Effect of Si, Mn, Be and Sr Addition on the Tensile Properties of 6061 Type Alloys: Role of Aging Treatment
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
3.1. General Remarks
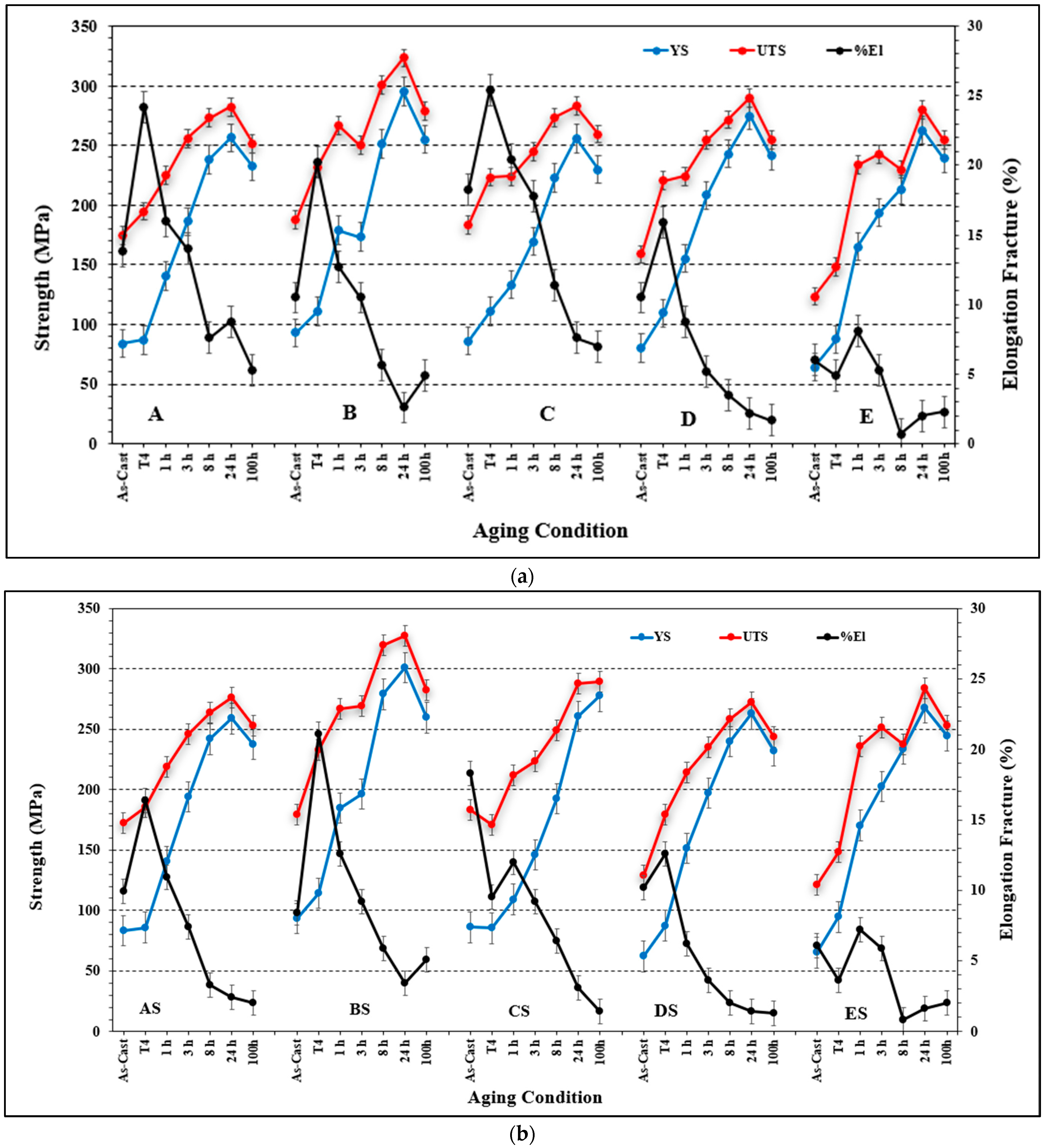
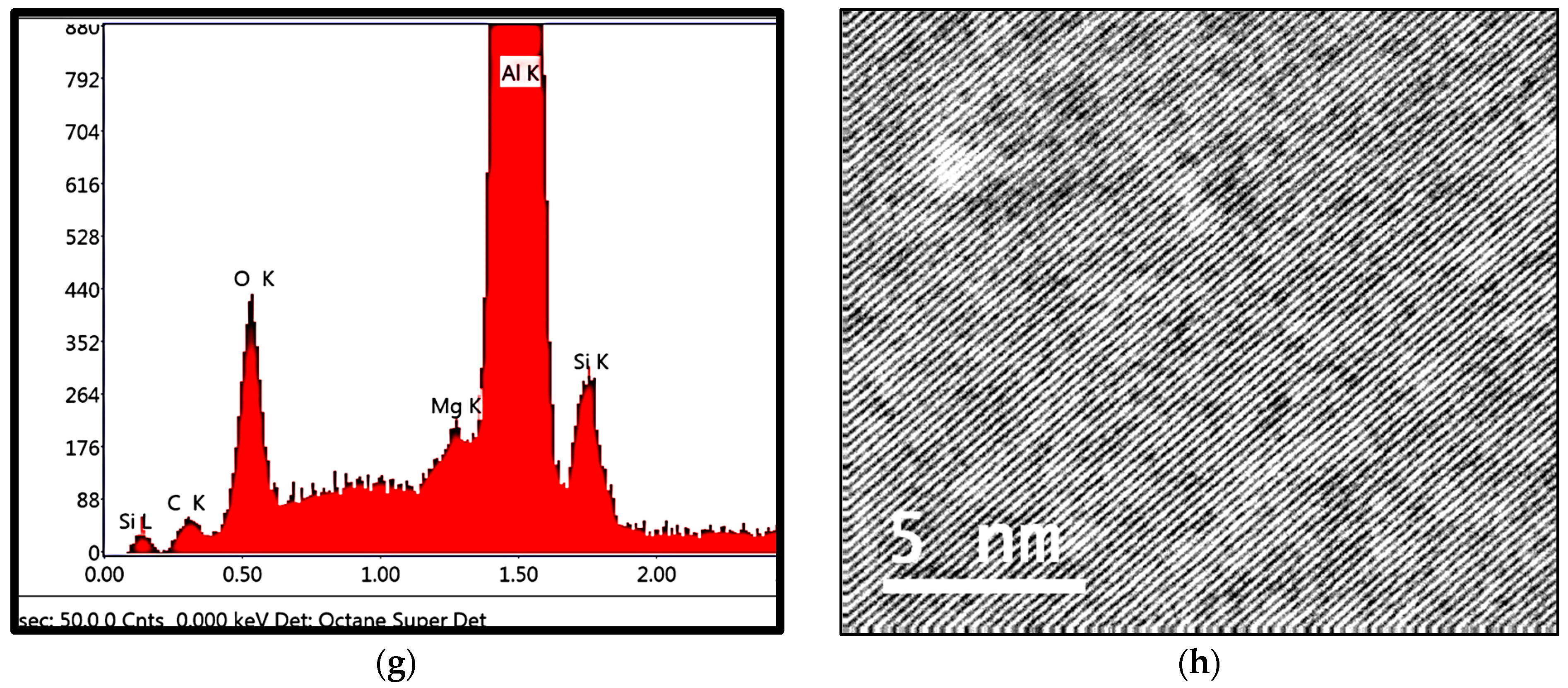
