Effect of Various Acid Solutions on the CO2 Dissolution Rate, Morphology, and Particle Size of Precipitated Calcium Carbonate Synthesized Using Seashells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
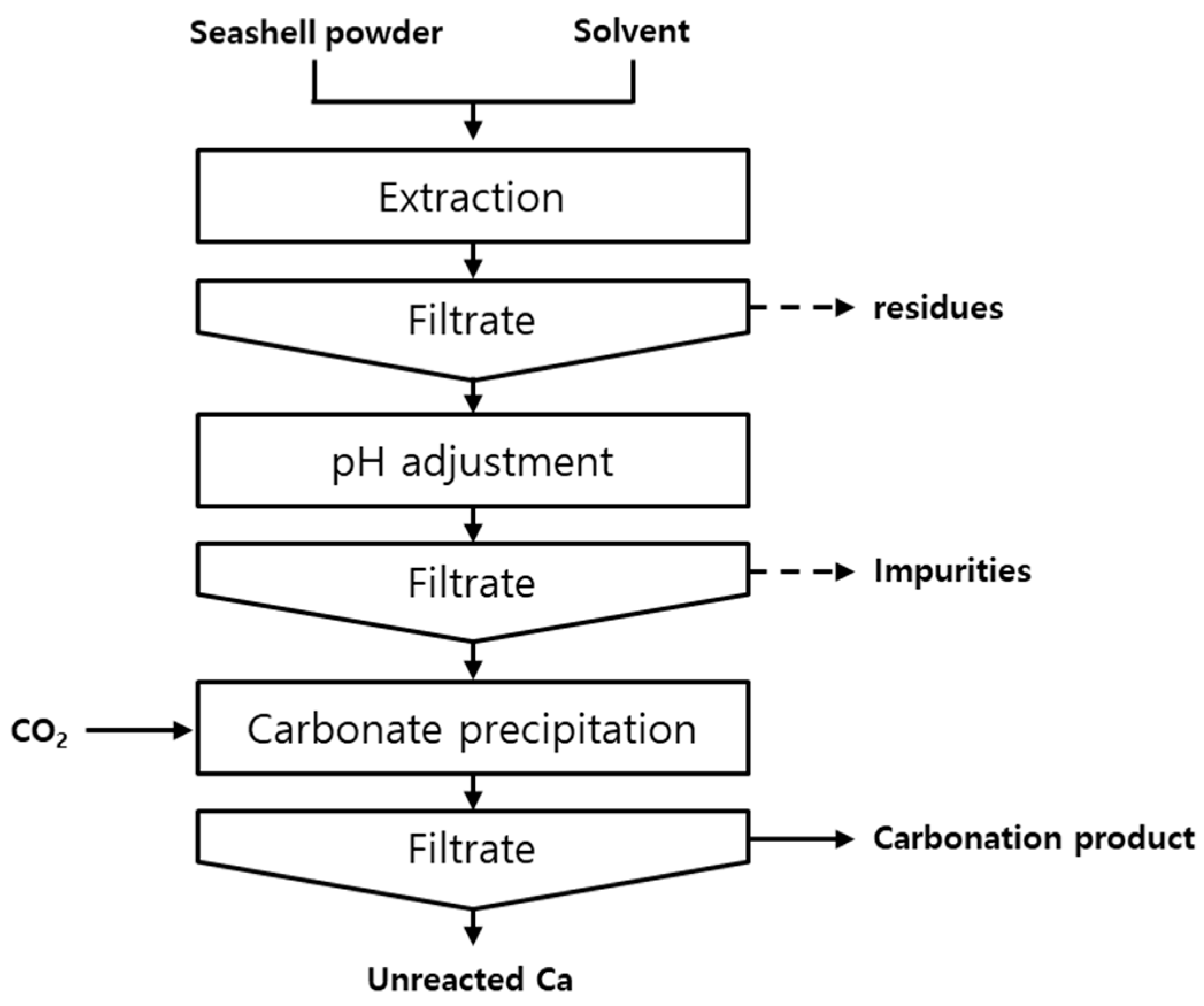
2.2. Methods
2.2.1. Dissolution
2.2.2. pH Adjustment
2.2.3. Synthesis of PCC
2.2.4. CO2 Dissolution Rate
2.3. Analysis
3. Results
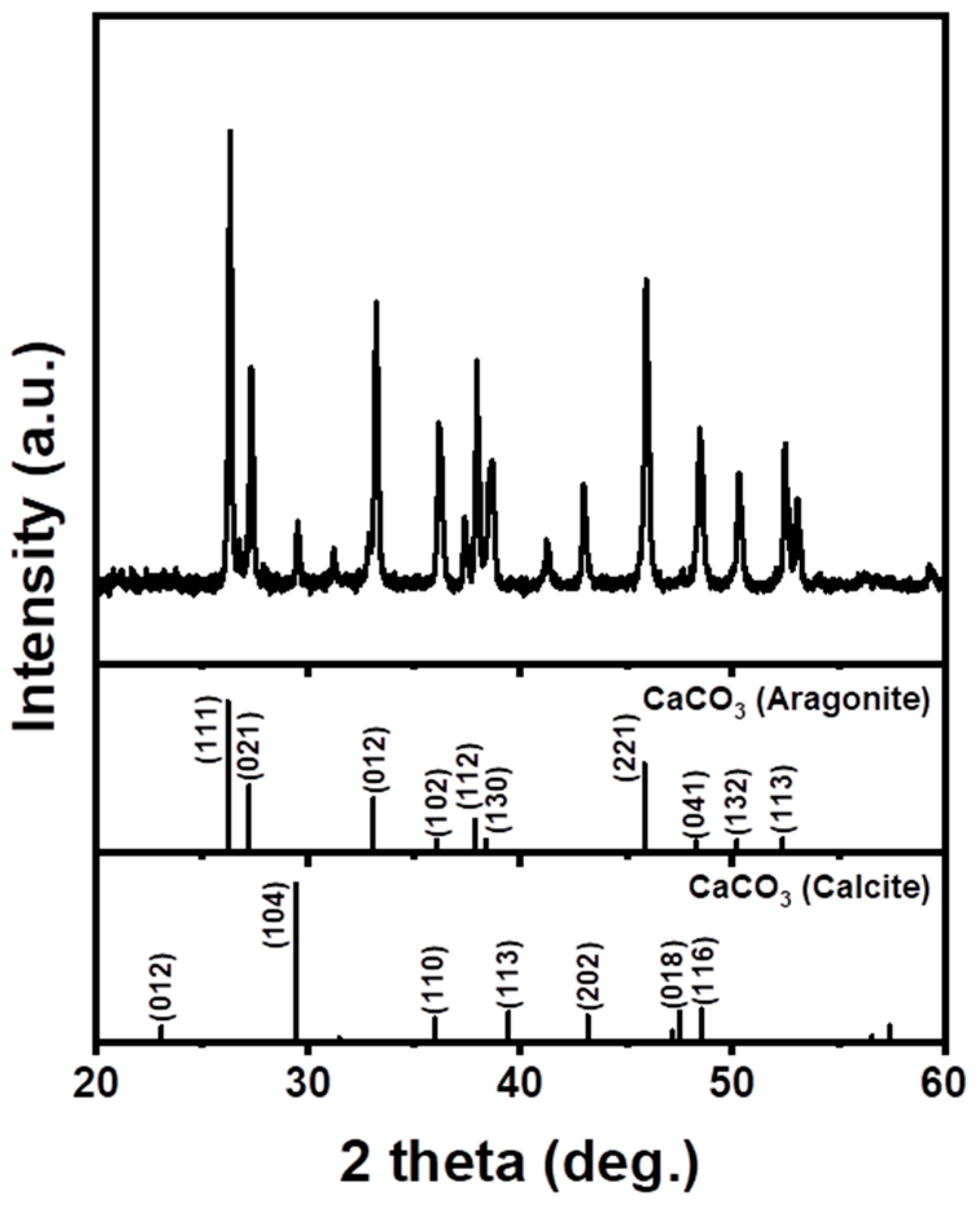
3.1. Seashell Analysis
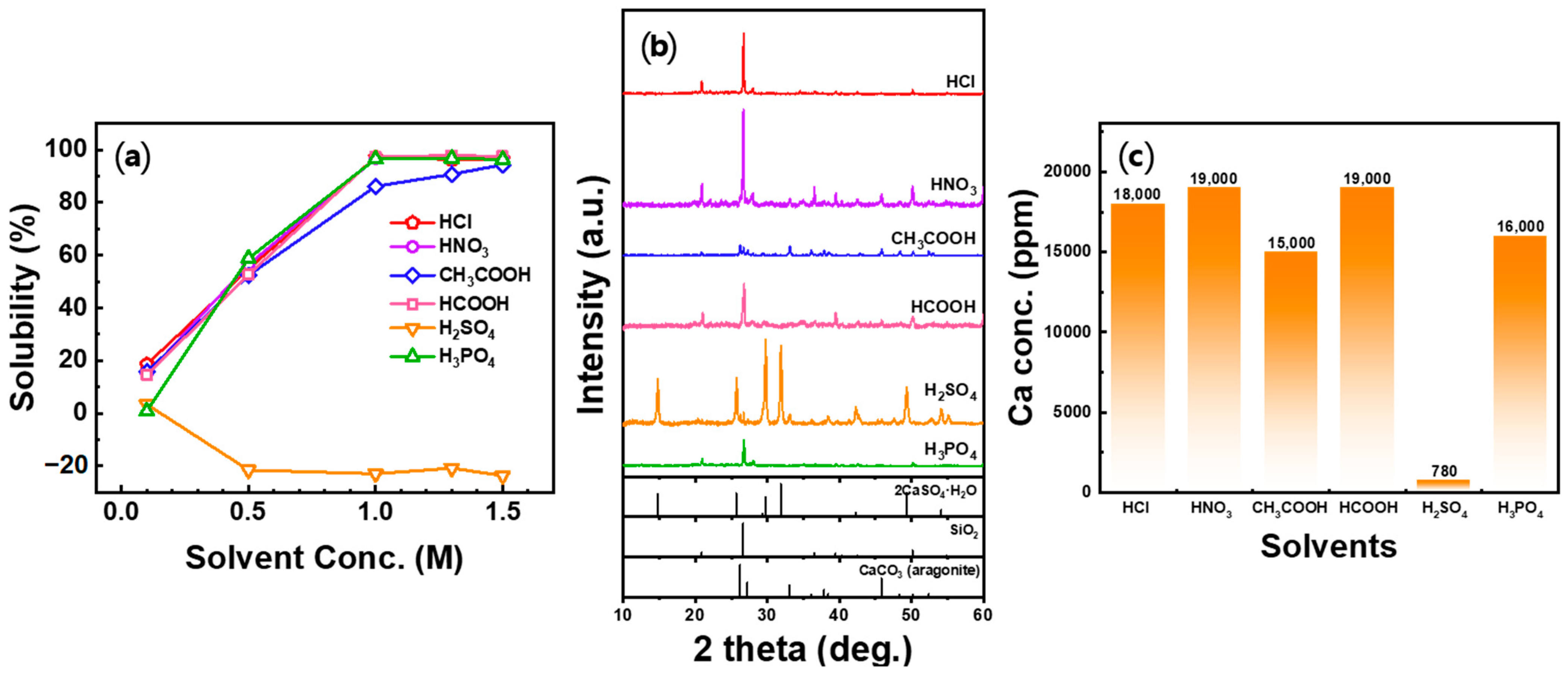
3.2. Ca2+ Dissolution from Seashell Powders
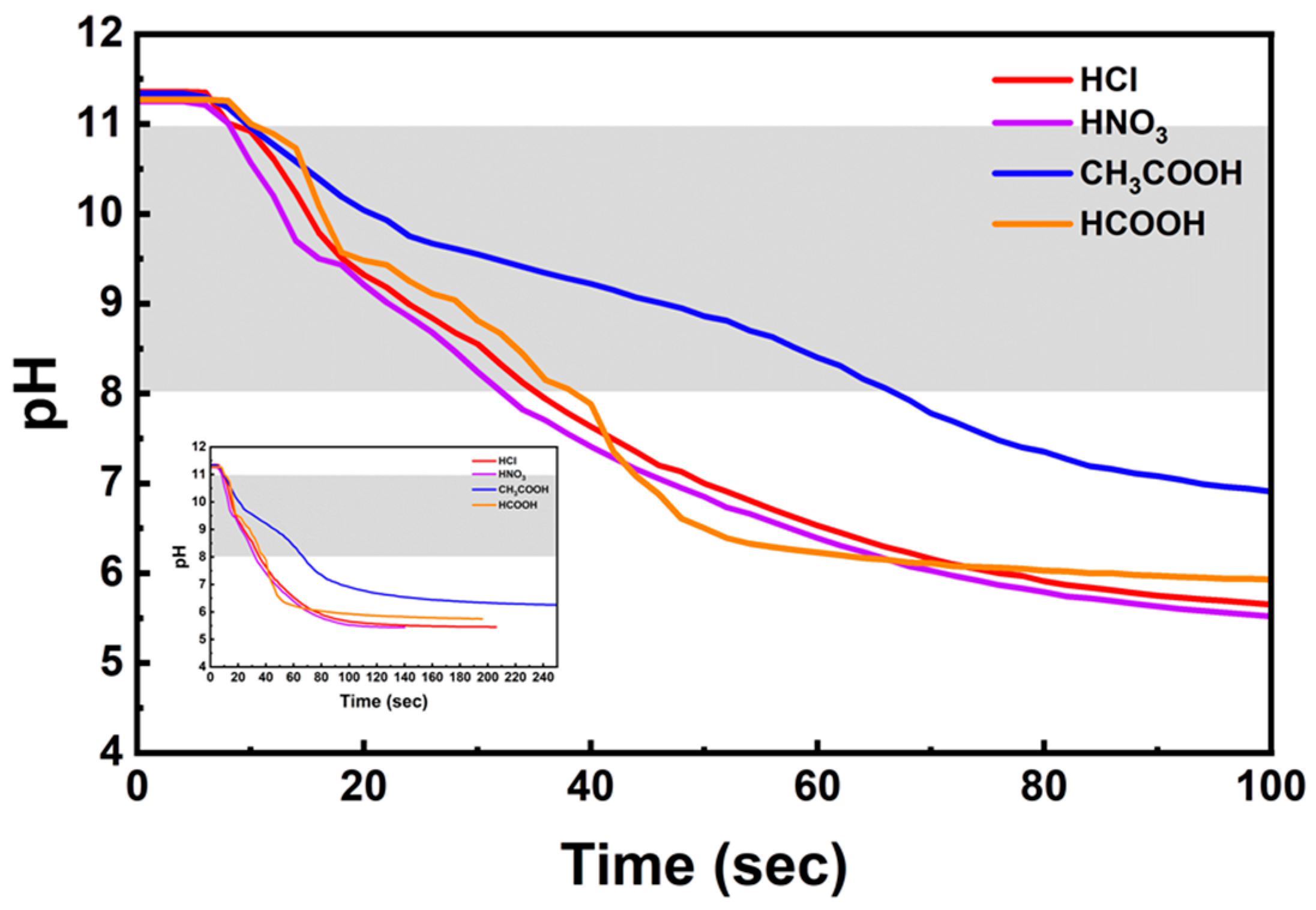
3.3. pH Adjustment
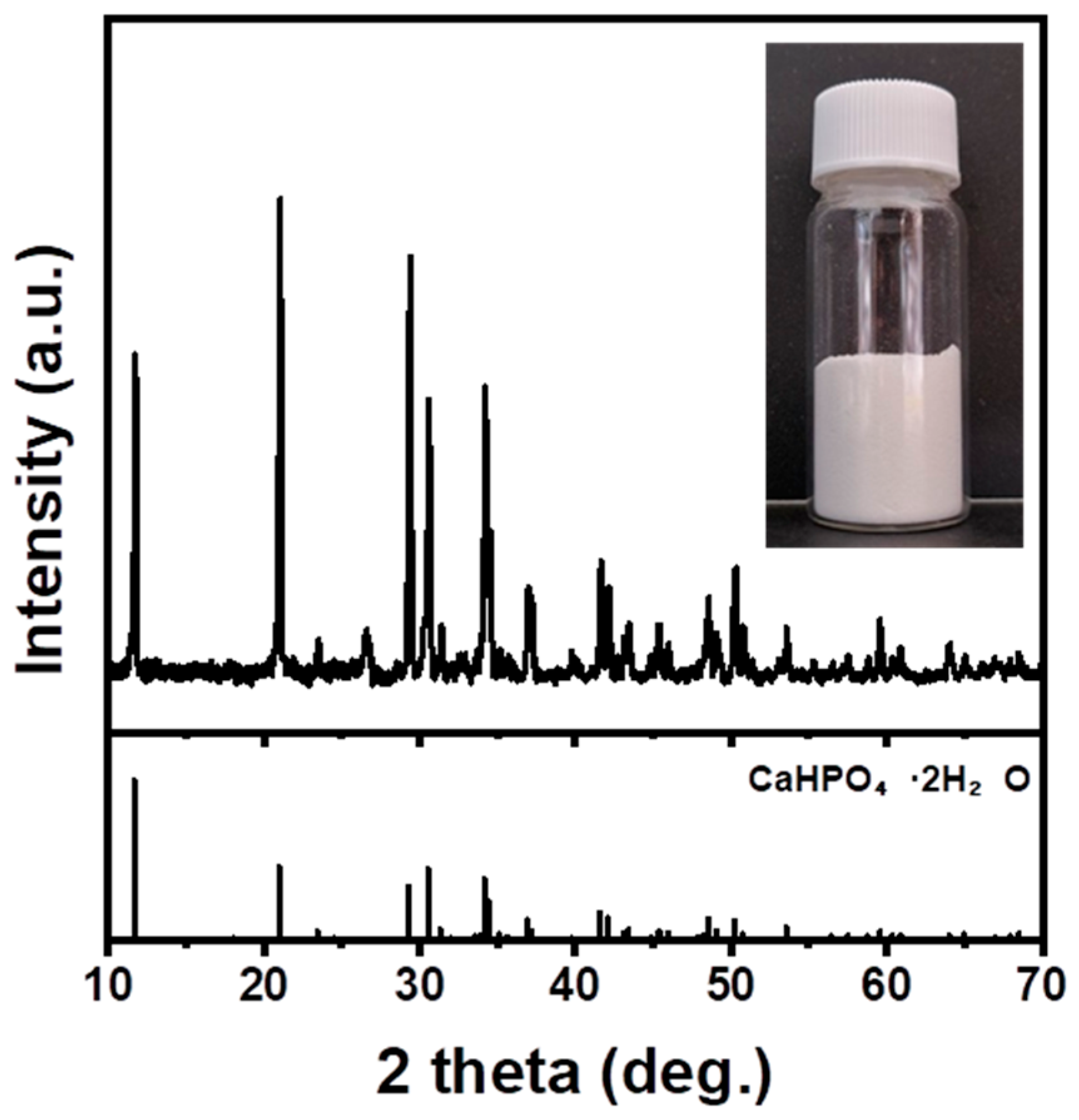
3.4. Carbonation
3.4.1. CO2 Dissolution Rate
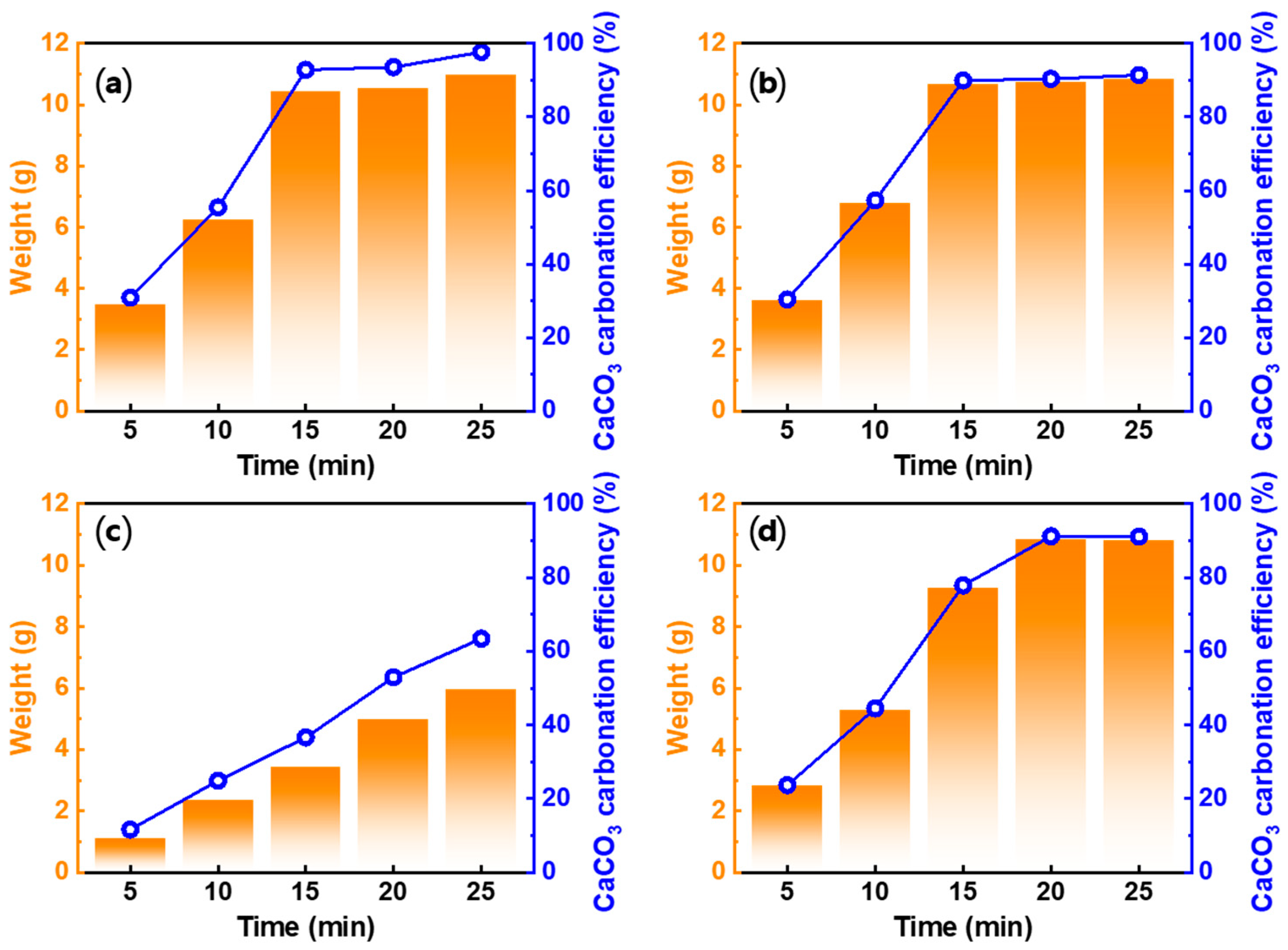
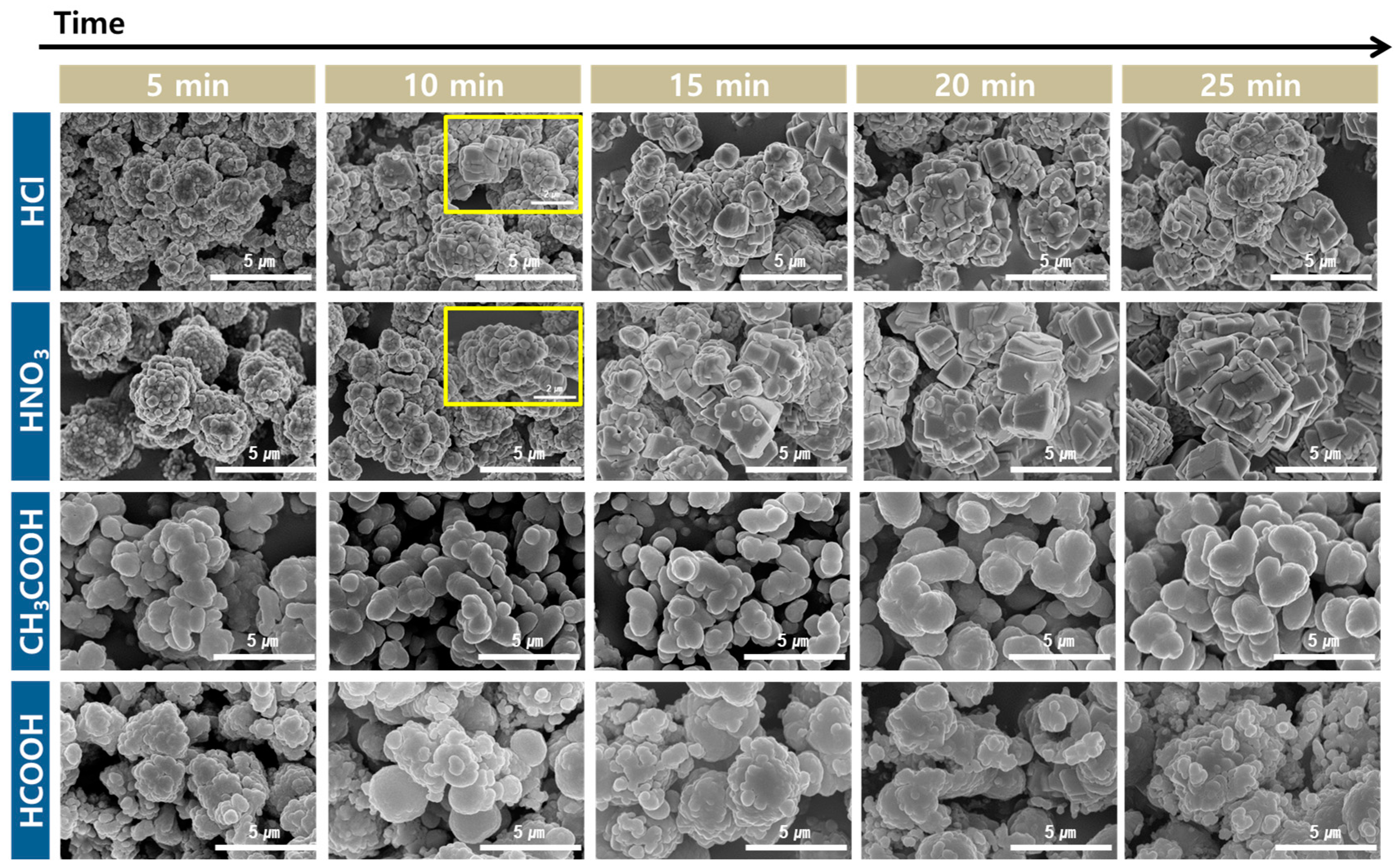
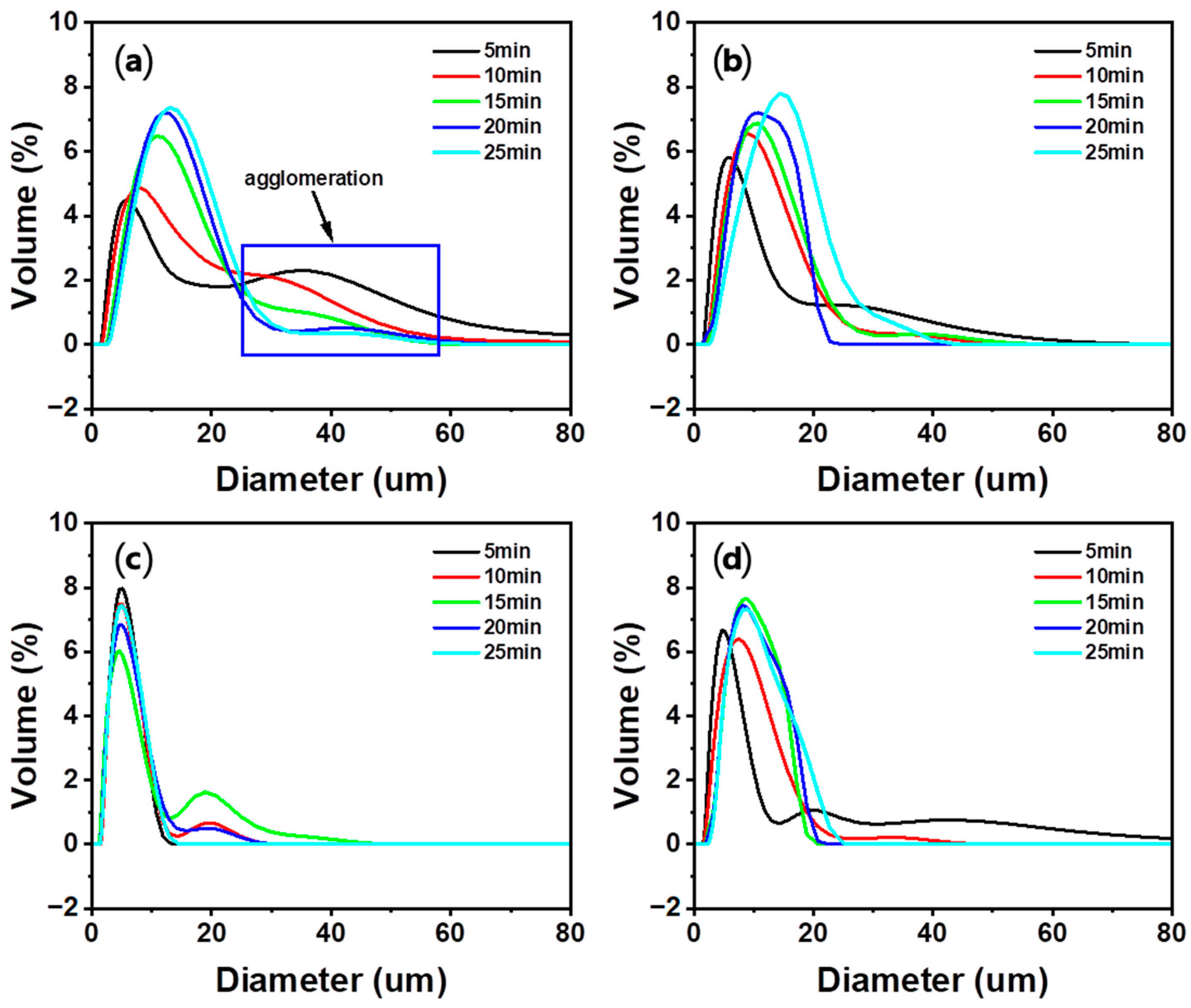
3.4.2. Effect of Carbonation Time
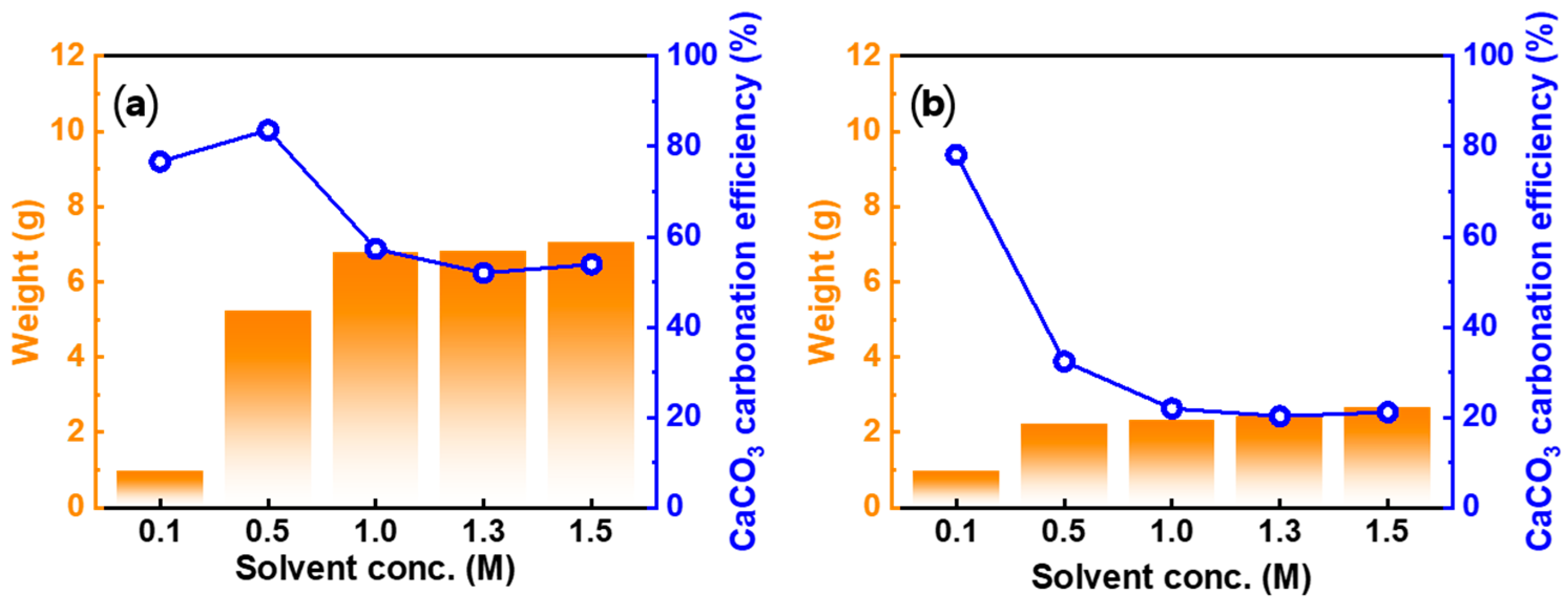
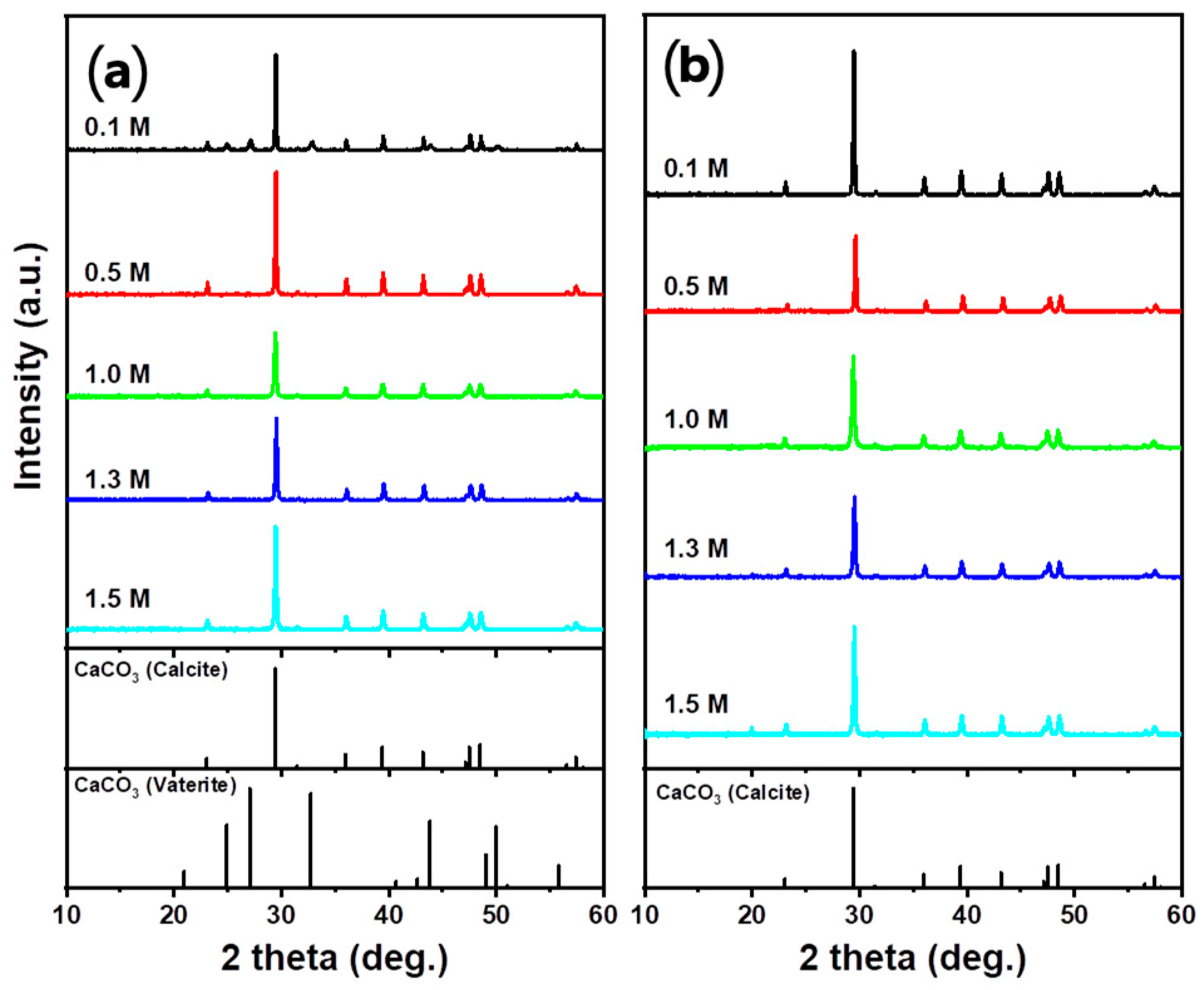
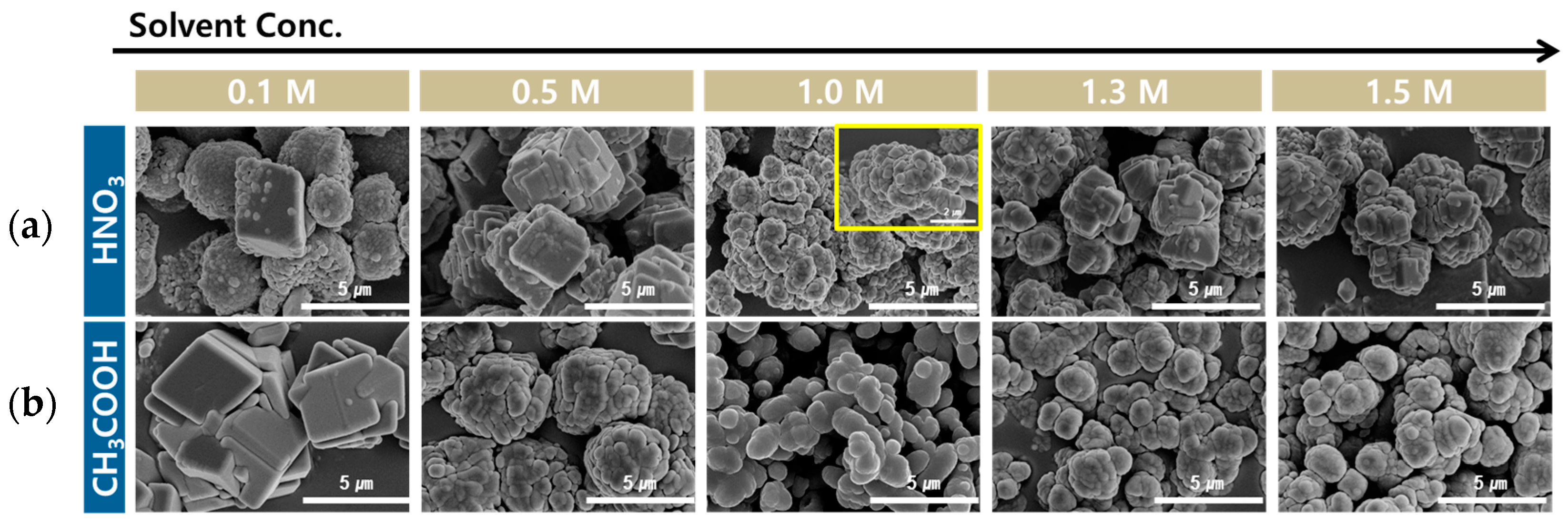
3.4.3. Effect of Acid Solution Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Lin, R.-S.; Wang, X.-Y. Effect of waste oyster shell powder on the properties of alkali-activated slag–waste ceramic geopolymers. J. Mater. Res. Technol. 2023, 22, 1768–1780. [Google Scholar] [CrossRef]
- Zhan, J.; Lu, J.; Wang, D. Review of shell waste reutilization to promote sustainable shellfish aquaculture. Rev. Aquac. 2022, 14, 477–488. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Nakatani, N.; Takamori, H.; Takeda, K.; Sakugawa, H. Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Bioresour. Technol. 2009, 100, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Cai, L.; Jiang, Y.; Han, Q. Eco-efficient, green, and scalable synthesis of 1, 2, 3-triazoles catalyzed by Cu (I) catalyst on waste oyster shell powders. ACS Sustain. Chem. Eng. 2014, 2, 765–771. [Google Scholar] [CrossRef]
- Yang, E.-I.; Yi, S.-T.; Leem, Y.-M. Effect of oyster shell substituted for fine aggregate on concrete characteristics: Part I. Fundamental properties. Cem. Concr. Res. 2005, 35, 2175–2182. [Google Scholar] [CrossRef]
