Microstructure, Mechanical Properties, and Corrosion Resistance of Ag–Cu Alloys with La2O3 Fabricated by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Methods
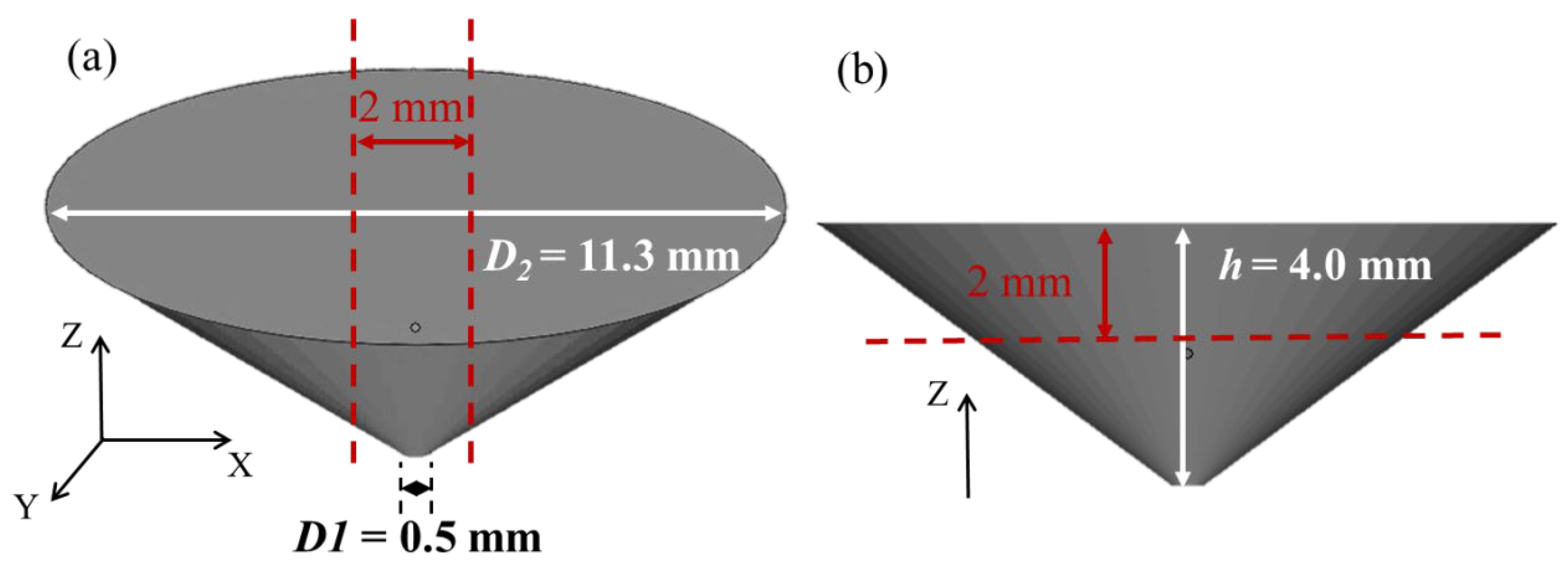
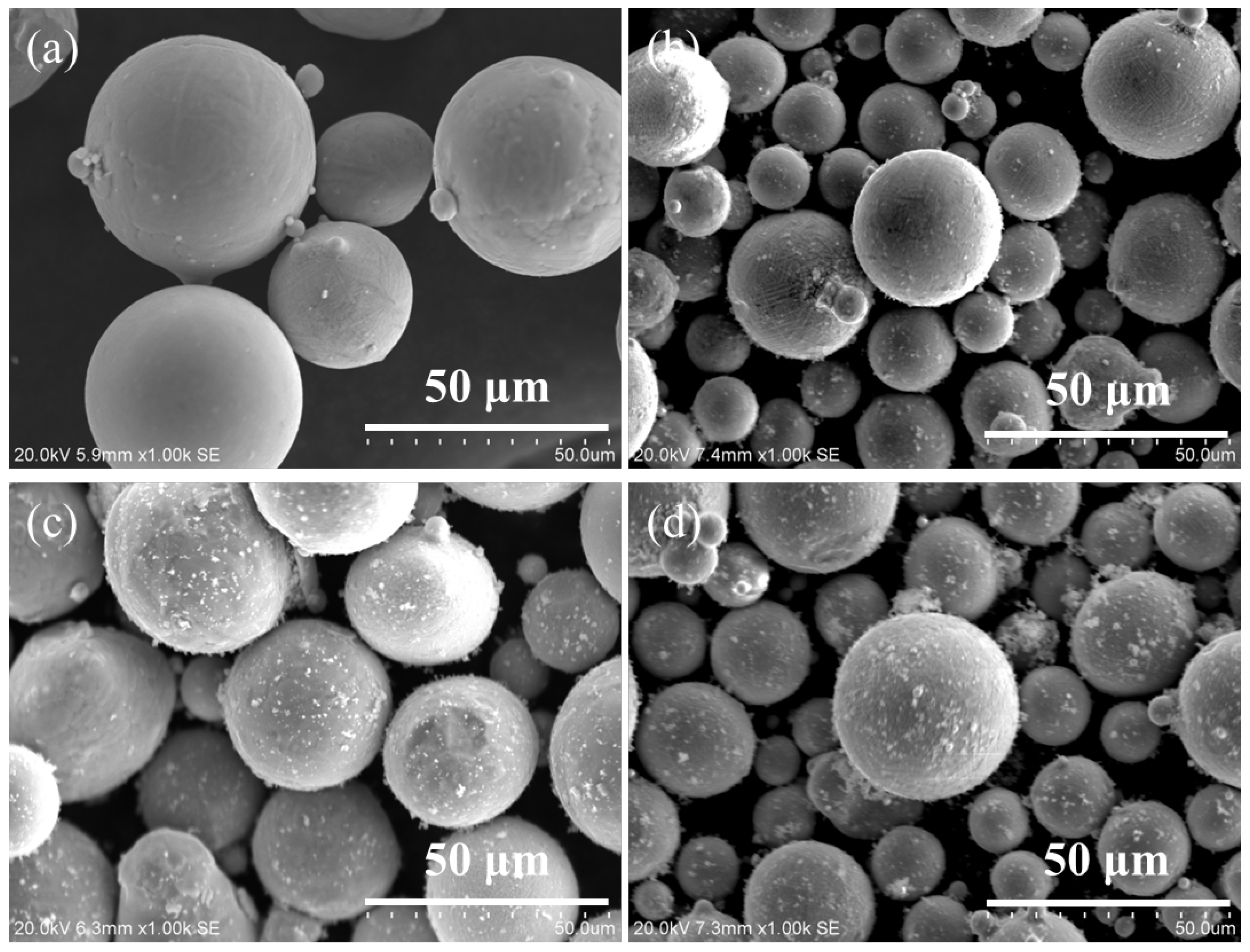
2.1. Preparation of Ag–Cu Alloys with Different La2O3 Contents by SLM
2.2. Characterizations
2.3. Mechanical Properties
2.4. Corrosion Behavior in Na2S Solution
