Nanolithographic Fabrication Technologies for Network-Based Biocomputation Devices
Abstract
:1. Introduction
2. Fundamentals of Network-Based Biocomputation (NBC): A Brief Description
2.1. Working Principle of NBC
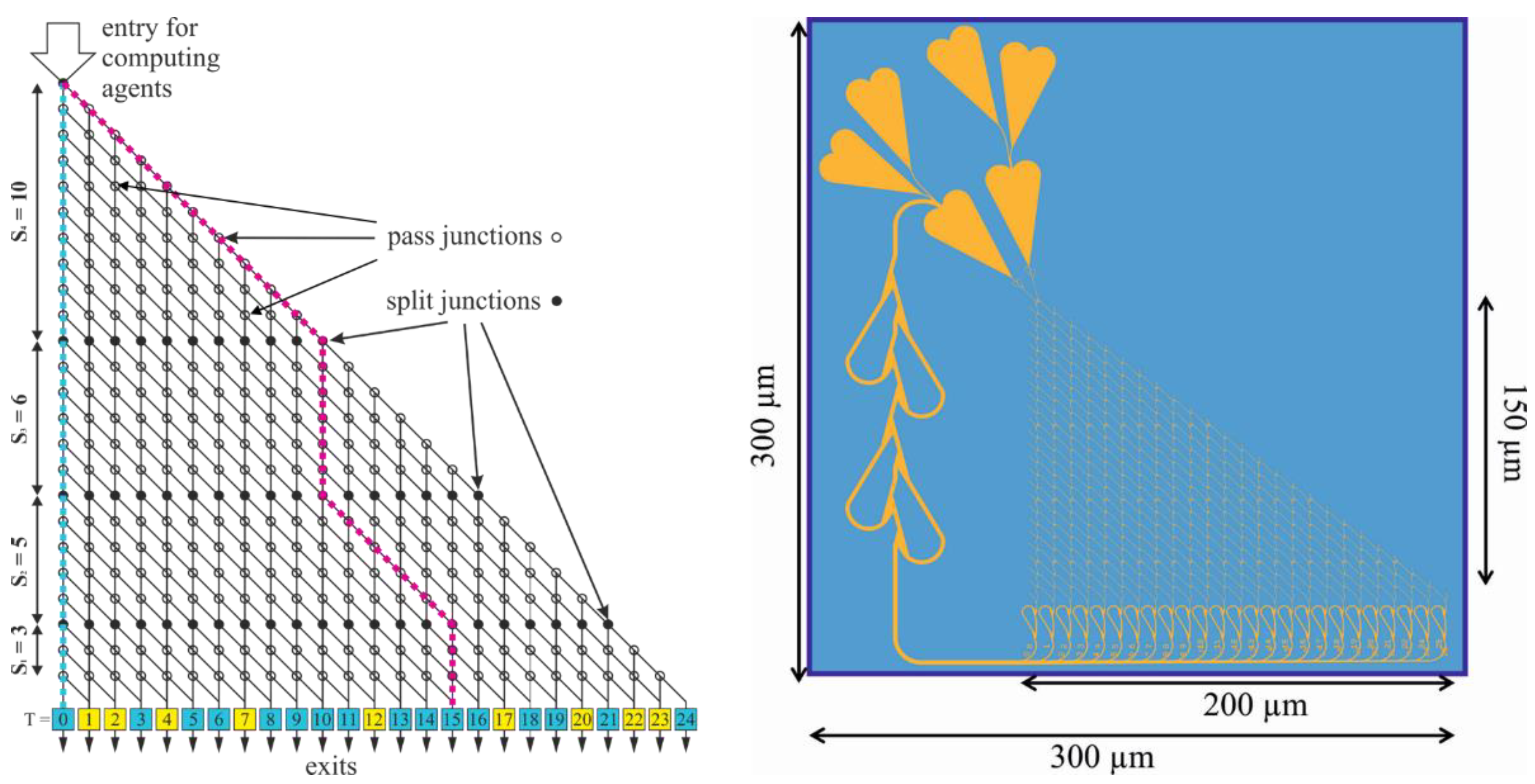
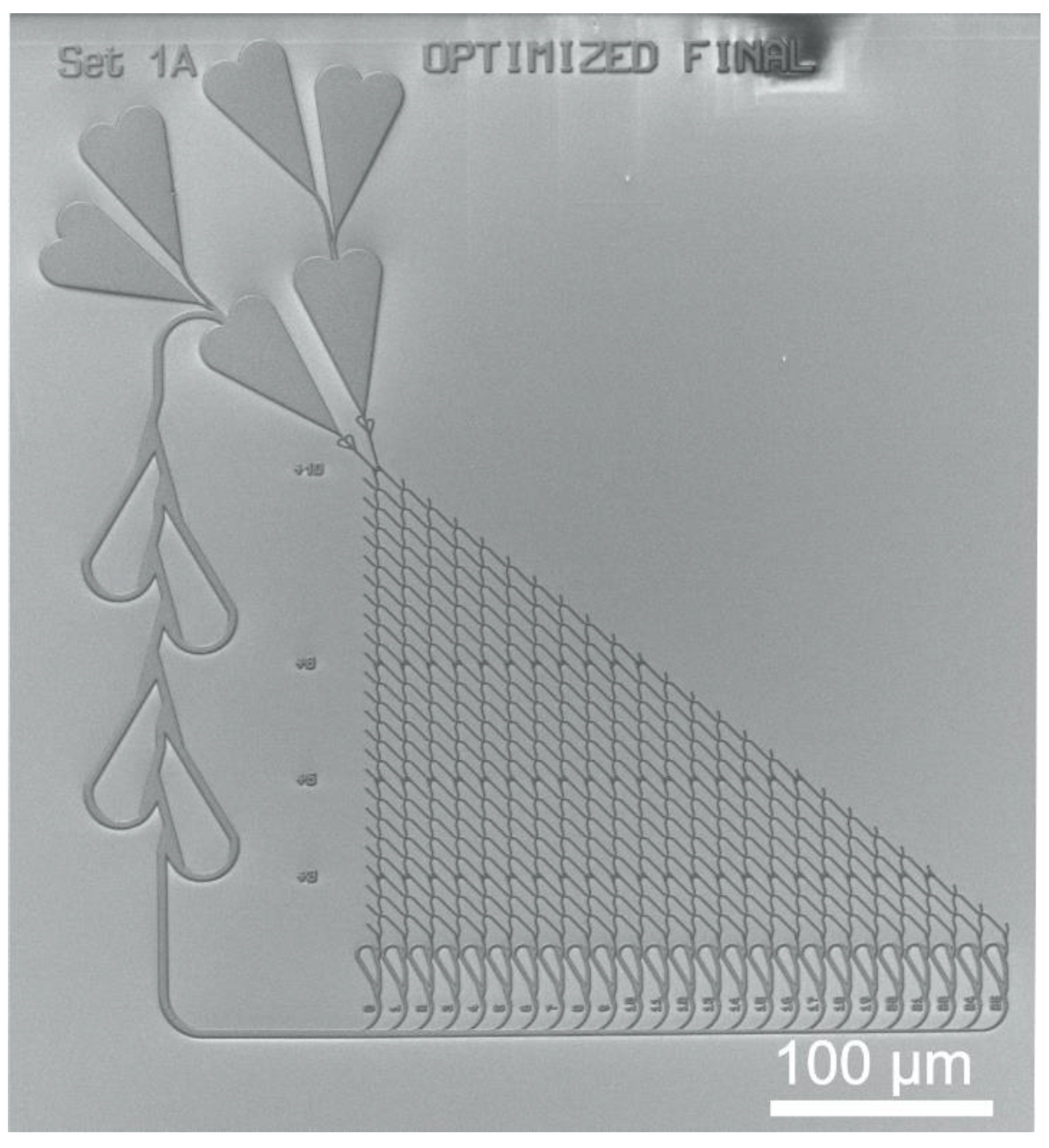
2.2. NBC Network Layout
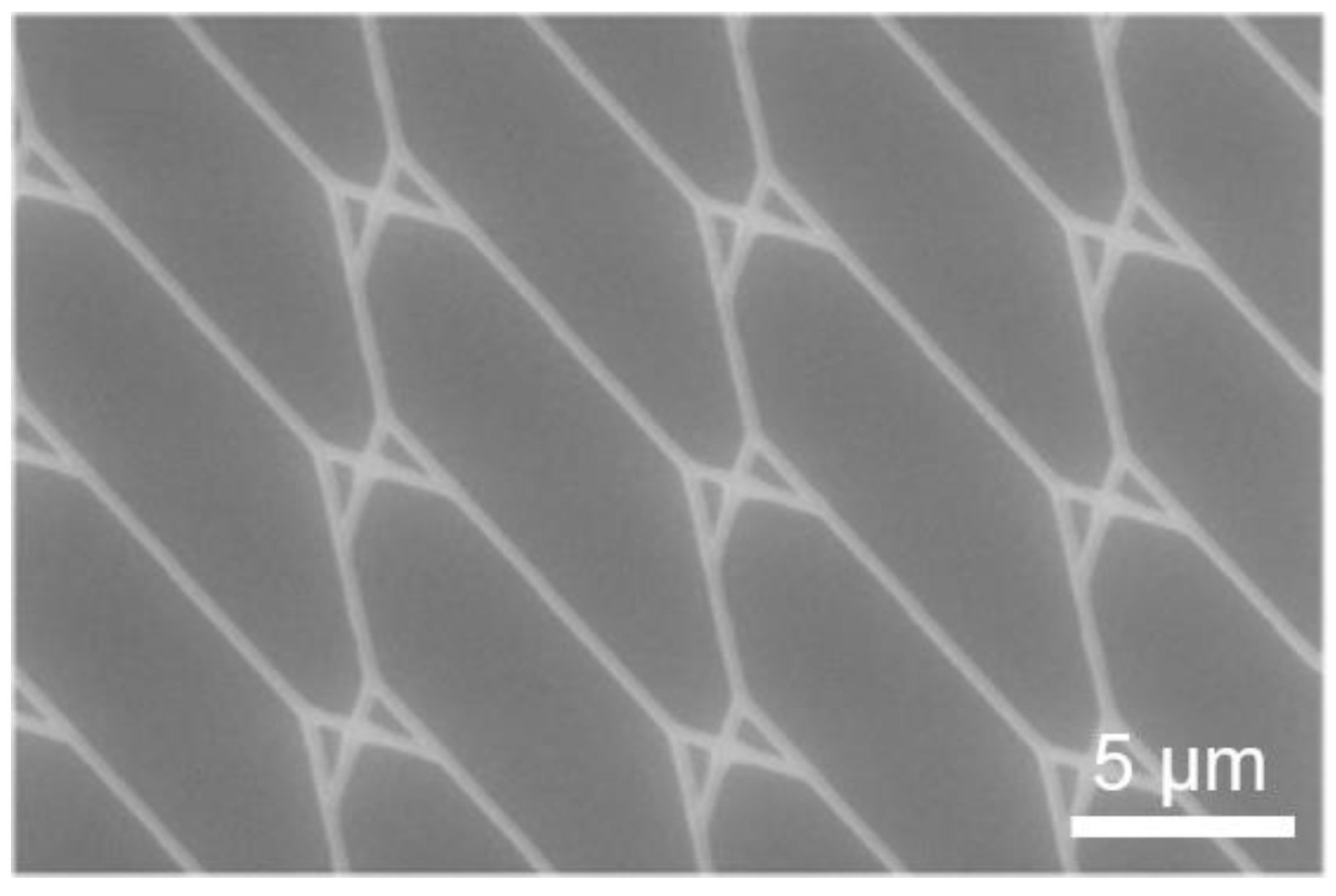
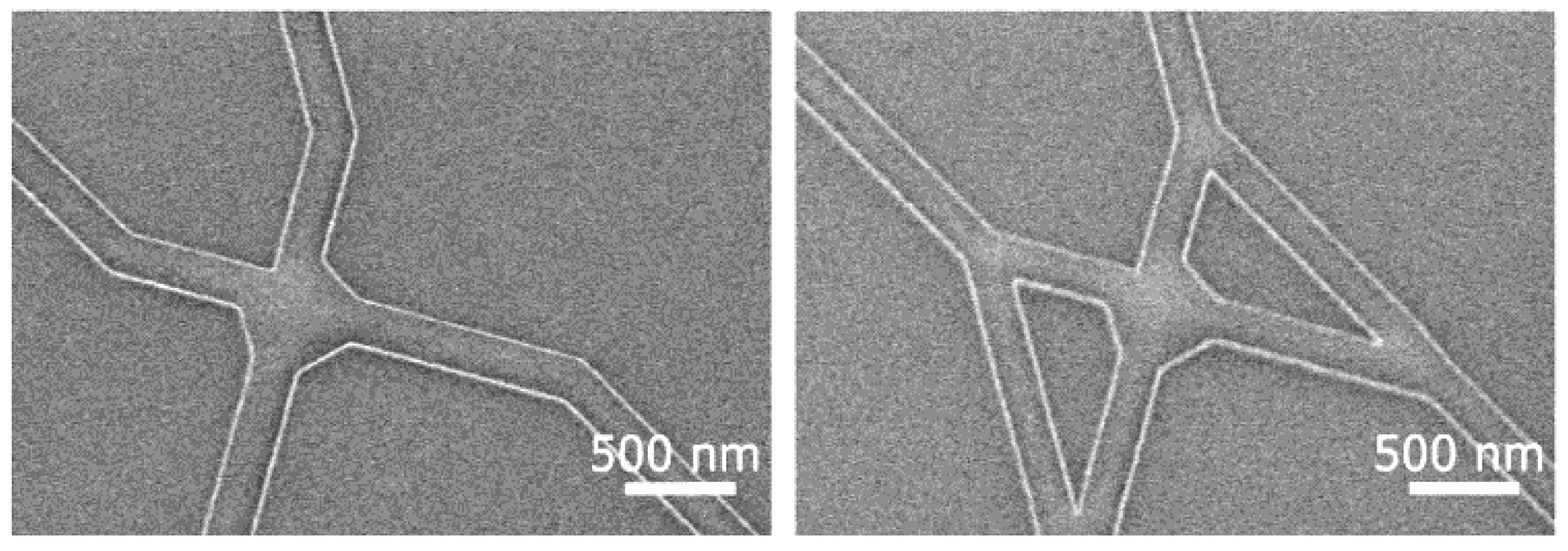
3. Nanofabrication Technologies for NBC
3.1. Microtubule-Kinesin System
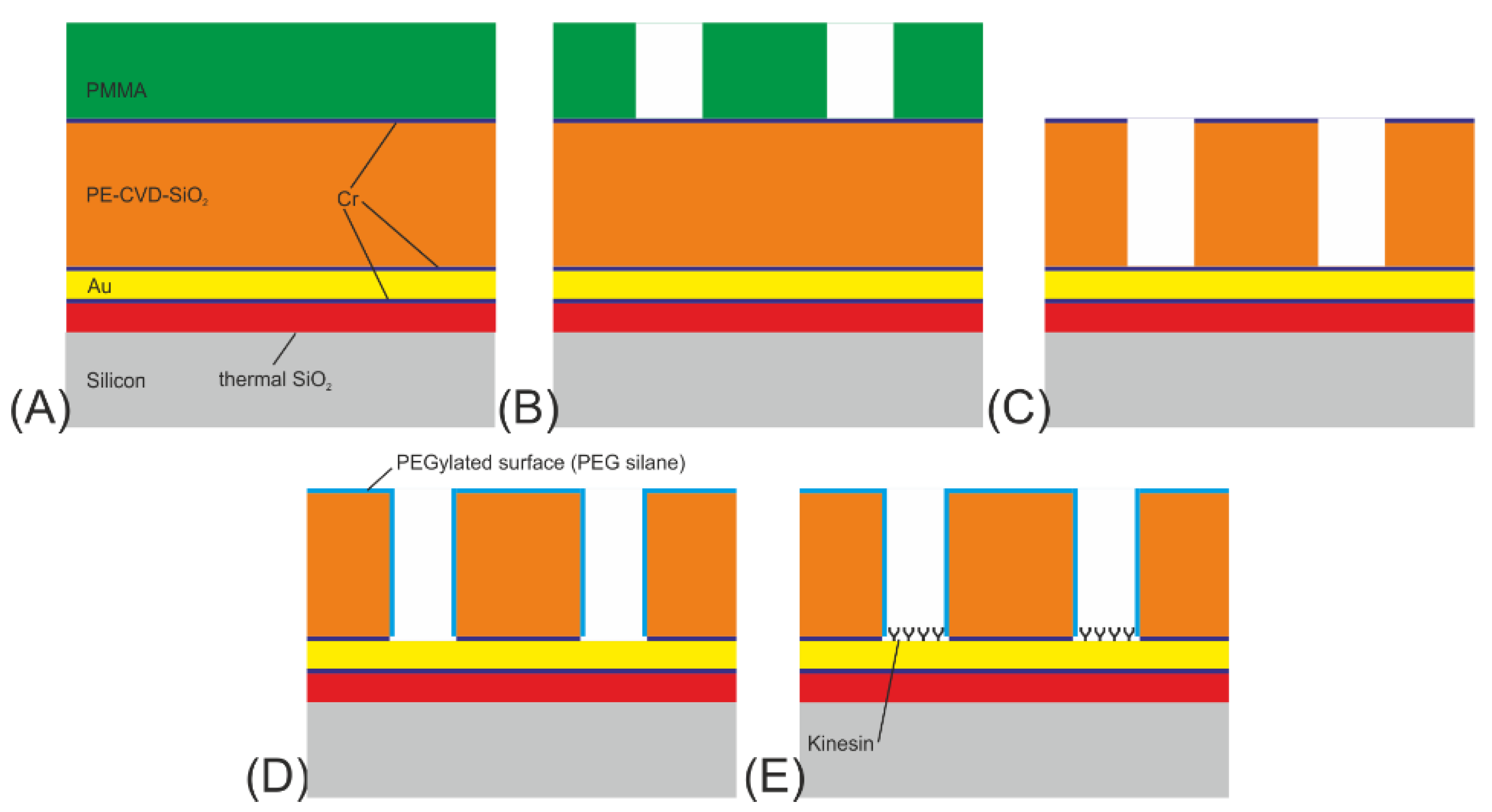
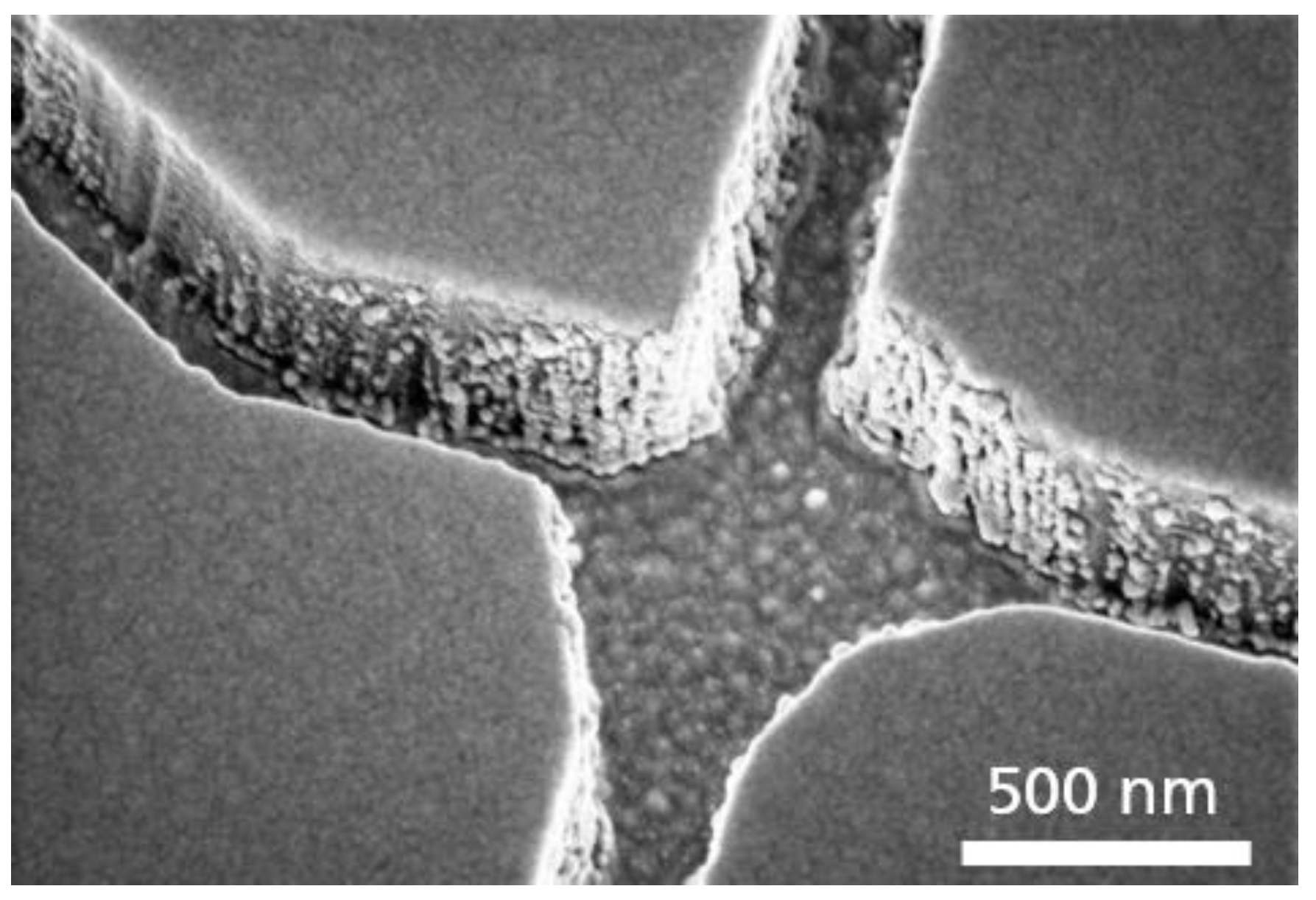
- The initial layer on the Si-substrate was a 100 nm thick SiO2 diffusion barrier layer made by dry thermal oxidation, carried out under an oxygen atmosphere with 3% HCl. An in situ sputter deposition followed, in which a 10 nm Cr adhesion layer, a 100 nm Au layer, and finally a 10 nm Cr layer were deposited onto the SiO2 diffusion barrier layer. In addition, a 10 nm thick Ti layer was investigated as an adhesion layer instead of the Cr layer. Next, a 500 nm SiO2 layer was deposited