Investigation of Preparation and Shrinkage Characteristics of Multi-Source Solid Waste-Based Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemical Composition of Solid Waste Materials
2.1.2. Phase Composition of Solid Waste Materials
2.1.3. Activity Evaluation of Solid Waste Materials
2.2. Methods
2.2.1. Box–Behnken Response Surface Experimental Design
2.2.2. The Synergistic Reaction Mechanism Test of SWCM
- (1)
- XRD analysis
- (2)
- XRF analysis
- (3)
- FT-IR analysis
- (4)
- TGA analysis
2.2.3. Mechanical Test of Stabilized Macadam Using SWCM
- (1)
- Mechanical properties test
- (2)
- Frost resistance test
- (3)
- Shrinkage test
3. Results and Analysis
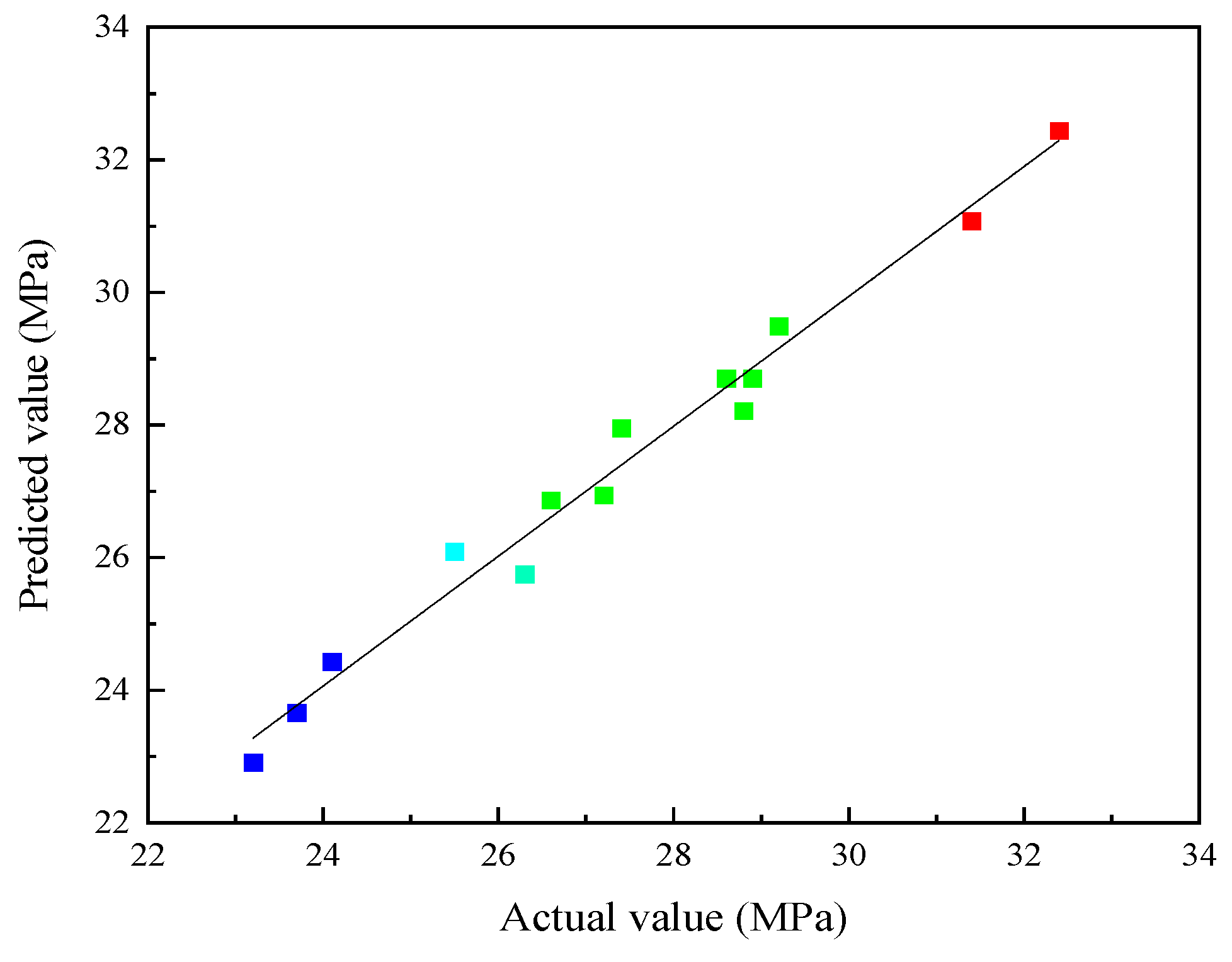
3.1. Analysis of Response Surface Method Results
3.2. Analysis on the Strength Mechanism of SWCM
3.2.1. XRD Analysis of SWCM
3.2.2. FT-IR Analysis of SWCM
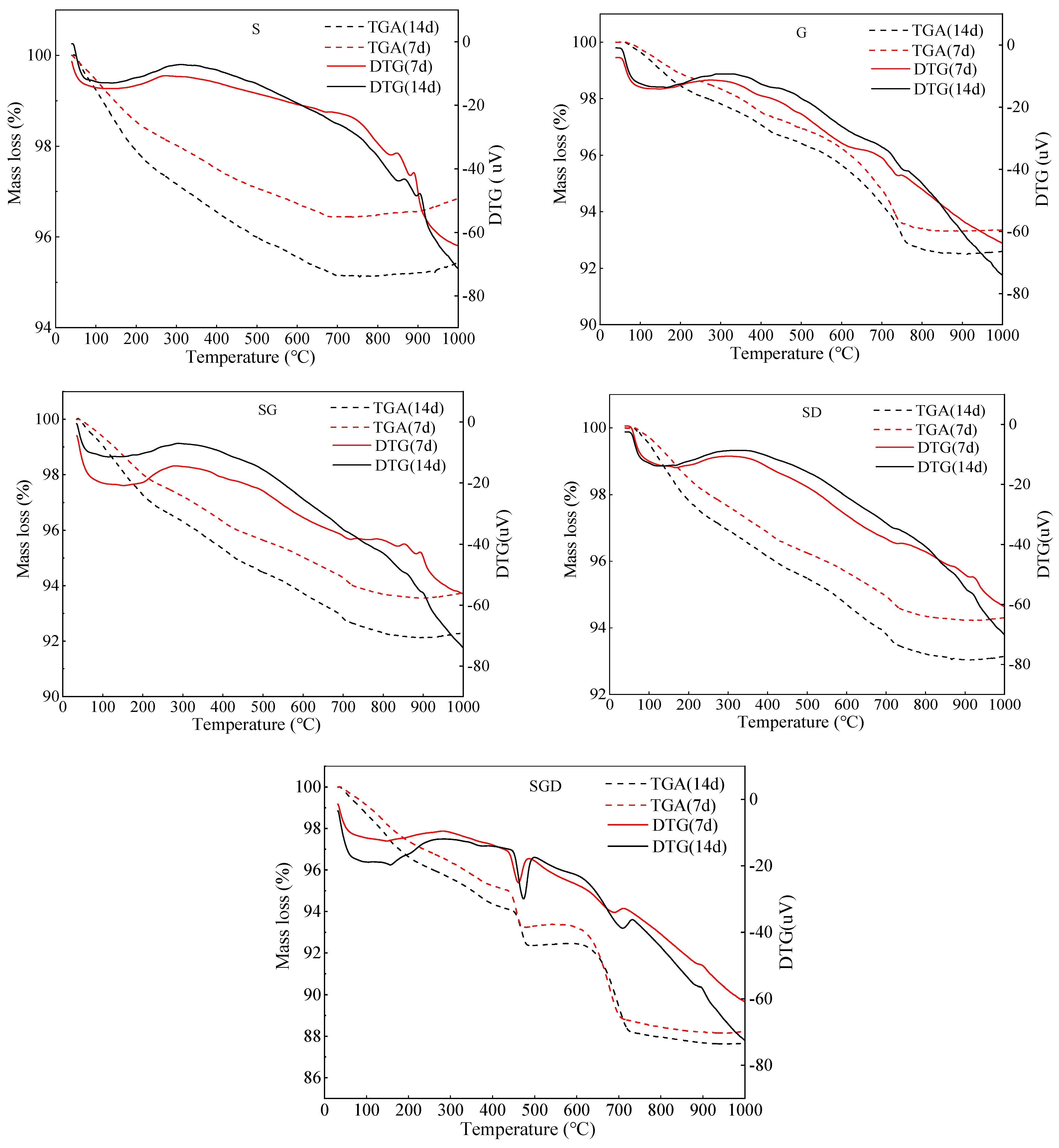
3.2.3. Thermogravimetric TG Analysis of SWCM
3.3. Analysis of Mechanical Properties of SWCM-SM
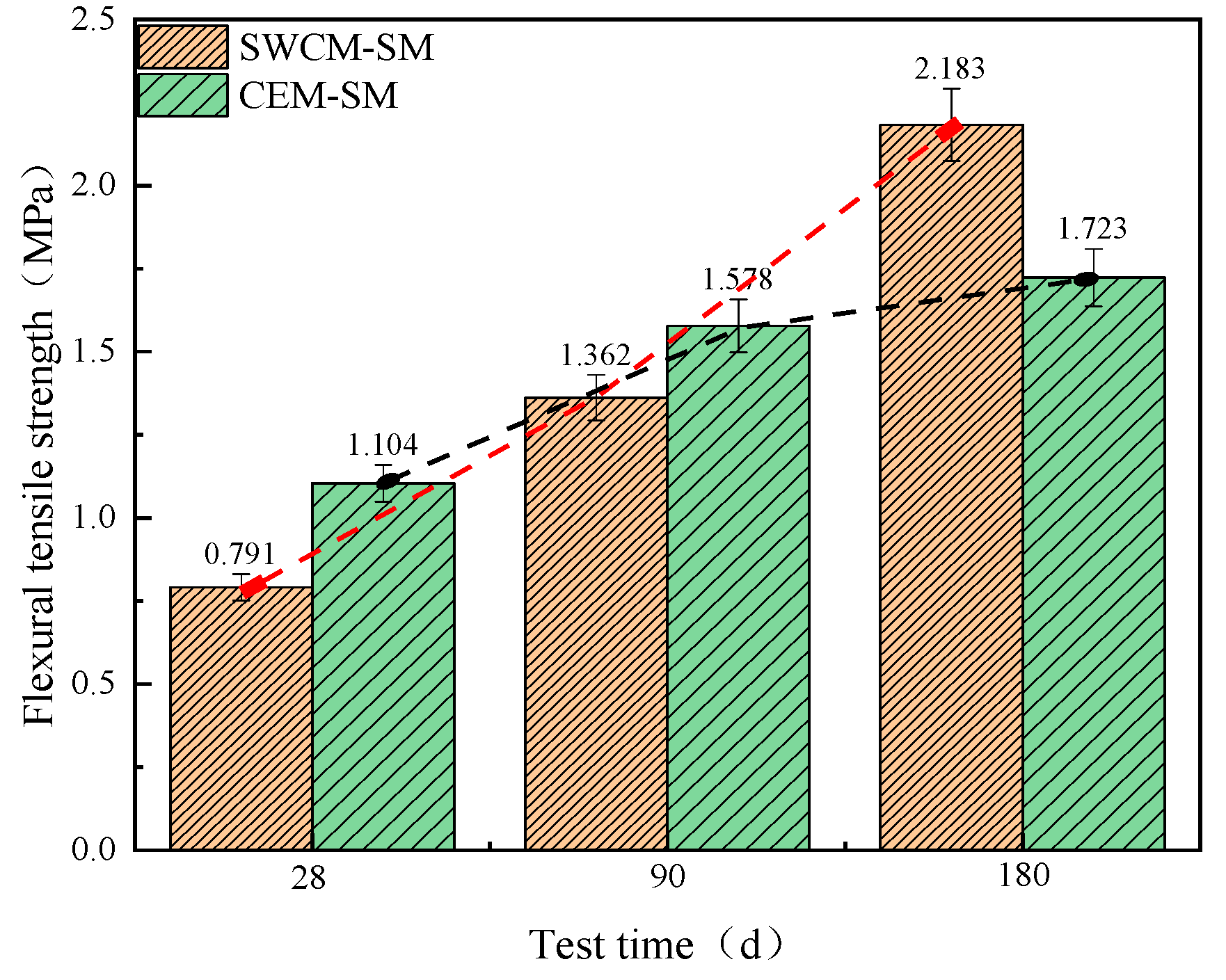
3.3.1. Analysis of Strength Growth Law
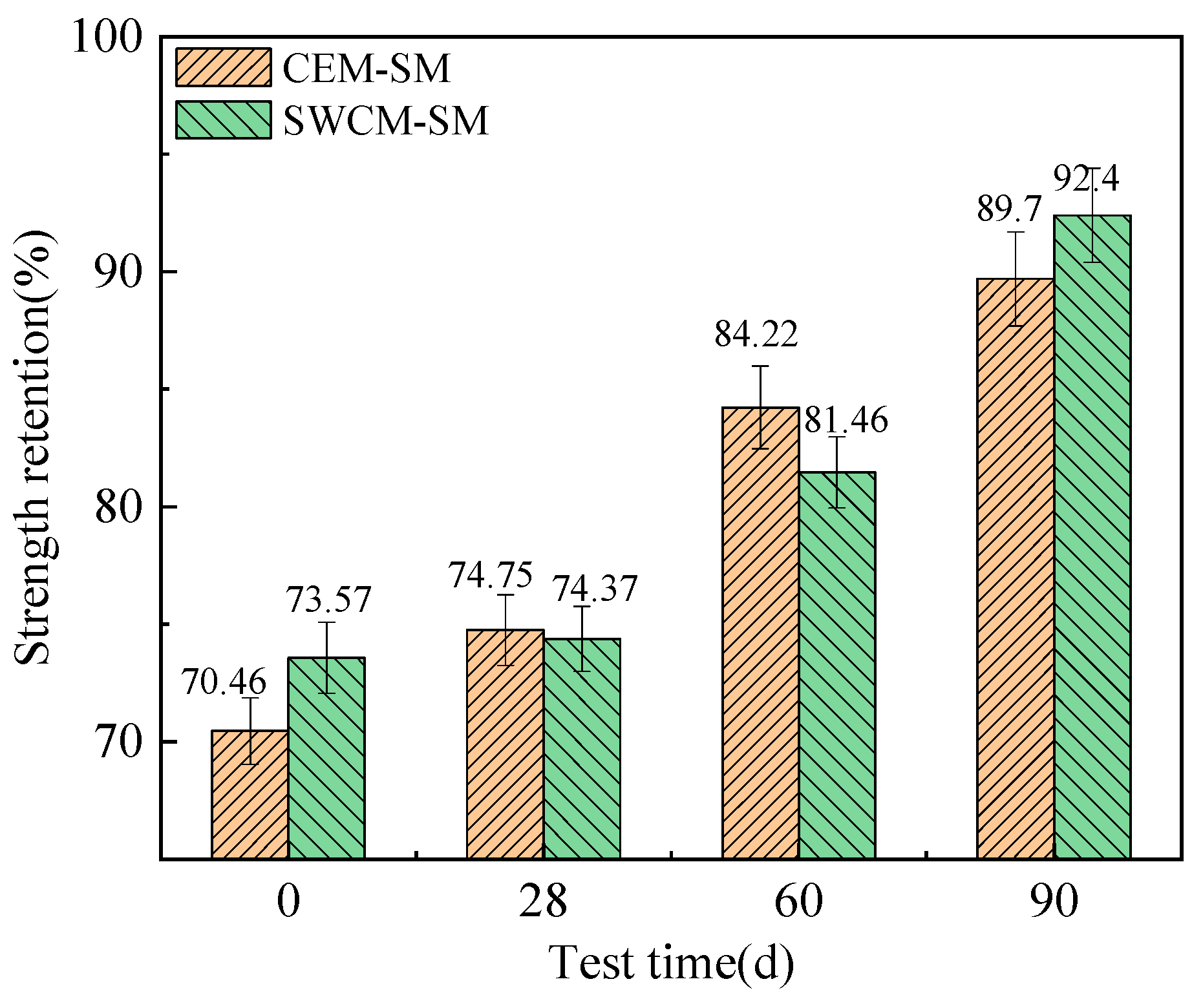
3.3.2. Frost Resistance Durability Analysis
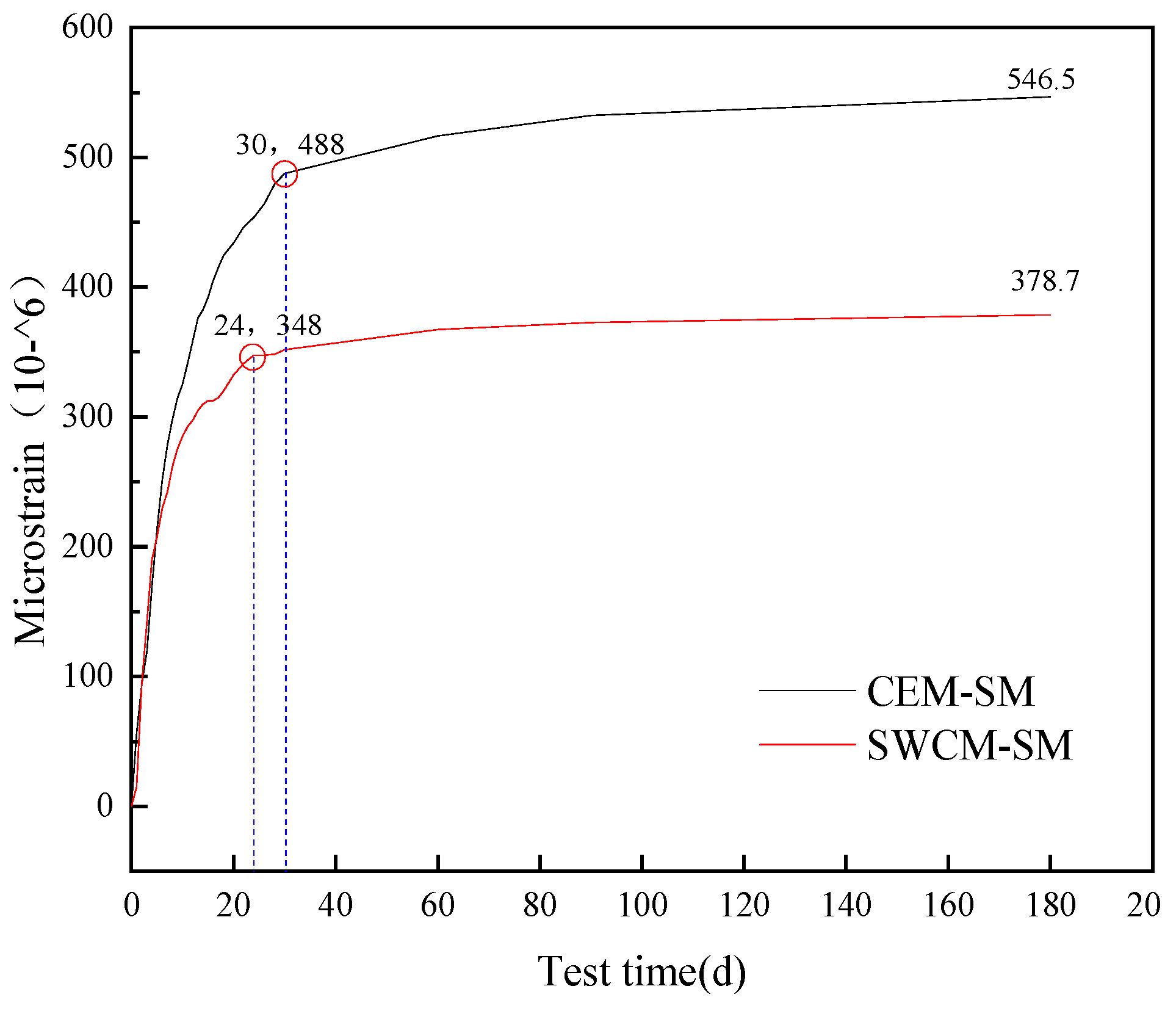
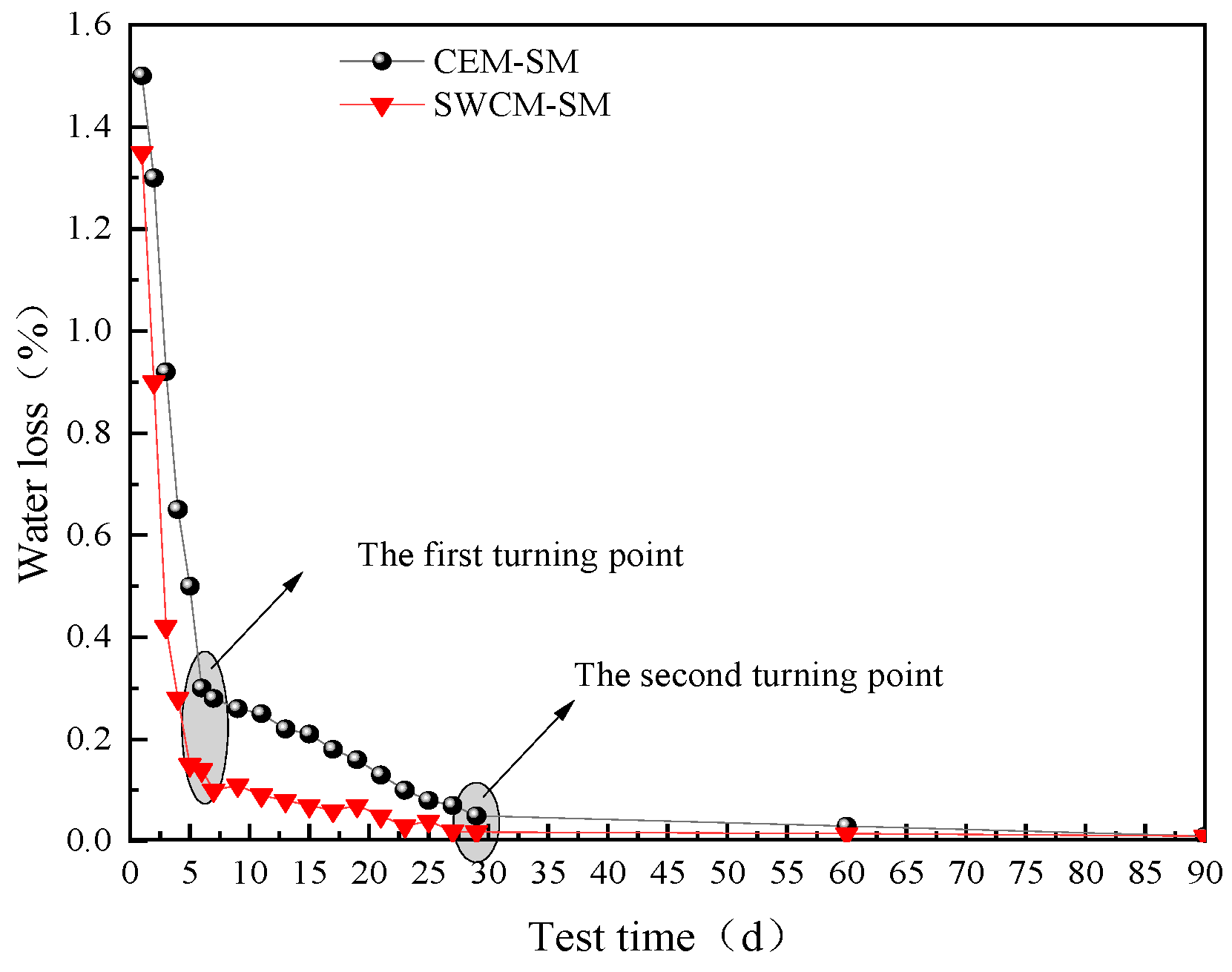
3.3.3. Shrinkage Characteristic Analysis
4. Conclusions
- (1)
- A response surface function model was established between 28 days compressive strength and multivariate solid waste, with a correlation of 0.9136. The optimal parameter combination of the SWCMs was calculated using this model, which included 60% slag content, 30% steel slag content, and 10% desulfurization gypsum content.
- (2)
- The active ingredients involved in the hydration reaction came from the amorphous calcium silicate aluminate active ingredients in the GGBS. Although SS had a higher calcium content, the active ingredient content in SS was lower. The active calcium component of SS promoted the early hydration reaction of the GGBS, and the FGD participated in the reaction of the cementitious system to generate ettringite crystals, improving the early strength.
- (3)
- The unconfined compressive strength and flexural tensile strength of the SWCM-SM exhibited characteristics of low early strength and high later strength. The frost resistance of the SWCM-SM was basically equivalent to that of the CEM-SM in the entire maintenance cycle.
- (4)
- The dry shrinkage strain of the SWCM-SM was reduced by 30.7% compared to the CEM-SM after 180 days of testing. The turning point for the growth of the dry shrinkage strain of the CEM-SM was 30 days, while that of the SWCM-SM was 24 days. The curing age during the construction process can be determined based on the turning point of the shrinkage strain.
