Sustainable Synthesis of Iron–Zinc Nanocomposites by Azadirachta indica Leaves Extract for RSM-Optimized Sono-Adsorptive Removal of Crystal Violet Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Adsorbate (CV Dye) Standards and Test Solutions by Standard Addition Method
2.3. Sustainable Fe–Zn Nanocomposites Synthesis
2.4. Nanocomposites Characterization
2.5. pH at Point of Zero Charge (pHPZC)
2.6. Ultrasonic Waves-Assisted Adsorption Experiments
2.7. Measurement of Adsorption Effectiveness
2.8. Central Composite Design (CCD)
2.9. Response Surface Methodology (RSM)
2.10. Adsorption Isotherms
2.10.1. Langmuir Adsorption Isotherms
2.10.2. Freundlich Adsorption Isotherm
2.10.3. Dubinin–Radushkevich Adsorption Isotherm
2.10.4. Temkin Adsorption Isotherm
2.10.5. Harkins–Jura Model
2.11. Kinetics Studies
2.11.1. Pseudo-First-Order Kinetic Model
2.11.2. Pseudo-Second-Order Kinetic Model
2.11.3. Intraparticle Diffusion Model
2.11.4. Elovich Model
2.12. Error Analysis
2.12.1. Chi-Square (χ2) Test
2.12.2. Root Mean Square Error (RMSE)
2.13. Thermodynamic Studies
2.14. CV Dye Recovery
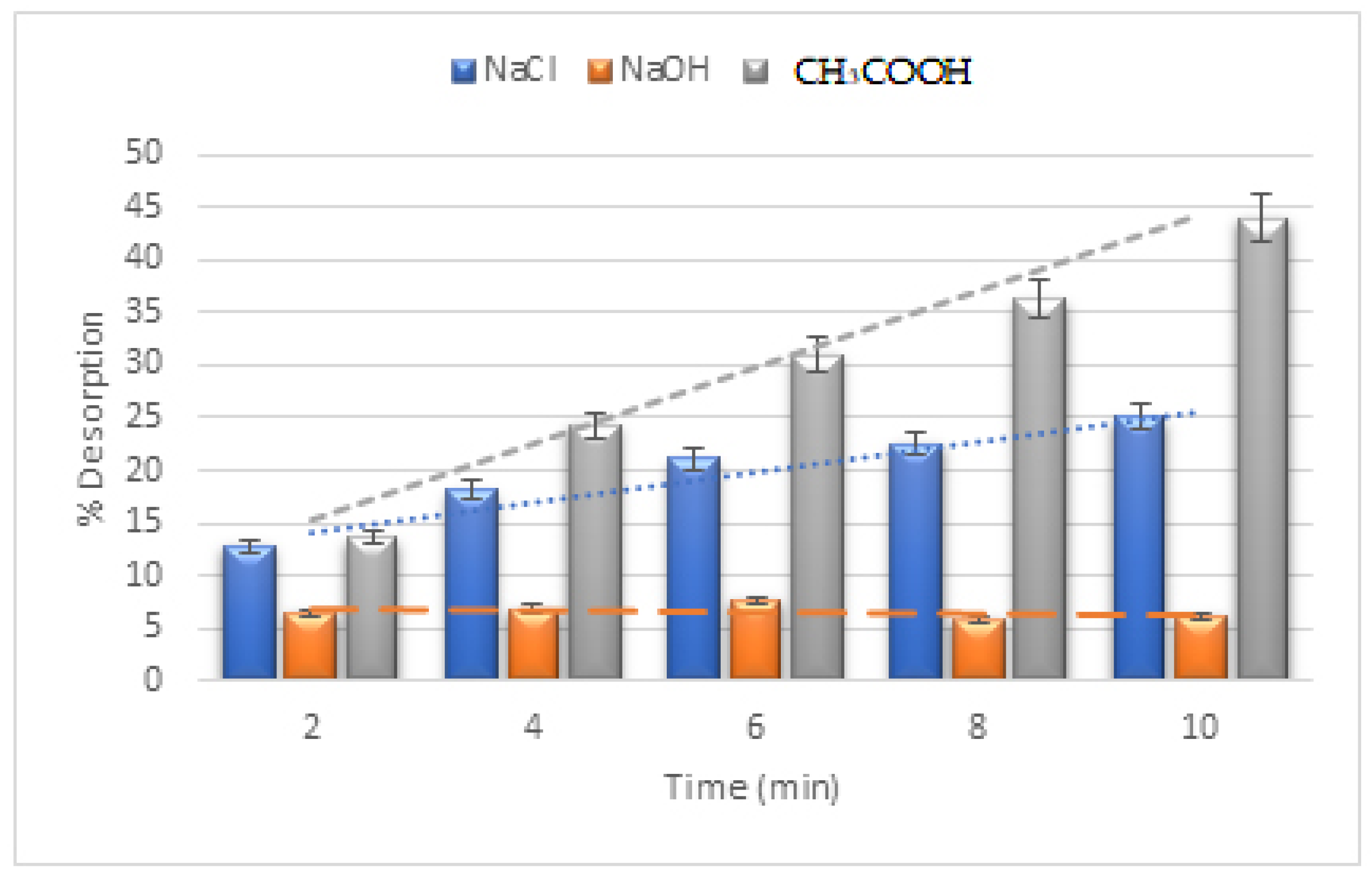
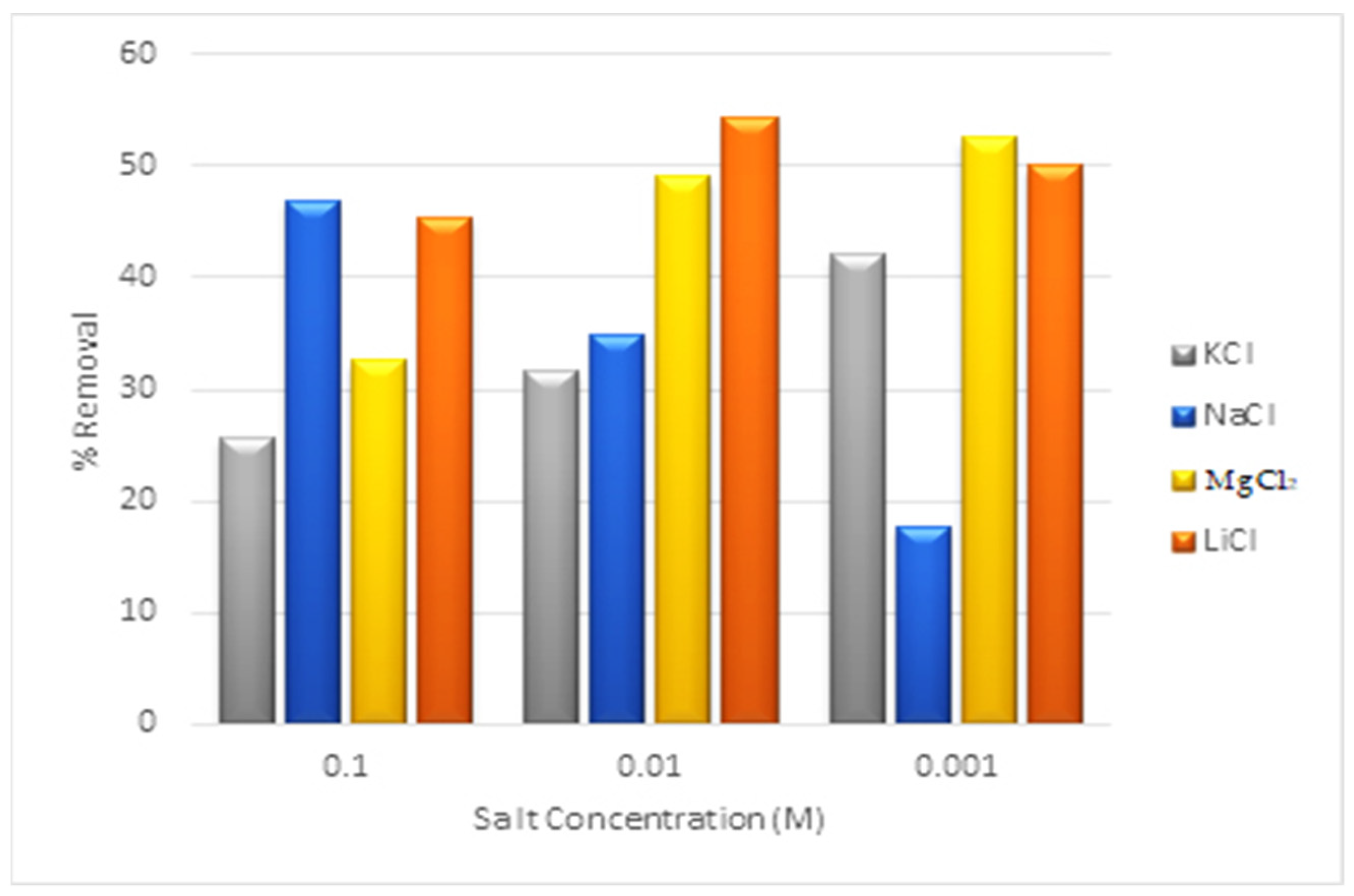
2.15. Interference Ions Effects
3. Results and Discussion
3.1. Characterization of Nanocomposites
3.1.1. Fourier Transformed Infrared Spectroscopy
3.1.2. Scanning Electron Microscopy
3.1.3. Energy Dispersive X-ray Spectroscopy
3.1.4. pH at Point of Zero Charge
3.2. Central Composite Design (CCD)
| S. No. | Time (min) | pH | Amount of Adsorbent (g) | CV Concentration (mg L−1) | % Removal |
|---|---|---|---|---|---|
| 01 | 6 | 7 | 0.015 | 5.0 | 34.90 |
| 02 | 4 | 9 | 0.010 | 20 | 36.84 |
| 03 | 6 | 7 | 0.015 | 15 | 76.06 |
| 04 | 4 | 5 | 0.010 | 20 | 27.07 |
| 05 | 8 | 5 | 0.010 | 20 | 51.87 |
| 06 | 4 | 5 | 0.010 | 10 | 19.02 |
| 07 | 8 | 5 | 0.020 | 10 | 68.10 |
| 08 | 2 | 7 | 0.015 | 15 | 38.27 |
| 09 | 4 | 5 | 0.020 | 10 | 13.84 |
| 10 | 8 | 9 | 0.010 | 20 | 58.08 |
| 11 | 6 | 7 | 0.015 | 15 | 74.77 |
| 12 | 8 | 5 | 0.010 | 10 | 22.91 |
| 13 | 6 | 7 | 0.015 | 15 | 77.48 |
| 14 | 6 | 7 | 0.025 | 15 | 85.33 |
| 15 | 4 | 9 | 0.020 | 20 | 41.95 |
| 16 | 4 | 5 | 0.020 | 20 | 37.70 |
| 17 | 6 | 7 | 0.015 | 15 | 73.98 |
| 18 | 6 | 7 | 0.015 | 15 | 75.98 |
| 19 | 4 | 9 | 0.010 | 10 | 21.52 |
| 20 | 8 | 9 | 0.020 | 10 | 23.21 |
| 21 | 8 | 9 | 0.010 | 10 | 65.64 |
| 22 | 6 | 11 | 0.015 | 15 | 82.65 |
| 23 | 10 | 7 | 0.015 | 15 | 63.80 |
| 24 | 6 | 7 | 0.015 | 25 | 39.35 |
| 25 | 6 | 7 | 0.005 | 15 | 80.02 |
