Abstract
In response to the trend of drug−resistant and super bacteria, the existing single antibacterial methods are not sufficient to kill bacteria, and the development of multifunctional antibacterial nanomaterials is urgent. Our study aims to construct copper−doped polydopamine−coated Ti3C2Tx (CuPDA@Ti3C2Tx) with an enhanced photothermal property and Fenton−like activity. The nanocomposite hydrogel consisting of CuPDA@Ti3C2Tx and alginate can improve the antioxidant activity of two−dimensional MXene nanosheets by coating them with a thin layer of PDA nanofilm. Meanwhile, Cu ions are adsorbed through the coordination of PDA−rich oxygen−containing functional groups and amino groups. Calcium ions were further used to crosslink sodium alginate to obtain antibacterial hydrogel materials with combined chemotherapy and photothermal therapy properties. The photothermal conversion efficiency of CuPDA@Ti3C2Tx is as high as 57.7% and the antibacterial rate of Escherichia coli reaches 96.12%. The photothermal effect leads to oxidative stress in bacteria, increases cell membrane permeability, and a high amount of ROS and copper ions enter the interior of the bacteria, causing protein denaturation and DNA damage, synergistically leading to bacterial death. Our study involves a multifunctional synergistic antibacterial nanodrug platform, which is conducive to the development of high−performance antibacterial agents and provides important research ideas for solving the problem of drug−resistant bacteria.
1. Introduction
Antibiotic resistance poses a serious threat to public health, economic growth, and global economic stability [1,2]. In response to the drug resistance of these bacteria, researchers have to synthesize new antibiotics. However, the average cycle time for humans to develop a new antibiotic is about 10 years. In general, most pathogenic bacteria reproduce every 20 min and may develop resistance within 2 years, leading to a dead cycle in new drug development [3,4]. Therefore, there is an urgent need for new antibacterial agents or treatment strategies to combat highly resistant bacteria. Previous work has found that nanomaterials have higher antibacterial properties due to their quantum confinement effect, small size effect, high surface area to volume ratio, and highly exposed surface atoms [5,6,7]. At present, metal−based [8,9,10,11,12], carbon−based [13,14], and polymer−based antibacterial nanomaterials [15], have displayed promising antibacterial properties.
On the basis of the photoconversion function of nanomaterials, local high heat can be generated upon infrared−light irradiation and can effectively kill bacteria [16,17,18]. Thus, the photothermal therapy (PTT) antibacterial strategy was developed and has attracted tremendous attention in the antibacterial field [18,19]. This treatment mechanism does not consider bacterial gene mutations and can avoid causing bacterial resistance. Compared to traditional antibiotic treatment strategies, the bacterial resistance caused by PTT is almost negligible [20]. The main obstacle of PTT is the development of nanomaterials with a high light−conversion efficiency [21]. MXene is a new multifunctional, two−dimensional nanomaterial, mainly used by the Gogotsi research group, and generally includes transition metal carbides, nitrides, and carbonitrides [22,23]. Recently, some studies have revealed the physical membrane destruction effect of MXene, which may exhibit a unique biological activity to kill pathogens [24]. On the one hand, the interaction between MXene (the sharp edges of nanosheets that act as “nanoknives”) and cell membranes may damage the integrity of bacterial membranes during physical contact, ultimately leading to cell death [25,26]. On the other hand, MXenes with zero band gaps have an excellent photothermal conversion efficiency, enabling them to act as photothermal agents for antibacterial applications [27,28,29]. Recently, Sun et al. constructed a photothermal nanoantibacterial material based on MXene to effectively kill methicillin−resistant Staphylococcus aureus (MRSA) by regulating the activity of lysozyme [30]. Wan et al. developed a sensor based on an antibacterial MXene hydrogel, which could effectively accelerate wound healing and achieve a human–computer interaction [31]. Wang et al. created a series of chitin/MXene composite sponges to prevent considerable blood loss and promote the healing process of bacteria−infected wounds [32].
However, Ti3C2Tx in the MXene family is unstable and is easily oxidized into TiO2 when stored in air in an ambient condition, especially at a relatively higher temperature, thus losing its photothermal function. Due to its high negative surface charge, it is usually difficult to electrostatically bind Ti3C2Tx onto anionic bacterial membranes to achieve effective physical destruction. Therefore, strategies, such as structural modification and drug compatibility, may be needed to simultaneously enhance the antibacterial activity and selectivity of Ti3C2Tx. Combination therapy is a widely adopted concept in bacterial therapy, which improves treatment efficiency by combining two different strategies compared to using a specific therapy alone. Therefore, the multifunctional nanoplatform based on photothermal, photodynamic, chemical antibacterial activities needs additional investigations for finally developing highly efficient broad−spectrum antibacterial nanoagents [33,34,35].
This paper presents a Ca2+ crosslinking alginate hydrogel film consisting of CuPDA−coated Ti3C2Tx, which possesses photothermal and chemical antibacterial activities. Ti3C2Tx with a zero band gap shows an excellent photothermal conversion efficiency. PDA belongs to the melanin class and is selected due to its film−forming coordination ability and biocompatible properties. Cu+ presents Fenton−like activity to produce •OH according to Equation (1): [36]. Alginate is an excellent hydrogel agent and its mechanical strength can be augmented by Ca2+ crosslinking. Thus, Cu ion−coordinated PDA is used to coat Ti3C2Tx to prevent its surface oxidation and enhance its biocompatibility and photothermal activity [37]. And the alginate−based hydrogel film containing CuPDA−coated Ti3C2Tx might possess synergistic photothermal and Fenton−like properties, and displays an excellent sterilization function. Therefore, this project is expected to provide important material design ideas and antibacterial mechanism research foundations for the development of high−performance synergistic antibacterial nanomaterials.
2. Materials and Methods
2.1. Materials
Ti3AlC2, dopamine hydrochloride (98.0%), dimethyl sulfoxide (99.7%), CuCl2, HCl, and LiF were purchased from Aladdin Co., Ltd. (Shanghai, China). Tris−base (C4H11NO3, 99.9%) was bought from Macklin Biochemical Co., Ltd. (Shanghai, China). E. coli and S. aureus were bought from Biobw Biotechnology Co., Ltd. (Beijing, China). PBS, nutrient broth (NB), and nutrient agar (NA) were acquired from Bio−Channel Biotechnology Co., Ltd. (Nanjing, Jiangsu, China).
2.2. Characterization
The nanosheet structure was determined using scanning electron microscopy (SEM) Quanta FEG (Thermo Fisher Scientific Inc., Waltham, MA, USA) and transmission electron microscopy (TEM) Tecnai G2 (Thermo Fisher Scientific Inc., Waltham, MA, USA), including the characterization of the nanomorphology and size of Ti3C2Tx and its composites. The phase of the sample was measured using X−ray diffraction (XRD) (Rigaku D/Max 2500 PC, Rigaku Corporation, Tokyo, Japan). Renishaw Invia Raman microscope (Beijing, China) was used to determine the functional groups of the sample, and X−ray photoelectron spectroscopy (XPS) using K−Alpha anode (Thermo Scientific, Waltham, MA, USA) was used to determine the surface elemental composition and valence of the sample. We measured the absorbance and photothermal conversion efficiencies of the sample using a UV−Vis−NIR spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) and an infrared thermal imager (FLIR E8, Oregon, Portland, OR, USA). F−320 fluorescence spectrophotometer (Tianjin, China) was used to determine •OH radicals using a terephthalic acid (TA) probe.
2.3. Synthesis of Ti3C2Tx Nanomaterials
In order to prepare mud−like Ti3C2Tx, this experiment used a mixed solution of concentrated hydrochloric acid and lithium fluoride to soak the Ti3AlC2, and the Al element was etched by in situ−generated HF. The mixture was stirred continuously at 35 °C for 24 h, with N2 bubbling to prevent oxidation. After the completion of the reaction, we washed the precipitate with a high quality of deionized water and measured the pH value until a gray−black turbid solution was obtained. The mixture was centrifuged at 3500 rpm for 5 min and treated with nitrogen gas. The upper layer of the turbid liquid was collected, washed multiple times, and vacuum−dried.
2.4. Synthesis of CuPDA@Ti3C2Tx
The Ti3C2Tx dispersion was prepared and coated with PDA on the surface using the alkaline polymerization method. The mixture was adjusted to a pH of 8.5 using a Tris. A dopamine monomer and copper chloride were added, and then static polymerization was maintained for 24 h in the presence of oxygen. The CuPDA@Ti3C2Tx powder was centrifuged and vacuum−dried for use.
2.5. Preparation of CuPDA@Ti3C2Tx Alginate Hydrogel
At 25 °C, 50 mg of sodium alginate was dispersed under ultrasound in the CuPDA@Ti3C2Tx solution (0.4 μg/mL). A total of 1 mL of the CaCl2 solution (50 μg/mL) was added to the suspension to start the crosslinking reaction. The CuPDA@Ti3C2Tx hydrogel was collected and purified with water three times, until no residue was detected in the spectral test.
2.6. Photothermal Property Calculation
The photothermal performance of the nanocomposites was measured under 808 nm near−infrared (NIR) laser irradiation [38]. The concentrations of various composite suspensions ranged from 100 to 500 μg/mL. Then, different concentrations of nanocomposite suspensions were irradiated with a near−infrared laser (808 nm, 1.0 W/cm2), and temperature−changed and thermal images were recorded every 30s using thermal imaging technology for 10 min. The photothermal conversion efficiency was calculated according to the following formula:
where η1 is the material’s photothermal conversion efficiency value, Tmax is the maximum temperature of the photothermal material dispersion or deionized water, Tsurr is the ambient temperature, A808 is the absorbance of the dispersion at 808 nm, Qdis is the heat emitted by the environment, C is the specific heat capacity of water, T is the real−time temperature of the photothermal material dispersion or deionized water during the temperature measurement, t is the time, τS is the slope of the cooling curve fitting line for the water dispersion or deionized water of the photothermal material, and I is the power density of the laser used.
2.7. Antibacterial Measurement
The colony counting method was used to determine the number of colony forming units (CFUs) for CuPDA@Ti3C2Tx under near−infrared irradiation. E. coli was incubated in fresh MHB in a shaking incubator at 37 °C. Logarithmic metaphase cells were diluted to 2 × 105 CFU/mL, and 500 μL of PDA@Ti3C2Tx nanocomposite was added. The mixture was shaken continuously at 37 °C for 1 h. Subsequently, cells from the Ti3C2Tx + NIR, PDA@Ti3C2Tx + NIR, and CuPDA@Ti3C2Tx + NIR groups were monitored using an infrared thermal imager. After another 5 h of cultivation, each group of bacterial suspension was continuously diluted 10 times and placed on an MHA plate, which was then incubated overnight at 37 °C. The bacterial colonies were counted and imaged on the board. All experiments were repeated three times. The staining method for live and dead bacteria was performed using Syto−9 to generate green fluorescence, which could characterize live bacteria, and propidium iodide (PI) could emit red fluorescence to characterize dead bacteria.
