different types of microcellular materials. Polimery 2007, 52, 3–12. [Google Scholar]
- Llewelyn, G.; Rees, A.; Griffiths, C.A.; Scholz, S.G. Advances in microcellular injection moulding. J. Cell. Plast. 2020, 56, 646–674. [Google Scholar] [CrossRef]
- Xu, J. Microcellular Injection Molding; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bula, K.; Szymańska, J.; Sterzyński, T.; Pasecki, A.; Wróblewski, R. Visualization of Particles Arrangement During Filling Stage of Polyamide 6–Metal Insert Injection Molding. Polym. Eng. Sci. 2019, 59, E271–E278. [Google Scholar] [CrossRef]
- Santharam, P.; Parenteau, T.; Charrier, P.; Taveau, D.; Le Saux, V.; Marco, Y. Complex fibers orientation distribution evaluation in short glass fiber-reinforced thermoplastic (PA66 GF50). MATEC Web Conf. 2018, 165, 22026. [Google Scholar] [CrossRef]
- Szewczykowski, P.P.; Skarżyński, Ł. Application of the X-ray micro computed tomography to the analysis of the structure of polymeric material. Polimery 2019, 64, 12–22. [Google Scholar] [CrossRef]
- Rolland, H.; Saintier, N.; Wilson, P.; Merzeau, J.; Robert, G. In situ X-ray tomography investigation on damage mechanisms in short glass fibre reinforced thermoplastics: Effects of fibre orientation and relative humidity. Compos. Part B Eng. 2017, 109, 170–186. [Google Scholar] [CrossRef]
- Rolland, H.; Saintier, N.; Robert, G. Damage mechanisms in short glass fibre reinforced thermoplastic during in situ microtomography tensile tests. Compos. Part B Eng. 2016, 90, 365–377. [Google Scholar] [CrossRef]
- Rolland, H.; Saintier, N.; Lenoir, N.; King, A.; Robert, G. Fatigue mechanisms description in short glass fibre reinforced thermoplastic by microtomographic observations. In Proceedings of the 21st European Conference on Fracture, ECF21, Catania, Italy, 20–24 June 2016; pp. 301–308. [Google Scholar]
- Rolland, H.; Saintier, N.; Raphaela, I.; Lenoir, N.; King, A.; Robert, G. Fatigue damage mechanisms of short fiber reinforced PA66 as observed by in-situ synchrotron X-ray microtomography. Compos. Part B Eng. 2018, 143, 217–229. [Google Scholar] [CrossRef]
- Quagliato, L.; Kim, Y.; Fonseca, J.H.; Han, D.; Yun, S.; Lee, H.; Park, N.; Lee, H.; Kim, N. The influence of fiber orientation and geometry-induced strain concentration on the fatigue life of short carbon fibers reinforced polyamide-6. Mater. Des. 2020, 190, 108569. [Google Scholar] [CrossRef]
- Cheng, Y.; Laiarinandrasana, L.; Helfen, L.; Proudhon, H.; Klinkova, O.; Baumbach, T.; Morgeneyer, T.F. 3D damage micromechanisms in polyamide 6 ahead of a severe notch studied by in situ synchrotron laminography. Macromol. Chem. Phys. 2016, 217, 701–715. [Google Scholar] [CrossRef]
- Mrzljak, S.; Delp, A.; Schlink, A.; Zarges, J.-C.; Hülsbusch, D.; Heim, H.-P.; Walther, F. Constant Temperature Approach for the Assessment of Injection Molding Parameter Influence on the Fatigue Behavior of Short Glass Fiber Reinforced Polyamide 6. Polymers 2021, 13, 1569. [Google Scholar] [CrossRef] [PubMed]
- Mofakhami, E.; Tencé-Girault, S.; Perrin, J.; Scheel, M.; Gervat, L.; Ovalle, C.; Laiarinandrasana, L.; Fayolle, B.; Miquelard-Garnier, G. Microstructure-mechanical properties relationships in vibration welded glass-fiber-reinforced polyamide 66: A high-resolution X-ray microtomography study. Polym. Test. 2020, 85, 106454. [Google Scholar] [CrossRef]
- Ayadi, A.; Nouri, H.; Guessasma, S.; Roger, F. An original approach to assess elastic properties of a short glass fibre reinforced thermoplastic combining X-ray tomography and finite element computation. Compos. Stuctures 2015, 125, 277–286. [Google Scholar] [CrossRef]