- Lu, J.; Cong, X.; Li, Y.; Hao, Y.; Wang, C. Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. J. Clean. Prod. 2018, 172, 1978–1985. [Google Scholar] [CrossRef]
- Prihanto, A.; Muryanto, S.; Ismail, R.; Jamari, J.; Bayuseno, A. Utilization of green mussel shell waste for calcium carbonate synthesis through the carbonation method with temperature variation. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2022; p. 012022. [Google Scholar]
- Lim, J.; Cho, H.; Kim, J. Optimization of wet flue gas desulfurization system using recycled waste oyster shell as high-grade limestone substitutes. J. Clean. Prod. 2021, 318, 128492. [Google Scholar] [CrossRef]
- Lim, J.; Choi, Y.; Kim, G.; Kim, J. Modeling of the wet flue gas desulfurization system to utilize low-grade limestone. Korean J. Chem. Eng. 2020, 37, 2085–2093. [Google Scholar] [CrossRef]
- Yuliatun, L.; Kunarti, E.S.; Widjijono, W.; Nuryono, N. Preparation of Precipitated Calcium Carbonate (PCC) from Shells through Dissolution, Carbonation, and Sonication. J. Aceh Phys. Soc. 2023, 12, 27–31. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Thenepalli, T.; Han, C.; Ahn, J.-W. Synthesis of aragonite-precipitated calcium carbonate from oyster shell waste via a carbonation process and its applications. Korean J. Chem. Eng. 2017, 34, 225–230. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Nam, S.Y.; Kim, C.; Ahn, J.W. Extraction of precipitated calcium carbonate from oyster shell waste and its applications. J. Energy Eng. 2018, 27, 51–58. [Google Scholar]
- Said, A.; Mattila, H.-P.; Järvinen, M.; Zevenhoven, R. Production of precipitated calcium carbonate (PCC) from steelmaking slag for fixation of CO2. Appl. Energy 2013, 112, 765–771. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Zevenhoven, R. Production of precipitated calcium carbonate from calcium silicates and carbon dioxide. Energy Convers. Manag. 2005, 46, 2954–2979. [Google Scholar] [CrossRef]