3. Results
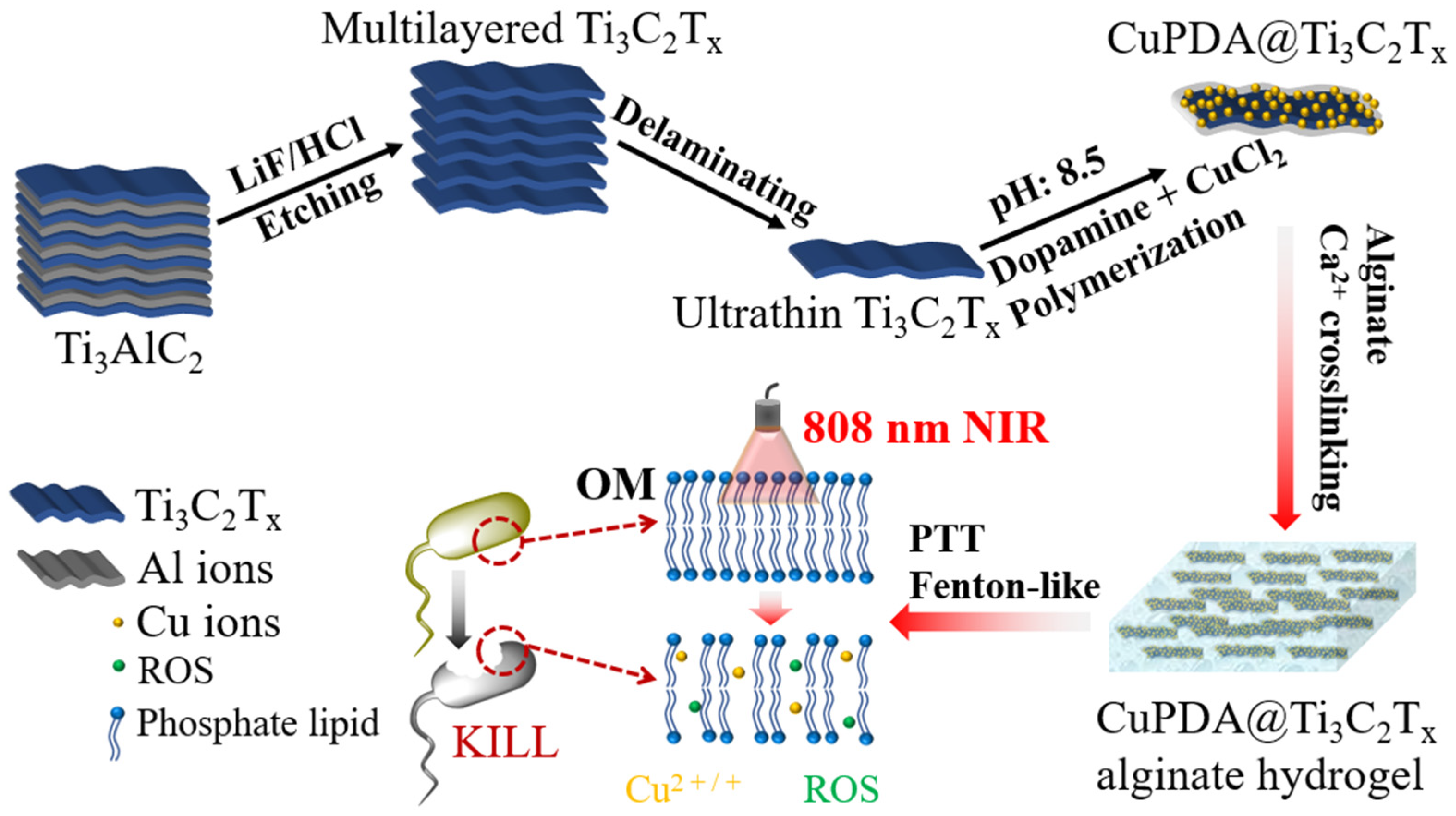
As shown in Figure 1, the design concept of this study was to use LiF and concentrated HCl routes to etch Ti3AlC2, create Ti3C2Tx nanosheets, and coat them with Cu−coordinated polydopamine to improve their water–oxygen stability while reducing cell toxicity [39,40]. The abundant oxygen−containing functional groups and amino coordination of polydopamine were utilized to adsorb Cu2+. Finally, Ca2+ ions crosslinked with sodium alginate hydrogel were used to prepare a composite antibacterial dressing, and the photothermal effect was used to improve the permeability of the bacterial membrane, promote the penetration capabilities of ROS and Cu ions, and synergistically improve its antibacterial performance.
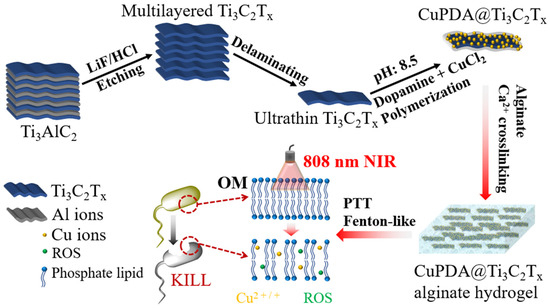
Figure 1.
Illustration of preparation procedure and antibacterial function of CuPDA@Ti3C2Tx nanocomposite hydrogel materials.
3.1. Synthesis and Characterization of Nanocomposites
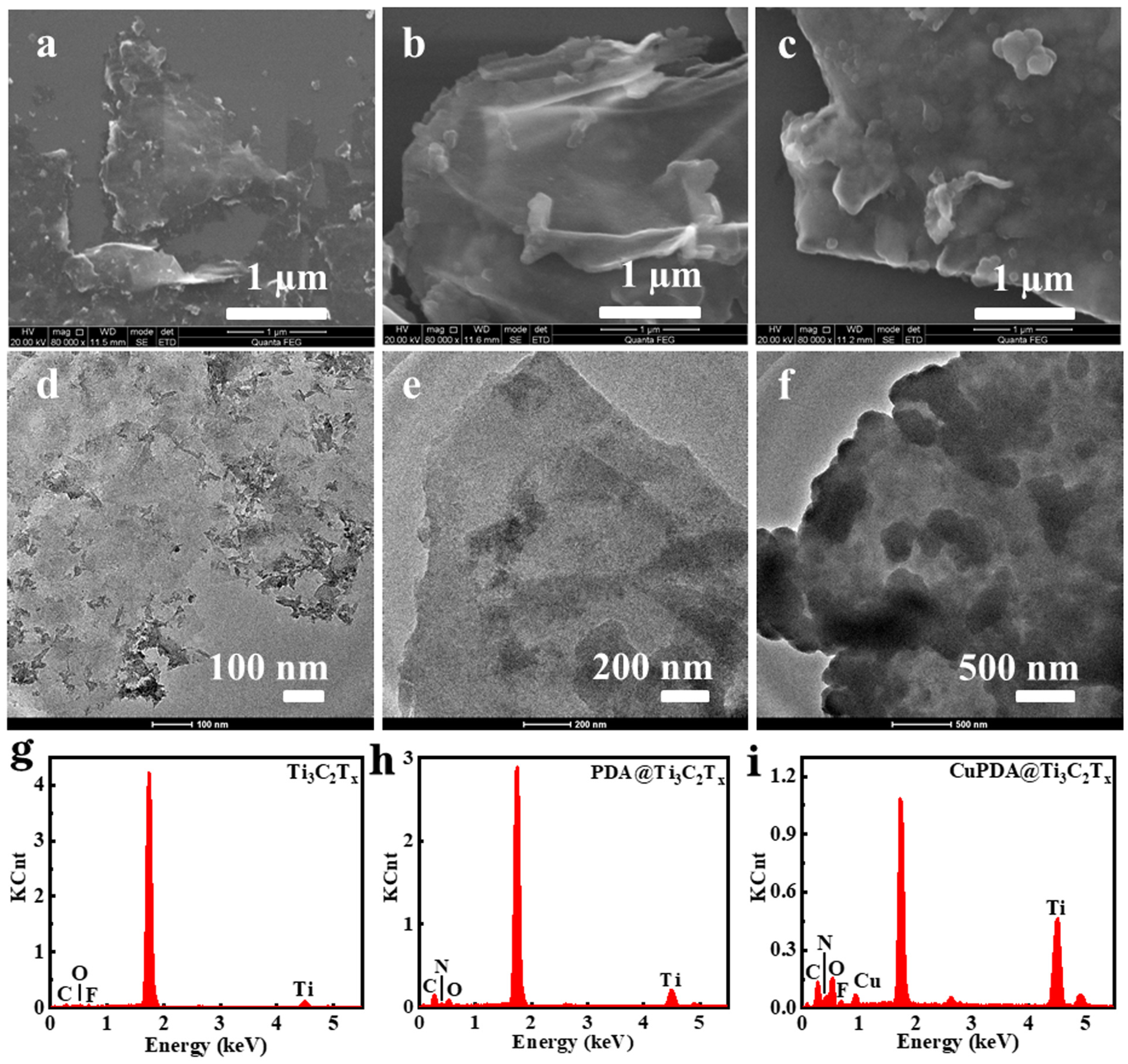
From the SEM images in Figure 2a, it can be seen that Ti3C2Tx exhibits a layered structure. In general, the sample exfoliated with HF exhibited the morphology of an accordion. However, the mixed exfoliation method of LiF and concentrated HCl could obtain the MXene mud, which was more suitable as a two−dimensional support for further loading other antibacterial agents. Figure 2b,c are the SEM images of PDA@Ti3C2Tx and CuPDA@Ti3C2Tx, which show a rough surface and increased thickness, indicating that the Ti3C2Tx material successfully coated the PDA polymer on its surface. As shown in Figure 2d,f, compared with the TEM image of Ti3C2Tx, there are some thin films and granular substances on the surface of the PDA@Ti3C2Tx and CuPDA@Ti3C2Tx materials, which may be caused by PDA or CuPDA coating.
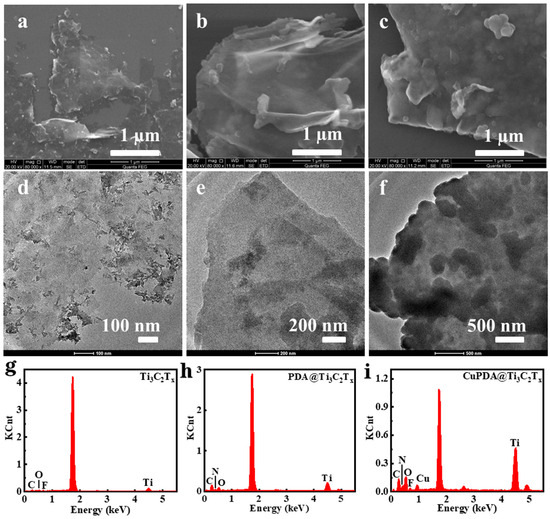
Figure 2.
SEM and TEM images of (a,d) Ti3C2Tx, (b,e) PDA@Ti3C2Tx, and (c,f) CuPDA@Ti3C2Tx. Corresponding EDS spectra for (g) Ti3C2Tx, (h) PDA@Ti3C2Tx and (i) CuPDA@Ti3C2Tx.
As shown in Figure 2g, there are characteristic peaks for Ti, C, O, and F in the energy dispersive spectrometer (EDS) spectrum of Ti3C2Tx, with element contents of 3.25%, 20.1%, 4.8%, and 1.3%, respectively. In the case of PDA@Ti3C2Tx in Figure 2h, there are characteristic peaks for Ti, C, O, and N in the EDS spectrum; the corresponding element contents are 4.2%, 38.3%, 9.0%, and 7.8%. After introducing Cu ions, CuPDA@Ti3C2Tx showed characteristic peaks of Ti, C, O, N, F, and Cu in the EDS spectrum, and the Cu content was ca. 1.5%. The N element came from PDA and Cu was produced due to the coordination between PDA and Cu, indicating that the surface of Ti3C2Tx was successfully coated by the Cu−coordinated PDA.