using plasma-enhanced chemical vapor deposition (PE-CVD) at 300 °C. Finally, a 10 nm Cr layer acting as a hard mask for the SiO2 patterning was sputter-deposited onto the surface (see Figure 2A).
- The wafer was subsequently spin-coated with PMMA (ALLRESIST AR-P 679.04), a positive-tone electron-beam resist, to a resist thickness of 400 nm and pre-baked at 180 °C for 5 min (see Figure 2a). For the exposure, a 50 kV e-beam lithography system (Vistec SB254) was used at a dose of 650 µC/cm2. The PMMA was developed for 60 s at room temperature in a solution of one part methyl isobutyl ketone (MIBK) and three parts of isopropanol (IPA), rinsed with IPA, and flushed in a conductance-controlled bath of deionized (DI) water. In the final step, all remaining surface humidity was removed in a commercial dryer. This left the wafer with a resist mask on top, ready to be used as an etch mask for the underlying chromium layer (see Figure 2B).
- The resilience of PMMA against plasma etching is quite low; therefore, the resist pattern was transferred into a Cr layer acting as a hard mask for the subsequent structuring of the SiO2-layer. Cr was used as mask material because it provides high pattern fidelity and smooth channel sidewalls, which is important for the next SiO2 etch step. Etching of the chromium hard mask was performed in a FHR MS-200-2-AE system using a mixture of Cl2 (100 sccm) and O2 (30 sccm) at a pressure of 32 Pa, a power of 300 W and a chuck temperature of 8 °C for 150 s. The patterning of the SiO2 channels was subsequently performed at an ICP Oxford Plasmalab System 100, using a gas flow of 10 sccm CHF3 and 18 sccm C4F8. The etching time was 180 s (see Figure 2C).
- In the following process step, the remaining resist residues and Cr on top of the SiO2 as well as on the channel floors were removed using O2 (1850 W) in an R3T STP2020 plasma reactor, and the SiO2 surface was passivated using PEG (see Figure 2D).
- Biofunctionalization of the Au surface with kinesin-1 motor proteins followed just before the experiment (see Figure 2E).