- Bak, Y.-C. Effect of Agitation and Additive on the Vaterite Contents of Precipitated Calcium Carbonate from Oyster Shell Waste. Clean Technol. 2023, 29, 95–101. [Google Scholar]
- Wongkaewphothong, B.; Kertbundit, C.; Srichonphaisan, P.; Julapong, P.; Homchuen, P.; Juntarasakul, O.; Maneeintr, K.; Tabelin, C.B.; Numprasanthai, A.; Saisinchai, S. The improvement in whiteness index of calcite tailings using attrition-scrubbing process. Sep. Sci. Technol. 2023, 58, 2077–2085. [Google Scholar] [CrossRef]
- Shin, E.S.; Lee, S.J.; Cho, K.J.; Cho, S.B. Whiteness Improvement of the Limestone Based on Mineralogical Characteristics. In Proceedings of the Annual Joint Conference, Mineralogical Society of Korea and Petrological Society of Korea, Chuncheon, Republic of Korea, 28–29 May 2009; pp. 43–46. [Google Scholar]
- Song, Y.-J.; Park, C.-H.; Cho, D.-S. Factors Affecting the Property of CaCO3 Precipitated from CaCl2-Na2CO3-H2O System. Resour. Recycl. 1996, 5, 32–41. [Google Scholar]
- Xiao, C.; Zhu, L.; Zhang, X.; Gao, R.; He, S.; Lv, Z.; Hu, C. Elemental impurities in pediatric calcium carbonate preparations-high throughput quantification and risk assessment. Front. Chem. 2021, 9, 682798. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Donatan, S.; Volodkin, D.V.; Tessarolo, F.; Antolini, R.; Möhwald, H.; Skirtach, A.G. Macromolecule Loading into Spherical, Elliptical, Star-Like and Cubic Calcium Carbonate Carriers. ChemPhysChem 2014, 15, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Ulkeryildiz, E.; Kilic, S.; Ozdemir, E. Nano-CaCO3 synthesis by jet flow. Colloids Surf. A Physicochem. Eng. Asp. 2017, 512, 34–40. [Google Scholar] [CrossRef]
- Ulkeryildiz, E.; Kilic, S.; Ozdemir, E. Rice-like hollow nano-CaCO3 synthesis. J. Cryst. Growth 2016, 450, 174–180. [Google Scholar] [CrossRef]
- Pukanszky, B. Influence of interface interaction on the ultimate tensile properties of polymer composites. Composites 1990, 21, 255–262. [Google Scholar] [CrossRef]
