Simultaneous Immobilization of Heavy Metals in MKPC-Based Mortar—Experimental Assessment
Abstract
:1. Introduction
2. Materials and Methods
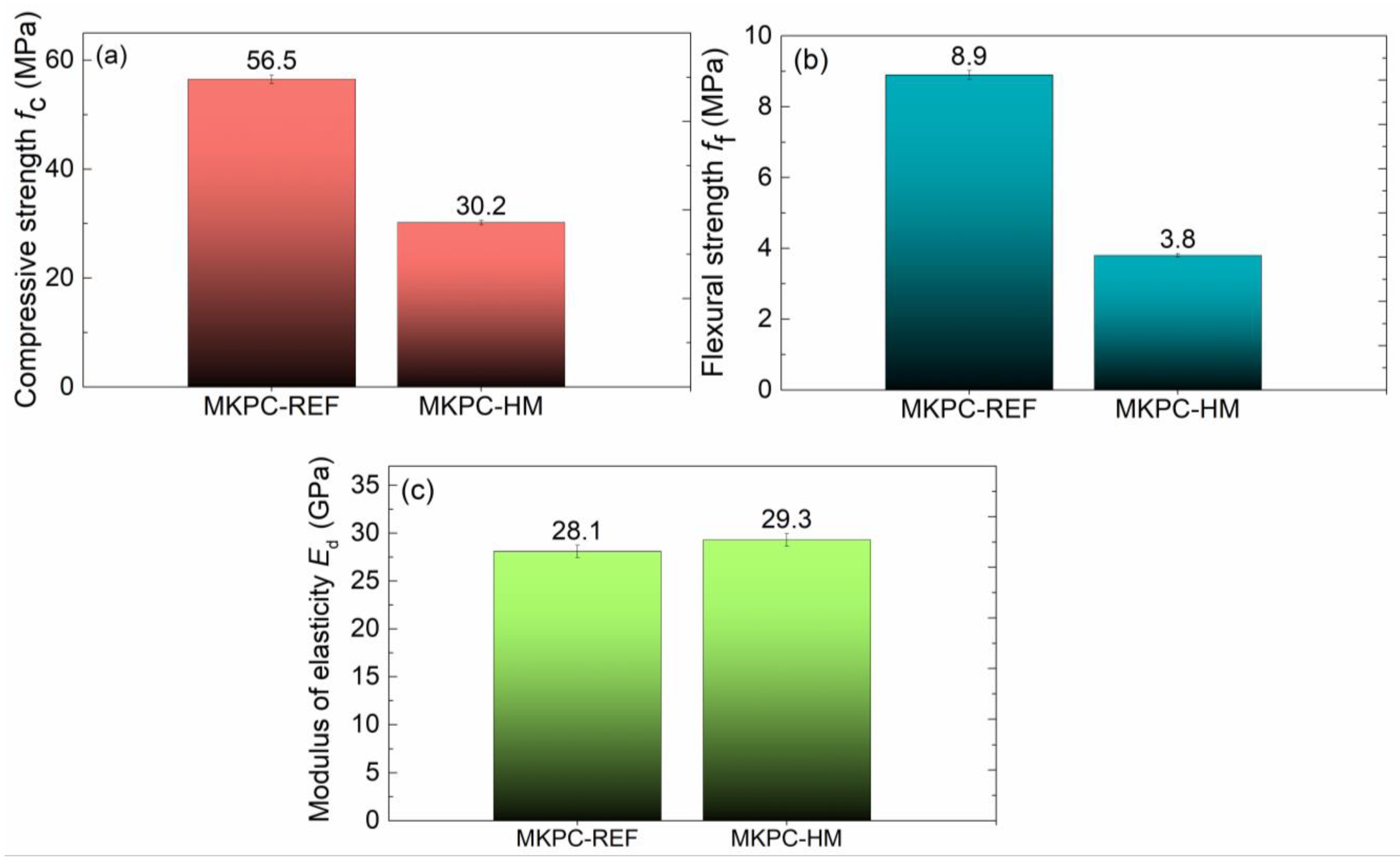
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Li, Z.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. J. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.E.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Marchant, B.P.; Saby, N.P.A.; Arrouays, D. A survey of topsoil arsenic and mercury concentrations across France. Chemosphere 2017, 181, 635–644. [Google Scholar] [CrossRef]
- Toth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Han, H.; Gao, C.; Wang, Y.; Dong, B.; Xu, Z. Organic amendments for in situ immobilization of heavy metals in soil: A review. Chemosphere 2023, 335, 139088. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, Y.; Yu, M.; Cheng, H.; Li, Z.; Xu, W. Mineral phase evolution and heavy metals migration during the hydrothermal treatment of municipal solid waste incineration fly ash. Fuel 2024, 357, 129790. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Res. Conserv. Manag. 2022, 1, 106261. [Google Scholar] [CrossRef]
- Silva-Sánchez, N. Environmental Impact of Roman Mining and Metallurgy and Its Correlation with the Archaeological Evidence: A European Perspective. Environ. Archeol. 2023. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Presser, T.S.; Sylvester, M.A.; Low, W.H. Bioaccumulation of selenium from natural geologic sources in western states and its potential consequences. Environ. Manag. 1994, 18, 423–436. [Google Scholar] [CrossRef]
- Varicca, D.; Tamburo, E.; Dongarrà, G.; Sposito, F. Trace elements in scalp hair of children chronically exposed to volcanic activity (Mt. Etna, Italy). Sci. Total Environ. 2014, 470–471, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Campos, I.; Vale, C.; Abrantes, N.; Keizer, J.J.; Pereira, P. Effects of wildfire on mercury mobilisation in eucalypt and pine forests. Catena 2015, 131, 149–159. [Google Scholar] [CrossRef]
- Zakari, S.; Jiang, X.; Zhu, X.; Liu, W.; Allakonon, M.G.B.; Singh, A.K.; Chen, C.; Zou, X.; Irénikatché Akponikpè, P.B.; Dossa, G.G.O.; et al. Influence of sulfur amendments on heavy metals phytoextraction from agricultural contaminated soils: A meta-analysis. Environ. Pollut. 2021, 288, 117820. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Q. Clarifying and quantifying the immobilization capacity of cement pastes on heavy metals. Cem. Concr. Res. 2022, 161, 106945. [Google Scholar] [CrossRef]
- Li, X. Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste material. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef]
- Golman, M.A.C.; da Silva, M.M.; Masuero, Ă.B. Stabilization and solidification of pb in cement matrices. J. Hazard. Mater. 2010, 179, 507–514. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef]
- Coruh, S.; Ergun, O.N. Leaching characteristics of copper flotation waste before and after vitrification. J. Environ. Manag. 2006, 81, 333–338. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Gao, M.; Guan, Y.; Wu, C.; Wang, Q.; Rao, Y.; Liu, S. Heavy metal leaching behaviour and long-term environmental risk assessment of cement-solidified municipal solid waste incineration fly ash in sanitary landfill. Chemosphere 2022, 300, 134571. [Google Scholar] [CrossRef]
- Gineys, N.; Aouad, G.; Damidot, D. Managing trace elements in Portland cement—Part I: Interactions between cement paste and heavy metals added during mixing as soluble salts. Cem. Concr. Compos. 2010, 32, 563–570. [Google Scholar] [CrossRef]
- Yu, Q.; Nagataki, S.; Lin, J.; Saeki, T.; Hisada, M. The leachability of heavy metals in hardened fly ash cement and cement-solidified fly ash. Cem. Concr. Res. 2005, 35, 1056–1063. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Tan, X.; Yu, P.; Xu, L. Review of the Interactions between Conventional Cementitious Materials and Heavy Metal Ions in Stabilization/Solidification Processing. Materials 2023, 16, 3444. [Google Scholar] [CrossRef]