3.2. Actin-Myosin System
- A 70 nm thick SiO2 layer was deposited onto the Si-substrate by atomic layer deposition (ALD), using pulses of bisdiethylaminosilane as a precursor in an oxygen plasma. The reason for applying this layer is twofold: to enable surface derivatization by TMCS to modify the surface hydrophobicity, and to enhance the contrast of fluorescently labeled actin filaments. The latter, known as fluorescence interference contrast (FLIC), enables signal enhancement through constructive interference of the emission signals of the fluorophores located on the filaments, excited by either direct light from the light source or light reflected by the Si surface. Subsequently, a layer of CSAR 62 (ALLRESIST AR-P 6200) was spin-coated onto the SiO2, to a thickness of around 360 nm and pre-baked at 180 °C for 2 min.
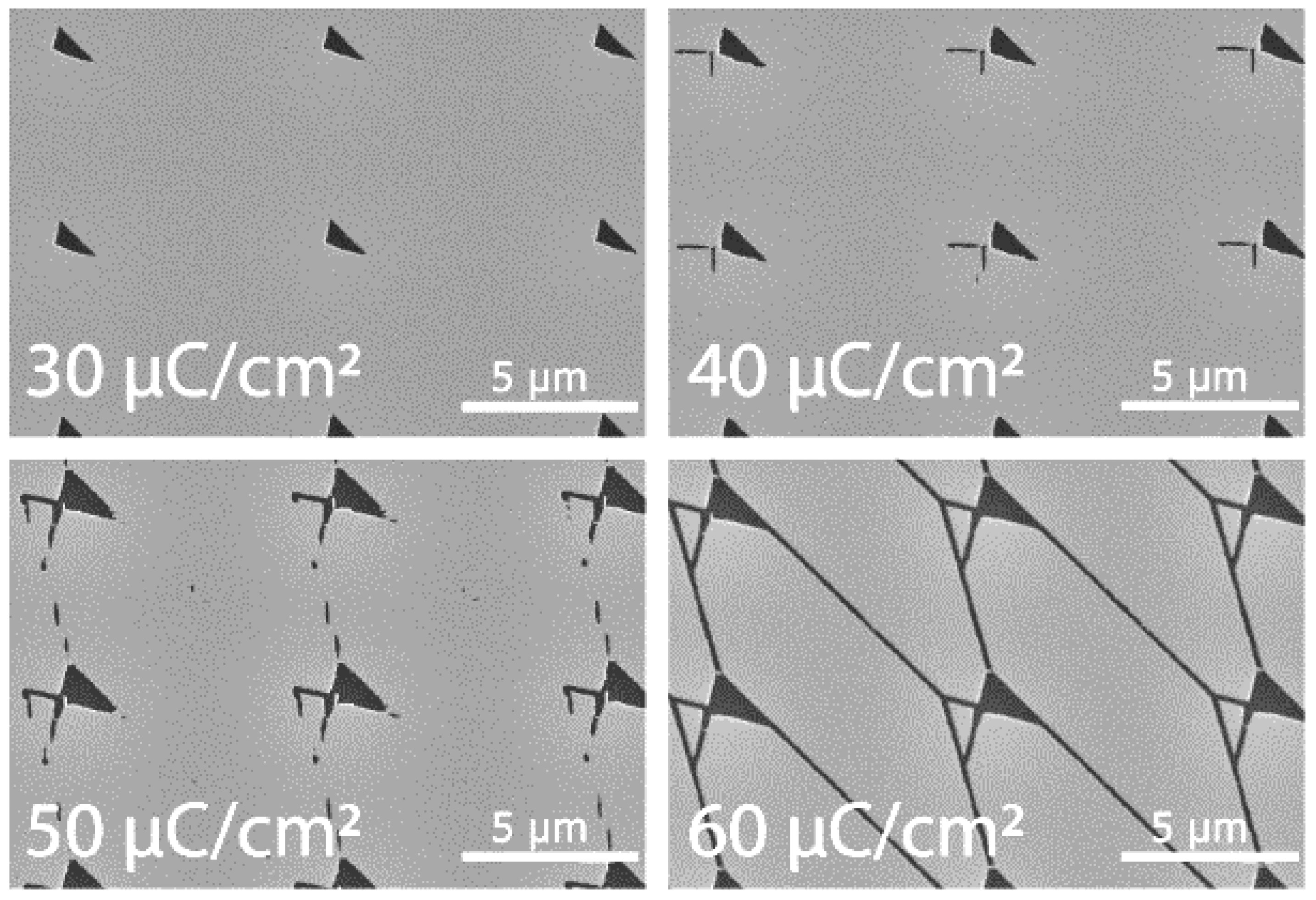
- The network was patterned by EBL (Raith150) at 50 kV with a dose of 60 µC/cm2. The CSAR 62 was then developed for 2 min in O-xylene, rinsed with IPA, and dried with N2 gas.