| 26 | 4 | 9 | 0.020 | 10 | 20.50 |
| 27 | 6 | 3 | 0.015 | 15 | 31.84 |
| 28 | 8 | 5 | 0.020 | 20 | 64.85 |
| 29 | 8 | 9 | 0.020 | 20 | 41.20 |
| 30 | 6 | 7 | 0.015 | 15 | 79.73 |
3.3. Analysis of Variances
3.4. Pareto Analysis
3.5. Normal Probability Plots
3.6. Response Surface Methodology (RSM)
3.7. Method Validation at the OOP
3.8. Isotherm Analysis
3.9. Kinetic Analysis
3.10. Thermodynamic Studies
3.11. CV dye Recovery
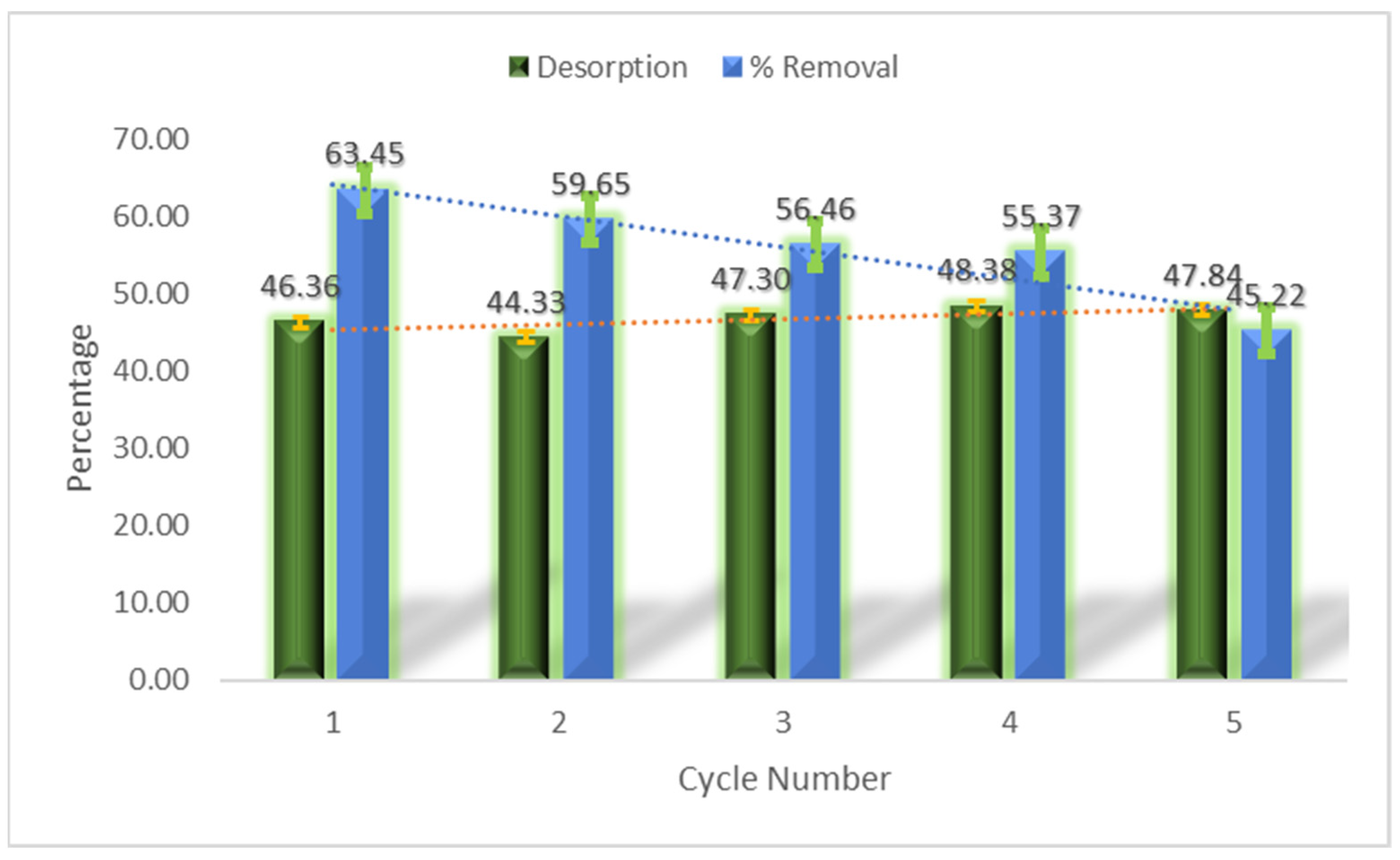
3.12. Regeneration of Adsorbent
3.13. Interference Ions Effect
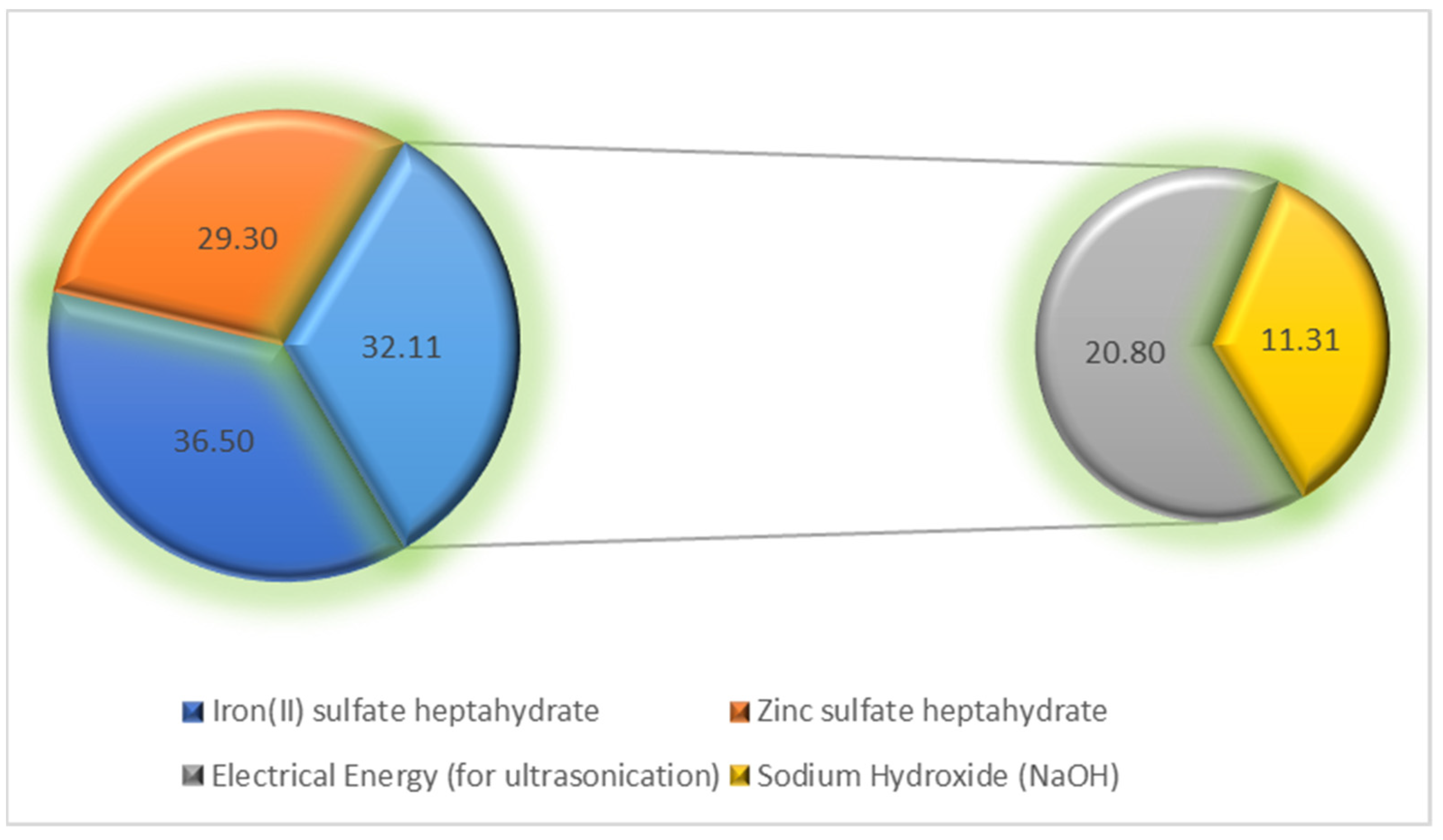
3.14. Operational Cost
3.15. Comparing the Capability of Adsorption with Earlier Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sultan, M.; Siddique, M.; Khan, R.; Fallatah, A.M.; Fatima, N.; Shahzadi, I.; Waheed, U.; Bilal, M.; Ali, A.; Abbasi, A.M. Ligustrum lucidum Leaf Extract-Assisted Green Synthesis of Silver Nanoparticles and Nano-Adsorbents Having Potential in Ultrasound-Assisted Adsorptive Removal of Methylene Blue Dye from Wastewater and Antimicrobial Activity. Materials 2022, 15, 1637. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Jagadeesh, D.; Prashantha, K.; Shabadi, R. Star-shaped sucrose-capped CaO nanoparticles from Azadirachta indica: A novel green synthesis. Inorg. Nano-Metal Chem. 2016, 47, 708–712. [Google Scholar] [CrossRef]
- Manikandan, R.; Manikandan, B.; Raman, T.; Arunagirinathan, K.; Prabhu, N.M.; Basu, M.J.; Perumal, M.; Palanisamy, S.; Munusamy, A. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Mankad, M.; Patil, G.; Patel, S.; Patel, D.; Patel, A. Green synthesis of zinc oxide nanoparticles using Azadirachta indica A. Juss. leaves extractand its antibacterial activity against Xanthomonas orzyae pv. oryzae. Ann. Phytomedicine 2016, 5, 76–86. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Christy, A.N. Green synthesis of platinum nanoparticles using Azadirachta indica—An eco-friendly approach. Mater. Lett. 2016, 170, 175–178. [Google Scholar] [CrossRef]
- Datta, L.P.; Chatterjee, A.; Acharya, K.; De, P.; Das, M. Enzyme responsive nucleotide functionalized silver nanoparticles with effective antimicrobial and anticancer activity. New J. Chem. 2017, 41, 1538–1548. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Sakthivel, R.; Mallika, J. A single pot green synthesis of ZnO nanoparticles using aqueous gum exudates of Azadirachta indica and its antifungal activity. Int. Res. J. Eng. Technol. 2016, 3, 300–306. [Google Scholar]