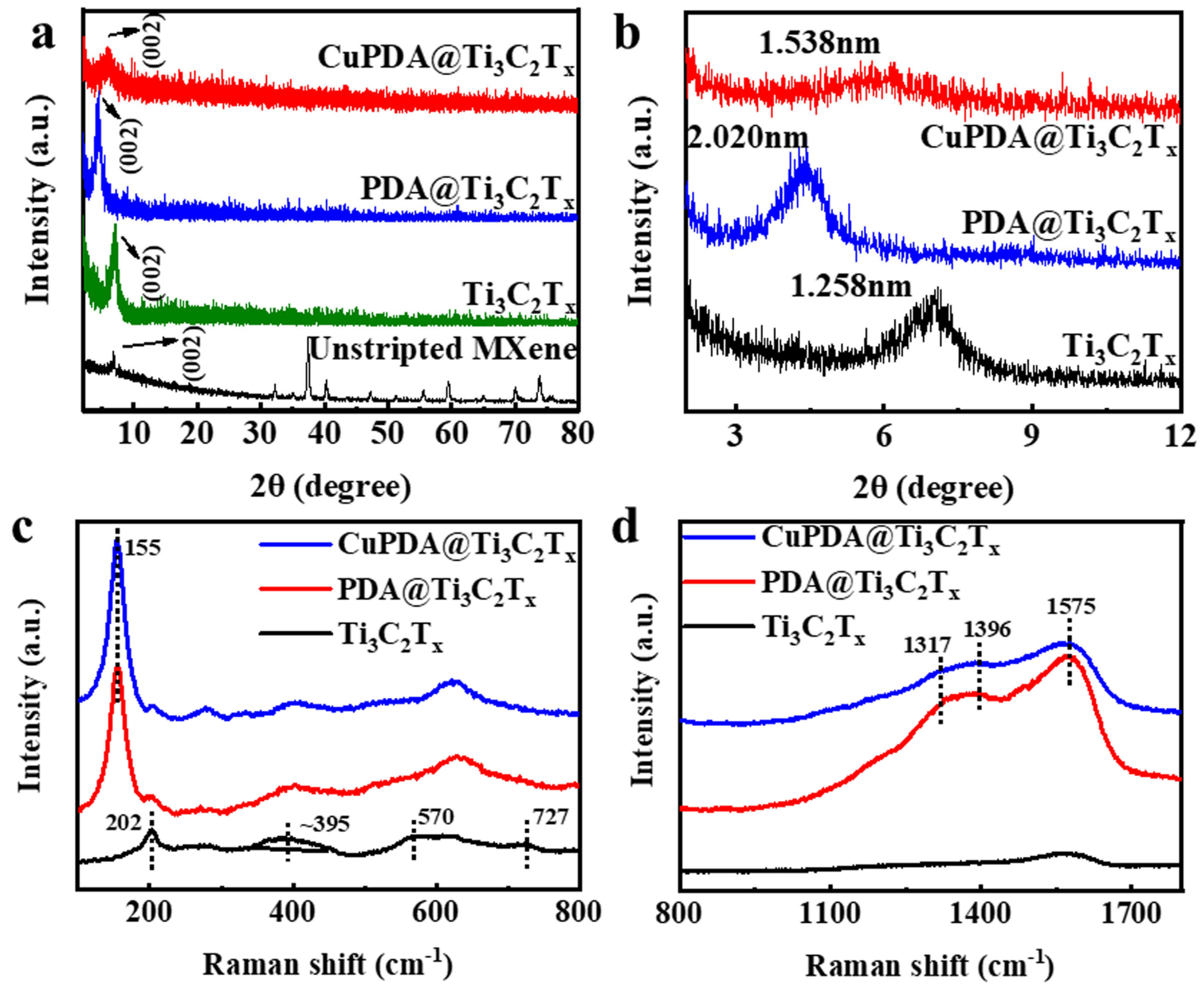
X−ray diffraction (XRD) further indicates the successful synthesis of exfoliated Ti3C2Tx, PDA@Ti3C2Tx, and CuPDA@Ti3C2Tx in Figure 3a,b, compared with unstripped Ti3AlC2, and the main characteristic peak appears at 2θ = 6° for the Ti3C2Tx nanosheets [41]. According to the Bragg equation, the interlayer spacing increased to 1.258 nm, confirming the successful delamination of Ti3AlC2 and the formation of the Ti3C2Tx nanosheets. The characteristic peak for PDA@Ti3C2Tx shifted to a low angle and the interlayer spacing increased to 2.020 nm, confirming the successful encapsulation of PDA between the adjacent Ti3C2Tx nanosheets. The addition of Cu ions reduced the interlayer distance to 1.538 nm, attributed to the electrostatic repulsion of positively charged Cu ions, which reduced the interlayer distance.
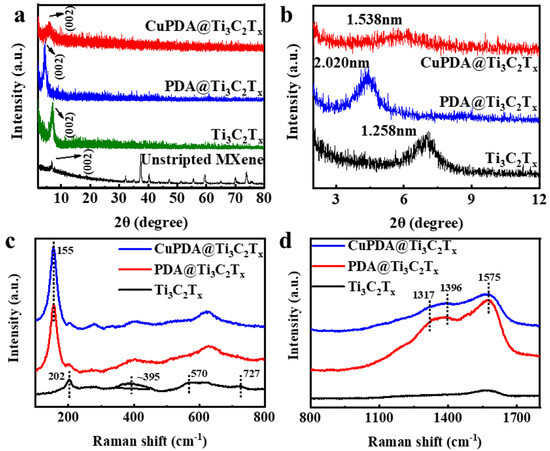
Figure 3.
(a) XRD patterns for unstripped Ti3AlC2 and exfoliated Ti3C2Tx, PDA@Ti3C2Tx, and CuPDA@Ti3C2Tx. (b) Enlarged XRD patterns in small angles for the calculation of the interlayer distance in corresponding materials. (c,d) Raman spectra of exfoliated Ti3C2Tx, PDA@Ti3C2Tx, and CuPDA@Ti3C2Tx in different wavenumber regions.
The Raman spectra further confirmed the composition of the antibacterial material. The peaks of 202, 395, and 570 cm−1 are attributed to −O, −F, and −OH, respectively, on the surface of Ti3C2Tx in Figure 3c. As seen in Figure 3d, three characteristic peaks can be observed for PDA@Ti3C2Tx and CuPDA@Ti3C2Tx, appearing at 1317, 1396, and 1575 cm−1, which belong to the −CH2NH2, C−C/C−N, and C−N indole groups of PDA, respectively [42]. Thus, the Raman results show the successful coating of PDA on the surface of Ti3C2Tx.
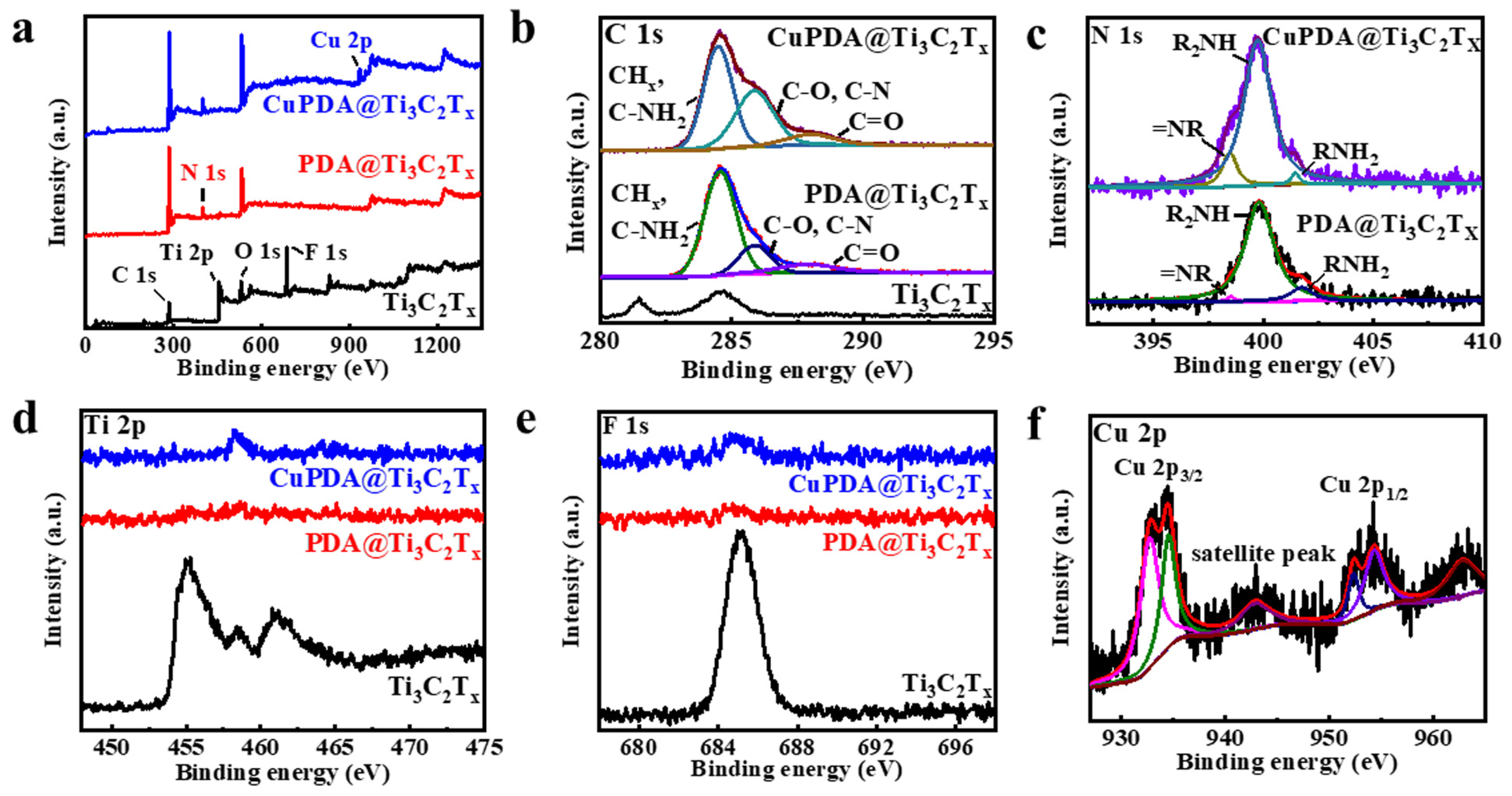
X−ray photoelectron spectroscopy (XPS) is a non−destructive measurement method that can analyze the elemental composition and valence state of elements. Figure 4a shows the full XPS spectra of each sample, and the characteristic peaks of C 1s, Ti 2p, O 1s, and F 1s can be observed, located at 284.6, 455.1, 529.6, and 685.1 eV, respectively, indicating that the Ti3C2Tx material has been successfully synthesized. It can be seen that the Al signal is not observable, further indicating that Al has been effectively etched from Ti3AlC2. In addition, the XPS full spectrum indicates that PDA@Ti3C2Tx contains 6.46% N, confirming a successful PDA−coating process. The N 1s spectrum (Figure 4b) indicates an increase in the characteristic peak area of =NR, possibly due to the N coordination effect between Cu and PDA [43]. As shown in Figure 4d,e, the Ti 2p and F 1s peaks significantly weaken, attributed to the XPS testing depth of only 5–10 nm, which cannot penetrate the PDA coating. This indicates that Ti3C2Tx nanosheets are almost completely encapsulated by PDA, which is beneficial for improving their antioxidant property. As seen in Figure 4f, the Cu ion 2p3/2 and 2p1/2 spin−orbit coupling splitting peaks appear at binding energies of 932.8 and 952.3 eV [44], indicating the presence of Cu+, while the splitting peaks at 934.5 and 954.3 eV indicate the presence of Cu2+. It is assumed that the Fenton−like activity of Cu+ will improve the antibacterial ability to some extent [1].
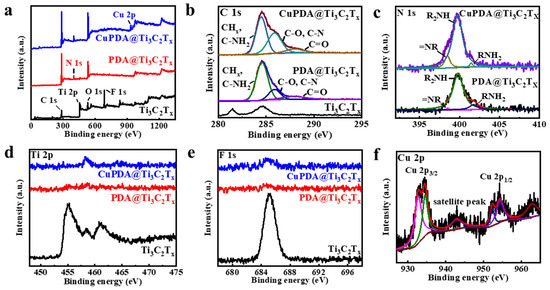
Figure 4.