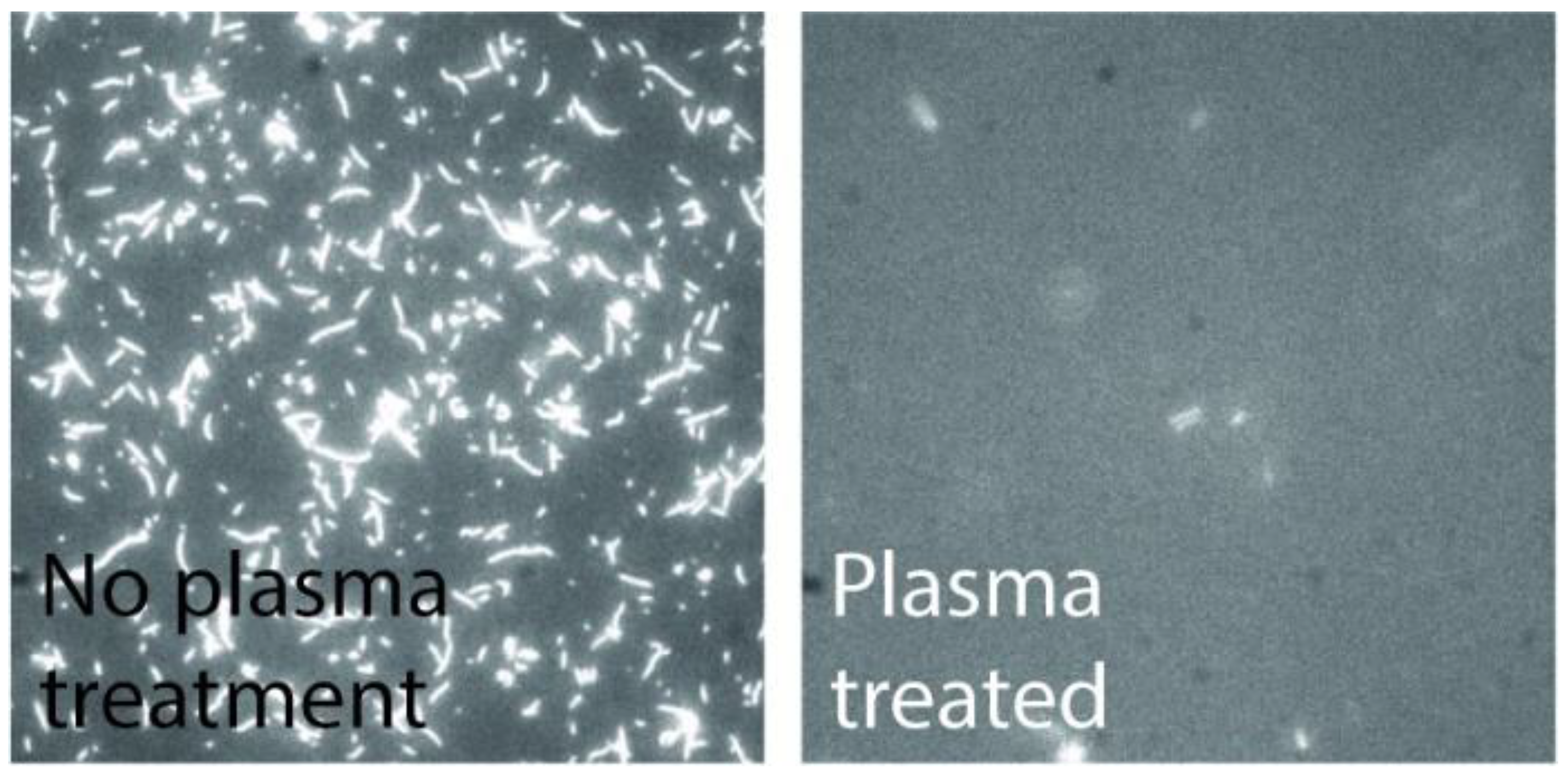
- To remove any resist residues and to activate the SiO2 surface with OH-groups, the devices were ashed in an oxygen plasma (750 W) for 15 s at 5 mbar. Once activated, the surface was derivatized with trimethyl chlorsilane (TMCS) by chemical vapor deposition as described in Ref. [29] at 200 mbar for 64 min at room temperature, providing a water contact angle of app. 75°. The plasma ashing has the additional benefit of making the surrounding resist surfaces and walls negatively charged and hydrophilic, which causes them to adsorb the motors in a non-functional form.
- Biofunctionalization of the SiO2 surface using myosin II motor proteins followed.