- El-eswed, B.I. Chemical evaluation of immobilization of wastes containing Pb, Cd, Cu and Zn in alkali-activated materials: A critical review. J. Environ. Chem. Eng. 2020, 8, 104194. [Google Scholar] [CrossRef]
- Khater, H.M.; Ghareib, M. Optimization of geopolymer mortar incorporating heavy metals in producing dense hybrid composites. J. Build. Eng. 2020, 32, 101684. [Google Scholar] [CrossRef]
- Fan, J.; Yan, J.; Zhou, M.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Wang, A.; Sun, D. Heavy metals immobilization of ternary geopolymer based on nickel slag, lithium slag and metakaolin. J. Hazard. Mater. 2023, 453, 131380. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Qi, Y.; Liu, Z. Characteristics and leaching behavior of MSWI fly ash in novel solidification/stabilization binders. Waste Manag. 2021, 131, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yang, J.; Liu, D.; Zhen, S.; Lin, N.; Zhou, Y. Solidification/stabilization of simulated cadmium-contaminated wastes with magnesium potassium phosphate cement. Environ. Eng. Res. 2016, 21, 15–21. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Q.; Yang, W.; Fang, L.; Liu, S.; Ma, R.; Guo, K.; Ma, N. Lead-chlorine synergistic immobilization mechanism in municipal solid waste incineration fly ash (MSWIFA)-based magnesium potassium phosphate cement. J. Hazard. Mater. 2023, 442, 130038. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, Z.; Yang, D.; Dong, J.; Cheng, X.; Yu, H. Immobilization of Zn(Ⅱ) and Cu(Ⅱ) in basic magnesium-sulfate-cementitious material system: Properties and mechanism. J. Hazard. Mater. 2023, 446, 130720. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wu, C.; Yu, H.; Li, Y.; Wen, J. Review of reactive magnesia-based cementitious materials: Current developments and potential applicability. J. Build. Eng. 2021, 40, 102342. [Google Scholar] [CrossRef]
- Marušiak, Š.; Kapicová, A.; Pivák, A.; Pavlíková, M.; Pavlík, Z. Magnesium Potassium Phosphate Cement-Based Derivatives for Construction Use: Experimental Assessment. Materials 2022, 15, 1896. [Google Scholar] [CrossRef] [PubMed]
- EN 1015-11; Methods of Test for Mortar Masonry—Part 11: Determination of Compressive and Flexural Strength of Hardened Mortar. CEN: Brussels, Belgium, 2019.
- EN 1015-18; Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillarity Action of Hardened Mortar. CEN: Brussels, Belgium, 2002.
- Feng, C.; Guimaraes, A.S.; Ramos, N.; Sun, L.X.; Gawin, D.; Konca, P.; Hall, C.; Zhao, J.H.; Hirsch, H.; Grunewald, J.; et al. Hygric properties of porous building materials (VI): A round robin campaign. Build. Environ. 2020, 185, 107242. [Google Scholar] [CrossRef]
- EN 12457-2; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 I/kg for Materials with Particle Size Below 4 mm (without or with Size Reduction). CEN: Brussels, Belgium, 2002.
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Chukanov, N.V. O29 brucite. In Infrared Spectra of Mineral Species; Geochemistra/Mineralogy; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar]
- Zhang, S.; Hui-Seng, S.; Shao-Wen, H.; Zhang, P. Dehydration characteristics of struvite-K pertaining to magnesium potassium phosphate cement system in non-isothermal condition. J. Therm. Anal. Calorim. 2013, 111, 35–40. [Google Scholar] [CrossRef]
- Chukanov, N.V. C94 magnesite. In Infrared Spectra of Mineral Species; Geochemistra/Mineralogy; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar]
- Chukanov, N.V. O168 periklas. In Infrared Spectra of Mineral Species; Geochemistra/Mineralogy; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar]
- Dong, H.; Gao, P.; Ye, G. Characterization and comparison of capillary pore structures of digital cement pastes. Mater. Struct. 2017, 50, 154. [Google Scholar] [CrossRef]
- Vyhláška, č. 8/2021 Sb. o Katalogu Odpadů a Posuzování Vlastností Odpadů (Katalog Odpadů), in Czech. Available online: https://www.zakonyprolidi.cz/cs/2021-8/zneni-20230101 (accessed on 3 December 2023).