(a) Full XPS spectra for Ti3C2Tx (black curve), PDA@Ti3C2Tx (red curve), and CuPDA@Ti3C2Tx (blue curve), and fine deconvoluted XPS spectra for (b) C 1s, (c) N 1s, (d) Ti 2p, (e) F 1s, and (f) Cu 2p.
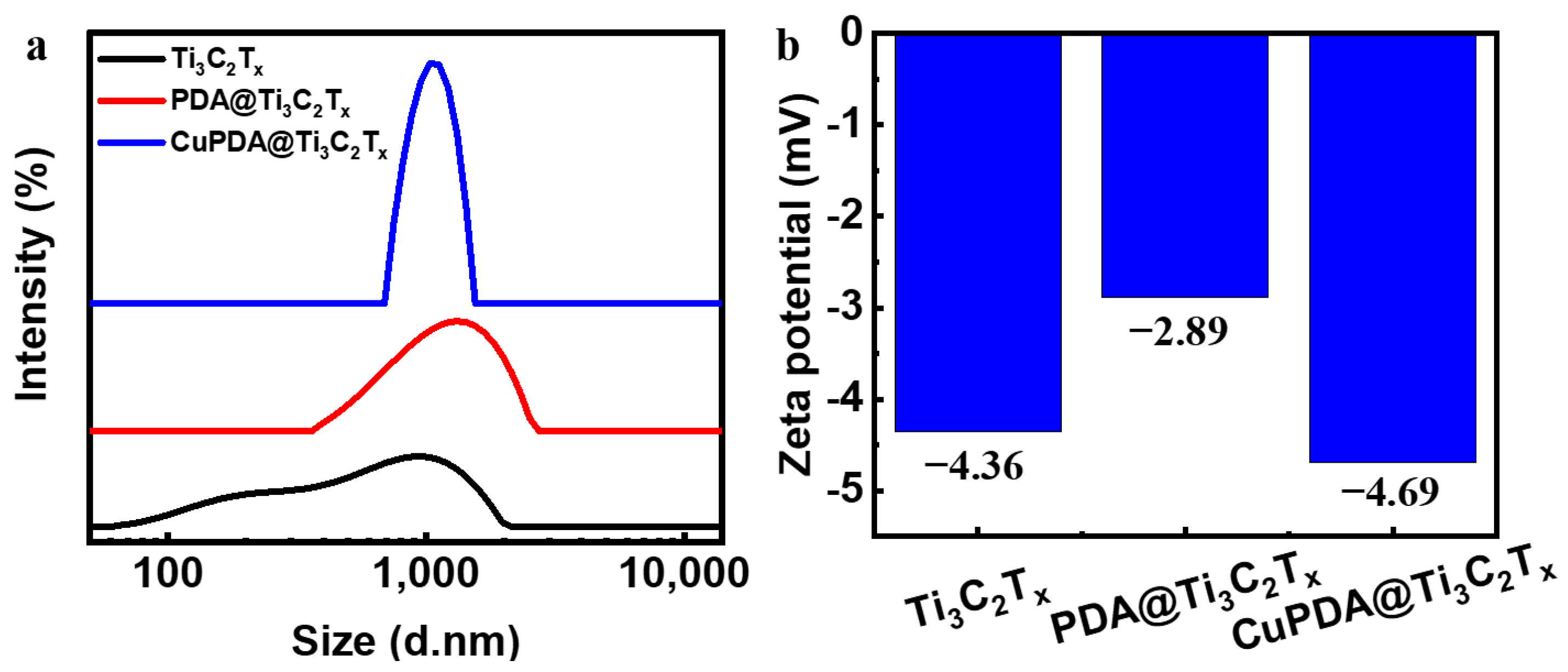
The dynamic light−scattering (DLS) measurement in Figure 5a shows that the average size of Ti3C2Tx nanosheets is about 1000 nm, and the hydration particle size increases to about 1200 nm after PDA coating. The zeta−potential results of the three materials in Figure 5b show that there is only a low level of negative charge on the surface.
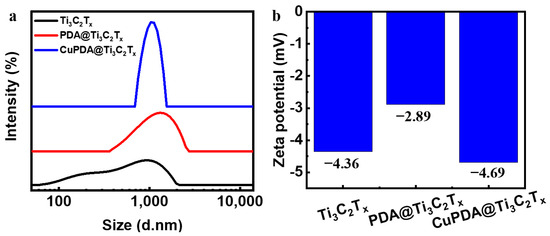
Figure 5.
(a) Particle size distribution diagram and (b) zeta−potential diagram for Ti3C2Tx, PDA@Ti3C2Tx, and CuPDA@Ti3C2Tx.
3.2. Photothermal Properties
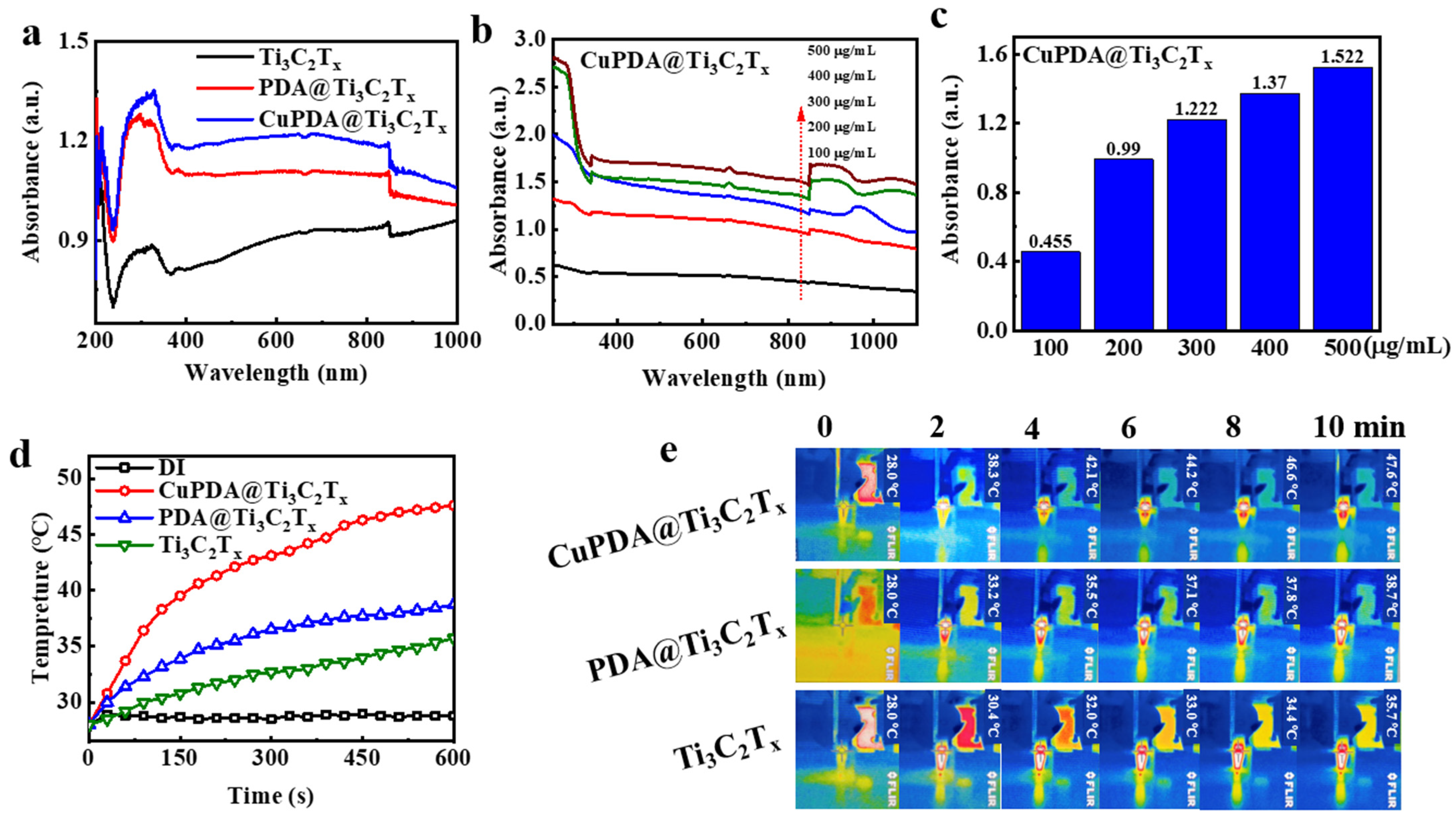
To verify the near−infrared (NIR) absorption performance of the material, we tested the DRS spectra of various photothermal materials. As shown in Figure 6a, the absorbance intensity of Ti3C2Tx at 808nm is approximately 0.934. After coating the PDA, its absorbance increased to 1.099. When further coordinated with copper ions, CuPDA@Ti3C2Tx had the highest absorbance intensity, reaching 1.19, which was conducive to improving the photothermal conversion performance. Figure 6b compares the UV−Vis−NIR absorbance spectra for CuPDA@Ti3C2Tx under different concentration conditions. When the dispersion concentration increased from 100 to 500 µg/mL, the higher the concentration, the greater the absorption intensity. As shown in Figure 6c, the absorbance of the composite suspension exhibits a concentration−dependent relationship at a wavelength of 808 nm.
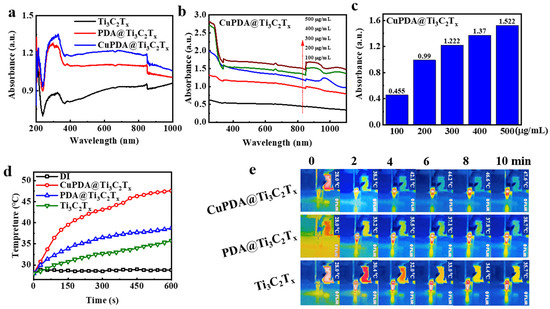
Figure 6.
(a) UV−Vis−NIR diffuse reflection spectra (DRS) of Ti3C2Tx (black curve), PDA@Ti3C2Tx (red curve), and CuPDA@Ti3C2Tx (blue curve). (b) UV−Vis−NIR absorbance spectra of CuPDA@Ti3C2Tx at various concentrations, arrow indicated the concentrations increase from 100 to 500 µg/mL, and (c) corresponding absorbance dependence histogram at 808 nm. (d) Solution temperature−rise curves for pure distilled water (DI), Ti3C2Tx, PDA@Ti3C2Tx, and CuPDA@Ti3C2Tx water dispersion at a concentration of 500 µg/mL. (e) Thermal imaging maps for Ti3C2Tx, PDA@Ti3C2Tx, and CuPDA@Ti3C2Tx dispersed solutions.
The potential of composite materials in photothermal antibacterial applications was verified by measuring the temperature evolution profile of laser irradiation. As shown in Figure 6d, the temperature of suspensions of various materials at 500 µg/mL rapidly increases under an 808 nm laser irradiation of 1 W/cm2, and the surface temperature of Ti3C2Tx increases to 35.7 ° C after 10 min of irradiation. However, the surface temperature of CuPDA@Ti3C2Tx can be raised to 47.6 ° C. In contrast, there is no significant temperature change in the temperature of water, indicating that CuPDA@Ti3C2Tx can efficiently and rapidly convert NIR light into thermal energy. Correspondingly, we recorded the thermal imaging maps of the dispersed solutions in the EP tubes during 10 min of 808 nm NIR irradiation (Figure 6e). It can be seen that the CuPDA@Ti3C2Tx dispersion exhibits a uniform higher−temperature distribution than Ti3C2Tx and PDA@Ti3C2Tx, indicating its better photothermal ability.