- Sharma, J.K.; Srivastava, P.; Ameen, S.; Akhtar, M.S.; Singh, G.; Yadava, S. Azadirachta indica plant-assisted green synthesis of Mn3O4 nanoparticles: Excellent thermal catalytic performance and chemical sensing behavior. J. Colloid Interface Sci. 2016, 472, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, A.; Harshini, E.; Marakathanandhini, B.D.; Kannan, S.R.; Muthukumaran, P. Catalytic Degradation of Reactive Red 120 by Copper Oxide Nanoparticles Synthesized by Azadirachta indica; Springer: Berlin/Heidelberg, Germany, 2017; pp. 95–102. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V.; Jain, M.; Kadirvelu, K. Preparation, characterization and potential use of flower shaped Zinc oxide nanoparticles (ZON) for the adsorption of Victoria Blue B dye from aqueous solution. Adv. Powder Technol. 2016, 27, 1180–1188. [Google Scholar] [CrossRef]
- Saad, M.; Tahir, H. Synthesis of carbon loaded γ-Fe2O3 nanocomposite and their applicability for the selective removal of binary mixture of dyes by ultrasonic adsorption based on response surface methodology. Ultrason. Sonochem. 2017, 36, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Tahir, H.; Ali, D. Green synthesis of Ag-Cr-AC nanocomposites by Azadirachta indica and its application for the simultaneous removal of binary mixture of dyes by ultrasonicated assisted adsorption process using Response Surface Methodology. Ultrason. Sonochem. 2017, 38, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.A.H.M.; Lim, L.B.L.; Usman, A.; Kooh, M.R.R. Efficient adsorption of malachite green dye using Artocarpus odoratissimus leaves with artificial neural network modelling. Desalin. Water Treat. 2018, 101, 313–324. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Thotagamuge, R.; Chau, Y.-F.C.; Mahadi, A.H.; Lim, C.M. Machine learning approaches to predict adsorption capacity of Azolla pinnata in the removal of methylene blue. J. Taiwan Inst. Chem. Eng. 2021, 132, 104134. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Aghaie, H.; Sheykhloie, H.; Vardini, M.T.; Etemadi, H. Synthesis of CarAlg/MMt nanocomposite hydrogels and adsorption of cationic crystal violet. Carbohydr. Polym. 2013, 98, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Lu, L. Magnetite/reduced graphene oxide nanocomposites: One step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; He, T.; Chen, L. Removal of crystal violet by clay/PNIPAm nanocomposite hydrogels with various clay contents. Appl. Clay Sci. 2014, 90, 1–5. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Akhbari, K.; Morsali, A. Dense coating of surface mounted CuBTC metal–organic framework nanostructures on silk fibers, prepared by layer-by-layer method under ultrasound irradiation with antibacterial activity. Ultrason. Sonochem. 2012, 19, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically assisted hydrothermal synthesis of activated carbon–HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason. Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef]
- Khanjani, S.; Morsali, A. Ultrasound-promoted coating of MOF-5 on silk fiber and study of adsorptive removal and recovery of hazardous anionic dye “congo red”. Ultrason. Sonochem. 2014, 21, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Bazrafshan, A. Ultrasound-assisted removal of Al3+ ions and Alizarin red S by activated carbon engrafted with Ag nanoparticles: Central composite design and genetic algorithm optimization. RSC Adv. 2015, 5, 59522–59532. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghaedi, M.; Dashtian, K.; Hajati, S. New ion-imprinted polymer-functionalized mesoporous SBA-15 for selective separation and preconcentration of Cr(iii) ions: Modeling and optimization. RSC Adv. 2015, 5, 105789–105799. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A. Ternary dye adsorption onto MnO2 nanoparticle-loaded activated carbon: Derivative spectrophotometry and modeling. RSC Adv. 2015, 5, 72300–72320. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Goudarzi, A. Synthesis and characterization of ZnO-nanorods loaded onto activated carbon and its application for efficient solid phase extraction and determination of BG from water samples by micro-volume spectrophotometry. New J. Chem. 2015, 39, 9407–9414. [Google Scholar]
- Hamza, W.; Fakhfakh, N.; Dammak, N.; Belhadjltaeif, H.; Benzina, M. Sono-assisted adsorption of organic compounds contained in industrial solution on iron nanoparticles supported on clay: Optimization using central composite design. Ultrason. Sonochem. 2020, 67, 105134. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total. Environ. 2014, 466–467, 210–213. [Google Scholar] [CrossRef]
- Saad, M.; Tahir, H.; Khan, J.; Hameed, U.; Saud, A. Synthesis of polyaniline nanoparticles and their application for the removal of Crystal Violet dye by ultrasonicated adsorption process based on Response Surface Methodology. Ultrason. Sonochem. 2017, 34, 600–608. [Google Scholar] [CrossRef]