- Cao, X.; Wang, W.; Ma, R.; Sun, S.; Lin, J. Solidification/stabilization of Pb2+ and Zn2+ in the sludge incineration residue-based magnesium potassium phosphate cement: Physical and chemical mechanisms and competition between coexisting ions. Environ. Pollut. 2019, 253, 171–180. [Google Scholar] [CrossRef]
- Rao, A.J.; Pagilla, K.R.; Wagh, A.S. Stabilization and solidification of metal-laden wastes by compaction and magnesium phosphate-based binder. J. Air Waste Manag. Assoc. 2000, 50, 1623–1631. [Google Scholar] [CrossRef]
- Buj, I.; Torras, J.; Rovira, M.; de Pablo, J. Leaching behaviour of magnesium phosphate cements containing high quantities of heavy metals. J. Hazard. Mater. 2010, 175, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Dai, J.-G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-J.; Wei, M.-L.; Reddy, K.R.; Jin, F.; Wu, H.-L.; Liu, Z.-B. New phosphate-based binder for stabilization of soils contaminated with heavy metals: Leaching, strength and microstructure characterization. J. Environ. Manag. 2014, 146, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Lai, X.; Shi, J.; Lu, Z. Effect of Zn2+ on the early hydration behavior of potassium phosphate based magnesium phosphate cement. Constr. Build. Mater. 2016, 129, 70–78. [Google Scholar] [CrossRef]