- Pukanszky, B. Particulate filled polypropylene: Structure and properties. In Polypropylene Structure, Blends and Composites: Volume 3 Composites; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–70. [Google Scholar]
- Kiss, A.; Fekete, E.; Pukánszky, B. Aggregation of CaCO3 particles in PP composites: Effect of surface coating. Compos. Sci. Technol. 2007, 67, 1574–1583. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, T.-J.; Kim, H.-J. Effects of nano-sized calcium carbonate on physical and optical properties of paper. J. Korea Tech. Assoc. Pulp Pap. Ind. 2014, 46, 1–10. [Google Scholar] [CrossRef]
- Song, K.; Bang, J.-H.; Chae, S.-C.; Kim, J.; Lee, S.-W. Phase and morphology of calcium carbonate precipitated by rapid mixing in the absence of additives. RSC Adv. 2022, 12, 19340–19349. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.; Liu, Y.; Zheng, C.; Zhan, Y. Synthesis and characterization of aragonite whiskers by a novel and simple route. J. Mater. Chem. 2001, 11, 1752–1754. [Google Scholar] [CrossRef]
- Zhou, G.-T.; Jimmy, C.Y.; Wang, X.-C.; Zhang, L.-Z. Sonochemical synthesis of aragonite-type calcium carbonate with different morphologies. New J. Chem. 2004, 28, 1027–1031. [Google Scholar] [CrossRef]
- Siva, T.; Muralidharan, S.; Sathiyanarayanan, S.; Manikandan, E.; Jayachandran, M. Enhanced polymer induced precipitation of polymorphous in calcium carbonate: Calcite aragonite vaterite phases. J. Inorg. Organomet. Polym. Mater. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Wray, J.L.; Daniels, F. Precipitation of calcite and aragonite. J. Am. Chem. Soc. 1957, 79, 2031–2034. [Google Scholar] [CrossRef]
- Littlewood, J.L.; Shaw, S.; Peacock, C.L.; Bots, P.; Trivedi, D.; Burke, I.T. Mechanism of enhanced strontium uptake into calcite via an amorphous calcium carbonate crystallization pathway. Cryst. Growth Des. 2017, 17, 1214–1223. [Google Scholar] [CrossRef]