3. Results and Discussion
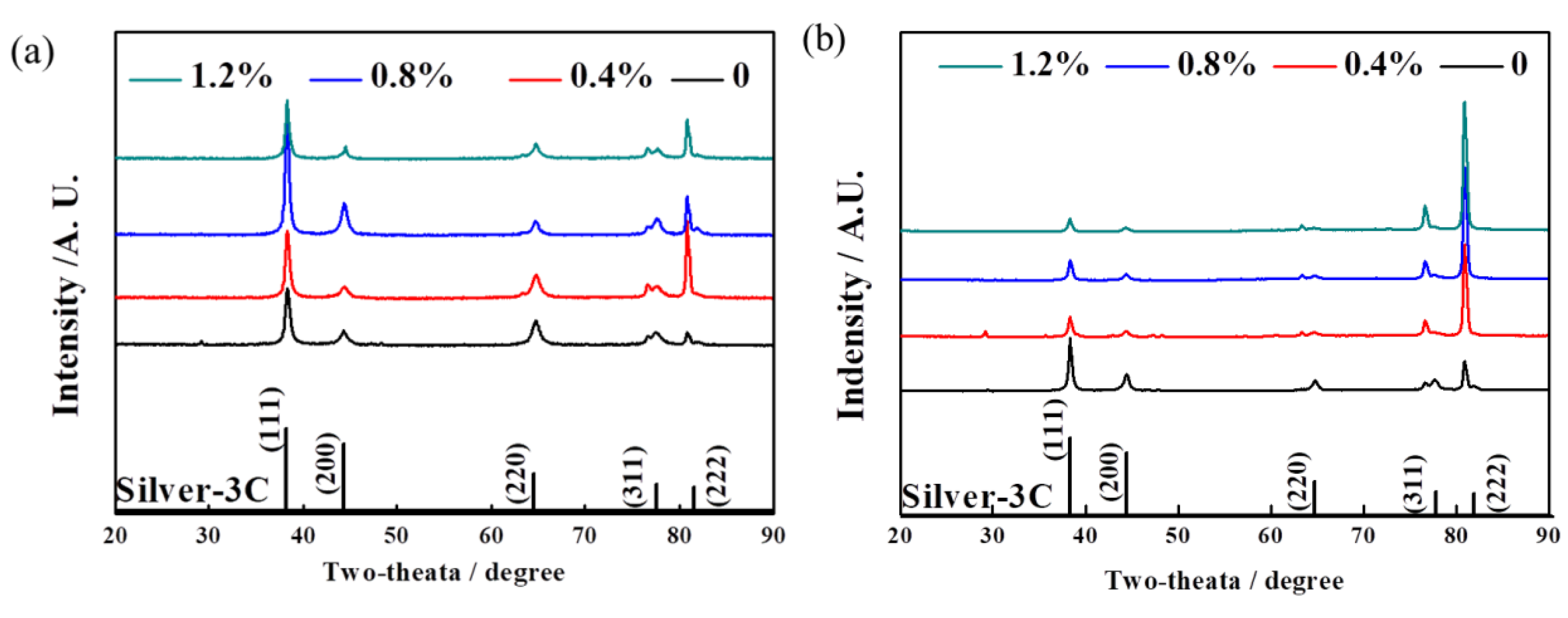
3.1. The Effect of La2O3 Contents on the Densities and Phase Compositions of Ag–Cu Samples Prepared by SLM
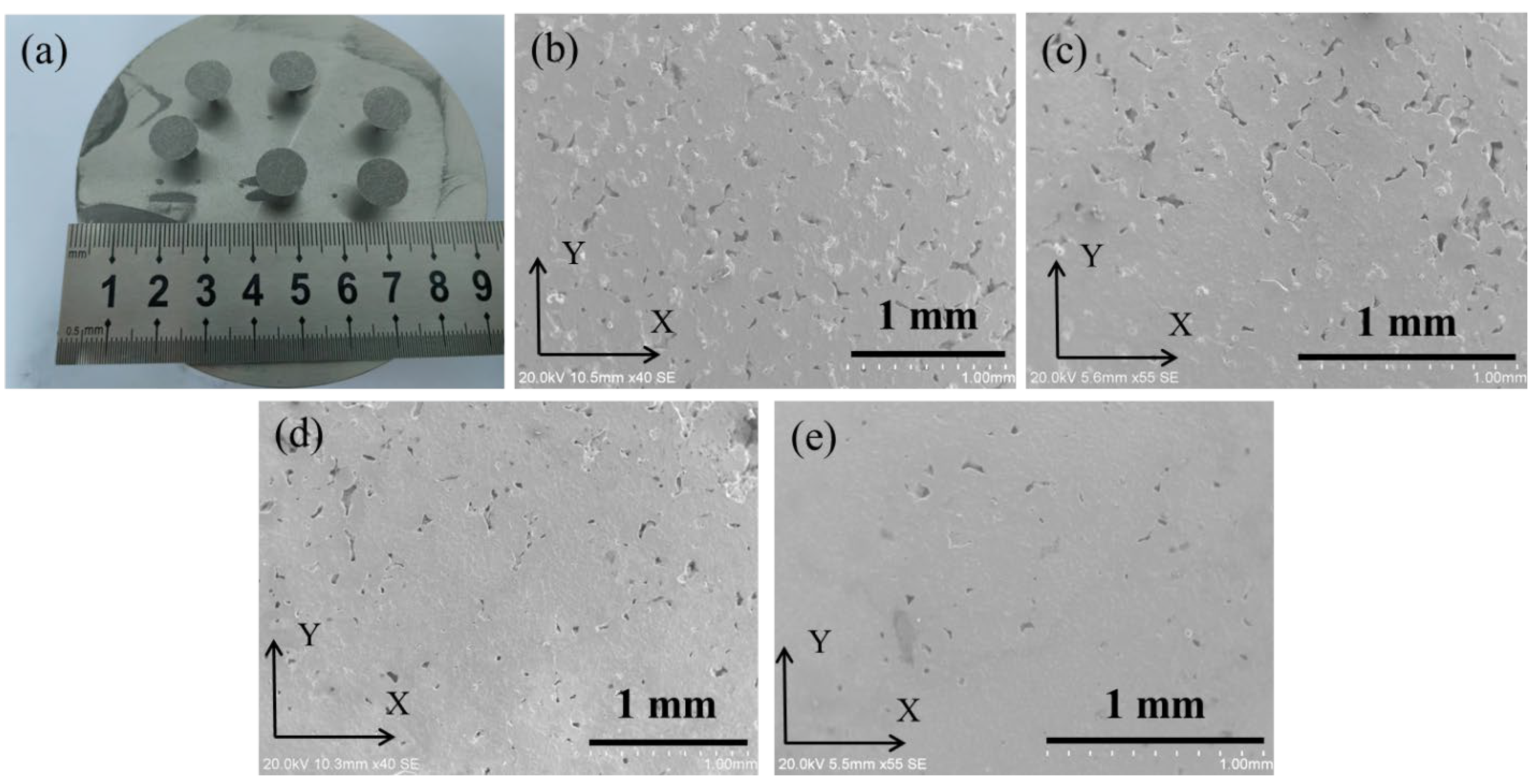
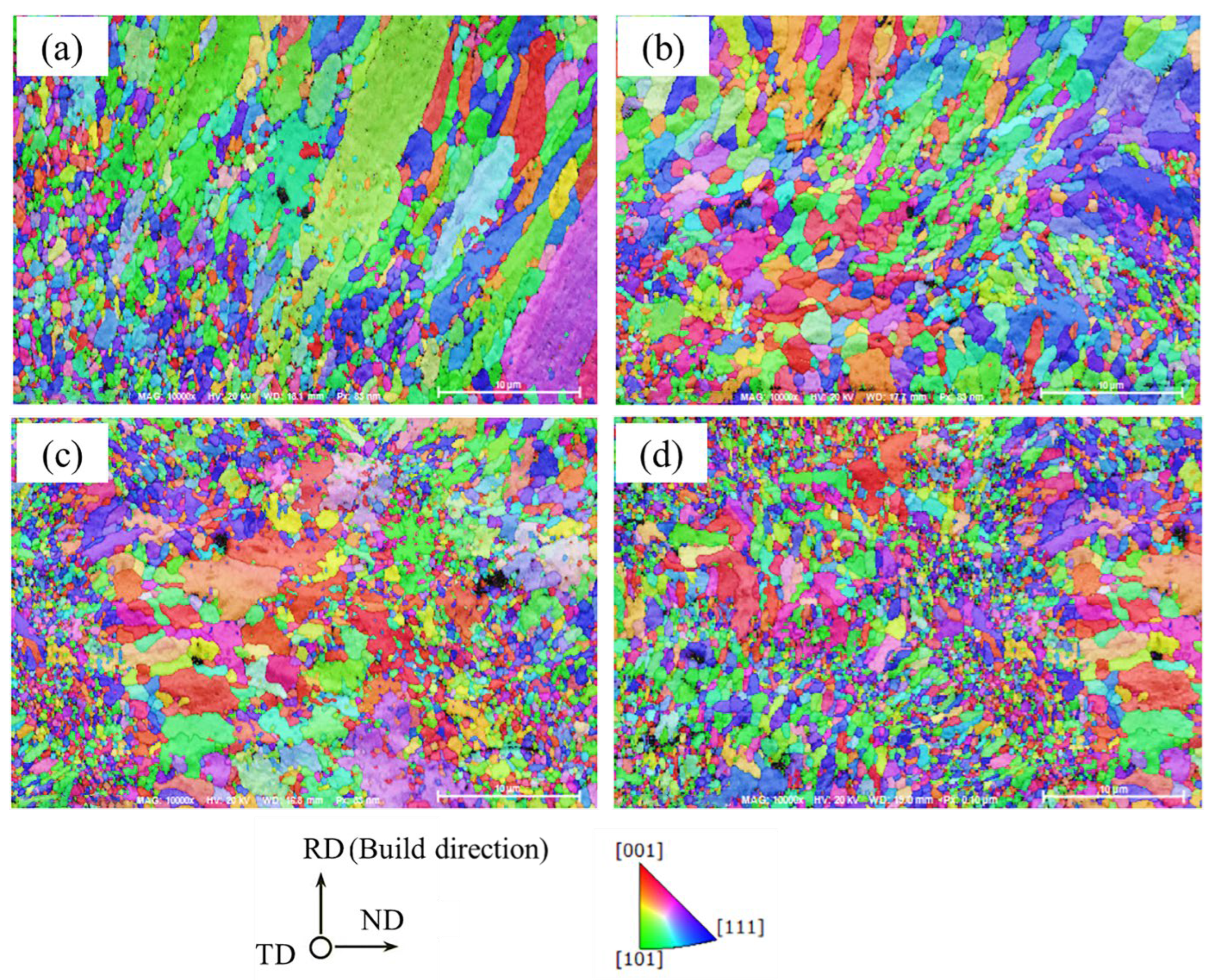
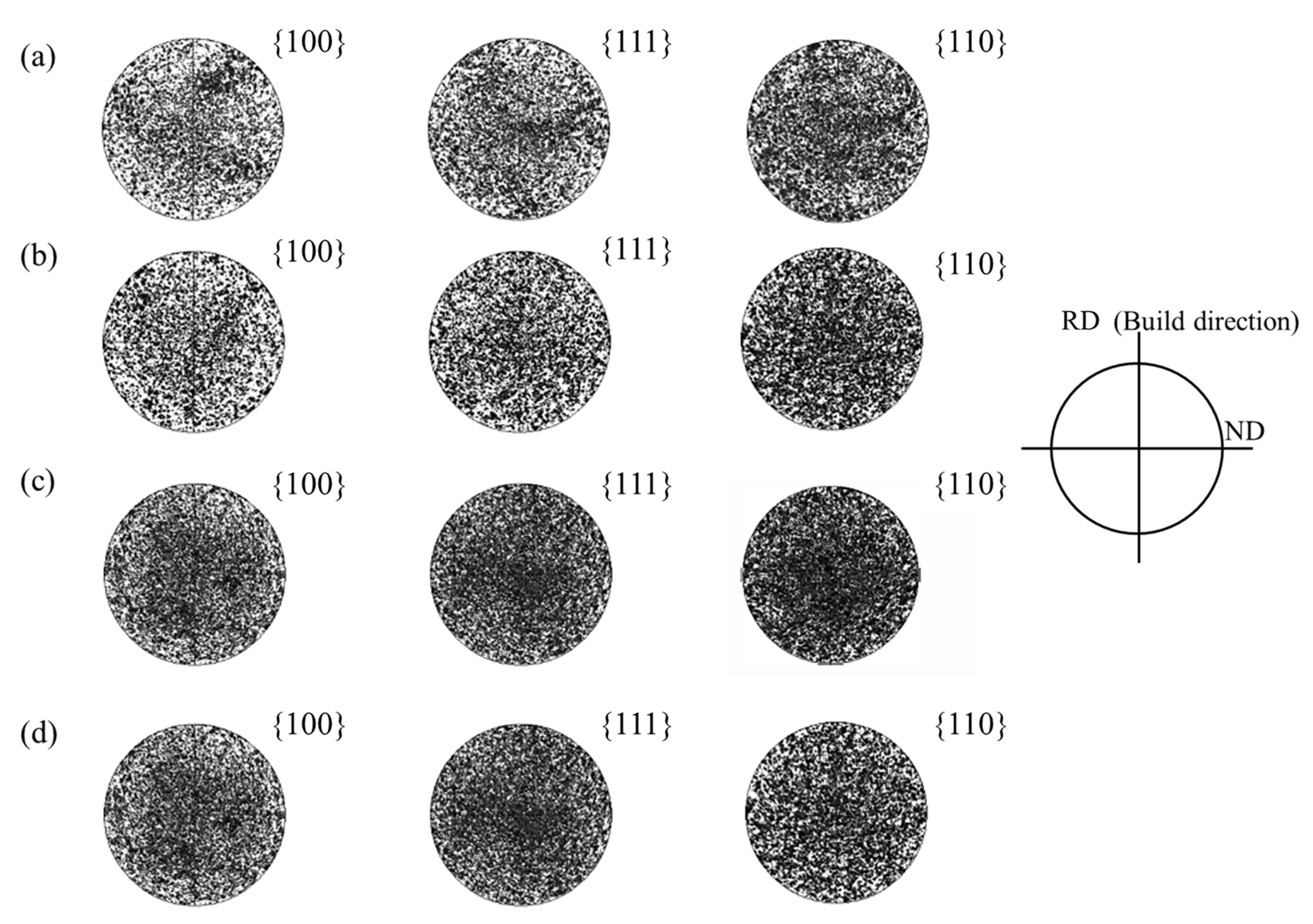
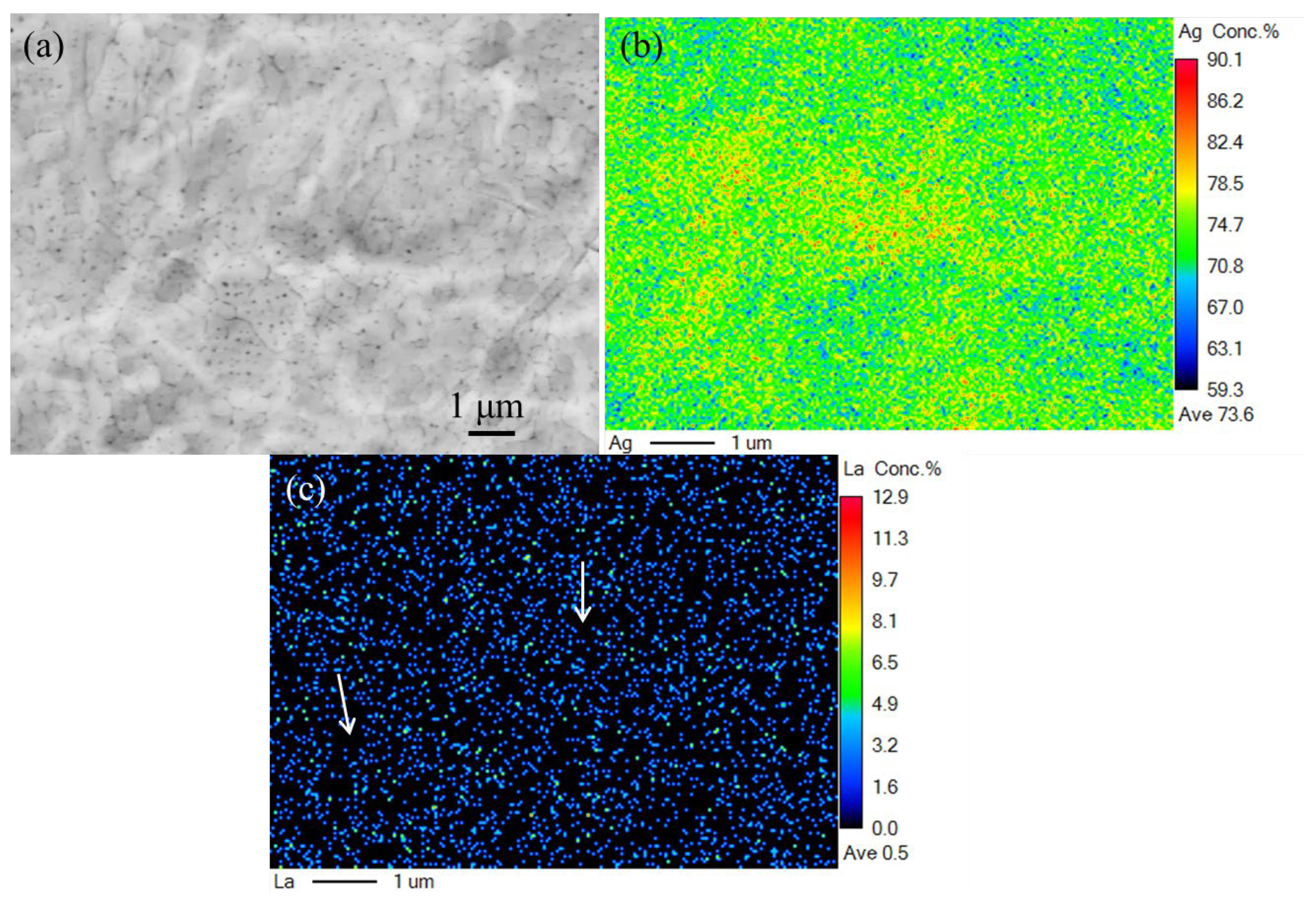