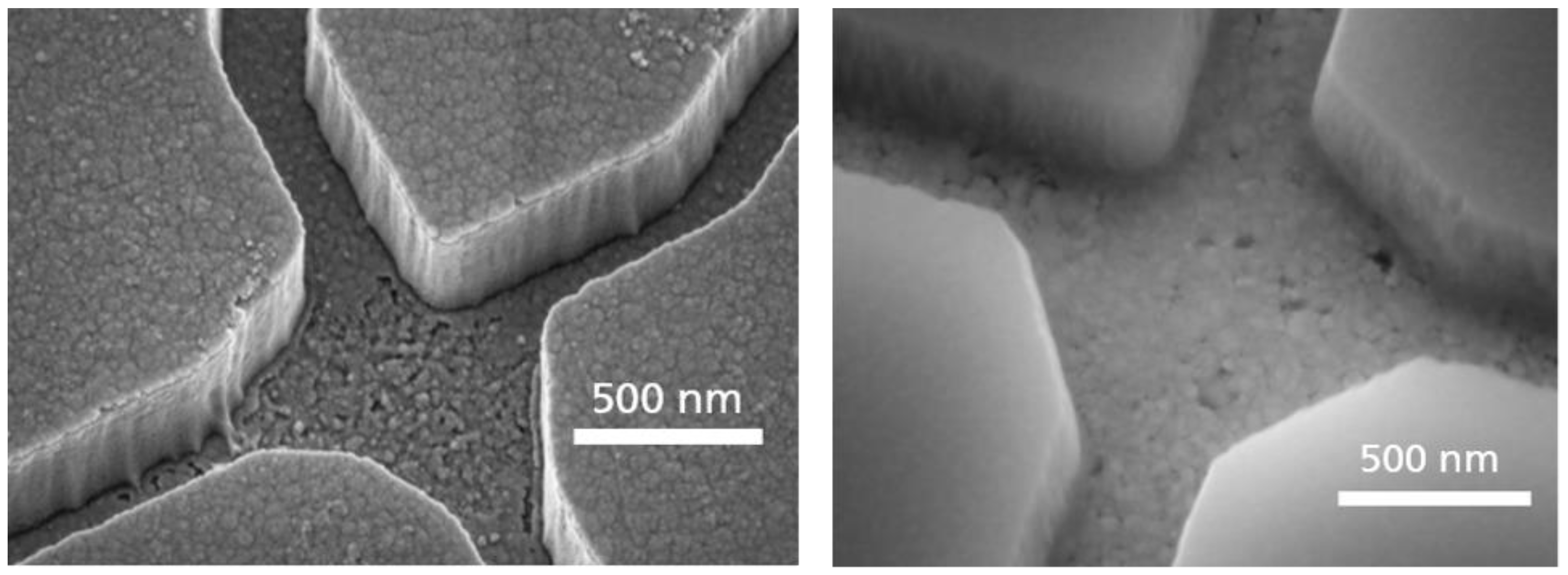
4. Results of the EBL Technology Optimizations for NBC and Their Discussion
- Fabrication of smooth and narrow channels that reliably guide motility and do not allow the filaments to make U-turns [33]
- Fabrication of devices that ensure continuous motility from the source of agents to the pool of possible solutions [34].
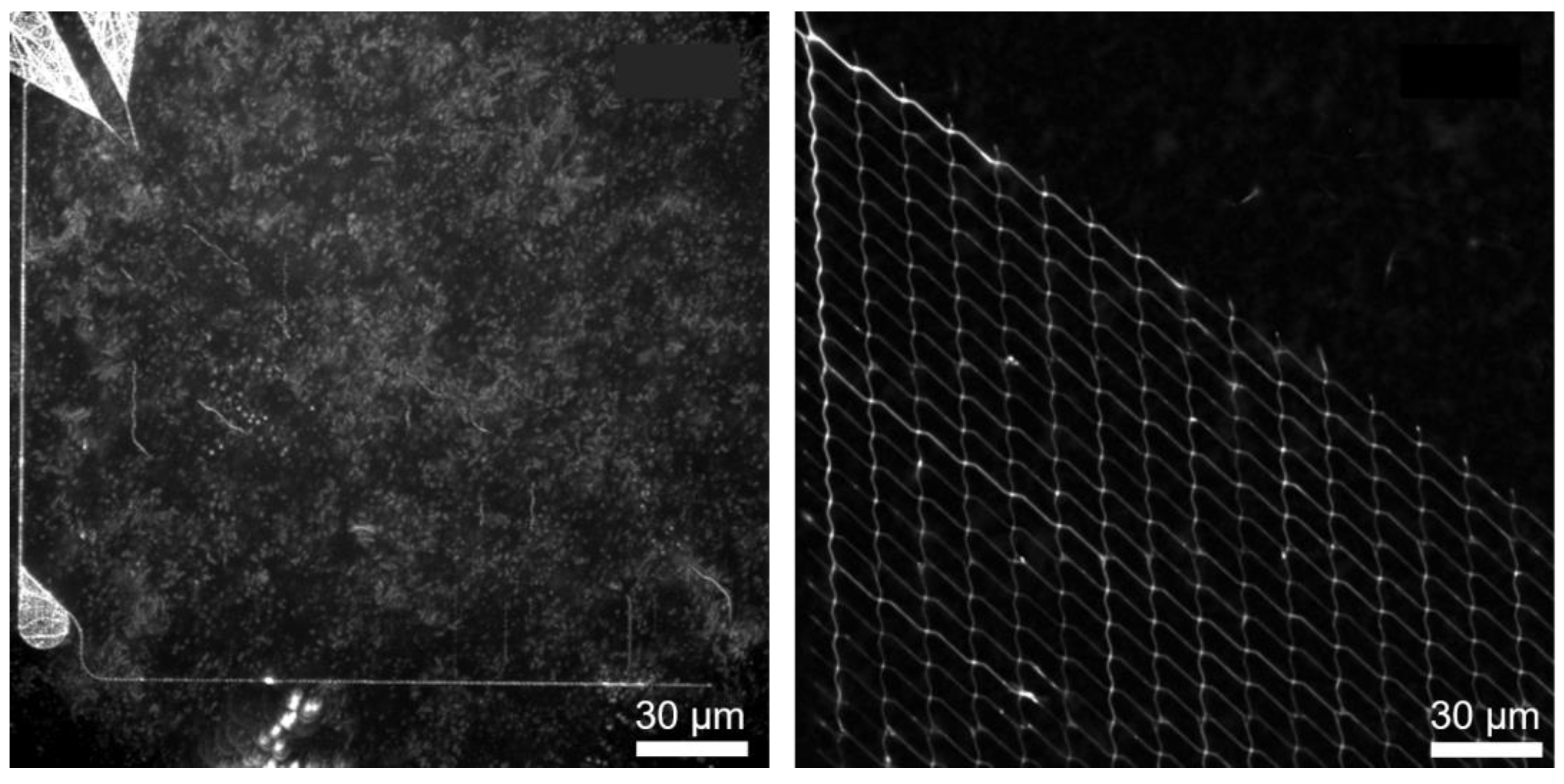
4.1. Microtubule-Kinesin System
4.2. Actin-Myosin System
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scudellari, M. DNA for data storage and computing. Proc. Natl. Acad. Sci. USA 2015, 112, 15771–15772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladd, T.D.; Jelezko, F.; Laflamme, R.; Nakamura, Y.; Monroe, C.; O’Brien, J.L. Quantum computers. Nature 2010, 464, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolau, D.V., Jr.; Nicolau, D.V., Sr.; Solana, G.; Hanson, K.L.; Filipponi, L.; Wang, L.; Lee, L. Molecular motors-based micro-and nano-biocomputation devices. Microelectron. Eng. 2006, 83, 1582–1588. [Google Scholar] [CrossRef]
- Nicolau, D.V., Jr.; Lard, M.; Korten, T.; van Delft, F.C.M.J.M.; Persson, M.; Bengtsson, E.; Månsson, A.; Diez, S.; Linke, H.; Nicolau, D.V., Sr. Parallel computation with molecular-motor-propelled agents in nanofabricated networks. Proc. Natl. Acad. Sci. USA 2016, 113, 2591–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundberg, M.; Bunk, R.; Albet-Torres, N.; Kvennefors, A.; Persson, F.; Montelius, L.; Nicholls, I.A.; Ghatnekar-Nilsson, S.; Omling, P.; Tågeud, S.; et al. Actin filament guidance on a chip: Toward high-throughput assays and lab-on-a-chip applications. Langmuir 2006, 22, 7286–7295. [Google Scholar] [CrossRef]
- Zhu, J.; Salhotra, A.; Meinecke, C.R.; Surendiran, P.; Lyttleton, R.; Reuter, D.; Kugler, H.; Diez, S.; Månsson, A.; Linke, H.; et al. Solving the 3-Satisfiability Problem Using Network-Based Biocomputation. Adv. Intell. Syst. 2022, 4, 2200202. [Google Scholar] [CrossRef]
- Nitzsche, B.; Bormuth, V.; Bräuer, C.; Howard, J.; Ionov, L.; Kerssemakers, J.; Korten, T.; Leduc, C.; Ruhnow, F.; Diez, S. Studying kinesin motors by optical 3D-nanometry in gliding motility assays. Methods Cell Biol. 2010, 95, 247–271. [Google Scholar]
- Korten, T.; Chaudhuri, S.; Tavkin, E.; Braun, M.; Diez, S. Kinesin-1 Expressed in Insect Cells Improves Microtubule in Vitro Gliding Performance, Long-Term Stability and Guiding Efficiency in Nanostructures. IEEE Trans. NanoBioscience 2016, 15, 62–69. [Google Scholar] [CrossRef]
- van Delft, F.C.M.J.M.; Ipolitti, G.; Nicolau, D.V., Jr.; Perumal, A.S.; Kăspar, O.; Kheireddine, S.; Wachsmann-Hogiu, S.; Nicolau, D.V., Sr. Something has to give: Scaling combinatorial computing by biological agents exploring physical networks encoding NP-complete problems. Interface Focus 2018, 8, 20180034. [Google Scholar] [CrossRef] [Green Version]
- Blaudeck, T.; Meinecke, C.R.; Reuter, D.; Steenhusen, S.; Jain, A.; Hermann, S.; Schulz, S.E.; Zenkevich, E.I.; Korten, T.; Linke, H. Biocomputation Using Molecular Agents Moving in Microfluidic Channel Networks: An Alternative Platform for Information Technology. In Cyber-Physical Systems: Intelligent Models and Algorithms, Springer Studies in Systems, Decision and Control; Kravets, A.G., Bolshakov, A.A., Shcherbakov, M., Eds.; Springer: Cham, Switzerland, 2022; Volume 417, pp. 15–27. [Google Scholar]
- Hanson, K.L.; Nicolau, D.V., Jr.; Filipponi, L.; Wang, L.S.; Lee, A.P.; Nicolau, D.V., Sr. Fungi use efficient algorithms for the exploration of microfluidic networks. Small 2006, 2, 1212–1220. [Google Scholar] [CrossRef]
- Blaudeck, T.; Meinecke, C.R.; Reuter, D.; Steenhusen, S.; Schulz, S.E.; Zenkevich, E.I.; Korten, T. Computation with biological molecules -an alternative approach in information technologies. Math. Methods Eng. Technol. 2021, 9, 56–60. [Google Scholar] [CrossRef]