- Kilic, S.; Toprak, G.; Ozdemir, E. Stability of CaCO3 in Ca(OH)2 solution. Int. J. Miner. Process. 2016, 147, 1–9. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-W.; Chae, S.; Bang, J.-H.; Lee, S.-W. CO2 sequestration technology through mineral carbonation: An extraction and carbonation of blast slag. J. CO2 Util. 2016, 16, 336–345. [Google Scholar] [CrossRef]
- Mun, M.; Cho, H.; Kwon, J. Study on characteristics of various extractants for mineral carbonation of industrial wastes. J. Environ. Chem. Eng. 2017, 5, 3803–3821. [Google Scholar] [CrossRef]
- Jo, H.; Young Jo, H.; Jang, Y.-N. Effect of extraction solutions on carbonation of cementitious materials in aqueous solutions. Environ. Technol. 2012, 33, 1391–1401. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Junin, R.; Hamidi, H.; Manan, M.; Daud, A.R.M. Mineral carbonation of red gypsum via pH-swing process: Effect of CO2 pressure on the efficiency and products characteristics. Chem. Eng. J. 2015, 264, 425–436. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Junin, R.; Mohammadian, E.; Hamidi, H.; Daud, A.R.M.; Manan, M. Extraction of calcium from red gypsum for calcium carbonate production. Fuel Process. Technol. 2015, 130, 12–19. [Google Scholar] [CrossRef]
- Rahmani, O. An experimental study of accelerated mineral carbonation of industrial waste red gypsum for CO2 sequestration. J. CO2 Util. 2020, 35, 265–271. [Google Scholar] [CrossRef]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, H.; Li, P.; Li, Y.; Yan, B. Preparation of CaMgAl-LDHs and mesoporous silica sorbents derived from blast furnace slag for CO2 capture. RSC Adv. 2019, 9, 6054–6063. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Wang, D.; Wang, Z.; Yu, Q. Hydrometallurgical leaching behavior and kinetic modeling of blast furnace slag in hydrochloric acid. J. Sustain. Metall. 2022, 8, 170–185. [Google Scholar] [CrossRef]