- Shafiee, A.; Baneshi, M.; Varma, R.S.; Mostafavi, E.; Iravani, S. Biosynthesized metallic nanocatalysts in the removal and degradation of pollutants. Mater. Lett. 2022, 326, 132911. [Google Scholar] [CrossRef]
- Eris, S.; Azizian, S. Analysis of adsorption kinetics at solid/solution interface using a hyperbolic tangent model. J. Mol. Liq. 2017, 231, 523–527. [Google Scholar] [CrossRef]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium uptake, isotherm and kinetic studies of Cd(II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Mahmood-Kashani, H.; Ahmadi, M. Adsorption of some cationic and anionic dyes on magnetite nanoparticles-modified activated carbon from aqueous solutions: Equilibrium and kinetics study. J. Iran. Chem. Soc. 2012, 10, 481–489. [Google Scholar] [CrossRef]
- Solisio, C.; Al Arni, S.; Converti, A. Adsorption of inorganic mercury from aqueous solutions onto dry biomass of Chlorella vulgaris: Kinetic and isotherm study. Environ. Technol. 2017, 40, 664–672. [Google Scholar] [CrossRef]
- Rêgo, T.; Cadaval, T.; Dotto, G.L.; Pinto, L. Statistical optimization, interaction analysis and desorption studies for the azo dyes adsorption onto chitosan films. J. Colloid Interface Sci. 2013, 411, 27–33. [Google Scholar] [CrossRef]
- Chang, G.; Bao, Z.; Zhang, Z.; Xing, H.; Su, B.; Yang, Y.; Ren, Q. Adsorption of 2-Butyl-2-ethyl-1,3-propanediol from Aqueous Solutions on Activated Carbon: Salt-Out Effect on Equilibrium, Kinetics, and Dynamics. Ind. Eng. Chem. Res. 2014, 53, 8592–8598. [Google Scholar] [CrossRef]
- Taghipour, T.; Karimipour, G.; Ghaedi, M.; Asfaram, A. Mild synthesis of a Zn(II) metal organic polymer and its hybrid with activated carbon: Application as antibacterial agent and in water treatment by using sonochemistry: Optimization, kinetic and isotherm study. Ultrason. Sonochem. 2018, 41, 389–396. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, A.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar] [CrossRef]
- Karthik, K.; Nikolova, M.P.; Phuruangrat, A.; Pushpa, S.; Revathi, V.; Subbulakshmi, M. Ultrasound-assisted synthesis of V2O5 nanoparticles for photocatalytic and antibacterial studies. Mater. Res. Innov. 2020, 24, 229–234. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Chai, P.V.; Takriff, M.S.; Benamor, A.; Mohammad, A.W. Optimization of nickel oxide nanoparticle synthesis through the sol–gel method using Box–Behnken design. Mater. Des. 2015, 86, 948–956. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J. Mater. Chem. A 2012, 1, 959–965. [Google Scholar] [CrossRef]