- (5)
- Early microstrain was mainly caused by the early shrinkage of the specimen due to water loss, and the water loss rate of the SWCM-SM was lower than that of the CEM-SM. A portion of the microstrain was contributed by the self-shrinkage generated by the hydration reaction of cementitious materials, and the self-shrinkage of the SWCM was smaller than that of cement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y. The hotspot and frontier of solid waste comprehensive utilization research—Big data visualization research based on scientific literature. Chem. Miner. Geol. 2022, 2, 183–192. [Google Scholar]
- Shen, S. Study on the Preparation Mechanism and Performance of Inorganic Polymerized Porous Materials in Multiple Solid Waste Systems; Hubei Univetity: Wuhan, China, 2022. [Google Scholar]
- Farhan, Z.; Johari, M.; Demirboga, R. Assessment of important parameters involved in the synthesis of geopolymer composites: A review. Constr. Build. Mater. 2020, 264, 120276. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Rasaki, S.; Zhang, B.; Guarecuco, R. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Cleanner Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Kiventera, J.; Lancellotti, I.; Catauro, M. Alkali activation as new option for gold mine tailing sinertization. J. Clean. Prod. 2018, 187, 76–84. [Google Scholar] [CrossRef]
- Bumanis, G.; Novais, R.; Carvalheiras, J. Metals removal from aqueous solutions by tailored porous waste-based granulated alkali-activated materials. Appl. Clay Slay Sci. 2019, 179, 105147.1–105147.9. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A.; Pawluk, A.; Pyzalski, M. The mineralsequestration of CO2 with theuse of flyash from the co-combustion of coal and biomass. Gospod. Surowcami Miner./Miner. Manag. 2017, 33, 143–156. [Google Scholar] [CrossRef][Green Version]
- Khalid, H.; Lee, N.; Park, S. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Maz. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Wen, N.; Qi, H. Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials. Chemosphere 2019, 223, 117–123. [Google Scholar]
- Qing, S.; Xiao, L.; Zeng, Z.; Chao, W.; Chun, X. Synergistic utilization of blast furnace slag with other industrial solid wastes in cement and concrete industry: Synergistic mechanisms, applications, and challenges. Green Energy Resour. 2023, 100012, 2949–7205. [Google Scholar]