- Ferrufino, G.L.A.A.; Okamoto, S.; Dos Santos, J.C.; de Carvalho, J.A., Jr.; Avila, I.; Luna, C.M.R.; Neto, T.G.S. CO2 sequestration by pH-swing mineral carbonation based on HCl/NH4OH system using iron-rich lizardite 1T. J. CO2 Util. 2018, 24, 164–173. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Maroto-Valer, M. Carbon dioxide capture and storage by pH swing aqueous mineralisation using a mixture of ammonium salts and antigorite source. Fuel 2013, 114, 153–161. [Google Scholar] [CrossRef]
- Wada, N.; Kanamura, K.; Umegaki, T. Effects of carboxylic acids on the crystallization of calcium carbonate. J. Colloid Interface Sci. 2001, 233, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-Q.; Liu, J.-H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.-H. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, L.; Feng, L.; He, Q.; Ji, L.; Yan, S. Insights into dual functions of amino acid salts as CO2 carriers and CaCO3 regulators for integrated CO2 absorption and mineralisation. J. CO2 Util. 2021, 48, 101531. [Google Scholar] [CrossRef]
- Grassmann, O.; Löbmann, P. Biomimetic nucleation and growth of CaCO3 in hydrogels incorporating carboxylate groups. Biomaterials 2004, 25, 277–282. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, J.-W.; Park, H.-S.; Park, C.-H. Synthesis peculiarity of the precipitated calcium carbonate polymorphs following variation of supersaturation in Ca(OH)2 and Na2CO3 reaction system. Geosystem Eng. 2004, 7, 95–102. [Google Scholar] [CrossRef]
- Hernández-Hernández, A.; Rodríguez-Navarro, A.B.; Gómez-Morales, J.; Jiménez-López, C.; Nys, Y.; García-Ruiz, J.M. Influence of model globular proteins with different isoelectric points on the precipitation of calcium carbonate. Cryst. Growth Des. 2008, 8, 1495–1502. [Google Scholar] [CrossRef]
- Kosanović, C.; Fermani, S.; Falini, G.; Kralj, D. Crystallization of calcium carbonate in alginate and xanthan hydrogels. Crystals 2017, 7, 355. [Google Scholar] [CrossRef]
- Mei, X.; Zhao, Q.; Li, Y.; Min, Y.; Liu, C.; Saxén, H.; Zevenhoven, R. Phase transition and morphology evolution of precipitated calcium carbonate (PCC) in the CO2 mineralization process. Fuel 2022, 328, 125259. [Google Scholar] [CrossRef]