- Lee, M.L.; Chen, H.; Chen, C.P.; Chu, L.C.; Wang, S.H.; Chen, C.Y.; Chen, Y.T.; Chang, K.M. Fabrication and characterization of cobalt nanoparticles on ZnO nanorods on a polished titanium substrate. Vacuum 2015, 118, 90–93. [Google Scholar] [CrossRef]
- Bognár, S.; Putnik, P.; Merkulov, D. Sustainable Green Nanotechnologies for Innovative Purifications of Water: Synthesis of the Nanoparticles from Renewable Sources. Nanomaterials 2022, 12, 263. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Sivakumar, T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Liu, F.; Su, H.; Jin, L.; Zhang, H.; Chu, X.; Yang, W. Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 2017, 505, 796–804. [Google Scholar] [CrossRef]
- Sui, T.; Ding, M.; Ji, C.; Yan, S.; Wei, J.; Wang, A.; Zhao, F.; Fei, J. Dispersibility and rheological behavior of functionalized silica nanoparticles as lubricant additives. Ceram. Int. 2018, 44, 18438–18443. [Google Scholar] [CrossRef]
- Saad, M.; Hajira, T.; Fakhra, S. Synthesis and characterization of SnO-CoO nanocomposites by bottom up approach and their efficacy for the treatment of dyes assisted simulated waste water. Int. J. Curren. Res. 2015, 7, 23542–23550. [Google Scholar]
- Mahato, J.K.; Gupta, S.K. Exceptional adsorption of different spectral indices of natural organic matter (NOM) by using cerium oxide nanoparticles (CONPs). Environ. Sci. Pollut. Res. 2021, 28, 45496–45505. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alkaim, A.F.; Al-Dujaili, A.H. Adsorption isotherm, kinetic modeling and thermodynamics of crystal violet dye on coconut husk-based activated carbon. Desalin. Water Treat. 2013, 53, 3656–3667. [Google Scholar] [CrossRef]
- Gholami, M.; Vardini, M.T.; Mahdavinia, G.R. Investigation of the effect of magnetic particles on the Crystal Violet adsorption onto a novel nanocomposite based on κ-carrageenan-g-poly(methacrylic acid). Carbohydr. Polym. 2016, 136, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Kumari, H.J.; Krishnamoorthy, P.; Arumugam, T.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, H.; Rahbar-Kelishami, A.; Norouzbeigi, R. Adsorption of malachite green and crystal violet cationic dyes from aqueous solution using pumice stone as a low-cost adsorbent: Kinetic, equilibrium, and thermodynamic studies. Desalin. Water Treat. 2015, 57, 12822–12831. [Google Scholar] [CrossRef]
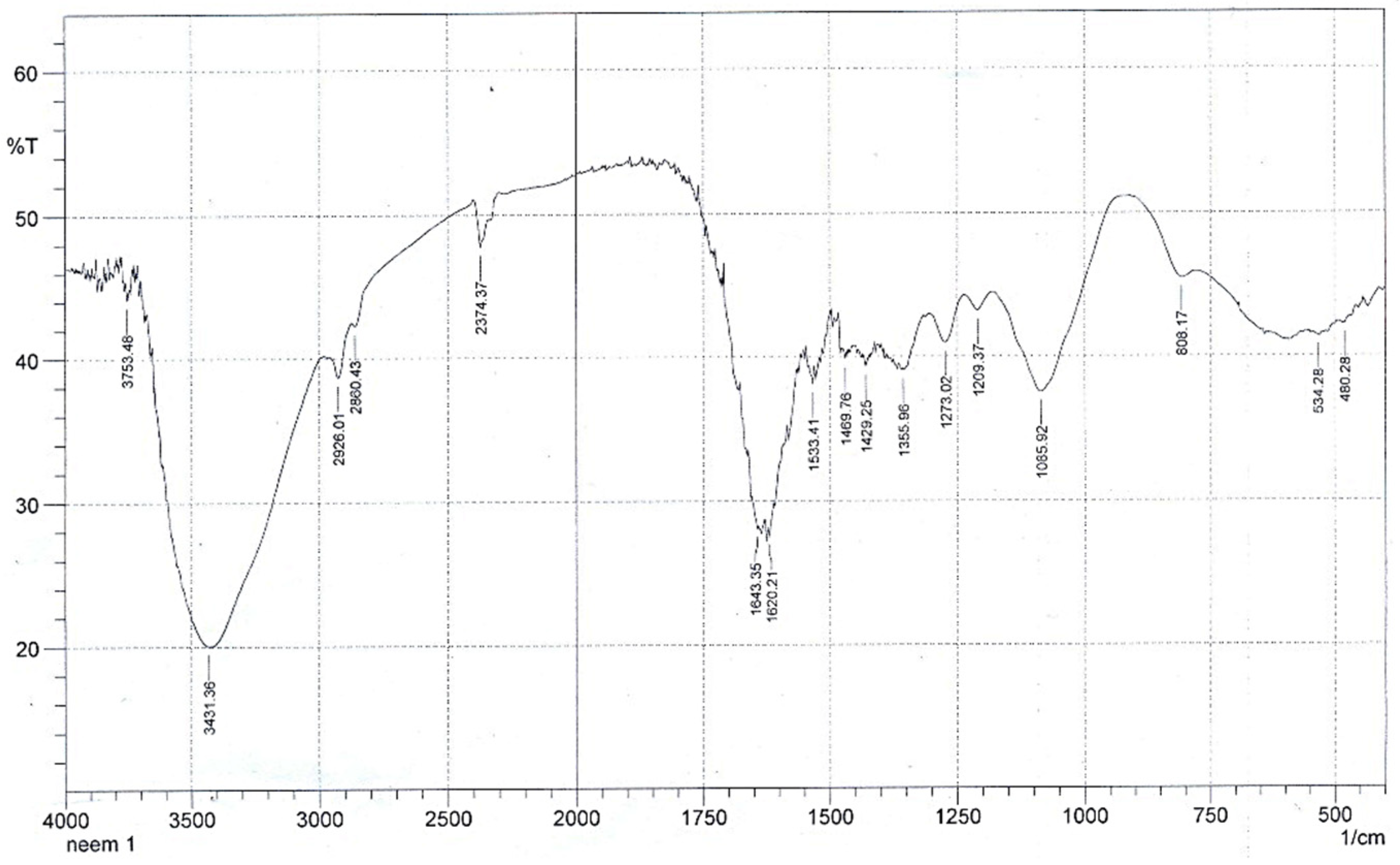