3.2. The Effect of La2O3 Content on the Microstructure of Ag–Cu Samples Prepared by SLM
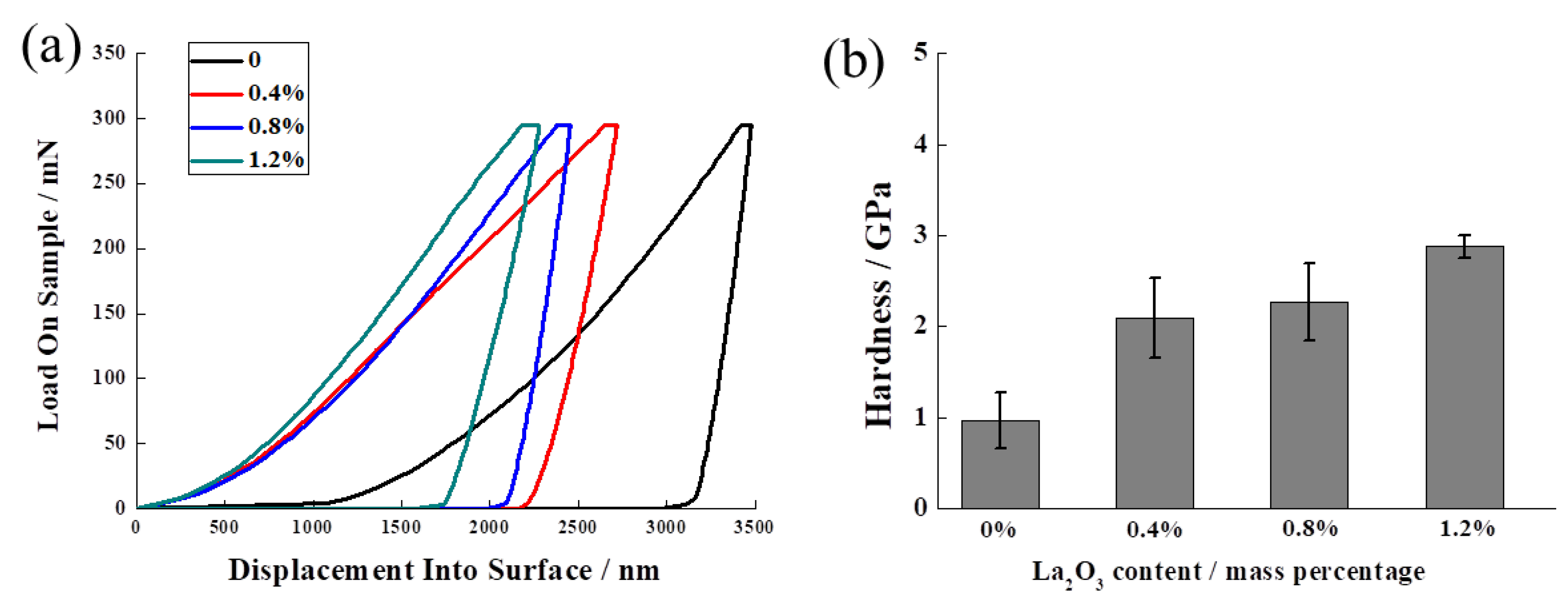
3.3. The Effect of La2O3 Content on the Mechanical Properties of Ag–Cu Samples Prepared by SLM
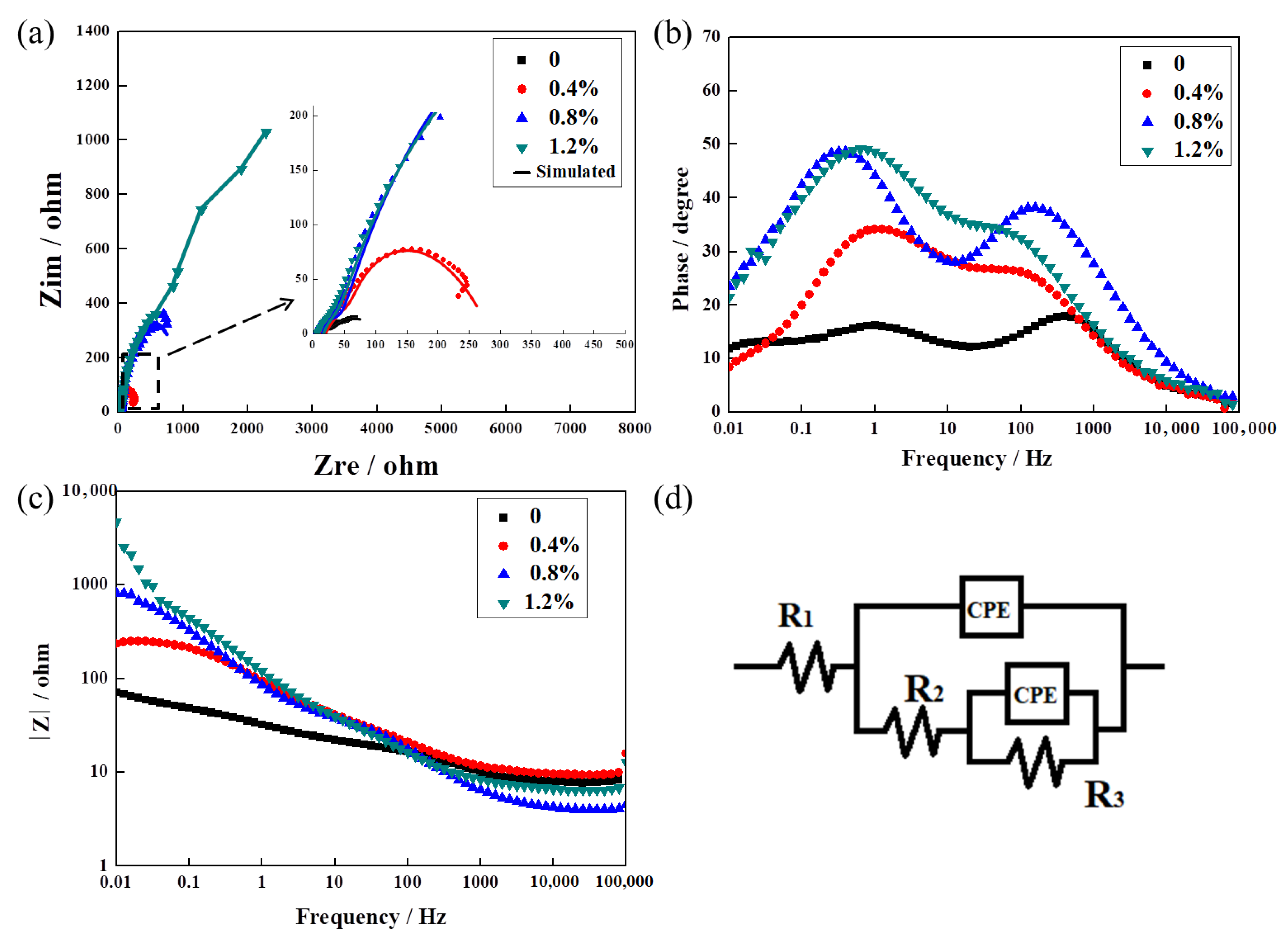
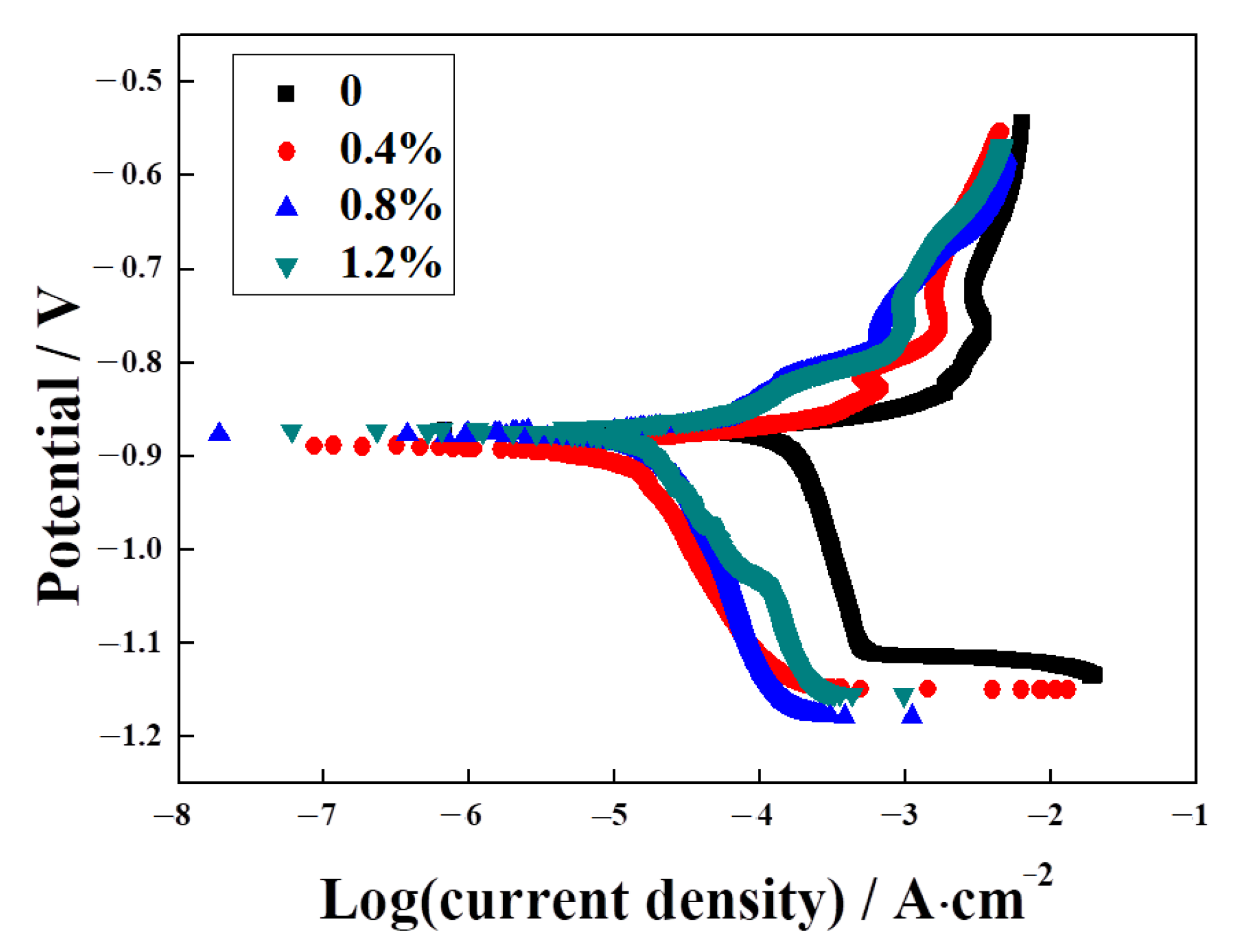
3.4. The Effect of La2O3 Content on the Corrosion Resistance of Ag–Cu Samples Prepared by SLM in 1% Na2S Solution
4. Conclusions
- The addition and mixture of La2O3 nano-particles to Ag–Cu alloy powders plays a positive role in improving laser absorptivity and reducing defects during the SLM process, leading to a significant increase in the density of Ag–Cu alloys, rising from 7.76 g/cm3 to 9.16 g/cm3.
- The phase composition of Ag–Cu alloys, as prepared by SLM, was found to be Silver-3C. The presence of La2O3 had no influence on this composition, but refined the grains of the Ag–Cu alloys by inhibiting the growth of columnar grains during the SLM process. No particular preferred orientation was observed in either the samples prepared with or without La2O3.
- There was an upwards trend in the hardness of the Ag–Cu alloys as the La2O3 content was increased from 0 to 1.2% (mass fraction). The average hardness significantly increased from 0.97 GPa to 2.88 GPa when 1.2% La2O3 was added, which was also higher than the ~1.11 GPa hardness of the sample with a similar chemical composition prepared by the powder metallurgy method, reported by a previous study [39]. This enhancement in hardness was mostly due to a reduction in pore defects and the refined grains caused by the effects of adding La2O3.