- Ghorbel, A.; Saintier, N.; Dhiab, A. Investigation of damage evolution in short glass fibers reinforced polyamide 6,6 under tensile loading using infrared thermography. In Proceedings of the 11th International Conference on the Mechanical Behavior of Materials (ICM11) Engineering Procedia, Como, Italy, 5–9 July 2011; p. 10. [Google Scholar]
- Gassoumi, M.; du Roscoat, S.R.; Casari, P.; Dumont, P.J.J.; Orgéas, L.; Jacquemin, F. Shear behavior of thermoformed woven- textile thermoplastic prepregs: An analysis combining bias-extension test and X-ray microtomography. In Proceedings of the 20th International Esaform Conference on Material Forming (ESAFORM 2017), Dublin, Ireland, 26–28 April 2017; AIP Publishing: College Park, MD, USA, 2017; Volume 1896, pp. 1–6. [Google Scholar]
- Malpot, A.; Touchard, F.; Bergamo, S. An investigation of the influence of moisture on fatigue damage mechanisms in a woven glass-fibre-reinforced PA66 composite using acoustic emission and infrared thermography. Compos. Part B Eng. 2017, 130, 11–20. [Google Scholar] [CrossRef]
- Madra, A.; El Hajj, N.; Benzeggagh, M. X-ray microtomography applications for quantitative and qualitative analysis of porosity in woven glass fiber reinforced thermoplastic. Compos. Sci. Technol. 2014, 95, 50–58. [Google Scholar] [CrossRef]
- Laiarinandrasana, L.; Klinkova, O.; Nguyen, F.; Proudhon, H.; Morgeneyer, T.F.; Ludwig, F. Three dimensional quantification of anisotropic void evolution in deformed semicrystalline Polyamide 6 International. J. Plast. 2016, 83, 19–36. [Google Scholar] [CrossRef]
- Laiarinandrasana, L.; Morgeneyer, T.F.; Proudhon, H.; N’guyen, F.; Maire, E. Effect of Multiaxial Stress State on Morphology and Spatial Distribution of Voids in Deformed Semicrystalline Polymer Assessed by X-ray Tomography. Macomolecules 2012, 45, 4658–4668. [Google Scholar] [CrossRef]
- Volpe, V.; Lanzillo, S.; Affinita, G.; Macchiarolo, I.; Pantani, R. Lightweight high-performance polymer composite for automotive applications. Polymers 2019, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Sykutera, D.; Czyżewski, P.; Szewczykowski, P. High-Performance of a Thick-Walled Polyamide Composite Produced by Microcellular Injection Molding. Materials 2021, 15, 4199. [Google Scholar] [CrossRef] [PubMed]
- Sykutera, D.; Szewczykowski, P.; Roch, M.; Wajer, Ł.; Grabowski, M.; Bieliński, M. Effect of nitrogen content on physical properties of glass fiber reinforced polyamide 6 prepared by microcellular injection molding. Polimery 2018, 11–12, 743–749. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Xie, P.; Gao, X.; Yang, W. Microstructure and Properties of Glass Fiber-Reinforced Polyamide/Nylon Microcellular Foamed Composites. Polymers 2020, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Román, A.J.; Chang, T.-C.; Yu, C.; Jiang, J.; Shotwell, D.; Chen, E.; Osswald, T.A.; Turng, L.-S. Cell morphologies, mechanical properties, and fiber orientation of glass fiber-reinforced polyamide composites: Influence of subcritical gas-laden pellet injection molding foaming technology. Phys. Fluids 2022, 34. [Google Scholar] [CrossRef]
- Kastner, C.; Steinbichler, G.; Kahlen, S.; Jerabek, M. Influence of process parameters on mechanical properties of physically foamed, fiber reinforced polypropylene parts. J. Applier Polym. Sci. 2019, 136, 47275. [Google Scholar] [CrossRef]
- Nobe, R.; Qiu, J.H.; Kudo, M.; Ito, K.; Kaneko, M. Effects of SCF content, injection speed, and CF content on the morphology and tensile properties of microcellular injection-molded CF/PP composites. Polym. Eng. Sci. 2019, 59, 1371–1380. [Google Scholar] [CrossRef]
- Ding, Y.; Hassan, M.H.; Bakker, O.; Hinduja, S.; Bartolo, P. A Review on Microcellular Injection Moulding. Materials 2021, 14, 4209. [Google Scholar] [CrossRef]
- PN-EN ISO 527-2:2012; Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. Polski Komitet Normalizacyjny: Warszawa, Poland, 2012.