- Surendiran, P.; Meinecke, C.R.; Salhotra, A.; Heldt, G.; Zhu, J.Y.; Månsson, A.; Diez, S.; Reuter, D.; Kugler, H.; Linke, H.; et al. Solving Exact Cover Instances with Molecular-Motor-Powered Network-Based Biocomputation. ACS Nanosci. Au 2022, 2, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Silvano, M.; Paolo, T. 4 Subset-sum problem. Knapsack problems: Algorithms and computer interpretations; Wiley-Interscience: Hoboken, NJ, USA, 1990; pp. 105–136. [Google Scholar]
- Korten, T.; Diez, S.; Linke, H.; Nicolau, D.V., Jr.; Kugler, H. Design of network-based biocomputation circuits for the exact cover problem. New J. Phys. 2021, 23, 085004. [Google Scholar] [CrossRef]
- van Delft, F.C.M.J.M.; Månsson, A.; Kugler, H.; Korten, T.; Reuther, C.; Zhu, J.Y.; Lyttleton, R.; Blaudeck, T.; Meinecke, C.R.; Reuter, D.; et al. Roadmap for network-based biocomputation. Nano Futures 2022, 6, 032002. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Korten, T.; Kugler, H.; van Delft, F.C.M.J.M.; Månsson, A.; Reuter, D.; Diez, S.; Linke, H. Physical requirements for scaling up network-based biocomputation. New J. Phys. 2021, 23, 105004. [Google Scholar] [CrossRef]
- Clemmens, J.; Hess, H.; Lipscomb, R.; Hanein, Y.; Boehringer, K.F.; Matzke, C.M.; Bachand, G.D.; Bunker, B.C.; Vogel, V. Mechanisms of Microtubule Guiding on Microfabricated Kinesin-Coated Surfaces: Chemical and Topographic Surface Patterns. Langmuir 2003, 19, 10967–10974. [Google Scholar] [CrossRef]
- Howard, J.; Hudspeth, A.J.; Vale, R.D. Movement of microtubules by single kinesin molecules. Nature 1989, 342, 154–158. [Google Scholar] [CrossRef]
- Reuther, C.; Mittasch, M.; Naganathan, S.R.; Grill, S.W.; Diez, S. Highly-Efficient Guiding of Motile Microtubules on Non-Topographical Motor Patterns. Nano Lett. 2017, 17, 5699–5705. [Google Scholar] [CrossRef] [Green Version]
- Reuther, C.; Steenhusen, S.; Meinecke, C.R.; Surendiran, P.; Salhotra, A.; Lindberg, F.W.; Månsson, A.; Linke, H.; Diez, S. Molecular motor-driven filament transport across three-dimensional, polymeric micro-junctions. New J. Phys. 2021, 23, 125002. [Google Scholar] [CrossRef]
- Uyeda, T.Q.P.; Kron, S.J.; Spudich, J.A. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J. Mol. Biol. 1990, 214, 699–710. [Google Scholar] [CrossRef]
- Salhotra, A.; Zhu, J.Y.; Surendiran, P.; Meinecke, C.R.; Lyttleton, R.; Ušaj, M.; Lindberg, F.W.; Norrby, M.; Linke, H.; Månsson, A. Prolonged function and optimization of actomyosin motility for upscaled network-based biocomputation. New J. Phys. 2021, 23, 085005. [Google Scholar] [CrossRef]
- Baum, M.; Meinecke, C.R.; Blaudeck, T.; Helke, C.; Reuter, D.; Hiller, K.; Hermann, S.; Schulz, S.E.; Kuhn, H. Bridging the gap: Perspectives of nanofabrication technologies for application-oriented research. J. Vac. Sci. Technol. B 2021, 39, 062805. [Google Scholar] [CrossRef]
- van den Heuvel, M.G.L.; Butcher, C.T.; Smeets, R.M.M.; Diez, S.; Dekker, C. High rectifying efficiencies of microtubule motility on kinesin-coated gold nanostructures. Nano Lett. 2005, 5, 1117–1122. [Google Scholar] [CrossRef]
- Castoldi, M.; Popov, A.V. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr. Purif. 2003, 32, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, M.; Balaz, M.; Bunk, R.; Rosengren-Holmberg, J.P.; Montelius, L.; Nicholls, I.A.; Omling, P.; Tågerud, S.; Månsson, A. Selective spatial localization of actomyosin motor function by chemical surface patterning. Langmuir 2006, 22, 7302–7312. [Google Scholar] [CrossRef] [PubMed]
- Månsson, A. Translational actomyosin research: Fundamental insights and applications hand in hand. J. Muscle Res. Cell Motil. 2012, 33, 219–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindberg, F.W.; Norrby, M.; Rahman, M.A.; Salhotra, A.; Takatsuki, H.; Jeppesen, S.; Linke, H.; Månsson, A. Controlled Surface Silanization for Actin-Myosin and Biocompatibility of New Polymer Resists. Langmuir 2018, 34, 8777–8784. [Google Scholar] [CrossRef]
- Rahman, M.A.; Reuther, C.; Lindberg, F.W.; Mengoni, M.; Salhotra, A.; Heldt, G.; Linke, H.; Diez, S.; Månsson, A. Regeneration of Assembled, Molecular-Motor-Based Bionanodevices. Nano Lett. 2019, 19, 7155–7163. [Google Scholar] [CrossRef]