- EIS and PD tests of the samples in 1% Na2S solution proved that the addition of La2O3 improved the corrosion resistance of Ag–Cu alloys prepared by SLM practically and efficaciously. This improvement was evident in the form of higher impedance values and lower corrosion current densities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Q.; Meng, K.; Guo, J. Research innovative application of silver material in modern jewelry design. MATEC Web Conf. 2018, 176, 02013. [Google Scholar] [CrossRef]
- Nisaratanaporn, S.; Nisaratanaporn, E. The Anti-tarnishing, Microstructure Analysis and Mechanical Properties of Sterling Silver with Silicon Addition. J. Met. Mater. Miner. 2003, 12, 13–18. [Google Scholar]
- Yang, F.; Zhou, S.; Li, J.; Zhang, M.; Tang, M. Influence of Trace Manganese Additive on Properties of Pure Silver. Precious Met. 2002, 23, 29–32. [Google Scholar]
- Xiong, W.; Hao, L.; Li, Y.; Tang, D.; Cui, Q.; Feng, Z.; Yan, C. Effect of selective laser melting parameters on morphology, microstructure, densification and mechanical properties of supersaturated silver alloy. Mater. Des. 2019, 170, 107697. [Google Scholar] [CrossRef]
- Praiphruk, S.; Lothongkum, G.; Nisaratanaporn, E.; Lohwongwatana, B. Investigation of Supersaturated Silver Alloys for High Hardness Jewelry Application. J. Met. Mater. Miner. 2013, 23, 67–73. [Google Scholar]
- Khan, A.K.; Robinson, J.S. Effect of silver on precipitation response of Al-Li-Cu-Mg alloys. Mater. Sci. Technol. 2008, 24, 1369–1377. [Google Scholar] [CrossRef]
- Wang, W.F.; Wu, Y.C.; Zong, Y.; Deng, S.S.; Chen, J. Effect of adding rare earth on the properties of Cu-Ag alloys treated by mechanical alloying. Rare Met. Mater. Eng. 2007, 36, 71–73. [Google Scholar]
- Cui, T.; Gu, X.; Jia, Z.; Yin, X.; Cao, Z.; Zhang, K. Corrosion Properties of Bulk Nanocrystalline Ag-25Ni Alloys Prepared by Different Processes in NaCl Solution. Mater. Rev. 2018, 32, 2798–2802. [Google Scholar]
- Jang, S.H.; Lim, J.H.; Kim, J.H.; Ji, B.K.; Joo, J.; Nah, W.; Volf, J.S.; Liu, H.K.; Apperley, M. Characterization of thermal conductivity and mechanical properties of Ag-alloy sheathed Bi(Pb)-Sr-Ca-Cu-O superconductor tape. IEEE Trans. Appl. Supercond. 2003, 13, 2956–2959. [Google Scholar] [CrossRef]
- Xiong, Z.H.; Qin, Z.Z.; Zhao, Q.; Chen, L.L. Role of Ag-alloy in the thermal stability of Ag-based ohmic contact to GaN (0001) surface. Scr. Mater. 2015, 99, 81–84. [Google Scholar] [CrossRef]
- Udoh, K.; Ohta, M.; Oki, K.; Hisatsune, K. Phase diagram of AuCu-Ag pseudobinary alloy. J. Phase Equilibria 2001, 22, 306–311. [Google Scholar] [CrossRef]
- Rapallo, A.; Rossi, G.; Ferrando, R.; Fortunelli, A.; Curley, B.C.; Lloyd, L.D.; Tarbuck, G.M.; Johnston, R.L. Global optimization of bimetallic cluster structures. I. Size-mismatched Ag–Cu, Ag–Ni, and Au–Cu systems. J. Chem. Phys. 2005, 122, 194308. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Nature and Strength of M-S Bonds (M = Au, Ag, and Cu) in Binary Alloy Gold Clusters. J. Phys. Chem. A 2010, 114, 9212–9221. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, W.; Yang, Q. The Studying Status of the Tarnish-resistant Silver Alloys for Jewelry. Mater. Rev. 2006, 20, 67–69. [Google Scholar]
- Lee, M.K.; Park, J.J.; Lee, J.G.; Rhee, C.K. Phase-dependent corrosion of titanium-to-stainless steel joints brazed by Ag–Cu eutectic alloy filler and Ag interlayer. J. Nucl. Mater. 2013, 439, 168–173. [Google Scholar] [CrossRef]
- Cai, Z.; Yao, Y.; Shi, X.; Chen, X.; Han, P. Influence of V Content on Microstructure and Processability of Ag–Cu Alloy. Hot Work. Technol. 2018, 47, 52–54. [Google Scholar]
- Yang, C.Y.; Xu, J.H.; Ding, W.F.; Chen, Z.Z.; Fu, Y.C. Effect of cerium on microstructure, wetting and mechanical properties of Ag–Cu–Ti filler alloy. J. Rare Earths 2009, 27, 1051–1055. [Google Scholar] [CrossRef]
- Robinson, J.; Stanford, M.; Arjunan, A. Correlation between selective laser melting parameters, pore defects and tensile properties of 99.9 % silver. Mater. Today Commun. 2020, 25, 101550. [Google Scholar] [CrossRef]
- Vikram, R.J.; Kollo, L.; Prashanth, K.G.; Suwas, S. Investigating the Structure, Microstructure, and Texture in Selective Laser-Melted Sterling Silver 925. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2021, 52, 5329–5341. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Zhang, B.; Liao, H.; Coddet, C. Effects of processing parameters on microstructure and mechanical property of selective laser melted Ti6Al4V. Mater. Des. 2012, 35, 120–125. [Google Scholar] [CrossRef]
- Leary, M.; Mazur, M.; Williams, H.; Yang, E.; Alghamdi, E.; Lozanovski, B.; Zhang, X.; Shidid, D.; Farahbod-Sternahl, L.; Witt, G. Inconel 625 lattice structures manufactured by selective laser melting (SLM): Mechanical properties, deformation and failure modes. Mater. Des. 2018, 157, 179–199. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium–titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Boley, C.D.; Khairallah, S.A.; Rubenchik, A.M. Calculation of laser absorption by metal powders in additive manufacturing. Appl. Opt. 2015, 54, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, Y.; Wei, X.M.; Khanlari, K.; Wang, Z.; Feng, Y.W.; Yang, X.S. Selective Laser Melting of Pure Ag and 925Ag Alloy and Their Thermal Conductivity. Crystals 2022, 12, 480. [Google Scholar] [CrossRef]