- Park, S.; Wu, S.; Liu, Z.; Pyo, S. The role of supplementary cementitious materials (SCMs) in ultra high performance concrete (UHPC): A review. Materials 2021, 14, 1472. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, J.; Kaur, M.; Eet, A. Compare the strength of fly ash based geopolymer concrete with demolition waste. Int. J. Civ. Eng. 2018, 5, 20–24. [Google Scholar] [CrossRef]
- Menglim, H.; Runglawan, R.; Suksun, H.; Arul, A.; Mehdi, M. Effect of wetting–drying cycles on compressive strength and microstructure of recycled asphalt pavement–Fly ash geopolymer. Constr. Build. Mater. 2017, 144, 624–634. [Google Scholar]
- Kumar, R.; Goyal, S.; Srivastava, A. A comprehensive study on the influence of supplementary cementitious materials on physico-mechanical, microstructural and durability properties of low carbon cement composites. Powder Technol. 2021, 394, 645–668. [Google Scholar]
- Pathak, K.; Pandey, V.; Murari, K.; Singh, P. Soil stabilisation using ground granulated blast furnace slag. Int. J. Eng. Res. Appl. 2014, 4, 164–171. [Google Scholar]
- Arulrajah, A.; Mohammadinia, A.; Phummiphan, I. Stabilization of recycled demolition aggregates by geopolymers comprising calcium carbide residue, fly ash and slag precursors. Constr. Build. Mater. 2016, 114, 864–873. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Ma, W. Investigation on the application of steel slag–fly ash–phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef]
- Teerawattanasuk, C.; Voottipruex, P. Comparison between cement and fly ash geopolymer for stabilized marginal lateritic soil as road material. Int. J. Pavement Eng. 2018, 20, 1264–1274. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Lin, H.; Wu, X.; Li, H.; Liu, Y.; Xu, F. Factors affecting the strength formation mechanism and water stability of geopolymer stabilized phosphogypsum in road construction. Coatings 2023, 13, 1652. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.; Zhang, H.; Wang, X.; Huo, J.; Zhang, T. Optimization design and characterization of slag cementitious composites containing carbide slag and desulfurized gypsum based on response surface methodology. J. Build. Eng. 2023, 77, 107441. [Google Scholar] [CrossRef]
- Duan, D.; Wu, H.; Wei, F.; Song, H.; Ma, Z.; Chen, Z.; Cheng, F. Preparation, characterization, and rheological analysis of eco-friendly geopolymer grouting cementitious materials based on industrial solid wastes. J. Build. Eng. 2023, 78, 107451. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, C.; Law, W.; Nasvi, M.; Setunge, S.; Dissanayake, R. Engineering properties of waste-based alkali activated concrete brick containing low calcium fly ash and rice husk ash: A comparison with traditional Portland cement concrete brick. J. Build. Eng. 2022, 46, 103810. [Google Scholar] [CrossRef]