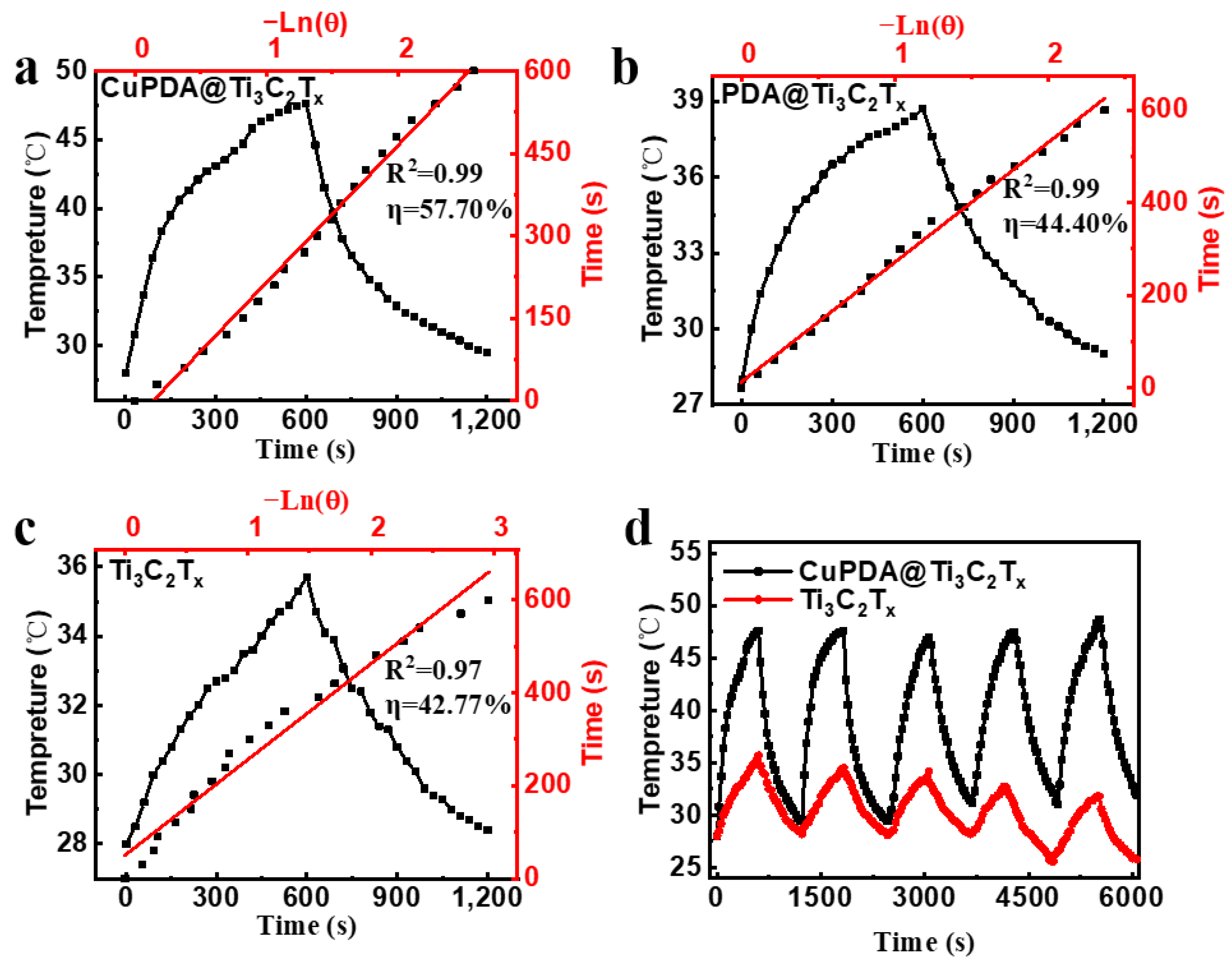
According to Equations (1)–(5), the photothermal conversion efficiencies of Ti3C2Tx and PDA@Ti3C2Tx are 42.77% (Figure 7a) and 44.4% (Figure 7b), while the photothermal conversion efficiency of CuPDA@Ti3C2Tx significantly improves, reaching 57.7% (Figure 7c), which is higher than most reported NIR photothermal conversion materials, such as Ti3C2Tx (30.6%) [45], MXene@EGCG (29.2%) [46], and Mo4VC4 (45.5%) [47]. To verify the photothermal stability of the material, the heating and cooling curves of the photothermal materials were tested (Figure 7d). The temperature of the CuPDA@Ti3C2Tx suspension remained stable at 47.6 °C after five cycles, while the temperature of the uncoated material decreased by 3.9 °C, indicating the potential of CuPDA@Ti3C2Tx as a durable photothermal agent for PTT.
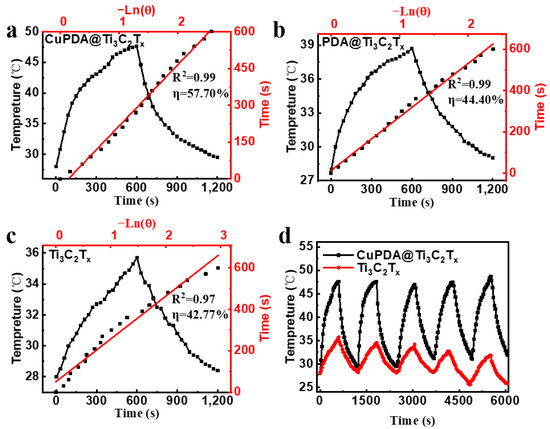
Figure 7.
Photothermal conversion efficiencies of (a) CuPDA@Ti3C2Tx, (b) PDA@Ti3C2Tx, and (c) Ti3C2Tx, red line is the linear fitting curve. (d) Stability curves of photothermal heating–cooling for CuPDA@Ti3C2Tx (black curve) and Ti3C2Tx (red curve).
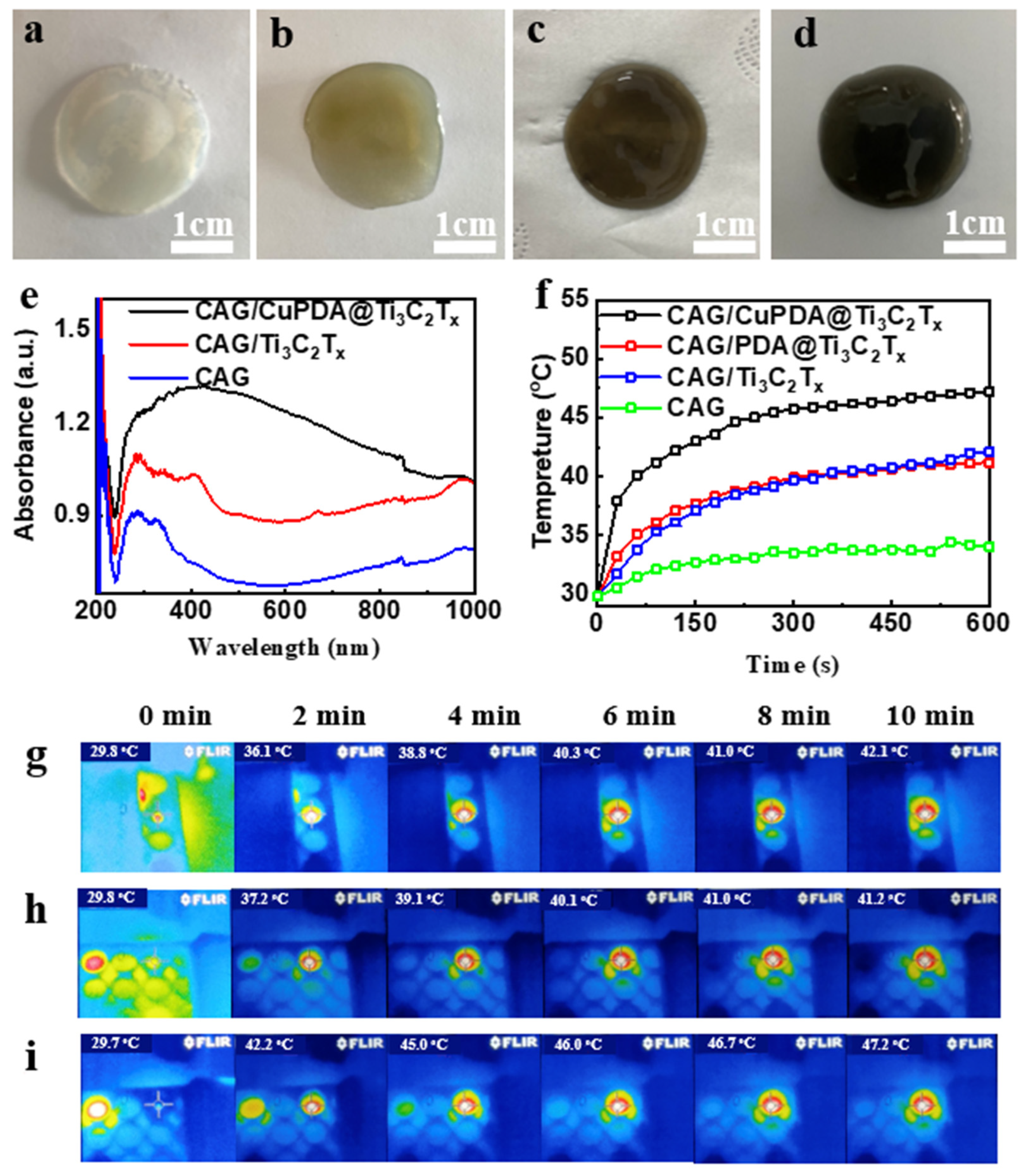
At 25 °C, 50 mg of sodium alginate was dispersed into Ti3C2Tx, PDA@ Ti3C2Tx, and CuPDA@ Ti3C2Tx solutions (500 μg/mL) under ultrasonication conditions. A total of 1 mL of CaCl2 solution (50 μg/mL) was added to the suspension to start the crosslinking reaction, as shown in Figure 8a–d. After 2 h, a stable gel film was formed and the color changed from colorless to light brown and finally to black. The total concentration of antibacterial nanomaterials in the gel was about 10 µg/mg; such a relatively low nanomaterial content might decrease the cytotoxicity. As shown in Figure 8e, the hydrogel has obvious absorption properties at 808 nm in the near−infrared region, and the CAG/CuPDA@Ti3C2Tx hydrogel has the strongest absorbance at 808 nm. The surface temperature of the blank CAG hydrogel increased to 34 °C after 10 min of irradiation with a 1 W/cm2 808 nm laser (Figure 8f). However, the surface temperature of the Ti3C2Tx hydrogel increased to 42.1 °C after 10 min of irradiation due to the absorption of Ti3C2Tx in the NIR region. After testing the temperature of the CAG/CuPDA@Ti3C2Tx hydrogel further, the surface temperature increased to 47.2 °C, which indicated that CAG/CuPDA@Ti3C2Tx could efficiently and rapidly convert near−infrared light into thermal energy, and the effect was better than that of CAG@Ti3C2Tx. At the same time, we also recorded the thermal imaging maps of CAG/Ti3C2Tx (Figure 8g), CAG/PDA@Ti3C2Tx (Figure 8h), and CAG/CuPDA@Ti3C2Tx (Figure 8i) hydrogels irradiated by an 808 nm NIR value for 10 min. The CAG/CuPDA@Ti3C2Tx hydrogel film showed the highest surface temperature and exhibited a uniform temperature distribution, consistent with the thermal imaging maps for the EP tubes.