- Jeon, I.K.; Qudoss, A.; Kim, H.G. Influence of carbonation curing on hydration and microstructure of magnesium potassium phosphate cement concrete. J. Build. Eng. 2021, 38, 102203. [Google Scholar] [CrossRef]
- Vandaperre, L.; Al-Tabbaa, A. Accelerated carbonation of reactive MgO cements. Adv. Cem. Res. 2007, 19, 67–79. [Google Scholar] [CrossRef]
- Zhang, R.; Bassim, N.; Panesar, D.K. Characterization of Mg components in reactive MgO—Portland cement blends during hydration and carbonation. J. CO2 Util. 2018, 27, 518–527. [Google Scholar] [CrossRef]

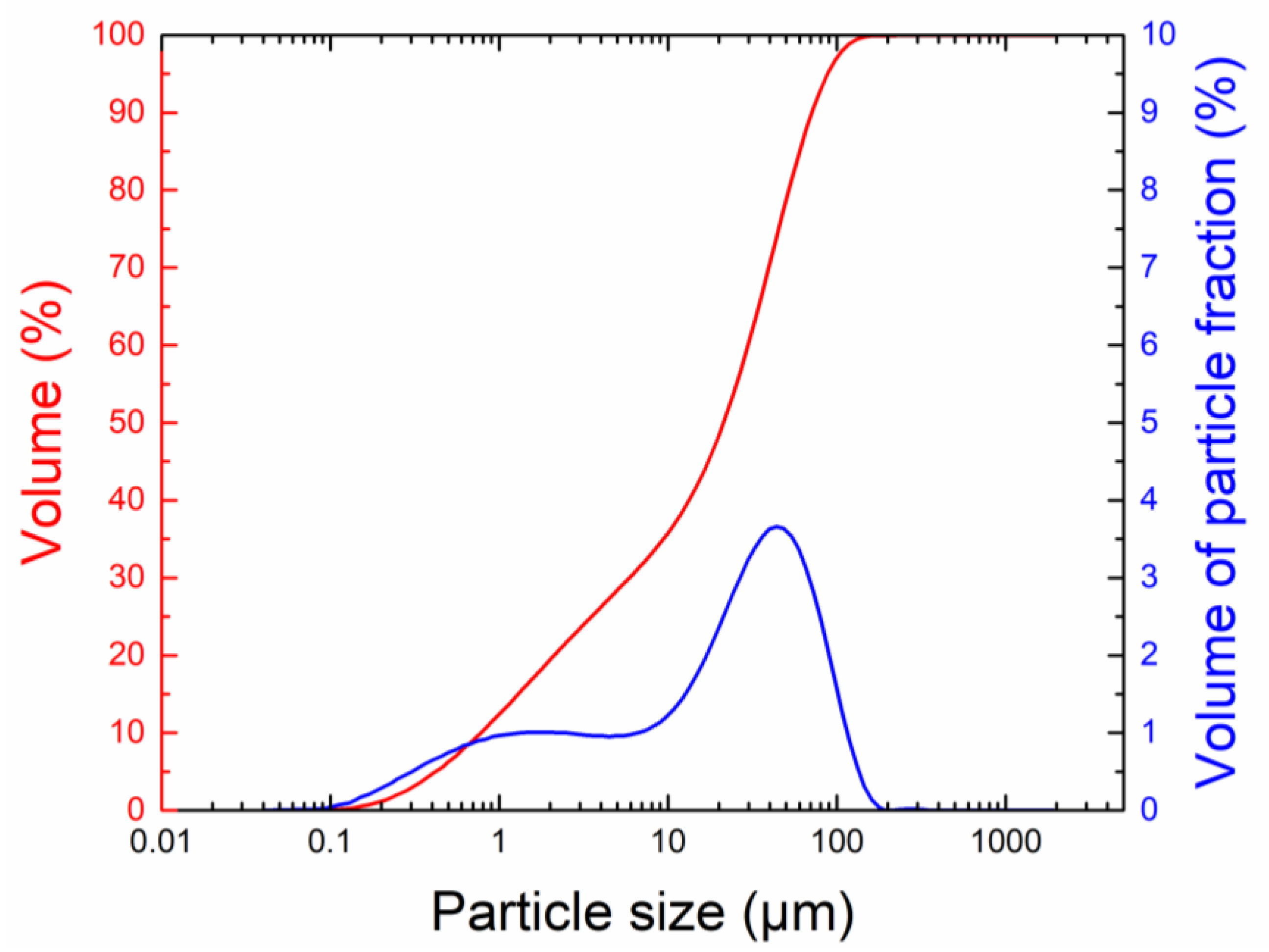
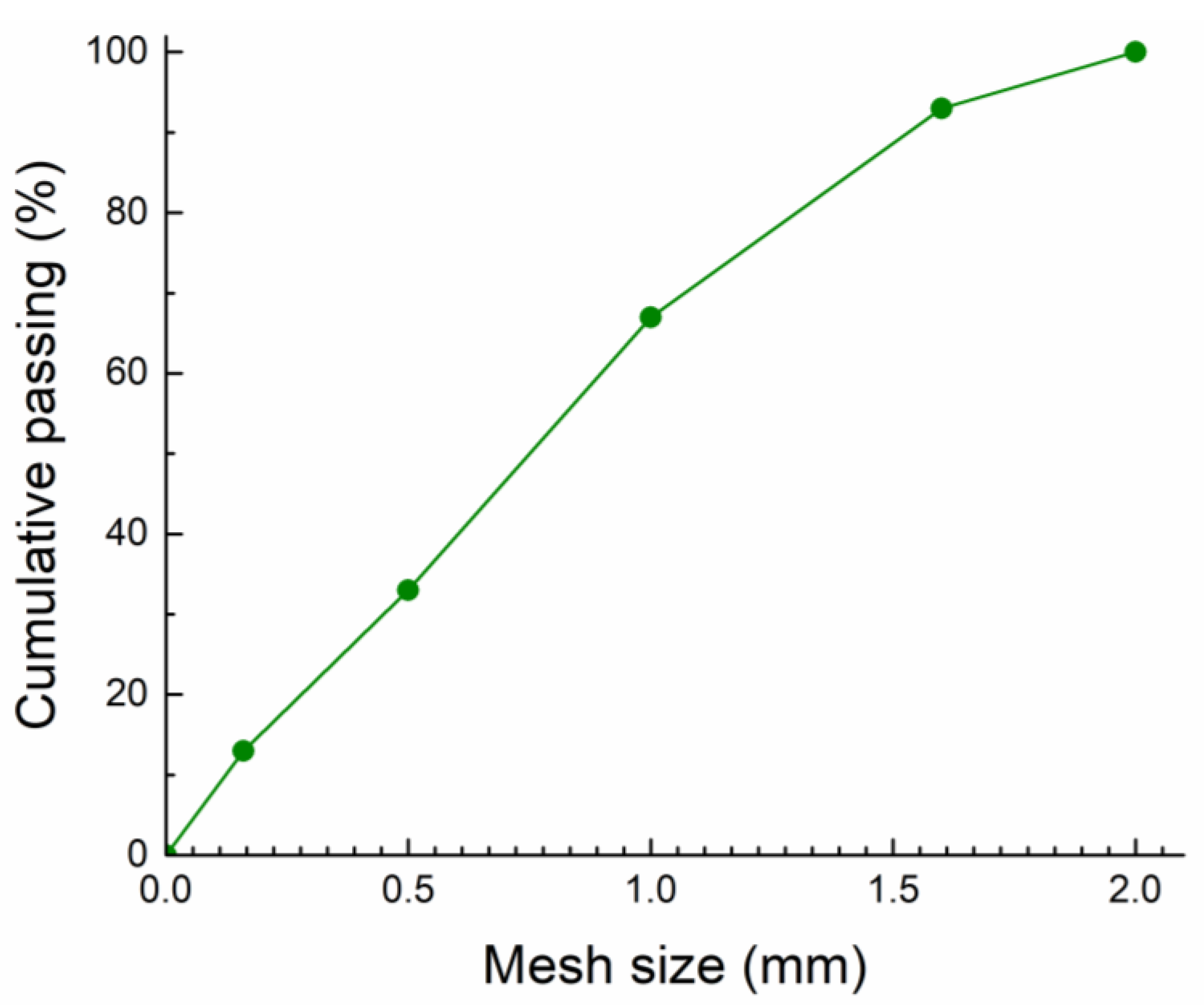

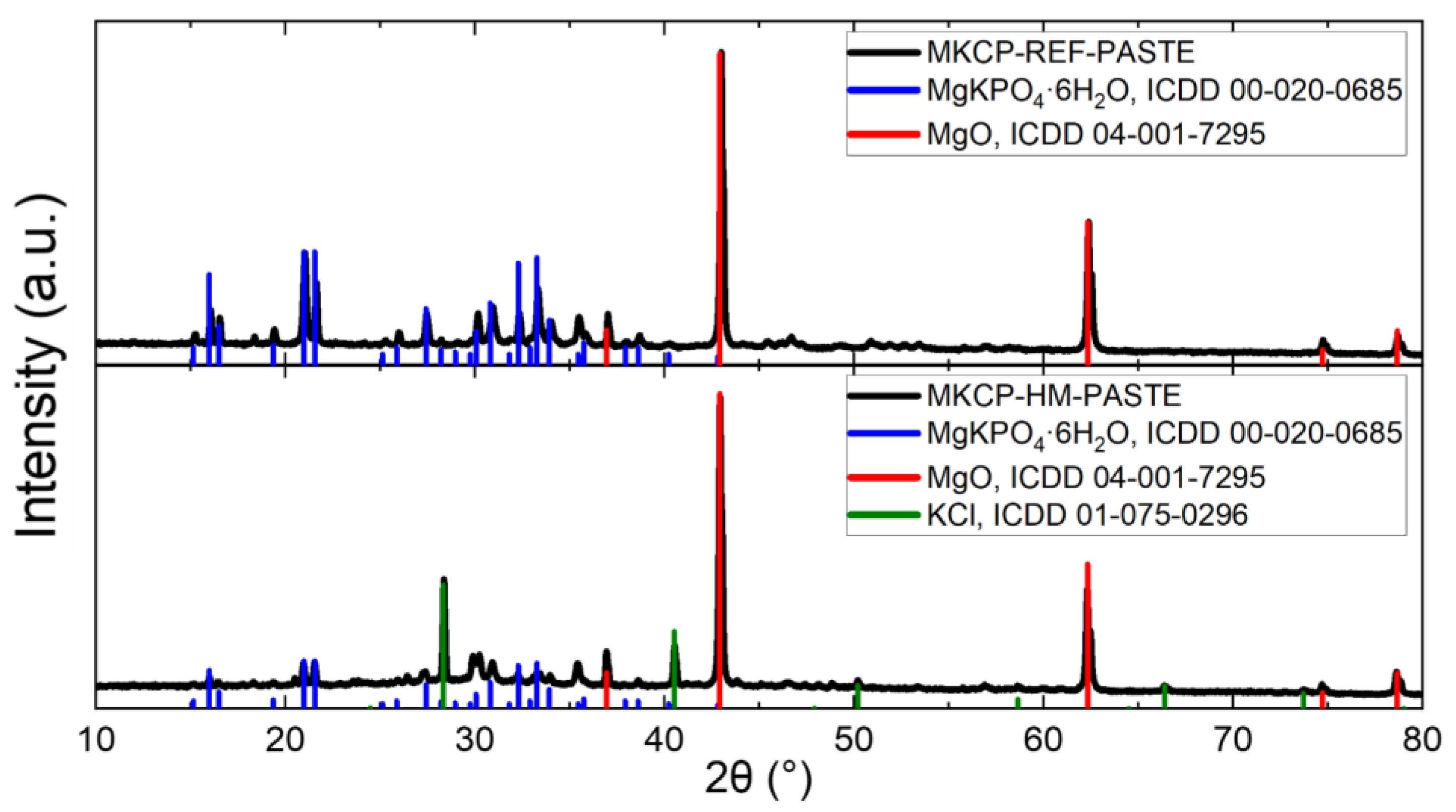
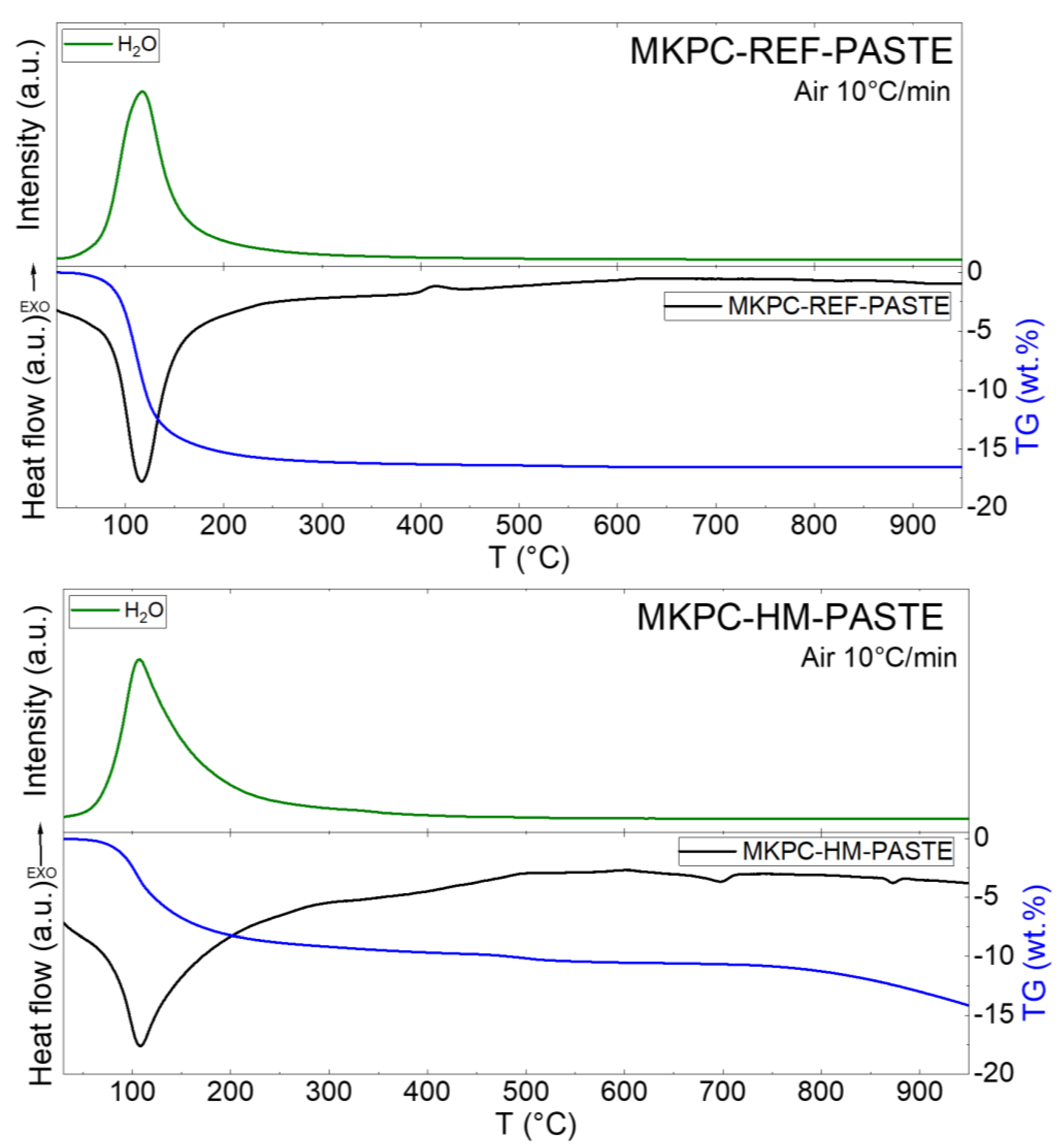
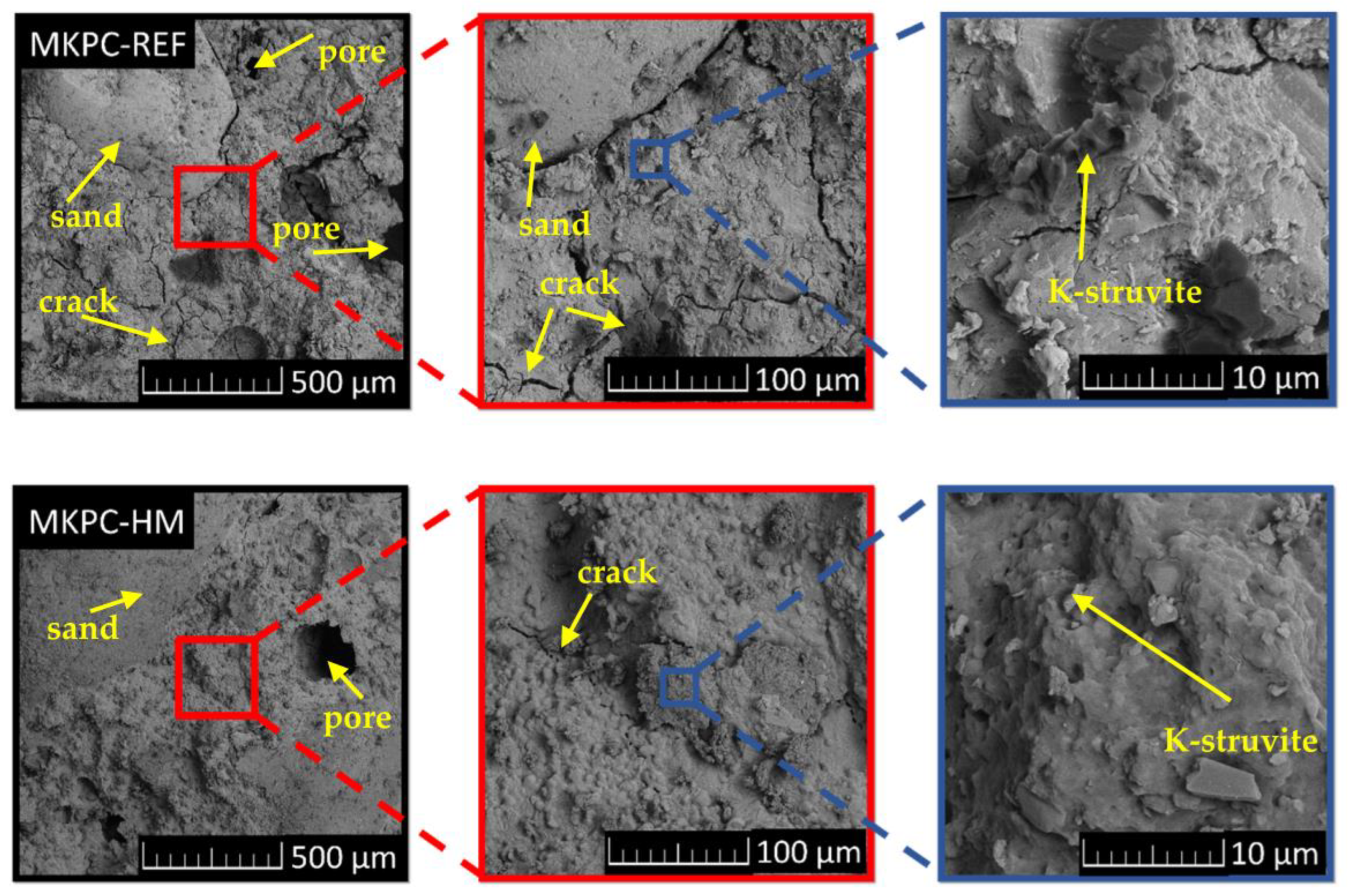

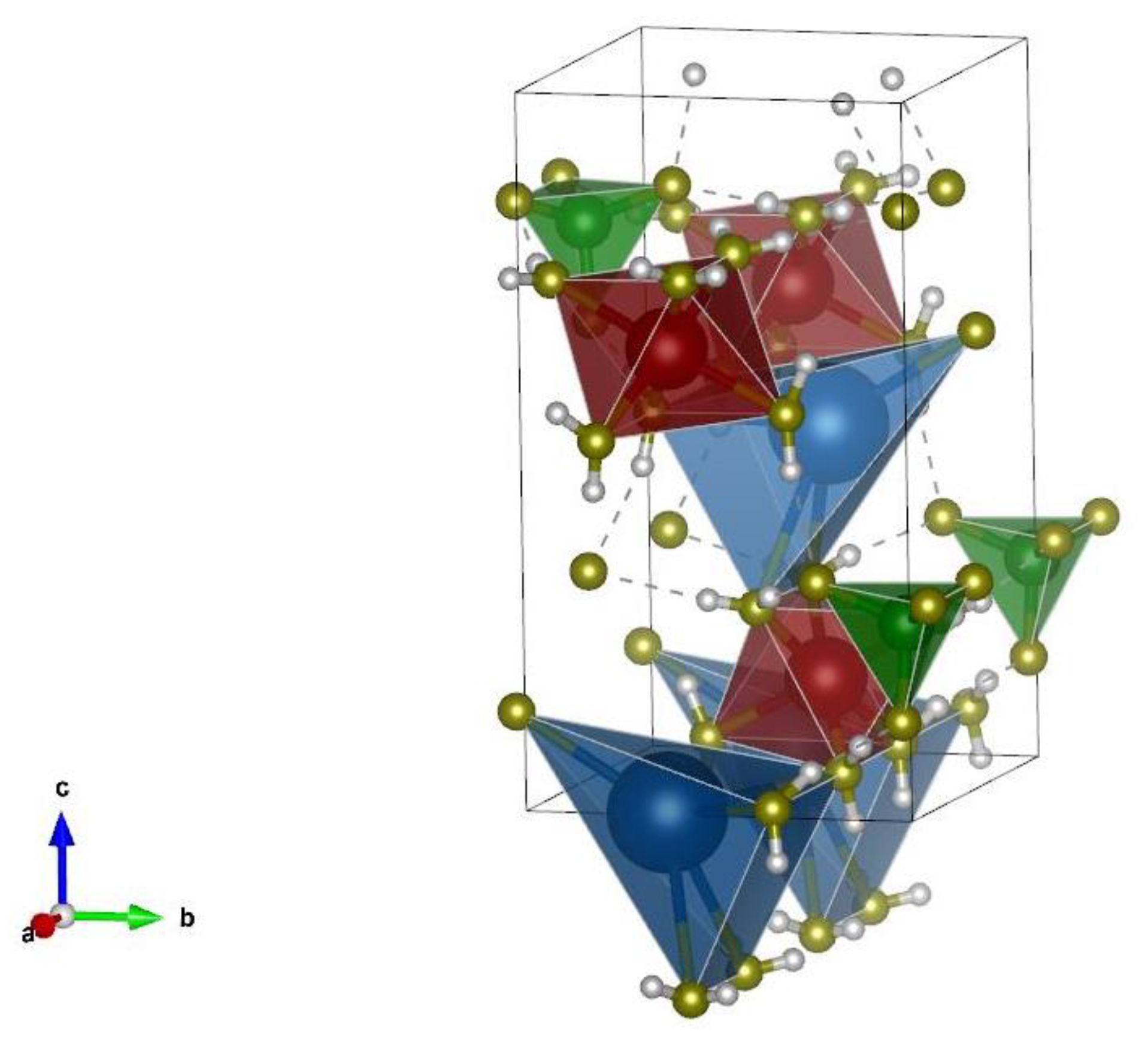

| Sample | MgO | KH2PO4 | Water | QS | Borax | ZnCl2 | BaCl2·2H2O | Pb(NO3)2 |
|---|---|---|---|---|---|---|---|---|
| MKPC-R | 843.1 | 272 | 229.3 | 3 × 305.7 | 45.9 | - | - | - |
| MKPC-HM | 843.1 | 272 | 229.3 | 3 × 305.7 | 45.9 | 47.8 | 40.8 | 36.7 |