- GB/T 18046-2017; Ground granulated blast furnace slag used for cement, mortar and concrete. The National Standards of the People’s Republic of China: Beijing, China, 2017.
- GB/T 17671-2021; Test method of cement mortar strength (ISO method). The National Standards of the People’s Republic of China: Beijing, China, 2021.
- GB/T 12957-2005; Test method for activity of industrial waste slag used as addition to cement. The National Standards of the People’s Republic of China: Beijing, China, 2005.
- GB/T 51003-2014; Technical Specification for Application of Mineral Admixtures. The National Standards of the People’s Republic of China: Beijing, China, 2014.
- JTG E51-2009; Test Specification for Inorganic Binder Stabilized Materials in Highway Engineering. Ministry of Transport of the People’s Republic of China: Beijing, China, 2009.
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, characterization and application of red mud, fly ash and desulfurized gypsum based eco-friendly road base materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Omur, T.; Kabay, N.; Miyan, N.; Özkan, H.; Özkan, Ç. The effect of alkaline activators and sand ratio on the physico- mechanical properties of blast furnace slag based mortars. J. Build. Eng. 2022, 58, 104998. [Google Scholar] [CrossRef]
- Durczak, K.; Pyzalski, M.; Brylewski, T.; Sujak, A. Effect of variable synthesis conditions on the formation of ye’elimite-aluminate-calcium (YAC) cement and its hydration in the presence of portland cement (OPC) and several accessory additives. Materials 2023, 16, 6052. [Google Scholar] [CrossRef] [PubMed]
- Pyzalski, M.; Dąbek, J.; Adamczyk, A.; Brylewski, T. Physicochemical study of the self-disintegration of calcium orthosilicate (β→γ) in the presence of the C12A7 aluminate phase. Materials 2021, 14, 6459. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Qiao, J.; Han, W.; Pan, B.; Jin, X.; Peng, H. Modification effect of Ca(OH)2 on the carbonation resistance of fly ash-metakaolin-based geopolymer. Materials 2023, 16, 2305. [Google Scholar] [CrossRef]
- Abadel, A.; Alghamdi, H.; Alharbi, R.; Alamri, M.; Khawaji, M.; Abdulaziz, A.; Nehdi, L. Investigation of alkali-activated slag-based composite incorporating dehydrated cement powder and red mud. Materials 2023, 16, 1551. [Google Scholar] [CrossRef]