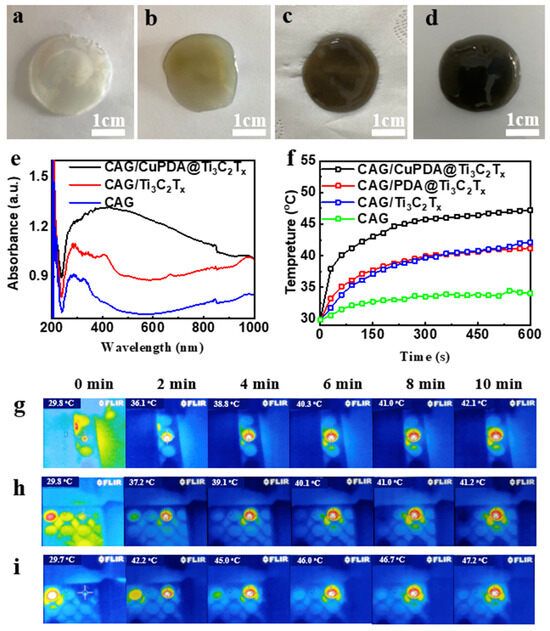
Figure 8.
Digital pictures of (a) CAG, (b) CAG/Ti3C2Tx, (c) CAG/PDA@Ti3C2Tx, and (d) CAG/CuPDA@Ti3C2Tx hydrogels. (e) Corresponding DRS and (f) surface temperature−rise curves. Thermal imaging maps for (g) CAG/Ti3C2Tx, (h) CAG/PDA@Ti3C2Tx, and (i) CAG/CuPDA@Ti3C2Tx hydrogels. Blue represents low temperature.
3.3. Antibacterial Performance and Mechanism
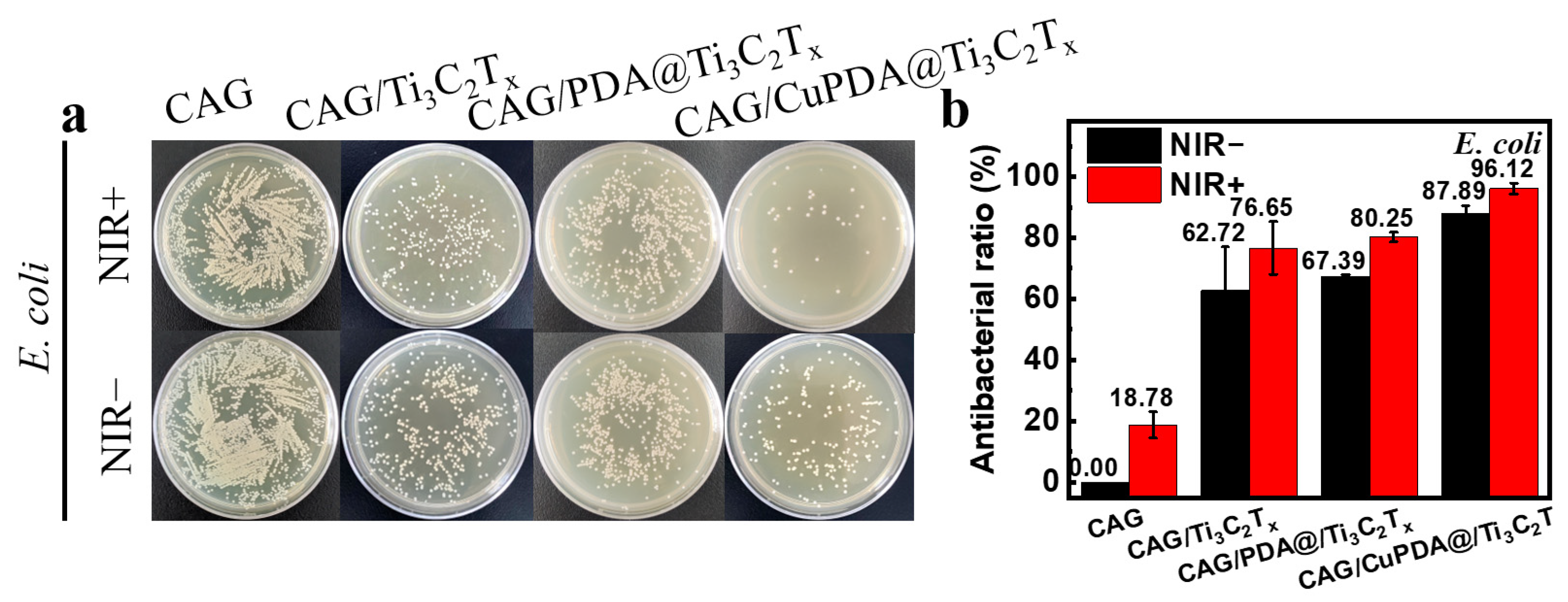
In this experiment, E. coli was selected to evaluate the antibacterial activity of a nanocomposite antibacterial hydrogel. As shown in Figure 9a, after co−culturing with bacteria at 37 °C for 6 h, the number of colonies in CAG, CAG/Ti3C2Tx, CAG/PDA@Ti3C2Tx, and CAG/CuPDA@Ti3C2Tx decreased sequentially, indicating that the CAG/CuPDA@Ti3C2Tx hydrogel presented stronger antibacterial properties. As expected, the number of bacterial colonies in CAG did not change significantly after light treatment. In order to compare the antibacterial properties of various materials, the antibacterial rate was calculated. As shown in Figure 9b, under near−infrared−light conditions, the antibacterial rate of the CAG hydrogel is only 18.78%. When mixed with the NIR−light−absorbing material, Ti3C2Tx, its antibacterial rate increased to 76.65%. Furthermore, Ti3C2Tx coated with PDA was mixed into the gel. Based on the spontaneous H2O2 generation of PDA and the photothermal function of the composite gel, its antibacterial rate increased to 80.25% [48]. It is worth mentioning that, with the introduction of Cu ions, the antibacterial efficiency of the CAG/CuPDA@Ti3C2Tx composite hydrogel group against E. coli reached 96.12%, indicating that our hydrogel presented excellent antibacterial properties due to the Fenton−like reaction and photothermal effect of copper ions.
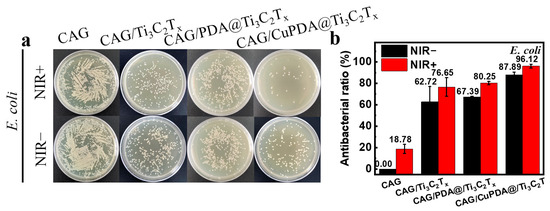
Figure 9.
(a) Plate—counting results after the co—cultivation of E. coli and (b) corresponding antibacterial rates for CAG, CAG/Ti3C2Tx, CAG/PDA@Ti3C2Tx, and CAG/CuPDA@Ti3C2Tx hydrogels.
4. Discussion
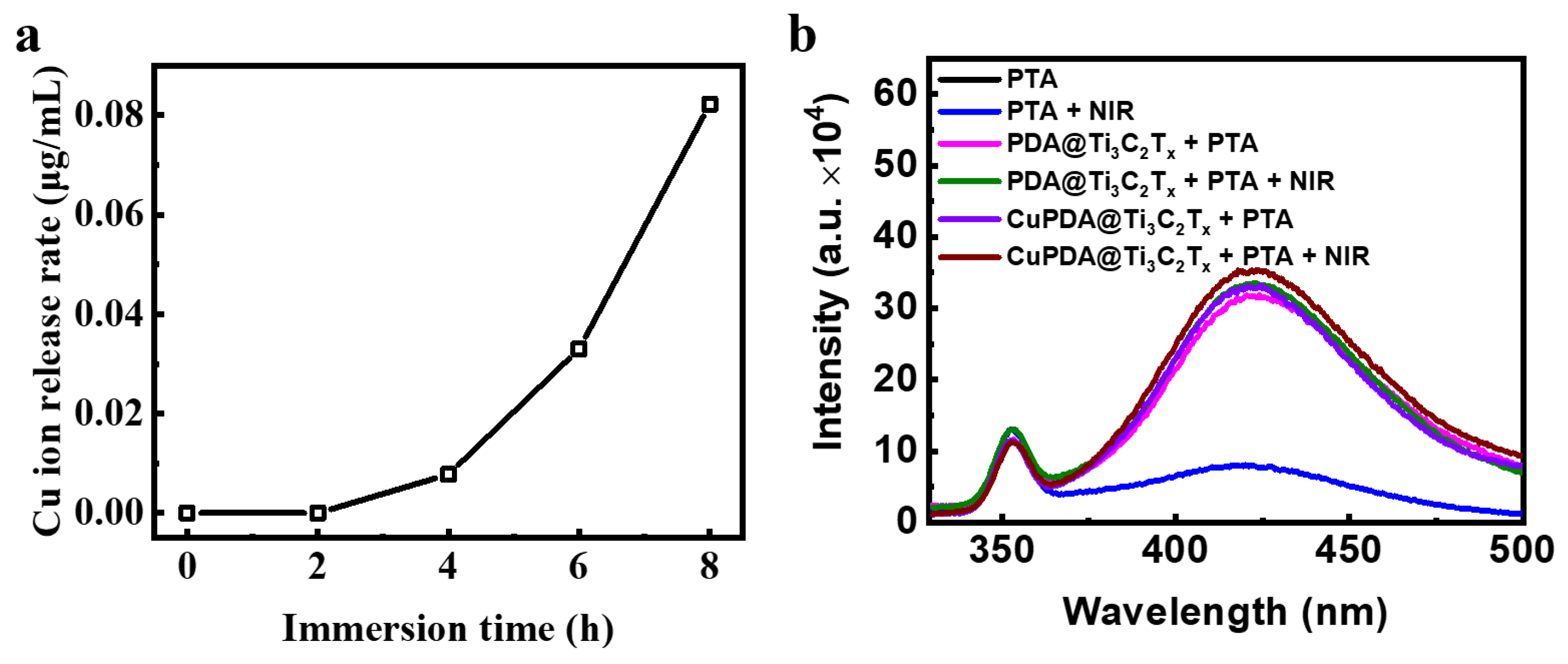
To explore the sterilization mechanism of hydrogels, the release of Cu ions was tested (Figure 10a). The results show that, after soaking them in PBS for 6 h, the concentration of Cu ions in the prepared solution reached 0.033 μg/mL, indicating that the composite hydrogel could release Cu ions. According to the XPS results, there are Cu+ ions that present Fenton−like activity to produce •OH. To verify the species of ROS, sodium terephthalate (PTA) was used as a fluorescence probe to detect the •OH in the solution [49]. PTA is a fluorescent reagent that undergoes a fluorescence enhancement reaction in the presence of •OH, and a stronger fluorescence intensity indicates the presence of more •OH. •OH radicals undergo an additional reaction with PTA, forming a stable form of 2−hydroxyterephthalic acid within the molecule. This stable form is more prone to fluorescence resonance energy transfer than terephthalic acid itself, thereby enhancing the fluorescence signal intensity. As shown in Figure 10b, under both light and dark conditions, the fluorescence intensity of the PTA solution at 425 nm was low and almost unchanged. After mixing CAG/PDA@Ti3C2Tx, the fluorescence intensity significantly increased, indicating that the nanomaterial could produce more •OH. According to the electrochemical potential of PDA, its oxidation potential to quinone is ca. 0.2 V, while the electrochemical potential of O2/H2O2 is ca. 0.5 V [50]. Therefore, in thermodynamics, PDA can spontaneously oxidize to generate H2O2. As expected, the CAG@CuPDA@Ti3C2Tx solution showed the highest fluorescence intensity, indicating that more •OH radicals were generated, which was related to the Fenton−like effect of Cu+ [51].