| MgO | Fe2O3 | Al2O3 | CaO | SiO2 | MnO | SO3 | K2O |
|---|---|---|---|---|---|---|---|
| 76.50 | 12.55 | 5.76 | 3.32 | 1.04 | 0.69 | 0.11 | 0.03 |
| Property, Parameter | Symbol | Unit | ECU (%) | Standard/Method |
|---|---|---|---|---|
| Bulk density | ρb | kg·m−3 | 1.4 | gravimetry |
| Specific density | ρs | kg·m−3 | 1.2 | helium pycnometry |
| Total open porosity | ψ | % | 2.0 | pycnometry/gravimetry |
| Flexural strength | ff | MPa | 1.4 | EN 1015-11 [35] |
| Compressive strength | fc | MPa | 1.4 | EN 1015-11 [35] |
| Young’s modulus | Ed | GPa | 2.3 | ultrasonic velocity |
| Water absorption coefficient | Aw | kg·m−2·s−1/2 | 1.2 | 1-D free water uptake test [36,37] |
| Water sorptivity | S | m2·s−1/2 | 1.2 | 1-D free water uptake test [36,37] |
| Sample | C | O | Na | Mg | Al | Si | P | K | Fe | Cl | Ca |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MKPC-REF | 5.4 | 43.2 | 0.7 | 15.0 | 1.1 | 12.2 | 6.8 | 12.2 | 2.3 | - | 1.2 |
| MKPC-HM | 8.6 | 15.5 | 0.5 | 3.2 | - | 0.9 | 1.0 | 38.6 | - | 31.7 | - |
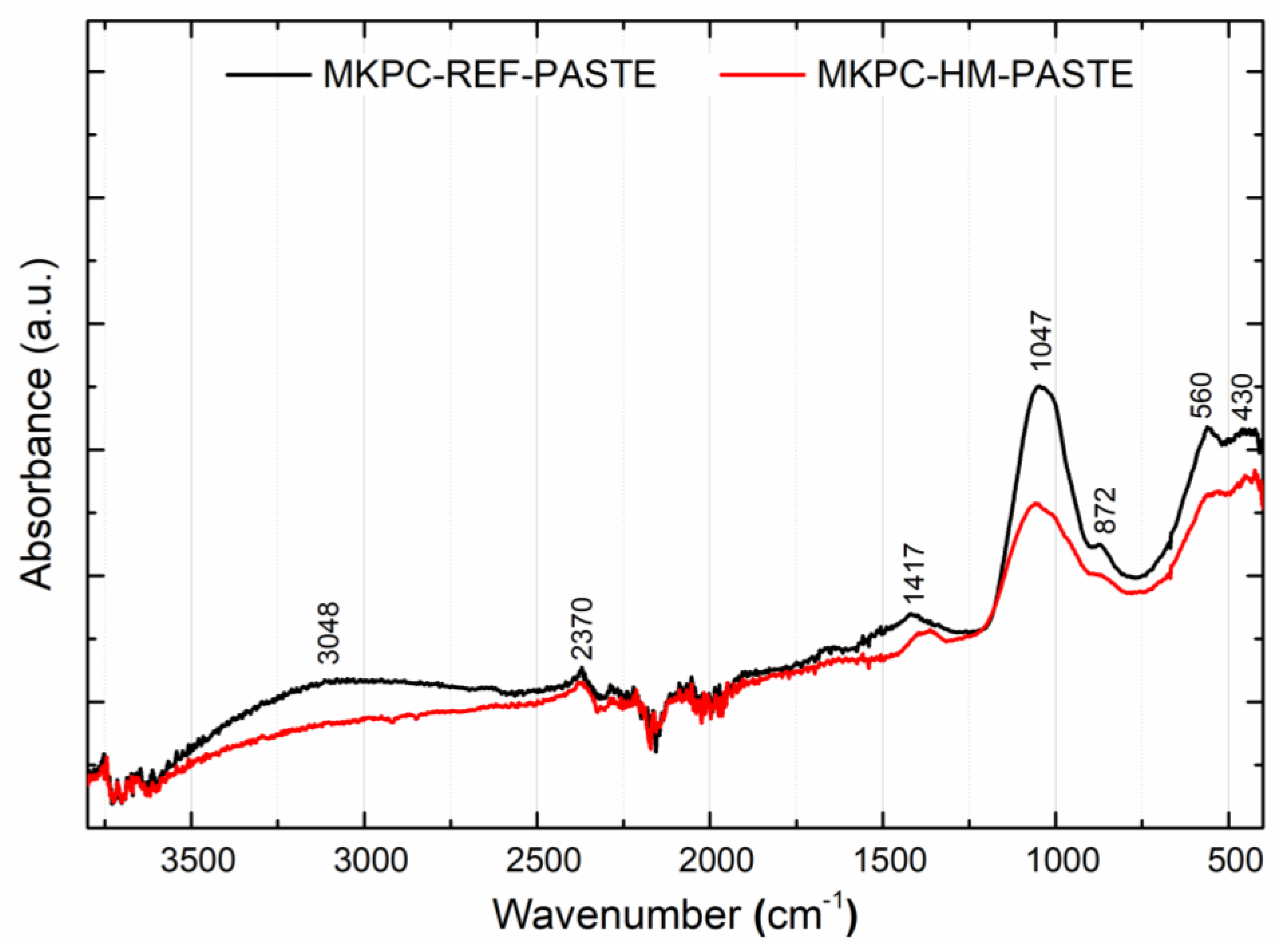
| Wavenumber/cm−1 | Assignment |
|---|---|
| 3048 | ν (-O-H) in water |