- Yan, X.; Cui, H.; Qin, Q.; Tang, W.; Zhou, X. Study on utilization of carboxyl group decorated carbon nanotubes and carbonation reaction for improving strengths and microstructures of cement paste. Nanomaterials 2016, 6, 153. [Google Scholar] [CrossRef]
- Gao, C.; Dong, Y.; Zhang, H.; Zhang, J. Utilization of distiller waste and residual mother liquor to prepare precipitated calcium carbonate. J. Clean. Prod. 2007, 15, 1419–1425. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Spanos, N.; Koutsoukos, P.G. The transformation of vaterite to calcite: Effect of the conditions of the solutions in contact with the mineral phase. J. Cryst. Growth 1998, 191, 783–790. [Google Scholar] [CrossRef]
- Babou-Kammoe, R.; Hamoudi, S.; Larachi, F.; Belkacemi, K. Synthesis of CaCO3 nanoparticles by controlled precipitation of saturated carbonate and calcium nitrate aqueous solutions. Can. J. Chem. Eng. 2012, 90, 26–33. [Google Scholar] [CrossRef]
- Bang, J.-H.; Jang, Y.N.; Kim, W.; Song, K.S.; Jeon, C.W.; Chae, S.C.; Lee, S.-W.; Park, S.-J.; Lee, M.G. Specific surface area and particle size of calcium carbonate precipitated by carbon dioxide microbubbles. Chem. Eng. J. 2012, 198, 254–260. [Google Scholar] [CrossRef]
- Shen, Y.; Hao, S.; Suonan, A.; Liu, Y.; Li, H.; Ma, W.; Zhao, L.; Zhang, Y. Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering. Crystals 2023, 13, 1432. [Google Scholar] [CrossRef]
- Yang, J.-H.; Shih, S.-M.; Wu, C.-I.; Yi-Der Tai, C. Preparation of high surface area CaCO3 for SO2 removal by absorption of CO2 in aqueous suspensions of Ca(OH)2. Powder Technol. 2010, 202, 101–110. [Google Scholar] [CrossRef]


| Ca | Si | Na | Al | Fe | Cl | Etc. | |
|---|---|---|---|---|---|---|---|
| Seashell powder | 94.41 | 1.86 | 0.86 | 0.85 | 0.61 | 0.53 | 0.88 |
| HCl | HNO3 | HCOOH | CH3COOH | |||||
|---|---|---|---|---|---|---|---|---|
| Fe | Al | Fe | Al | Fe | Al | Fe | Al | |
| Before | 5.48 | 5.22 | 8 | 12 | 0.95 | 3.5 | - | 1.9 |