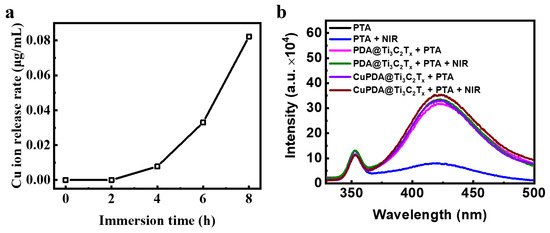
Figure 10.
(a) Cu ion release curve for CAG@CuPDA@Ti3C2Tx. (b) Fluorescence spectra in the presence of PTA with and without NIR irradiation.
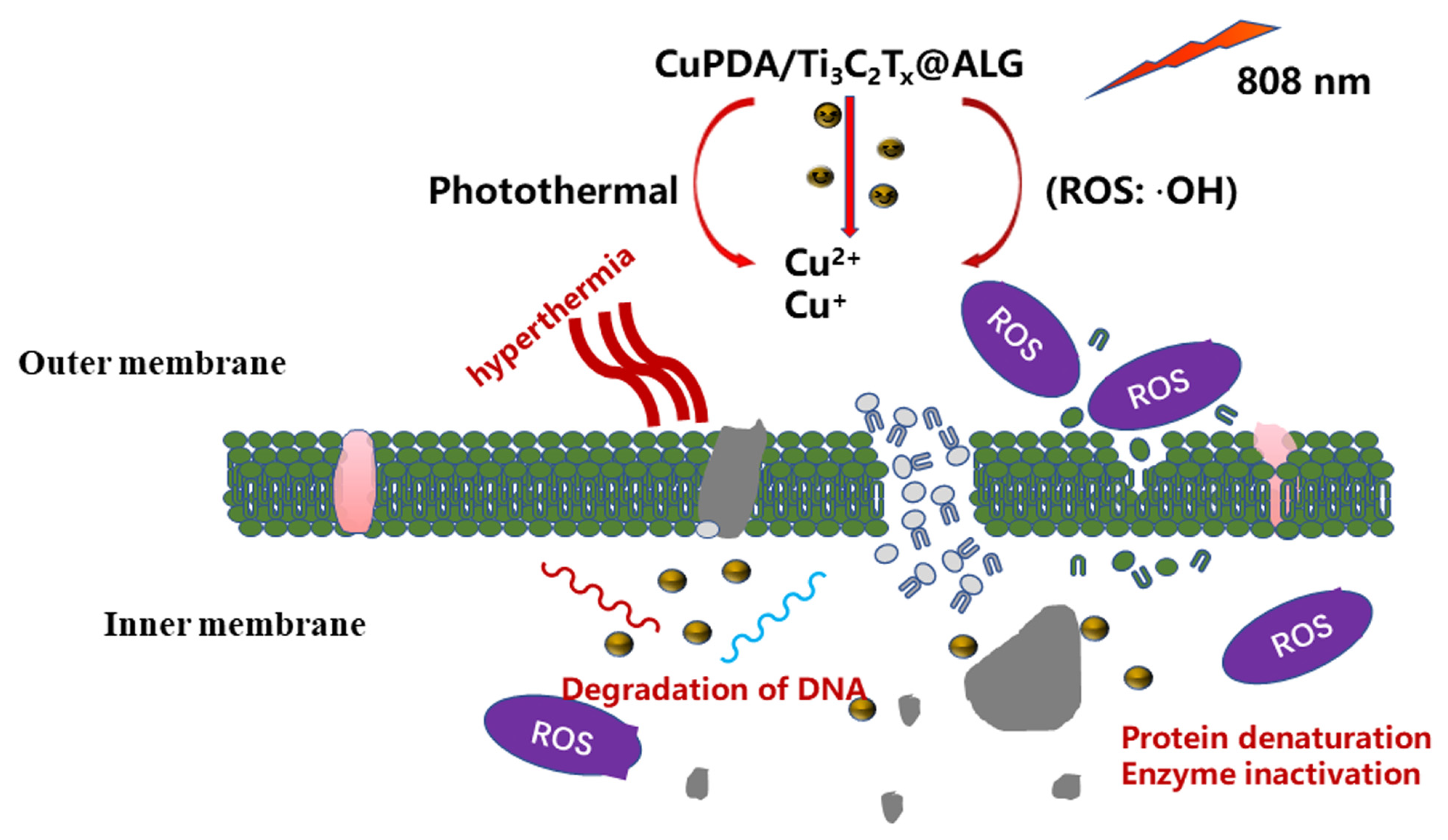
In brief, the combination of copper ion chemical antibacterial and PTT antibacterial therapies can effectively produce antibacterial effects through NIR irradiation [52]. As shown in Figure 11, when exposed to 808 nm NIR irradiation, CAG@CuPDA@Ti3C2Tx can convert light energy into heat energy, resulting in a thermal effect that alters the structure of bacterial cell membranes. This alteration leads to changes in bacterial cell membrane permeability, and in extreme cases, high heat levels can directly cause a bacterial membrane rupture [53,54]. At the same time, a certain number of H2O2 molecules are produced due to the spontaneous self−oxidation of PDA in the presence of O2. Then, the Cu+ Fenton−like reaction, as described above, is used to catalyze H2O2 into more oxidative form of •OH. Due to the increase in the outer−membrane permeability caused by photothermal treatment, Cu2+ and Cu+ ions, as well as •OH, enter the interior of the cells, damaging the proteins, DNA, and other structures. Therefore, this damage affects the normal physiological activities of bacteria and leads to bacterial lysis and death.
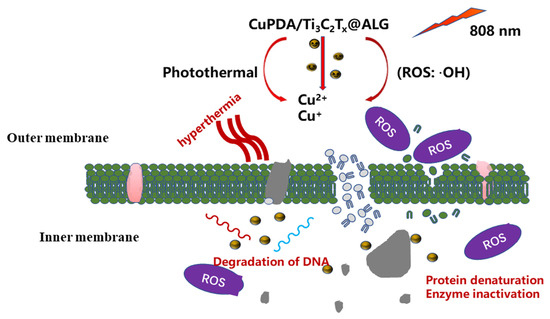
Figure 11.
Proposed antibacterial mechanism of the CAG@CuPDA@Ti3C2Tx upon irradiation.
5. Conclusions
In conclusion, we reported the Fenton−like and photothermal effects of the CAG hydrogel, and also studied the synergistic antibacterial effect of its chemical kinetics and photothermal effect. After coating the surface of Ti3C2Tx with PDA, its antioxidant activity can be significantly improved, which is beneficial for obtaining high photothermal stability. At the same time, the spontaneous oxidation reaction of the catechol structure in PDA can be utilized to generate H2O2. Therefore, PDA can generate Cu+ ions by coordinating the Cu ions with abundant chemical functional groups, and its Fenton−like reaction can be used to reduce H2O2 to produce •OH. Compared with pure Ti3C2Tx, our antibacterial material exhibited greater photothermal stability in five heating−cooling cycle experiments. This photothermal effect can improve the permeability of the bacterial outer membrane, and the Cu ions and •OH generated by Fenton−like reactions can easily enter the interior of the bacteria to induce damage. It is believed that metal−doped PDA coating can solve the key problem of enhancing the effective ROS production capacity of CDT and provide guidance for the design of multifunctional antibacterial materials.
Author Contributions
Z.F.: conceptualization, writing—review and editing, methodology. Q.Z.: data curation, writing—original draft, investigation. W.Z.: software, investigation, data curation. J.W.: formal analysis and investigation. Y.L.: software, investigation. M.Y.: software and investigation. Y.Q.: conceptualization, writing—review and editing, methodology, supervision, writing—review and editing. Z.M.: writing—review and editing and methodology. S.L.: data curation, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National College Students’ Innovation and Entrepreneurship Training Program (grant number: 202310213133), the National Science Fund for Distinguished Young Scholars (grant number: 51825202), and the Foundation for Heilongjiang Touyan Innovation Team (HITTY−20190036).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wood, S.J.; Kuzel, T.M.; Shafikhani, S.H. Pseudomonas aeruginosa: Infections, Animal Modeling, and Therapeutics. Cells 2023, 12, 199. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Hojjati-Najafabadi, A.; Pei, R. Mxene-Laden Bacteriophage: A New Antibacterial Candidate to Control Bacterial Contamination in Water. Chemosphere 2022, 290, 133383. [Google Scholar] [CrossRef]
- Brown, D. Antibiotic Resistance Breakers: Can Repurposed Drugs Fill the Antibiotic Discovery Void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Rojas-Andrade, M.D.; Chata, G.; Rouholiman, D.; Liu, J.L.; Saltikov, C.; Chen, S.W. Antibacterial Mechanisms of Graphene-Based Composite Nanomaterials. Nanoscale 2017, 9, 994–1006. [Google Scholar] [CrossRef]
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K.; Aprem, A.S.; Sisupal, S.B. MoS2 Based Nanomaterials: Advanced Antibacterial Agents for Future. J. Control. Release 2022, 348, 158–185. [Google Scholar] [CrossRef]
- Abbas, M.; Ovais, M.; Atiq, A.; Ansari, T.M.; Xing, R.; Spruijt, E.; Yan, X. Tailoring Supramolecular Short Peptide Nanomaterials for Antibacterial Applications. Coord. Chem. Rev. 2022, 460, 214481. [Google Scholar] [CrossRef]
- Packialakshmi, J.S.; Kang, J.; Jayakumar, A.; Park, S.; Chang, Y.; Kim, J.T. Insights into the Antibacterial and Antiviral Mechanisms of Metal Oxide Nanoparticles Used in Food Packaging. Food Packag. Shelf Life 2023, 40, 101213. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Karatasios, I.; Kanellopoulos, N.K.; Prombona, A.; Karanikolos, G.N. Ag and Cu Monometallic and Ag/Cu Bimetallic Nanoparticle-Graphene Composites with Enhanced Antibacterial Performance. ACS Appl. Mater. Interfaces 2016, 8, 27498–27510. [Google Scholar] [CrossRef]
- Cruces, E.; Arancibia-Miranda, N.; Manquián-Cerda, K.; Perreault, F.; Bolan, N.; Azócar, M.I.; Cubillos, V.; Montory, J.; Rubio, M.A.; Sarkar, B. Copper/Silver Bimetallic Nanoparticles Supported on Aluminosilicate Geomaterials as Antibacterial Agents. ACS Appl. Nano Mater. 2022, 5, 1472–1483. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Ku, J.W.K.; Zhang, H.; Salim, T.; Oo, G.; Zinn, A.A.; Boothroyd, C.; Tang, R.M.Y.; Gan, C.L.; Gan, Y.-H.; et al. Copper-Nanoparticle-Coated Fabrics for Rapid and Sustained Antibacterial Activity Applications. ACS Appl. Nano Mater. 2022, 5, 12876–12886. [Google Scholar] [CrossRef]
- Moskvitina, E.; Kuznetsov, V.; Moseenkov, S.; Serkova, A.; Zavorin, A. Antibacterial Effect of Carbon Nanomaterials: Nanotubes, Carbon Nanofibers, Nanodiamonds, and Onion-Like Carbon. Materials 2023, 16, 957. [Google Scholar] [CrossRef]
- Plachá, D.; Muñoz-Bonilla, A.; Škrlová, K.; Echeverria, C.; Chiloeches, A.; Petr, M.; Lafdi, K.; Fernández-García, M. Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials. Nanomaterials 2020, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Kim, J.; Jang, E.-S. Recent Progress in Multifunctional Conjugated Polymer Nanomaterial-Based Synergistic Combination Phototherapy for Microbial Infection Theranostics. Coord. Chem. Rev. 2022, 470, 214701. [Google Scholar] [CrossRef]
- Wang, B.; Feng, G.; Seifrid, M.; Wang, M.; Liu, B.; Bazan, G.C. Antibacterial Narrow-Band-Gap Conjugated Oligoelectrolytes with High Photothermal Conversion Efficiency. Angew. Chem.-Int. Edit. 2017, 56, 16063–16066. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.J.; Sharker, S.M.; In, I.; Park, S.Y. Iron Oxide@Pedot-Based Recyclable Photothermal Nanoparticles with Poly(Vinylpyrrolidone) Sulfobetairies for Rapid and Effective Antibacterial Activity. ACS Appl. Mater. Interfaces 2015, 7, 9469–9478. [Google Scholar] [CrossRef]
- Bi, X.; Bai, Q.; Liang, M.; Yang, D.; Li, S.; Wang, L.; Liu, J.; Yu, W.W.; Sui, N.; Zhu, Z. Silver Peroxide Nanoparticles for Combined Antibacterial Sonodynamic and Photothermal Therapy. Small 2022, 18, 2104160. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.-T.; Kuo, T.-R. Copper Sulfide with Morphology-Dependent Photodynamic and Photothermal Antibacterial Activities. J. Colloid Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef]
- Younis, M.R.; An, R.B.; Yin, Y.C.; Wang, S.J.; Ye, D.J.; Xia, X.H. Plasmonic Nanohybrid with High Photothermal Conversion Efficiency for Simultaneously Effective Antibacterial/Anticancer Photothermal Therapy. ACS Appl. Bio Mater. 2019, 2, 3942–3953. [Google Scholar] [CrossRef]