| 2370 | ν (-O-H) of water molecules clustered in crystalline form in K-struvite (MgKPO4·6H2O) |
| 1417 | ν (-C=O) in magnesite (MgCO3) |
| 1047 | ν (-P=O) in phosphates (PO43−) |
| 872 | ν (K-O-P-, Mg-O-P-) in K-struvite (MgKPO4·6H2O) |
| 560, 430 | ν (Mg=O, Mg-O-H) in periclase (MgO) and brucite (Mg(OH)2) |
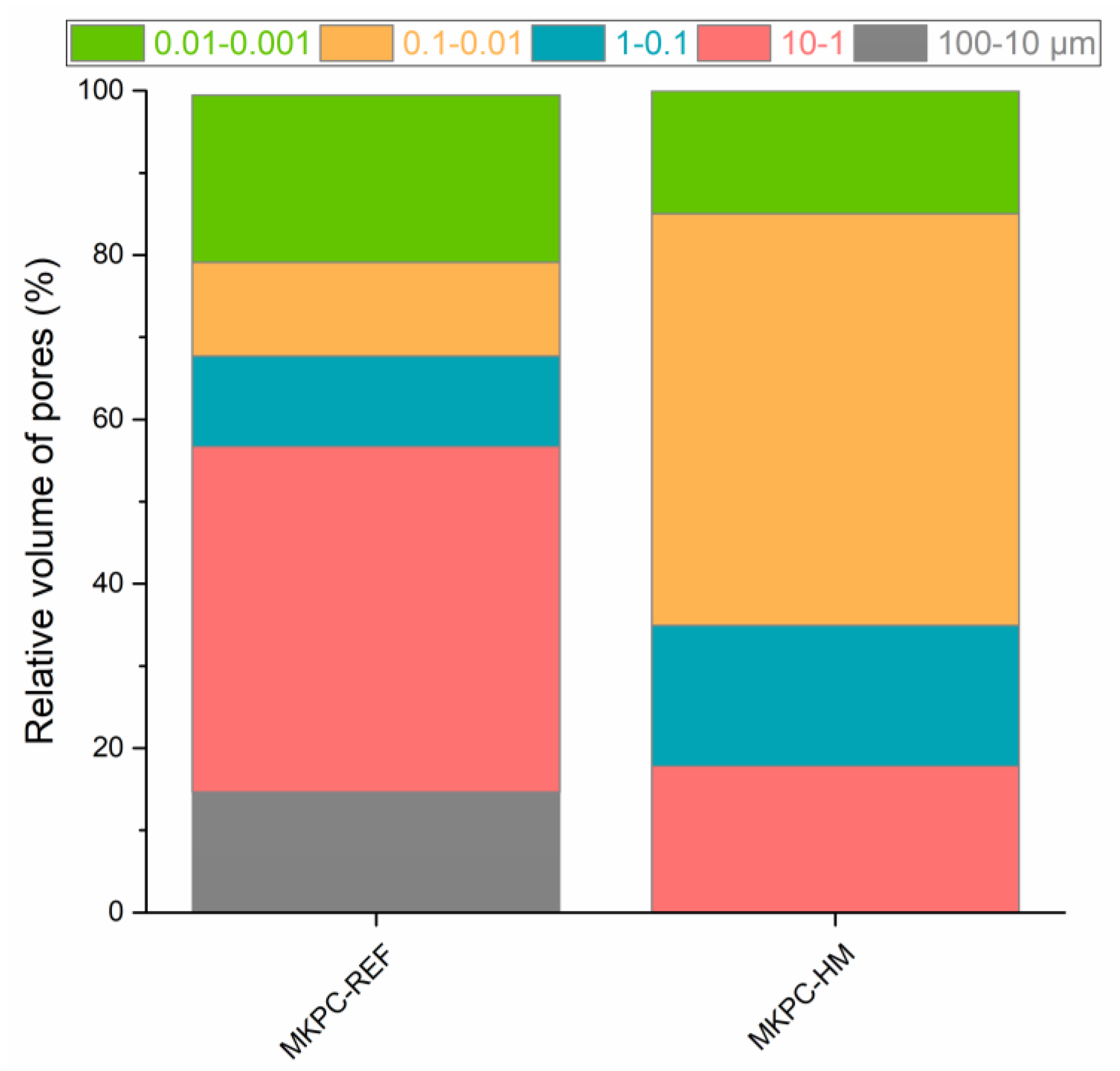
| Mortar | Bulk Density ρb (kg·m−3) | Specific Density ρs (kg·m−3) | Total Open Porosity φ (%) | Total Pore Volume (cm3·g−1) | Average Pore Diameter (μm) |
|---|---|---|---|---|---|
| MPC-REF | 2247 ± 31 | 2867 ± 34 | 21.6 ± 0.4 | 0.0988 | 0.0249 |
| MPC-HM | 2231 ± 31 | 2780 ± 33 | 19.7 ± 0.4 | 0.0795 | 0.0224 |
| Mortar | Water Absorption Coefficient Aw ×10−3 (kg·m−2·s−1/2) | Sorptivity S ×10−6 (m·s−1/2) |
|---|---|---|
| MPC-REF | 9.98 ± 0.12 | 10.0 ± 0.1 |
| MPC-HM | 4.26 ± 0.05 | 4.0 ± 0.1 |
| Solvent, Leachate | pH (-) | Limit Value of pH [39] (-) | Electrical Conductivity (μS·cm−1) |
|---|---|---|---|
| Distilled water (D) | 7.00 | - | 6.0 |
| Rainwater (R) | 6.41 | - | 31.2 |
| MKPC-R-D | 11.18 | 5.5 < 11.18 < 13.00 | 3800.0 |
| MKPC-R-R | 11.14 | 5.5 < 11.14 < 13.00 | 3780.0 |
| MKPC-HM-D | 10.07 | 5.5 < 10.07 < 13.00 | 8280.0 |