| After (pH 11) | - | 0.83 | - | 0.53 | - | - | - | - |
| HCl | HNO3 | CH3COOH | HCOOH | |
|---|---|---|---|---|
| Ca | 97.86 | 97.43 | 99.26 | 99.04 |
| Si | 0.09 | 0.06 | 0.08 | 0.09 |
| Na | - | - | - | - |
| Al | 1.72 | 2.23 | 0.27 | 0.64 |
| Fe | - | - | - | - |
| Sr | - | 0.12 | 0.16 | 0.12 |
| Cl | 0.12 | - | - | - |
| etc. | 0.21 | 0.16 | 0.23 | 0.10 |
| pH/s | |
|---|---|
| HCl | 0.113 |
| HNO3 | 0.122 |
| CH3COOH | 0.052 |
| HCOOH | 0.107 |
| HCl | HNO3 | CH3COOH | HCOOH | |
|---|---|---|---|---|
| 5 min | 8.31 | 6.67 | 4.93 | 5.59 |
| 10 min | 9.46 | 9.06 | 5.06 | 7.23 |
| 15 min | 11.11 | 9.96 | 4.91 | 8.64 |
| 20 min | 11.71 | 10.12 | 5.03 | 8.74 |
| 25 min | 12.41 | 12.95 | 4.98 | 9.02 |
| HNO3 | CH3COOH | |
|---|---|---|
| 0.1 M | 13.93 | 15.99 |
| 0.5 M | 9.13 | 7.64 |
| 1.0 M | 9.06 | 5.06 |
| 1.3 M | 9.43 | 6.70 |
| 1.5 M | 9.96 | 6.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.J.; Lee, S.; Kim, Y.; Ryu, Y.B. Effect of Various Acid Solutions on the CO2 Dissolution Rate, Morphology, and Particle Size of Precipitated Calcium Carbonate Synthesized Using Seashells. Materials 2023, 16, 7665. https://doi.org/10.3390/ma16247665
Yun YJ, Lee S, Kim Y, Ryu YB. Effect of Various Acid Solutions on the CO2 Dissolution Rate, Morphology, and Particle Size of Precipitated Calcium Carbonate Synthesized Using Seashells. Materials. 2023; 16(24):7665. https://doi.org/10.3390/ma16247665
Chicago/Turabian StyleYun, Yu Jeong, Siwoo Lee, Yangdo Kim, and Young Bok Ryu. 2023. "Effect of Various Acid Solutions on the CO2 Dissolution Rate, Morphology, and Particle Size of Precipitated Calcium Carbonate Synthesized Using Seashells" Materials 16, no. 24: 7665. https://doi.org/10.3390/ma16247665
APA StyleYun, Y. J., Lee, S., Kim, Y., & Ryu, Y. B. (2023). Effect of Various Acid Solutions on the CO2 Dissolution Rate, Morphology, and Particle Size of Precipitated Calcium Carbonate Synthesized Using Seashells. Materials, 16(24), 7665. https://doi.org/10.3390/ma16247665