- Mo, S.; Song, Y.; Lin, M.; Wang, J.; Zhang, Z.; Sun, J.; Guo, D.; Liu, L. Near-Infrared Responsive Sulfur Vacancy-Rich Cus Nanosheets for Efficient Antibacterial Activity Via Synergistic Photothermal and Photodynamic Pathways. J. Colloid Interface Sci. 2022, 608, 2896–2906. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Huang, Z.D.; Shuck, C.E.; Liang, G.J.; Gogotsi, Y.; Zhi, C.Y. Mxene Chemistry, Electrochemistry and Energy Storage Applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of Mxenes at Every Step, from Their Precursors to Single Flakes and Assembled Films. Prog. Mater Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Seidi, F.; Arabi Shamsabadi, A.; Dadashi Firouzjaei, M.; Elliott, M.; Saeb, M.R.; Huang, Y.; Li, C.; Xiao, H.; Anasori, B. Mxenes Antibacterial Properties and Applications: A Review and Perspective. Small 2023, 19, 2206716. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.J.; Qin, X.J.; Su, Z.L.; Gou, X.; Yang, Z.M.; Wang, H.S. Research on the Antibacterial Properties of Mxene-Based 2D-2D Composite Materials Membrane. Nanomaterials 2023, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Hu, H.; Han, J.; Zhao, Z. Double Transition-Metal Tivctx Mxene with Dual-Functional Antibacterial Capability. Mater. Lett. 2022, 308, 131100. [Google Scholar] [CrossRef]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx Mxene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef]
- Sana, S.S.; Santhamoorthy, M.; Haldar, R.; Raorane, C.J.; Iravani, S.; Varma, R.S.; Kim, S.-C. Recent Advances on Mxene-Based Hydrogels for Antibacterial and Drug Delivery Applications. Process Biochem. 2023, 132, 200–220. [Google Scholar] [CrossRef]
- Kumar, R.; Agarwal, S.; Pal, S.; Verma, A.; Prajapati, Y.K. Refractive Index Sensing Using Mxene Mediated Surface Plasmon Resonance Sensor in Visible to near Infrared Regime. Measurement 2024, 224, 113682. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Sun, D.-W.; Pu, H.; Wei, Q. Bio-Interface Engineering of Mxene Nanosheets with Immobilized Lysozyme for Light-Enhanced Enzymatic Inactivation of Methicillin-Resistant Staphylococcus Aureus. Chem. Eng. J. 2023, 452, 139078. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Lian, L.; Liu, K.; Lu, M.; Chen, Y.; Zhang, L.; Zhang, X.; Wan, P. Flexible Accelerated-Wound-Healing Antibacterial Mxene-Based Epidermic Sensor for Intelligent Wearable Human-Machine Interaction. Adv. Funct. Mater. 2022, 32, 2208141. [Google Scholar] [CrossRef]
- Li, S.; Gu, B.; Li, X.; Tang, S.; Zheng, L.; Ruiz-Hitzky, E.; Sun, Z.; Xu, C.; Wang, X. Mxene-Enhanced Chitin Composite Sponges with Antibacterial and Hemostatic Activity for Wound Healing. Adv. Healthc. Mater. 2022, 11, 2102367. [Google Scholar] [CrossRef]
- Chidre, P.; Chavan, A.; Hulikunte Mallikarjunaiah, N.; Chandrakanth Revanasiddappa, K. Nanomaterials: Potential Broad Spectrum Antimicrobial Agents. Nat. Prod. Commun. 2023, 18, 1934578 × 221106904. [Google Scholar]
- Azmy, A.; Zhao, X.; Angeli, G.K.; Welton, C.; Raval, P.; Wojtas, L.; Zibouche, N.; Manjunatha Reddy, G.N.; Trikalitis, P.N.; Cai, J.; et al. One-Year Water-Stable and Porous Bi(Iii) Halide Semiconductor with Broad-Spectrum Antibacterial Performance. ACS Appl. Mater. Interfaces 2023, 15, 42717–42729. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, C.F.; Díaz-Barrios, A.; Campaña, K.O.; Romani, E.C.; Quiroz, F.; Nardecchia, S.; Debut, A.; Vizuete, K.; Niebieskikwiat, D.; Ávila, C.E.; et al. Broad-Spectrum Antimicrobial Znmintpc Encapsulated in Magnetic-Nanocomposites with Graphene Oxide/Mwcnts Based on Bimodal Action of Photodynamic and Photothermal Effects. Pharmaceutics 2022, 14, 705. [Google Scholar] [CrossRef]
- Ma, S.; Luo, X.; Ran, G.; Zhou, Z.; Xie, J.; Li, Y.; Li, X.; Yan, J.; Cai, W.; Wang, L. Copper Stabilized Bimetallic Alloy Cu–Bi by Convenient Strategy Fabrication: A Novel Fenton-Like and Photothermal Synergistic Antibacterial Platform. J. Clean. Prod. 2022, 336, 130431. [Google Scholar] [CrossRef]
- Kumar, S.; Aftab, S.; Singh, T.; Kumar, M.; Kumar, S.; Seo, Y. Charge Storage Improvement in Uniformly Grown TiO2 on Ti3C2Tx Mxene Surface. J. Alloys Compd. 2023, 968, 172181. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, Y.; Lin, L.; He, Q.; Hu, H.; Luo, R.; Xian, D.; Wu, J.; Shi, Y.; Zeng, F.; et al. Titanium Carbide Mxene-Based Hybrid Hydrogel for Chemo-Photothermal Combinational Treatment of Localized Bacterial Infection. Acta Biomater. 2022, 142, 113–123. [Google Scholar] [CrossRef]
- Shuck, C.E.; Sarycheva, A.; Anayee, M.; Levitt, A.; Zhu, Y.Z.; Uzun, S.; Balitskiy, V.; Zahorodna, V.; Gogotsi, O.; Gogotsi, Y. Scalable Synthesis of Ti3C2Tx Mxene. Adv. Eng. Mater. 2020, 22, 1901241. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A Review on Mxene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Lu, F.; Shi, D.; Tan, P.; Han, Y. A Novel Infrared Electrochromic Device Based on Ti3C2Tx Mxene. Chem. Eng. J. 2022, 450, 138324. [Google Scholar] [CrossRef]
- Gkogkos, G.; Panariello, L.; Grammenou, E.; Cornwell, M.A.; Afrashtehpour, A.; MacRobert, A.J.; Parkin, I.P.; Gavriilidis, A. Synthesis of PEG-PPG-PEG Templated Polydopamine Nanoparticles under Intensified Conditions: Kinetics Investigation, Continuous Process Design and Demonstration for Photothermal Application. Chem. Eng. J. 2023, 467, 143350. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Williams, M.D.; Aktuglu, M.; Falkinham, J.O., III; Ducker, W.A. Porous Antimicrobial Coatings for Killing Microbes within Minutes. ACS Appl. Mater. Interfaces 2023, 15, 15120–15128. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Batsalova, T.; Dzhambazov, B.; Naumkin, A. XPS Study of Silver and Copper Nanoparticles Demonstrated Selective Anticancer, Proapoptotic, and Antibacterial Properties. Surf. Interface Anal. 2022, 54, 189–202. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.G.; Yu, L.D.; Chen, Y.; Shi, J.L. Two-Dimensional Ultrathin Mxene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Liao, T.; Chen, Z.; Kuang, Y.; Ren, Z.; Yu, W.; Rao, W.; Li, L.; Liu, Y.; Xu, Z.; Jiang, B.; et al. Small-Size Ti3C2Tx Mxene Nanosheets Coated with Metal-Polyphenol Nanodots for Enhanced Cancer Photothermal Therapy and Anti-Inflammation. Acta Biomater. 2023, 159, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, X.; Feng, J.; Wang, D.; Obeng, E.; Yu, C.; Song, Y.; Shen, J.; Li, Z. Engineering a New Member of Mxenes M5C4 Phases Nanoplatforms as Synergistically Photothermal and Chemodynamic Therapeutics for Methicillin-Resistant Staphylococcus Aureus. Chem. Eng. J. 2023, 466, 143004. [Google Scholar] [CrossRef]
- Leem, J.; Vallez, L.; Gill, T.M.; Zheng, X. Machine Learning Assisted Analysis of Electrochemical H2O2 Production. ACS Appl. Energy Mater. 2023, 6, 3953–3959. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Ma, F.; Yang, X.; Liu, Y.; Zhang, X.; Guo, S.; Wang, Z.; Yang, S.; Zhao, R. Atomic Copper(I)-Carbon Nitride as a Peroxidase-Mimic Catalyst for High Selective Detection of Perfluorooctane Sulfonate. Chem. Eng. J. 2022, 435, 134966. [Google Scholar] [CrossRef]
- Liu, H.; Qu, X.; Tan, H.Q.; Song, J.L.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C.S. Role of Polydopamine’s Redox-Activity on Its Pro-Oxidant, Radical-Scavenging, and Antimicrobial Activities. Acta Biomater. 2019, 88, 181–196. [Google Scholar] [CrossRef]
- Roy, S.; Hasan, I.; Guo, B. Recent Advances in Nanoparticle-Mediated Antibacterial Applications. Coord. Chem. Rev. 2023, 482, 215075. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Nain, A.; Lin, Y.-F.; Tseng, Y.-T.; Li, Y.-J.; Sangili, A.; Srivastava, P.; Yu, H.-L.; Huang, Y.-F.; Huang, C.-C.; et al. Self-Redox Reaction Driven in Situ Formation of Cu2O/Ti3C2Tx Nanosheets Boost the Photocatalytic Eradication of Multi-Drug Resistant Bacteria from Infected Wound. J. Nanobiotech. 2022, 20, 235. [Google Scholar] [CrossRef] [PubMed]
- Borjihan, Q.; Meng, S.; Bai, H.; Chen, T.; Hu, X.; Xiao, D.; Shi, L.; Dong, A. Active Iodine Regulated in Cow Dung Biochar-Based Hydrogel Combined with PDT/PTT for MRSA Infected Wound Therapy. Mater. Design 2023, 231, 112051. [Google Scholar] [CrossRef]
- Awad, M.; Thomas, N.; Barnes, T.J.; Prestidge, C.A. Nanomaterials Enabling Clinical Translation of Antimicrobial Photodynamic Therapy. J. Control. Release 2022, 346, 300–316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).