| MKPC-HM-R | 10.07 | 5.5 < 10.07 < 13.00 | 8280.0 |
| Solvent, Leachate | Concentration of K (mg·L−1) | Concentration of Mg (mg·L−1) |
|---|---|---|
| Distilled water (D) | n.d. | 0.0013 |
| Rainwater (R) | 0.8812 | 0.5103 |
| MKPC-R-D | 804.4099 | 13.3010 |
| MKPC-R-R | 886.5471 | 63.0548 |
| MKPC-HM-D | 3979.1110 | 116.6638 |
| MKPC-HM-R | 2161.0330 | 184.2604 |
| Sample | Concentration of Pb2+ (mg·L−1) | Concentration Limit [39] (mg·L−1) | Immobilization Ratio (%) |
|---|---|---|---|
| MKPC-R-D | 0.0001 | 5 | n.d. |
| MKPC-R-R | 0.0435 | 5 | n.d. |
| MKPC-HM-D | 1.3134 | 5 | 99.86 |
| MKPC-HM-R | 11.3471 | 5 | 98.76 |
| Sample | Concentration of Zn2+ (mg·L−1) | Concentration Limit [39] (mg·L−1) | Immobilization Ratio (%) |
|---|---|---|---|
| MKPC-R-D | 0.0749 | 20 | n.d. |
| MKPC-R-R | 0.0163 | 20 | n.d. |
| MKPC-HM-D | 1.2323 | 20 | 99.87 |
| MKPC-HM-R | 10.3513 | 20 | 98.87 |
| Sample | Concentration of Ba2+ (mg·L−1) | Concentration Limit [39] (mg·L−1) | Immobilization Ratio (%) |
|---|---|---|---|
| MKPC-R-D | 0.0005 | 30 | n.d. |
| MKPC-R-R | 0.0365 | 30 | n.d. |
| MKPC-HM-D | 2.4331 | 30 | 99.74 |