- PN-EN ISO 179-2:2020-12; Plastics—Determination of Charpy Impact Properties—Part 2: Instrumented Impact Test. Polski Komitet Normalizacyjny: Warszawa, Poland, 2020.
- Nowinka, B.; Czyżewski, P.; Sykutera, D.; Marciniak, D. Analysis of microcellular injection molding with use of FEM simulation. AIP Conf. Proc. 2022, 2611, 11. [Google Scholar]
- Sykutera, D.; Czyżewski, P.; Szewczykowski, P. The Microcellular Structure of Injection Molded Thick-Walled Parts as Observed by In-Line Monitoring. Materials 2020, 13, 5464. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, F.; Ravalico, C. Threshold Identification for Micro-Tomographic Damage Characterisation in a Short-Fibre-Reinforced Polymer. Strain 2015, 51, 171–179. [Google Scholar] [CrossRef]
- Cosmi, F.; Bernasconi, A. Micro-CT investigation on fatigue damage evolution in short fibre reinforced polymers. Compos. Sci. Technol. 2013, 79, 70–76. [Google Scholar] [CrossRef]
- Cieszko, M.; Szczepański, Z.; Gadzala, P. Determination of bone porosity based on histograms of 3D μCT images. J. Mater. Sci. 2015, 50, 948–959. [Google Scholar] [CrossRef]
- Ding, Y.; Vyas, C.; Bakker, O.; Hinduja, S.; Bartolo, P. Modelling and Simulation of MuCell®: The Effect of Key Processing Parameters on Cell Size and Weight Reduction. Polymers 2022, 14, 4215. [Google Scholar] [CrossRef]
- A Guide to the MeCell® Microcellular Foam Injection Molding Process–T SERIES, Trexel. 2015. Available online: https://ja.trexel.com/wp-content/uploads/2019/08/MuCell-Processing-Guide-English-082015.pdf (accessed on 1 November 2023).