- Richardson, G.; Brough, R.; Groves, W.; Dobson, M. The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (CSH) phase. Cem. Concr. Res. 1994, 24, 813–829. [Google Scholar] [CrossRef]
- Chen, B.; Pang, L.; Zhou, Z.; Chang, Q.; Fu, P. Study on the hydration properties of a ternary cementitious material system containing activated gold tailings and granulated blast furnace slag. J. Build. Eng. 2023, 63, 105574. [Google Scholar] [CrossRef]

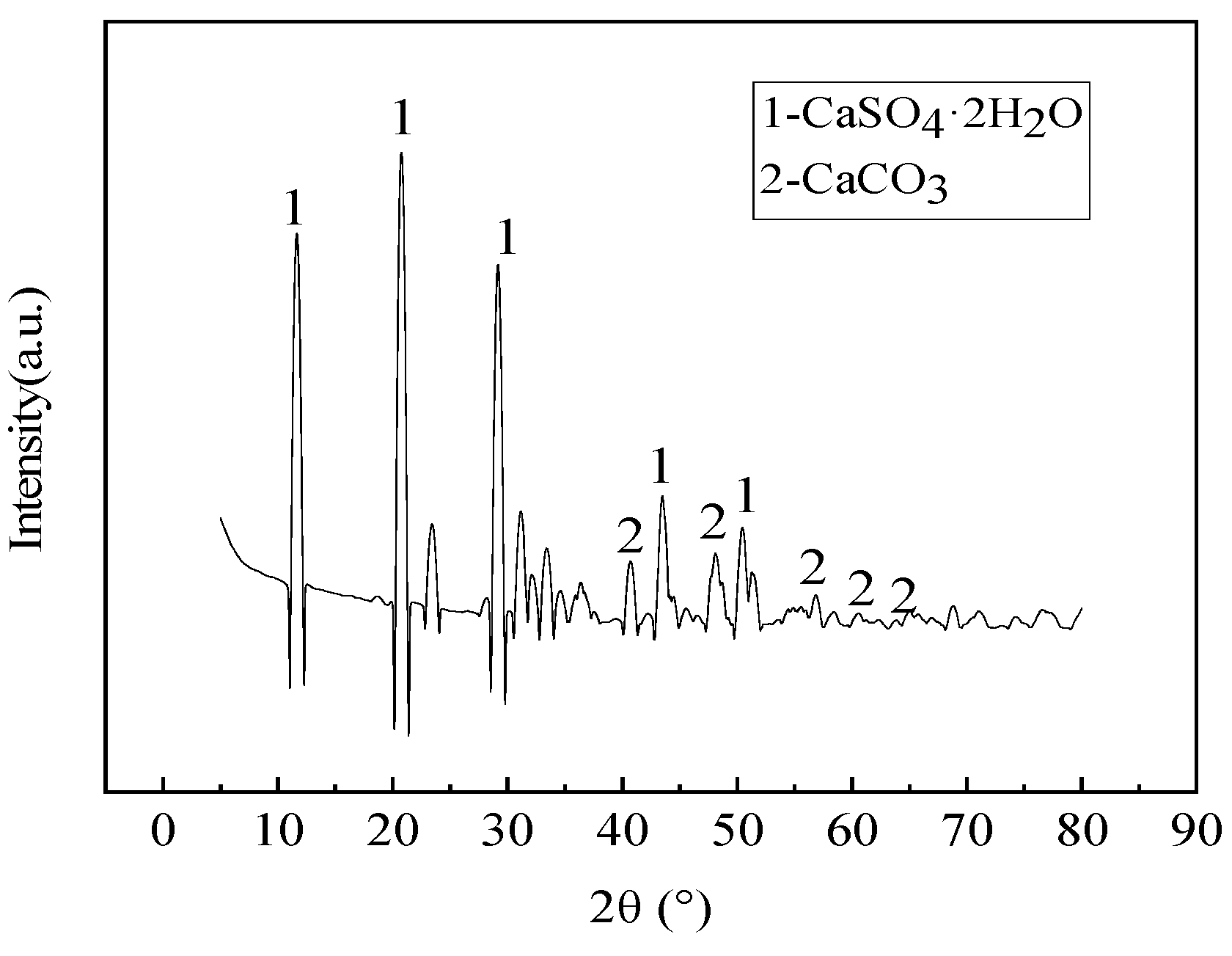
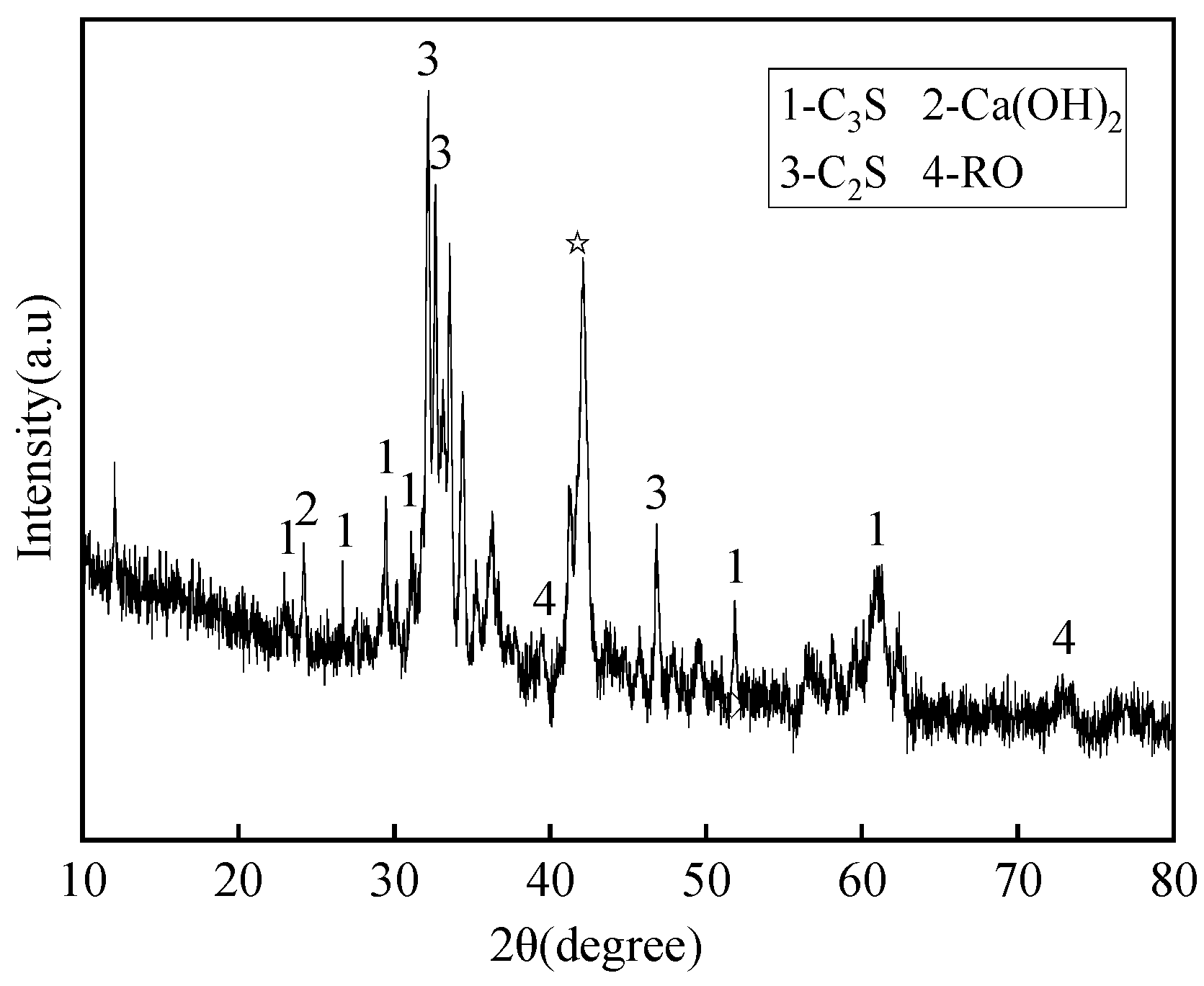
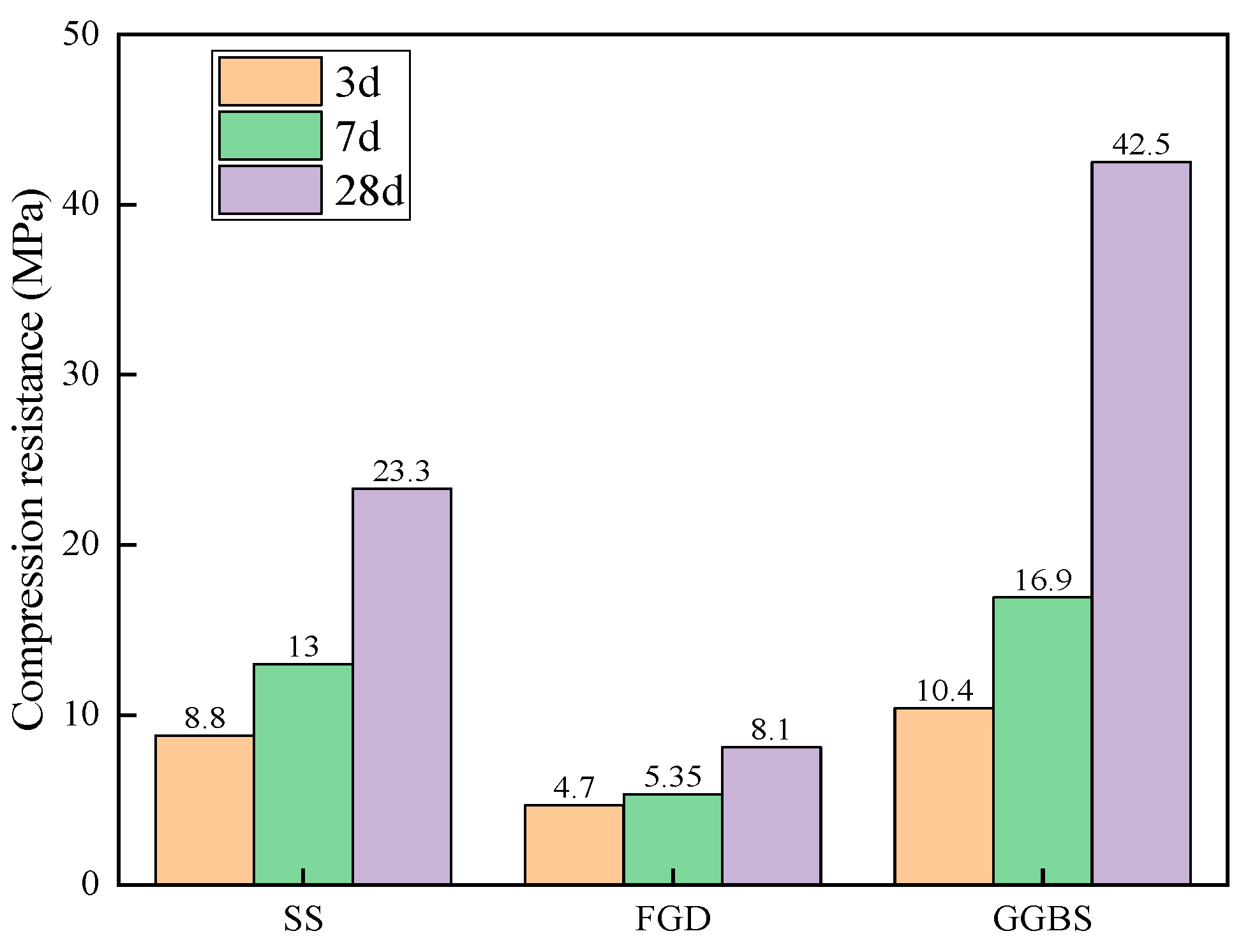
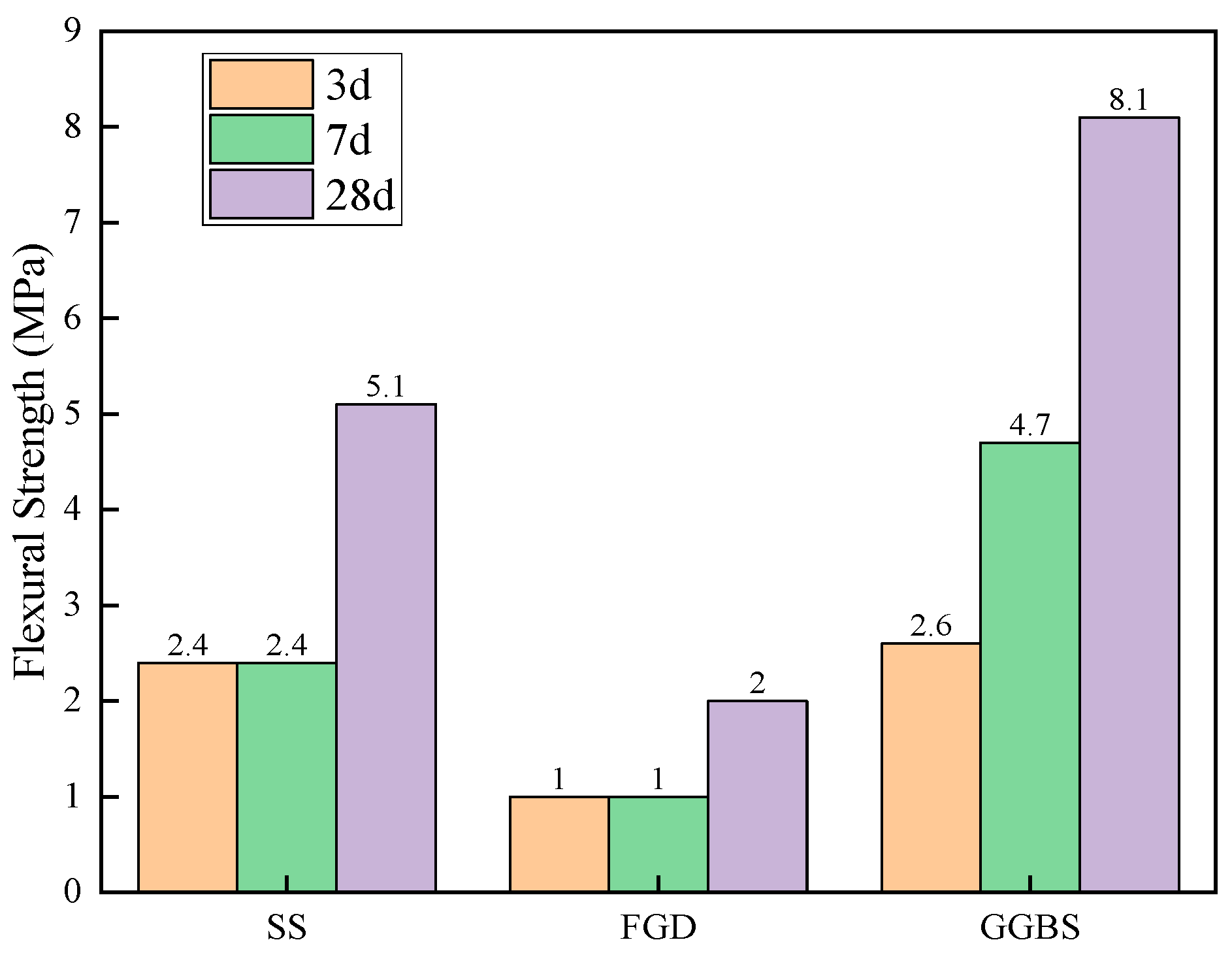
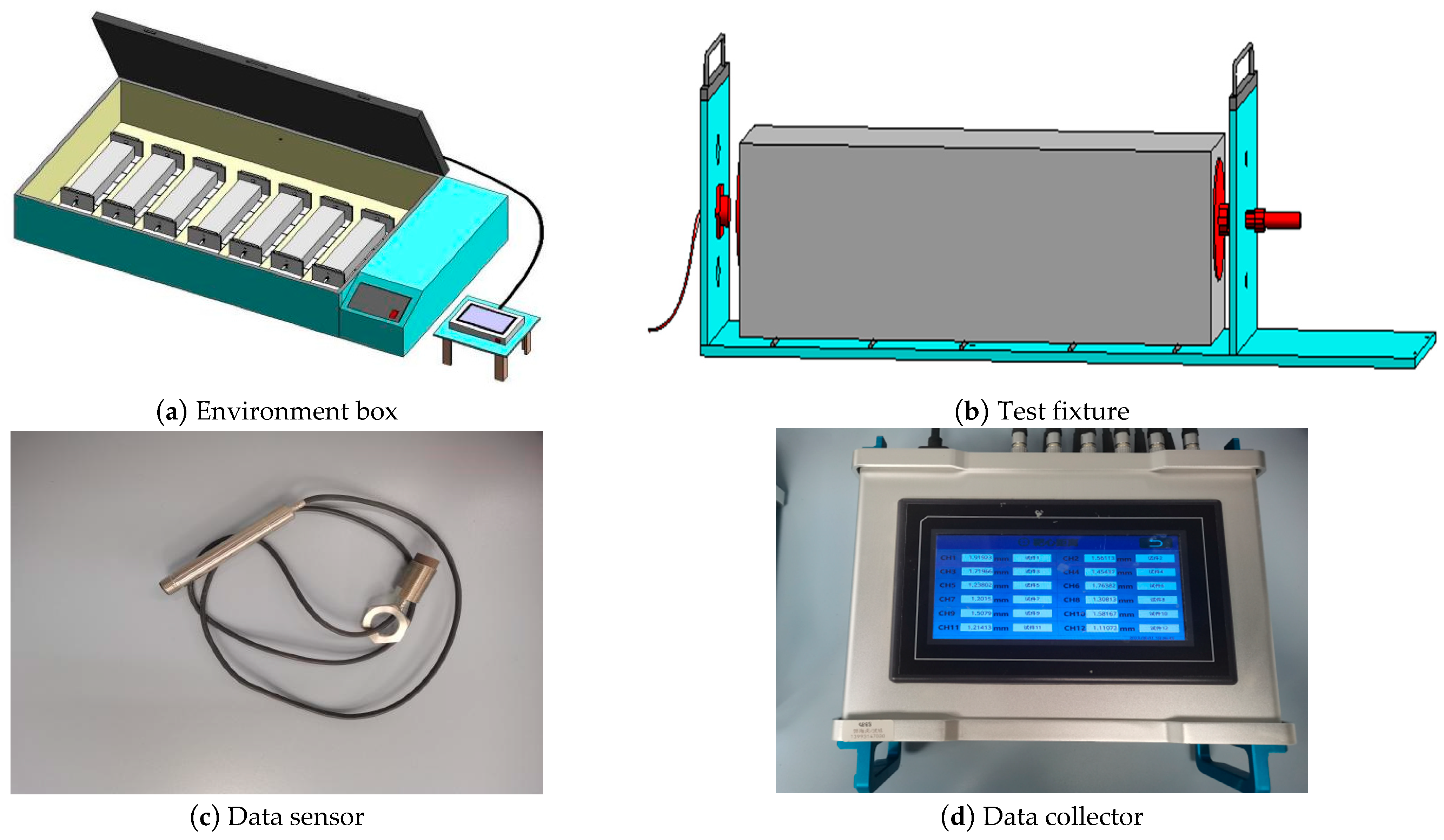






| Material | Mass Fraction/% | |||||||
|---|---|---|---|---|---|---|---|---|
| CaO | Al2O3 | SiO2 | Na2O | MgO | Fe2O3 | SO3 | Other | |
| GGBS | 35.62 | 11.12 | 36.85 | 0.56 | 9.05 | 0.47 | 0.11 | 6.22 |
| SS | 40.03 | 6.16 | 25.53 | 0.16 | 9.03 | 12.19 | 3.58 | 3.32 |
| FGD | 41.96 | 0.71 | 1.55 | 0.38 | 0.22 | 0.35 | 53.30 | 1.53 |
| Code Value | Code Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 | 40 | 50 | 60 |
| X2 | 20 | 30 | 40 |
| X3 | 5 | 10 | 15 |
| Evaluation Index | Correlation Evaluation |
|---|---|
| Complex correlation coefficient () | 0.9811 |
| Correction correlation coefficient () | 0.9670 |
| Predictive correlation coefficient () | 0.9057 |
| Coefficient of variation (CV) | 7.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Li, B.; Wei, D.; Guo, F.; Ji, H. Investigation of Preparation and Shrinkage Characteristics of Multi-Source Solid Waste-Based Cementitious Materials. Materials 2023, 16, 7522. https://doi.org/10.3390/ma16247522
Wu X, Li B, Wei D, Guo F, Ji H. Investigation of Preparation and Shrinkage Characteristics of Multi-Source Solid Waste-Based Cementitious Materials. Materials. 2023; 16(24):7522. https://doi.org/10.3390/ma16247522
Chicago/Turabian StyleWu, Xu, Bo Li, Dingbang Wei, Fucheng Guo, and Haidong Ji. 2023. "Investigation of Preparation and Shrinkage Characteristics of Multi-Source Solid Waste-Based Cementitious Materials" Materials 16, no. 24: 7522. https://doi.org/10.3390/ma16247522
APA StyleWu, X., Li, B., Wei, D., Guo, F., & Ji, H. (2023). Investigation of Preparation and Shrinkage Characteristics of Multi-Source Solid Waste-Based Cementitious Materials. Materials, 16(24), 7522. https://doi.org/10.3390/ma16247522