| MKPC-HM-R | 4.2469 | 30 | 99.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlík, Z.; Záleská, M.; Pavlíková, M.; Pivák, A.; Nábělková, J.; Jankovský, O.; Jiříčková, A.; Chmel, O.; Průša, F. Simultaneous Immobilization of Heavy Metals in MKPC-Based Mortar—Experimental Assessment. Materials 2023, 16, 7525. https://doi.org/10.3390/ma16247525
Pavlík Z, Záleská M, Pavlíková M, Pivák A, Nábělková J, Jankovský O, Jiříčková A, Chmel O, Průša F. Simultaneous Immobilization of Heavy Metals in MKPC-Based Mortar—Experimental Assessment. Materials. 2023; 16(24):7525. https://doi.org/10.3390/ma16247525
Chicago/Turabian StylePavlík, Zbyšek, Martina Záleská, Milena Pavlíková, Adam Pivák, Jana Nábělková, Ondřej Jankovský, Adéla Jiříčková, Oskar Chmel, and Filip Průša. 2023. "Simultaneous Immobilization of Heavy Metals in MKPC-Based Mortar—Experimental Assessment" Materials 16, no. 24: 7525. https://doi.org/10.3390/ma16247525
APA StylePavlík, Z., Záleská, M., Pavlíková, M., Pivák, A., Nábělková, J., Jankovský, O., Jiříčková, A., Chmel, O., & Průša, F. (2023). Simultaneous Immobilization of Heavy Metals in MKPC-Based Mortar—Experimental Assessment. Materials, 16(24), 7525. https://doi.org/10.3390/ma16247525