- Wu, H.; Haugen, V.; Wintermantel, E. Supercritical CO2 in injection molding can produce open porous polyurethane scaffolds–A parameter study. J. Cell. Plast. 2012, 48, 141–159. [Google Scholar] [CrossRef]
- Llewelyn, G.; Rees, A.; Griffiths, C.; Jacobi, M. A Design of Experiment Approach for Surface Roughness Comparisons of Foam Injection- Moulding Methods. Materials 2020, 13, 2358. [Google Scholar] [CrossRef]
- Błędzki, A.; Kirschling, H.; Rohleder, M.; Chate, A. Correlation between injection moulding processing parameters and mechanical properties of microcellular polycarbonate. J. Cell. Plast. 2012, 48, 301–340. [Google Scholar] [CrossRef]







| Sample Thickness, mm | Material | Density Reduction, % |
|---|---|---|
| 2 | PA6 30GF (Akulon K224-HG6) (DSM, Heerlen, The Netherlands) | 5.1 |
| 4 | PA66 30GF (Technyl AR 130GF) (Solvay, Brussels, Belgia) | 5.5 |
| 6 | PA66 30GF (Technyl AR 130-1) (Rhodia, La Defence, France) | 5.3 |
| 8.4 | PA66 30GF (Technyl AR 130-1) (Rhodia, La Defence, France) | 5.3 |
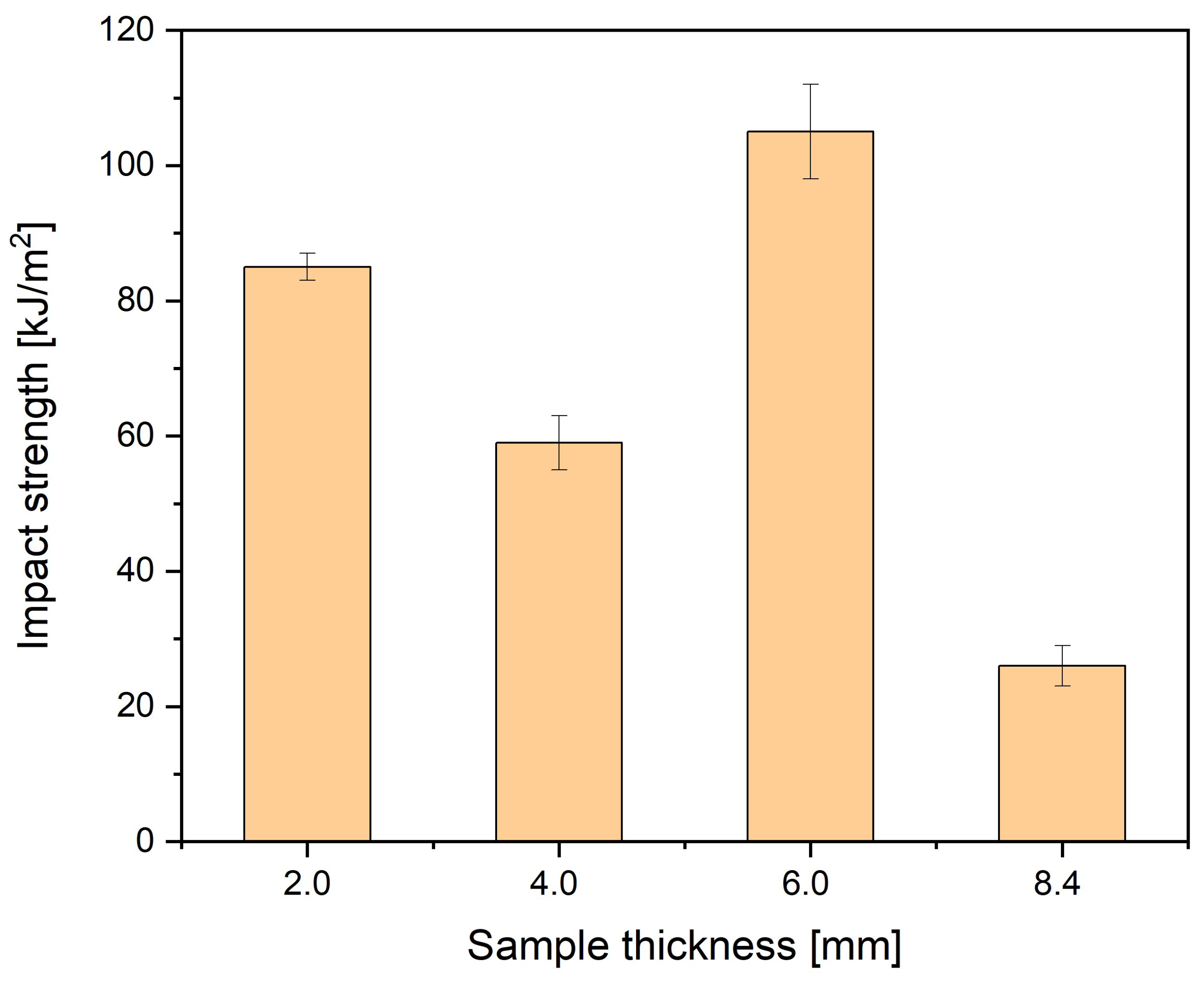
| Sample Thickness, mm | Density, kg/m³ | MFI, g/10 min | Tensile Modulus, MPa | Stress at Break, MPa | Charpy Impact Strength, kJ/m2 |
|---|---|---|---|---|---|