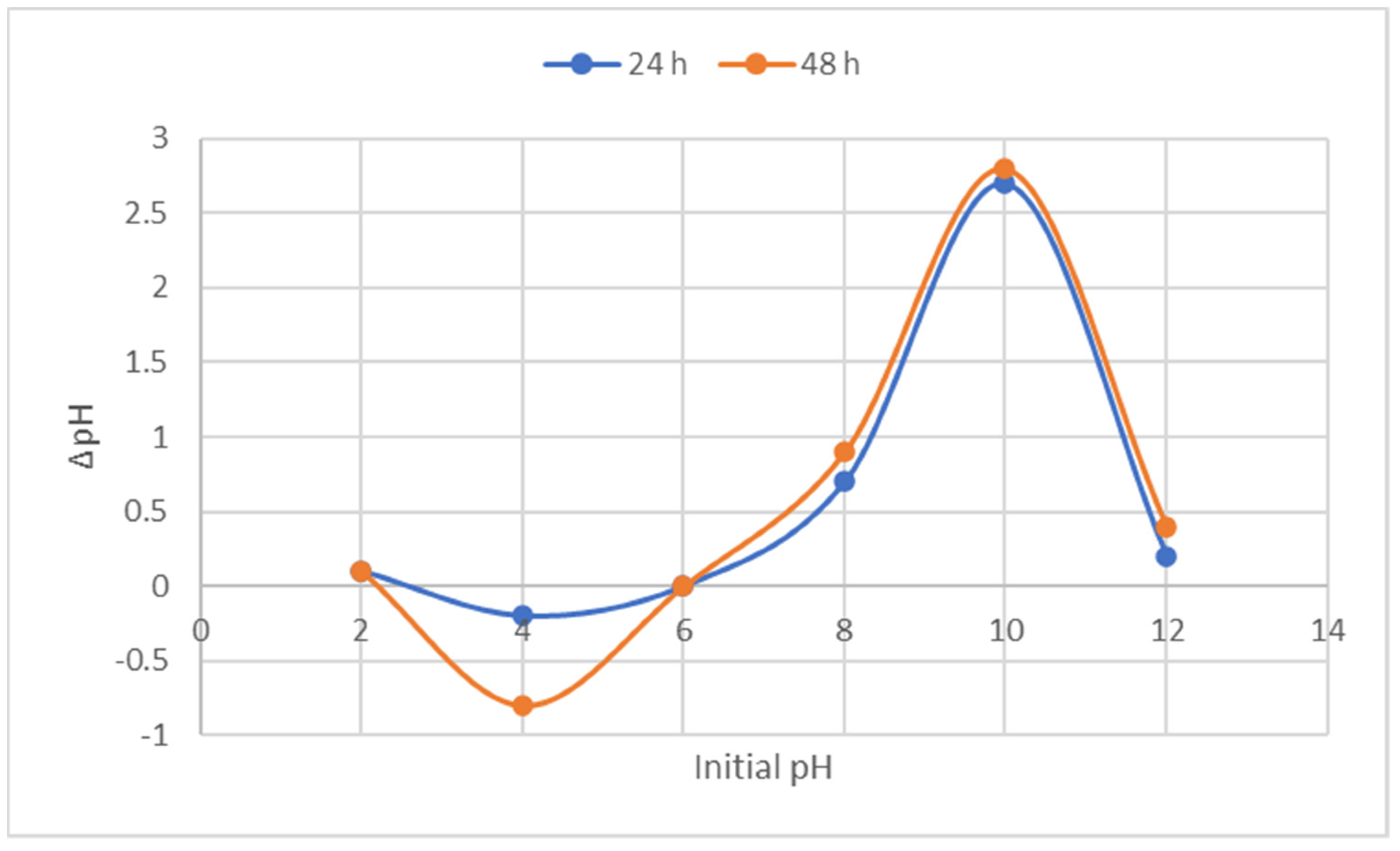

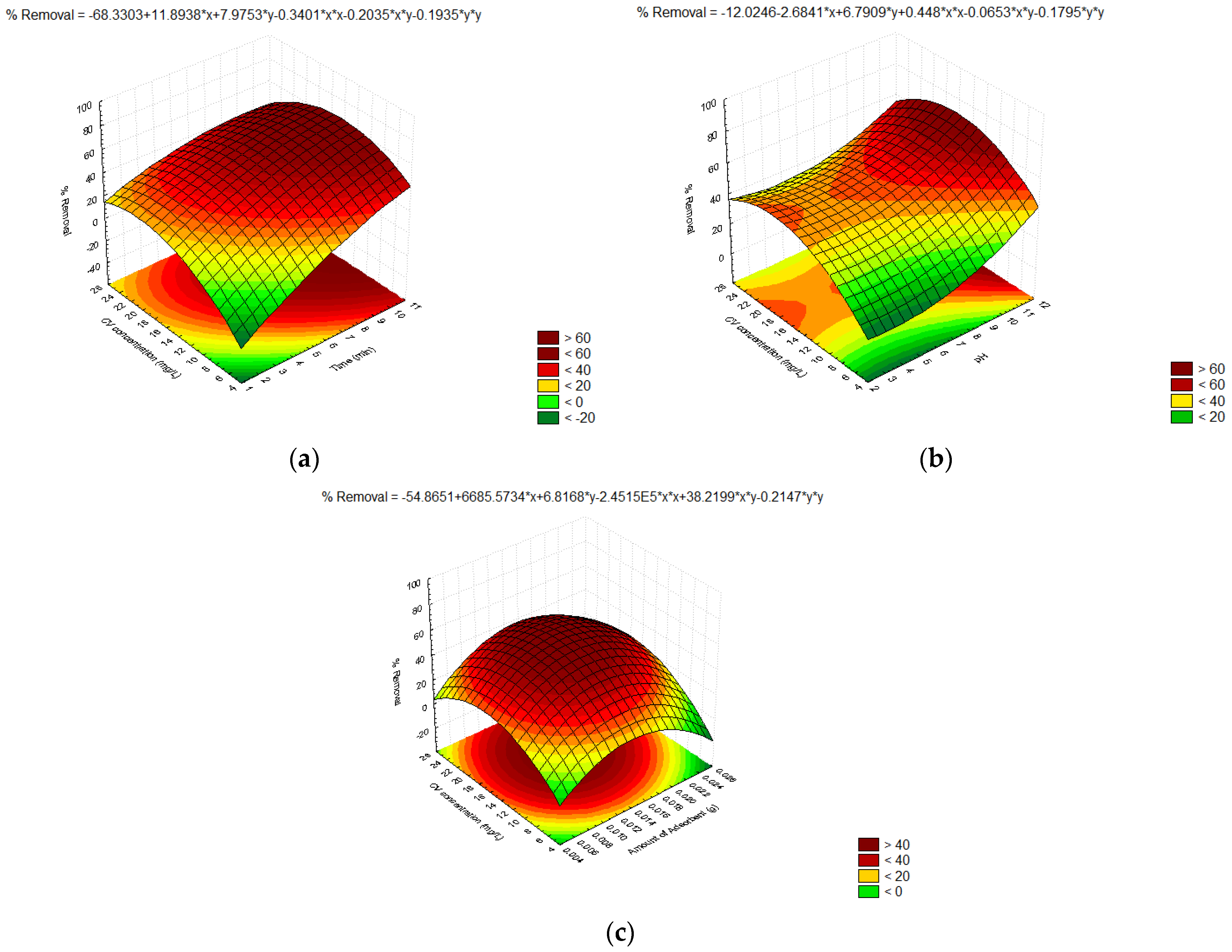
| Source | SS | DF | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 11,300 | 14 | 806.8 | 2.795 | 0.02872 |
| Time | 2175 | 1 | 2175 | 7.536 | 0.01503 |
| pH | 461.3 | 1 | 461.3 | 1.598 | 0.2254 |
| Amount of adsorbent | 15.10 | 1 | 15.10 | 0.0523 | 0.8222 |
| CV concentration | 539.1 | 1 | 539.1 | 1.868 | 0.1919 |
| AB | 114.3 | 1 | 114.3 | 0.3959 | 0.5387 |
| AC | 7.104 | 1 | 7.104 | 0.0246 | 0.8774 |
| AD | 66.26 | 1 | 66.26 | 0.2296 | 0.6388 |
| BC | 882.8 | 1 | 882.8 | 3.059 | 0.1007 |
| BD | 6.818 | 1 | 6.818 | 0.0236 | 0.8799 |
| CD | 14.61 | 1 | 14.61 | 0.0506 | 0.8250 |
| AA | 2438 | 1 | 2438 | 8.448 | 0.01085 |
| BB | 1702 | 1 | 1702 | 5.896 | 0.02823 |
| CC | 63.25 | 1 | 63.25 | 0.219 | 0.6464 |
| DD | 4569 | 1 | 4569 | 15.83 | 0.00121 |
| Residual | 4329 | 15 | 288.6 | * | * |
| Lack of Fit | 4308 | 10 | 430.8 | 102.6 | 0.00004 |
| Pure Error | 21.00 | 5 | 4.201 | * | * |
| Cor Total | 15,620 | 29 | * | * | * |
| Optimum Parameters | Responses | |||
|---|---|---|---|---|
| OOP | Replicates’ Responses at OOP | % RSD9 at OOP | ||
| Time (min) | 8.00 | 79.72 | 1.648 | |
| pH | 9.00 | 77.48 | ||
| Amount of adsorbent (mg) | 10.0 | 79.43 | ||
| CV concentration (mg L−1) | 25.0 | 78.64 | ||
| Predicted response at OOP | 80.39 | 78.85 | ||
| %R2 | 72.29 | 78.58 | ||
| %R2 Adjusted | 46.43 | 78.10 | ||
| CCD Standard Deviation | 7.180 | 75.07 | ||
| 78.10 | ||||
| Model | Parameter | Temperature (K) | ||
|---|---|---|---|---|
| 308 | 313 | 318 | ||
| Langmuir | Qo (mg g−1) | −41.23 | 1483 | −1.927 |
| KL (L g−1) | −112.5 | 7.474 | −146.2 | |
| %R2 | 0.3968 | 0.4634 | 97.00 | |
| χ2 | 36.01 | 4.188 | 39.62 | |
| RMSE | 21.07 | 6.689 | 27.87 | |
| Freundlich | KF (mg g−1) (L mg−1)1/n | 2.901 | 10.75 | 0.002031 |
| N | 0.5293 | 0.9758 | 0.1947 | |
| %R2 | 80.60 | 92.75 | 89.71 | |
| χ2 | 21.55 | 4.316 | 12.52 | |
| RMSE | 16.79 | 7.139 | 10.27 | |
| Dubinin-Radushkevich | Qs (mg g−1) | 6.466 | 10.12 | 0.0295 |
| (mol2 kJ−2) (×−10−8) | 10.30 | 8.256 | 2.453 | |
| E (kJ mol−1) | 2.203 | 2.461 | 1.428 | |
| %R2 | 75.15 | 82.10 | 90.73 | |
| χ2 | 25.50 | 9.449 | 11.50 | |
| RMSE | 17.29 | 10.46 | 9.710 | |
| Temkin | A (L g−1) | 0.4752 | 1.382 | 0.2495 |
| B | 77.64 | 25.43 | 65.02 | |
| %R2 | 90.25 | 91.47 | 61.53 | |
| χ2 | 8.300 | 2.500 | 2.293 | |
| RMSE | 11.36 | 5.443 | 12.54 | |
| Harkins–Jura Model | A | 76.57 | 73.49 | 1.456 |
| B (×−10−2) | 75.18 | 59.66 | 80.29 | |