- Gu, D.; Meiners, W.; Wissenbach, K. Poprawe R, Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Tolochko, N.K.; Khlopkov, Y.V.; Mozzharov, S.E.; Ignatiev, M.B.; Laoui, T.; Titov, V.I. Absorptance of powder materials suitable for laser sintering. Rapid Prototyp. J. 2000, 6, 155–161. [Google Scholar] [CrossRef]
- Khan, M.; Dickens, P. Selective laser melting (SLM) of pure gold. Gold Bull. 2010, 43, 114–121. [Google Scholar] [CrossRef]
- Shuai, C.J.; Xue, L.F.; Gao, C.D.; Yang, Y.W.; Peng, S.P.; Zhang, Y.R. Selective laser melting of Zn-Ag alloys for bone repair: Microstructure, mechanical properties and degradation behaviour. Virtual Phys. Prototyp. 2018, 13, 146–154. [Google Scholar] [CrossRef]
- Robinson, J.; Arjunan, A.; Stanford, M.; Lyall, I.; Williams, C. Effect of silver addition in copper-silver alloys fabricated by laser powder bed fusion in situ alloying. J. Alloys Compd. 2021, 857, 157561. [Google Scholar] [CrossRef]
- Zhao, G.M.; Wang, K.L. Effect of La2O3 on resistance to high-temperature oxidation of laser clad ferrite-based alloy coatings. Surf. Coat. Technol. 2005, 190, 249–254. [Google Scholar] [CrossRef]
- Ashutosh, G.; Dilshat, U.T.; Vladislav, V.K.; Aleksey, A.Y.; Jose, M.F.F. The effect of Cr2O3 addition on crystallization and properties of La2O3-containing diopside glass-ceramics. Acta Mater. 2008, 56, 3065–3076. [Google Scholar]
- Yuehui, W.; Ji, Z.; Ting, W.; Shen, J.; Cui, X. Microstructure and dielectric properties of BaTiO3-La2O3-MgO ceramic. Rare Met. Mater. Eng. 2006, 35, 165–168. [Google Scholar]
- Joubert, M.F.; Kazanskii, S.A.; Guyot, Y.; Gacon, J.C.; Rivoire, J.Y.; Pedrini, C. A new microwave resonant technique for studying rare earth photoionization thresholds in dielectric crystals under laser irradiation. Opt. Mater. 2003, 24, 137–141. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Wang, T.W.; Meng, X.F.; Shan, X.B.; Xu, Z.Z. Influence of composition of Sm2O3-containing rare earth glass on its absorption spectrum. J. Rare Earths 2005, 23, 295–298. [Google Scholar]
- Stanford, N.; Atwell, D.; Barnett, M.R. The effect of Gd on the recrystallisation, texture and deformation behaviour of magnesium-based alloys. Acta Mater. 2010, 58, 6773–6783. [Google Scholar] [CrossRef]
- Fu, H.G.; Xiao, Q.; Kuang, J.C.; Jiang, Z.Q.; Xing, J.D. Effect of rare earth and titanium additions on the microstructures and properties of low carbon Fe-B cast steel. Mater. Sci. Eng. A 2007, 466, 160–165. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, Q.B.; Sun, M.L.; Wei, X.G.; Zhu, Y.M. Rare earth elements modification of laser-clad nickel-based alloy coatings. Appl. Surf. Sci. 2001, 174, 191–200. [Google Scholar] [CrossRef]
- Tyurina, S.A.; Chavushyan, S.L.; Makarova, A.V.; Khvostov, R.E.; Yudin, G.A. Research and Analysis of Methods for Preventing Silver Alloys from Tarnishing. Inorg. Mater. Appl. Res. 2021, 12, 1615–1622. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, S.; Sun, S.; Wang, S.; Xie, M.; Hu, J. Preparation and Performances Analysis of a New Ag–CuO–La2O3 Electrical Contact Material. Precious Met. 2015, 36, 9–13. [Google Scholar]

| Element | Cu | Ge | K | Si | Ca | Ag |
|---|---|---|---|---|---|---|
| Mass fraction (%) | 5.626 | 0.893 | 0.431 | 0.242 | 0.138 | Bal. |
| Laser Power | Layer Thickness | Scanning Speed | Focus Offset | Oxygen Content |
|---|---|---|---|---|
| 60 W | 20 μm | 1000 mm/s | 0 mm | <0.3% |
| La2O3 Content (Mass Fraction) | 0% | 0.4% | 0.8% | 1.2% |
|---|---|---|---|---|
| Density (g/cm3) | 7.76 ± 0.22 | 8.14 ± 0.26 | 8.55 ± 0.33 | 9.16 ± 0.55 |
| La2O3 Content (wt%) | R1 (Ω/cm2) | CPE (10−4 F∙cm2) | n1 | R2 (Ω/cm2) | CPE (10−3 F∙cm2) | n2 | R3 (Ω/cm2) |
|---|---|---|---|---|---|---|---|
| 0 | 7.77 ± 0.73 | 1.82 ± 0.25 | 0.82 ± 0.05 | 7.74 ± 1.02 | 2.32 ± 0.03 | 0.38 ± 0.01 | 86.91 ± 6.65 |
| 0.4% | 9.90 ± 2.87 | 0.22 ± 0.03 | 0.68 ± 0.03 | 23.44 ± 3.36 | 1.46 ± 0.05 | 0.81 ± 0.05 | 0.35 ± 0.08 × 103 |
| 0.8% | 9.57 ± 1.43 | 5.39 ± 0.83 | 0.71 ± 0.02 | 39.50 ± 5.56 | 3.36 ± 0.18 | 0.73 ± 0.02 | 1.04 ± 0.21 × 103 |
| 1.2% | 7.25 ± 1.69 | 6.54 ± 0.99 | 0.75 ± 0.06 | 47.54 ± 9.31 | 1.64 ± 0.41 | 0.77 ± 0.01 | 4.09 ± 0.30 × 103 |
| La2O3 Content (wt%) | 0 | 0.4 | 0.8 | 1.2 |
|---|---|---|---|---|
| Ecorr (V) | −0.87 ± 0.015 | −0.88 ± 0.040 | −0.89 ± 0.045 | −0.87 ± 0.055 |
| icorr (μA∙cm−2) | 126.35 ± 17.33 | 40.80 ± 2.16 | 11.23 ± 2.68 | 9.62 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zheng, H.; Ma, X.; Sheng, Y.; Zeng, D.; Yuan, J. Microstructure, Mechanical Properties, and Corrosion Resistance of Ag–Cu Alloys with La2O3 Fabricated by Selective Laser Melting. Materials 2023, 16, 7670. https://doi.org/10.3390/ma16247670
Zhao X, Zheng H, Ma X, Sheng Y, Zeng D, Yuan J. Microstructure, Mechanical Properties, and Corrosion Resistance of Ag–Cu Alloys with La2O3 Fabricated by Selective Laser Melting. Materials. 2023; 16(24):7670. https://doi.org/10.3390/ma16247670
Chicago/Turabian StyleZhao, Xueyang, Haiyan Zheng, Xin Ma, Yinying Sheng, Dahai Zeng, and Junping Yuan. 2023. "Microstructure, Mechanical Properties, and Corrosion Resistance of Ag–Cu Alloys with La2O3 Fabricated by Selective Laser Melting" Materials 16, no. 24: 7670. https://doi.org/10.3390/ma16247670
APA StyleZhao, X., Zheng, H., Ma, X., Sheng, Y., Zeng, D., & Yuan, J. (2023). Microstructure, Mechanical Properties, and Corrosion Resistance of Ag–Cu Alloys with La2O3 Fabricated by Selective Laser Melting. Materials, 16(24), 7670. https://doi.org/10.3390/ma16247670