| 2 | 1350 | 30 | 6000 | 110 | 110 |
| 4 | 1370 | 34 | 6000 | 95 | 65 |
| 6 | 1370 | 34 | 6300 | 95 | 35 |
| 8.4 | 1370 | 34 | 6300 | 95 | 35 |
| IM Parameters | 2 mm | 4 mm | 6 mm | 8.4 mm |
|---|---|---|---|---|
| filling pressure, MPa | 112.9 | 70 | 70 | 70 |
| melt temperature, °C | 285 | 285 | 285 | 285 |
| holding pressure, MPa | 20 | 16 | 14 | 14 |
| holding time, s | 0.3 | 0.3 | 0.3 | 0.3 |
| mold temperature, °C | 90 | 90 | 90 | 90 |
| cooling time, s | 28 | 30 | 50 | 50 |
| Parameter | 2 mm | 4.2 mm | 6 mm + 8.4 mm |
|---|---|---|---|
| Solid mesh elements | 1,038,216 | 78,387 | 141,605 |
| Surface mesh elements | 196,920 | 14,162 | 26,806 |
| Mesh size (mm) | 2.72 | 2.475 | 2.475 |
| Mesh boundary layers | 3 | 3 | 3 |
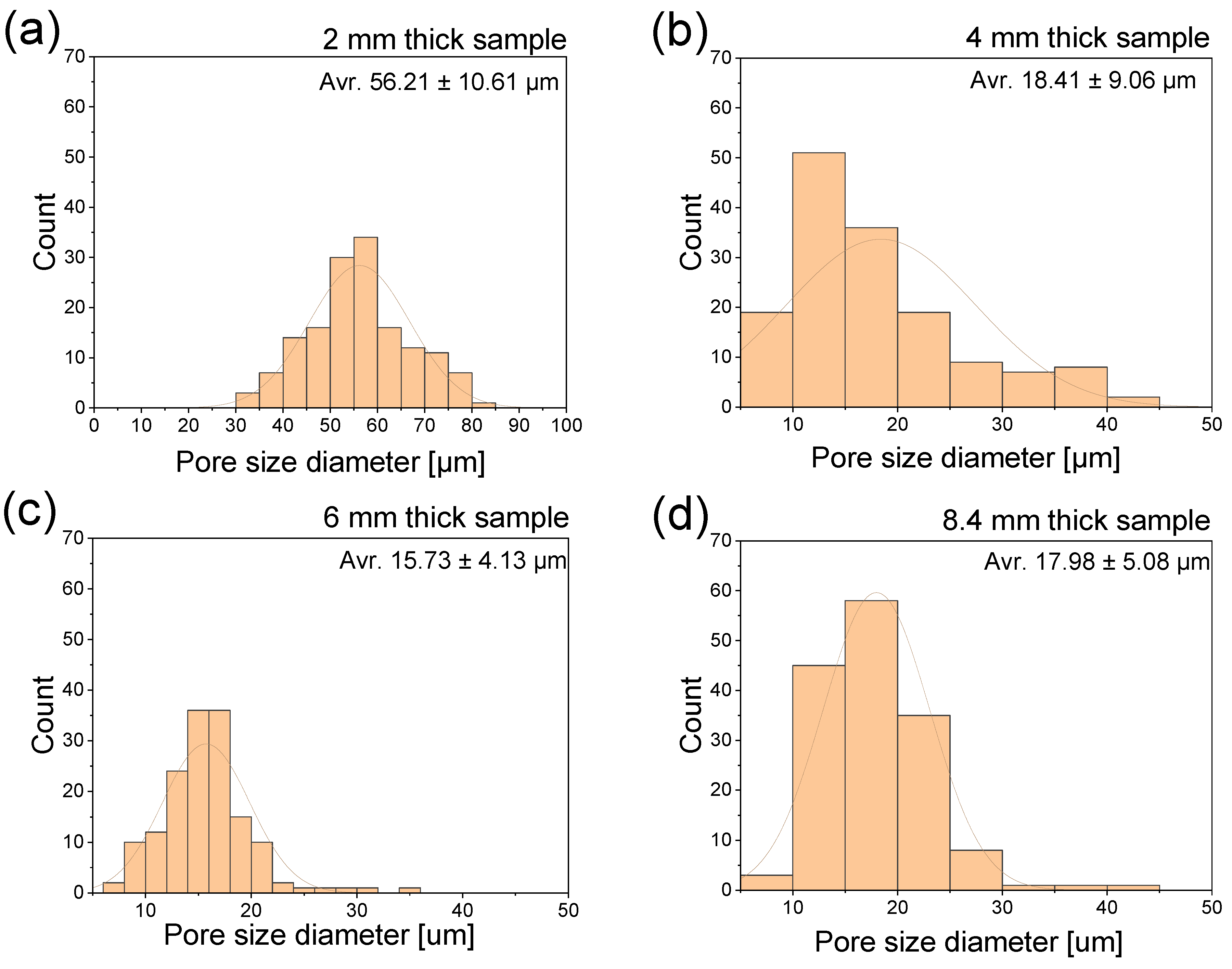
| Sample Thickness, mm | Skin Layer Thickness (by SEM), mm | Avr. Pore Size (by SEM), µm | Avr. Pore Size (by µCT), µm | Pore Size in the Core Part (by Moldex3D®), µm |
|---|---|---|---|---|
| 2 | ~0.5 | 56.21 ± 10.61 | 32.59 | 74.21 |
| 4 | ~0.7 | 18.41 ± 9.06 | 28.37 | 71.44 |
| 6 | ~0.8 | 15.73 ± 4.13 | 16.87 | 44.59 |