| %R2 | 52.12 | 75.06 | 95.96 | |
| χ2 | 1666 | 451.0 | 18.89 | |
| RMSE | 69.28 | 34.48 | 12.42 | |
| Model | Parameter | Values |
|---|---|---|
| Pseudo-First-Order Kinetic Model | k1 (min−1) | 0.2750 |
| Qe (mg g−1) | 8.660 | |
| %R2 | 61.32 | |
| χ2 | 836.4 | |
| RMSE | 369.7 | |
| Pseudo-Second-Order Kinetic Model | k2 (g g−1) (L mg−1)1/n | 52.67 |
| Qe (mg g−1) | 80.89 | |
| %R2 | 99.93 | |
| χ2 | 0.1116 | |
| RMSE | 1.661 | |
| Intraparticle Diffusion Model | kid (mg g−1 min−0.5) | 3.627 |
| C (mg g−1) | 68.59 | |
| %R2 | 76.92 | |
| χ2 | 0.06649 | |
| RMSE | 1.291 | |
| Elovich Model | a (g g−1 min−1) (104) | 89.75 |
| b (mg g−1) (10−2) | 27.212 | |
| %R2 | 75.10 | |
| χ2 | 63.65 | |
| RMSE | 32.80 |
| Concentration (mg L−1) | Enthalpy (ΔH) (kJmol−1 K−1) | Entropy (ΔS) (Jmol−1) | ΔG (kJ/mol) | ||
|---|---|---|---|---|---|
| 308 K | 313 K | 318 K | |||
| 5 | −65.94 | −196.8 | −1.475 | −2.159 | 0.1102 |
| 10 | −37.22 | −96.23 | −2.645 | −2.887 | −2.256 |
| 15 | −22.14 | −43.55 | −3.092 | −3.182 | −2.966 |
| 20 | −0.8586 | 27.32 | −3.249 | −3.440 | −3.345 |
| 25 | −1.089 | 28.59 | −3.444 | −3.549 | −3.546 |
| Adsorbent | CV Adsorption Capacity (mg g−1) | References |
|---|---|---|
| Fe–Zn nanocomposites | 40.20 | This research |
| Magnetic κ-carrageenan-g-poly (methacrylic acid) | 28.24 | [52] |
| Coconut husk-based activated carbon | 25.74 | [51] |
| TLAC/Chitosan composite | 12.50 | [53] |
| Pumice Stone | 6.990 | [54] |
| clay/poly(N-isopropylacrylamide) hydrogel | 4.710 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, H.; Saad, M.; Attala, O.A.; El-Saoud, W.A.; Attia, K.A.; Jabeen, S.; Zeb, J. Sustainable Synthesis of Iron–Zinc Nanocomposites by Azadirachta indica Leaves Extract for RSM-Optimized Sono-Adsorptive Removal of Crystal Violet Dye. Materials 2023, 16, 1023. https://doi.org/10.3390/ma16031023
Tahir H, Saad M, Attala OA, El-Saoud WA, Attia KA, Jabeen S, Zeb J. Sustainable Synthesis of Iron–Zinc Nanocomposites by Azadirachta indica Leaves Extract for RSM-Optimized Sono-Adsorptive Removal of Crystal Violet Dye. Materials. 2023; 16(3):1023. https://doi.org/10.3390/ma16031023
Chicago/Turabian StyleTahir, Hajira, Muhammad Saad, Osama A. Attala, Waleed A. El-Saoud, Kamal A. Attia, Shaista Jabeen, and Jahan Zeb. 2023. "Sustainable Synthesis of Iron–Zinc Nanocomposites by Azadirachta indica Leaves Extract for RSM-Optimized Sono-Adsorptive Removal of Crystal Violet Dye" Materials 16, no. 3: 1023. https://doi.org/10.3390/ma16031023