- Persson, M.; Gullberg, M.; Tolf, C.; Lindberg, A.M.; Månsson, A.; Kocer, A. Transportation of Nanoscale Cargoes by Myosin Propelled Actin Filaments. PLoS ONE 2013, 8, e55931. [Google Scholar] [CrossRef] [Green Version]
- Rasband, W.S. ImageJ User Guide; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2012. Available online: https://imagej.nih.gov/ij/ (accessed on 13 August 2018).
- Bunk, R.; Sundberg, M.; Månsson, A.; Nicholls, L.A.; Omling, P.; Tågerud, S.; Montelius, L. Guiding motor-propelled molecules with nanoscale precision through silanized bi-channel structures. Nanotechnology 2005, 16, 710–717. [Google Scholar] [CrossRef]
- Hiratsuka, Y.; Tada, T.; Oiwa, K.; Kanayama, T.; Uyeda, T.Q.P. Controlling the direction of kinesin-driven microtubule movements along microlithographic tracks. Biophys. J. 2001, 81, 1555–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, H.; Fischer, T.; Agarwal, A.; Katira, P.; Finger, I.; Mobley, E.; Tucker, R.; Kerssemakers, J.; Diez, S. Biomolecular motors challenge imaging and enable sensing. Proc. SPIE 2008, 6865, 686505. [Google Scholar]
- Vikhorev, P.G.; Vikhoreva, N.N.; Sundberg, M.; Balaz, M.; Albet-Torres, N.; Bunk, R.; Kvennefors, A.; Liljesson, K.; Nicholls, I.A.; Nilsson, L.; et al. Diffusion Dynamics of Motor-Driven Transport: Gradient Production and Self-Organization of Surfaces. Langmuir 2008, 24, 13509–13517. [Google Scholar] [CrossRef] [PubMed]
- Clemmens, J.; Hess, H.; Doot, R.; Matzke, C.M.; Bachand, G.D.; Vogel, V. Motor-protein “roundabouts”: Microtubules moving on kinesin-coated tracks through engineered networks. Lab Chip 2004, 4, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, F.; Steenhusen, S.; Houbertz, R.; Asche, T.S.; Nickel, J.; Nolte, S.; Tucher, N.; Josten, P.; Obel, K.; Wolter, H.; et al. Two-photon polymerization of inorganic-organic polymers for biomedical and microoptical applications. In Optically Induced Nanostructures Biomedical and Technical Applications; König, K., Ostendorf, A., Eds.; Walter de Gruyter: Berlin, Germany, 2015; Chapter 5. [Google Scholar]
- Steenhusen, S.; Burmeister, F.; Groß, M.; Domann, G.; Houbertz, R.; Nolte, S. Heterogeneous microoptical structures with sub-micrometer precision. Thin Solid Films 2018, 668, 74–80. [Google Scholar] [CrossRef]
- Heldt, G.; Meinecke, C.R.; Steenhusen, S.; Korten, T.; Groß, M.; Domann, G.; Lindberg, F.; Reuter, D.; Diez, S.; Linke, H.; et al. Approach to combine electron-beam lithography and two-photon polymerization for enhanced nanochannels in network-based biocomputation devices. In Proceedings of the SPIE 34th European Mask and Lithography Conference, Grenoble, France, 18–20 June 2018; pp. 175–182. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinecke, C.R.; Heldt, G.; Blaudeck, T.; Lindberg, F.W.; van Delft, F.C.M.J.M.; Rahman, M.A.; Salhotra, A.; Månsson, A.; Linke, H.; Korten, T.; et al. Nanolithographic Fabrication Technologies for Network-Based Biocomputation Devices. Materials 2023, 16, 1046. https://doi.org/10.3390/ma16031046
Meinecke CR, Heldt G, Blaudeck T, Lindberg FW, van Delft FCMJM, Rahman MA, Salhotra A, Månsson A, Linke H, Korten T, et al. Nanolithographic Fabrication Technologies for Network-Based Biocomputation Devices. Materials. 2023; 16(3):1046. https://doi.org/10.3390/ma16031046
Chicago/Turabian StyleMeinecke, Christoph R., Georg Heldt, Thomas Blaudeck, Frida W. Lindberg, Falco C. M. J. M. van Delft, Mohammad Ashikur Rahman, Aseem Salhotra, Alf Månsson, Heiner Linke, Till Korten, and et al. 2023. "Nanolithographic Fabrication Technologies for Network-Based Biocomputation Devices" Materials 16, no. 3: 1046. https://doi.org/10.3390/ma16031046
APA StyleMeinecke, C. R., Heldt, G., Blaudeck, T., Lindberg, F. W., van Delft, F. C. M. J. M., Rahman, M. A., Salhotra, A., Månsson, A., Linke, H., Korten, T., Diez, S., Reuter, D., & Schulz, S. E. (2023). Nanolithographic Fabrication Technologies for Network-Based Biocomputation Devices. Materials, 16(3), 1046. https://doi.org/10.3390/ma16031046