| 8.4 | ~1.2 | 17.98 ± 5.08 | 23.95 | 60.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczykowski, P.; Sykutera, D.; Czyżewski, P.; Cieszko, M.; Szczepański, Z.; Nowinka, B. Structure Analysis and Its Correlation with Mechanical Properties of Microcellular Polyamide Composites Reinforced with Glass Fibers. Materials 2023, 16, 7501. https://doi.org/10.3390/ma16237501
Szewczykowski P, Sykutera D, Czyżewski P, Cieszko M, Szczepański Z, Nowinka B. Structure Analysis and Its Correlation with Mechanical Properties of Microcellular Polyamide Composites Reinforced with Glass Fibers. Materials. 2023; 16(23):7501. https://doi.org/10.3390/ma16237501
Chicago/Turabian StyleSzewczykowski, Piotr, Dariusz Sykutera, Piotr Czyżewski, Mieczysław Cieszko, Zbigniew Szczepański, and Bartosz Nowinka. 2023. "Structure Analysis and Its Correlation with Mechanical Properties of Microcellular Polyamide Composites Reinforced with Glass Fibers" Materials 16, no. 23: 7501. https://doi.org/10.3390/ma16237501
APA StyleSzewczykowski, P., Sykutera, D., Czyżewski, P., Cieszko, M., Szczepański, Z., & Nowinka, B. (2023). Structure Analysis and Its Correlation with Mechanical Properties of Microcellular Polyamide Composites Reinforced with Glass Fibers. Materials, 16(23), 7501. https://doi.org/10.3390/ma16237501