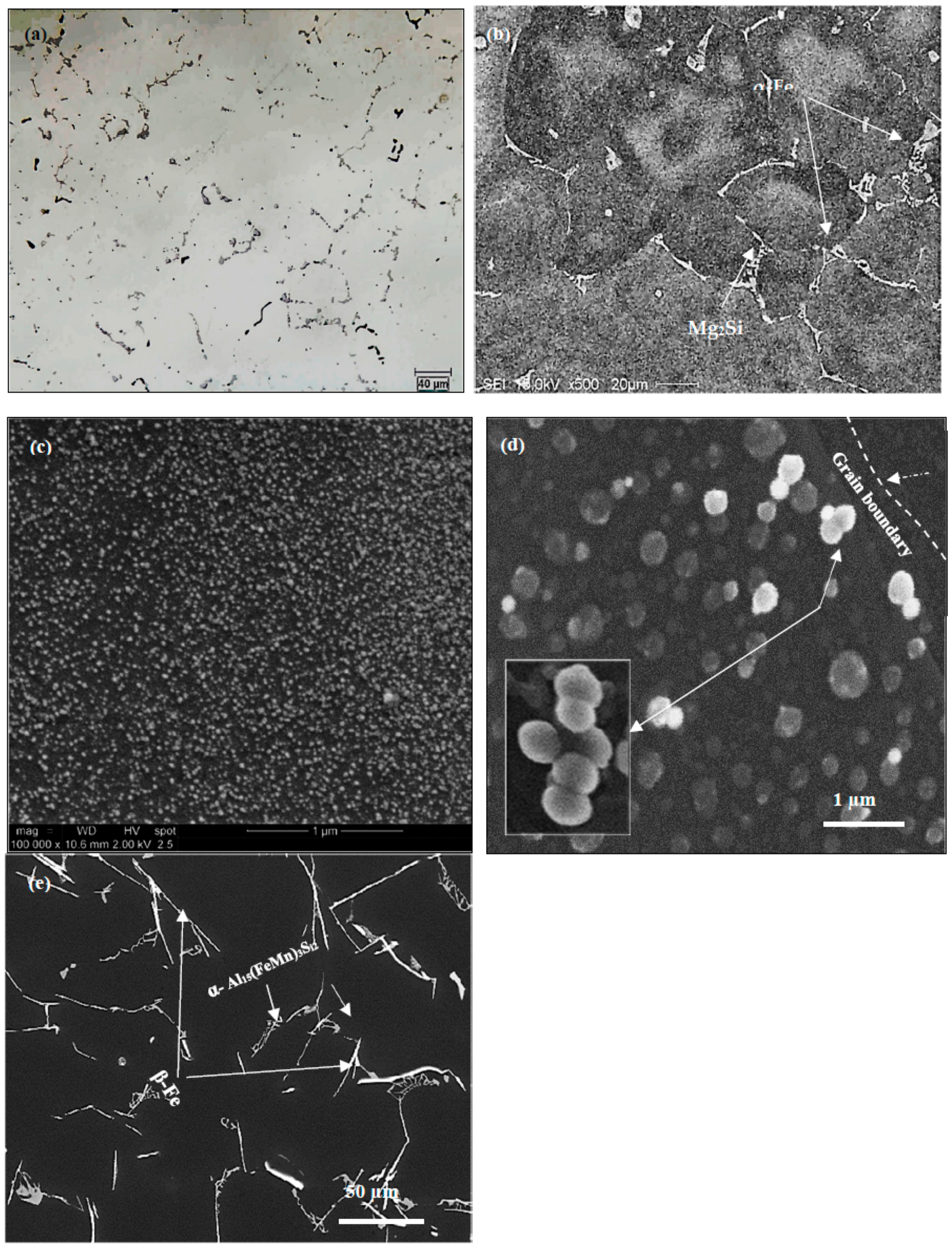
3.2. Tensile Properties and Microstructural Characterization
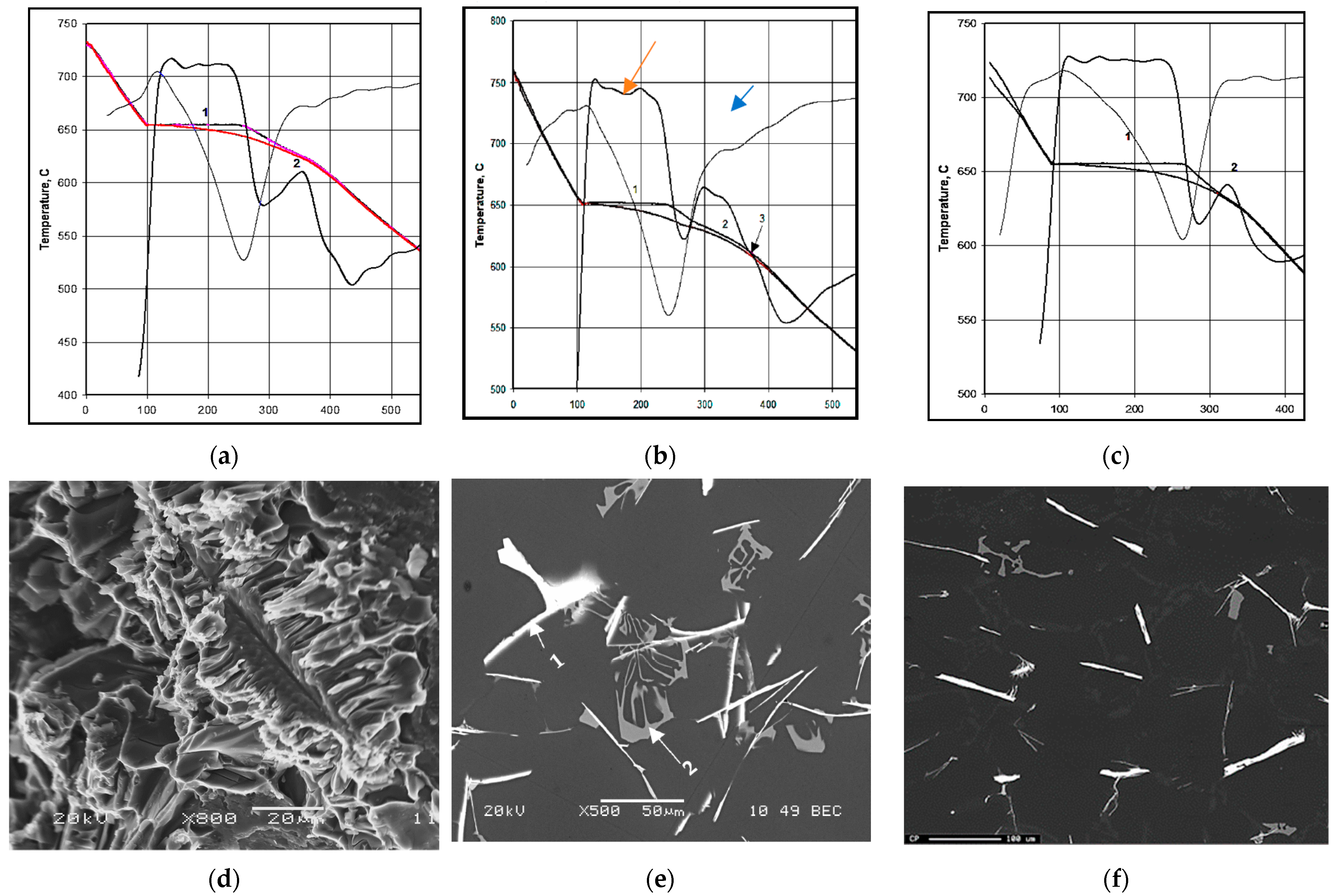
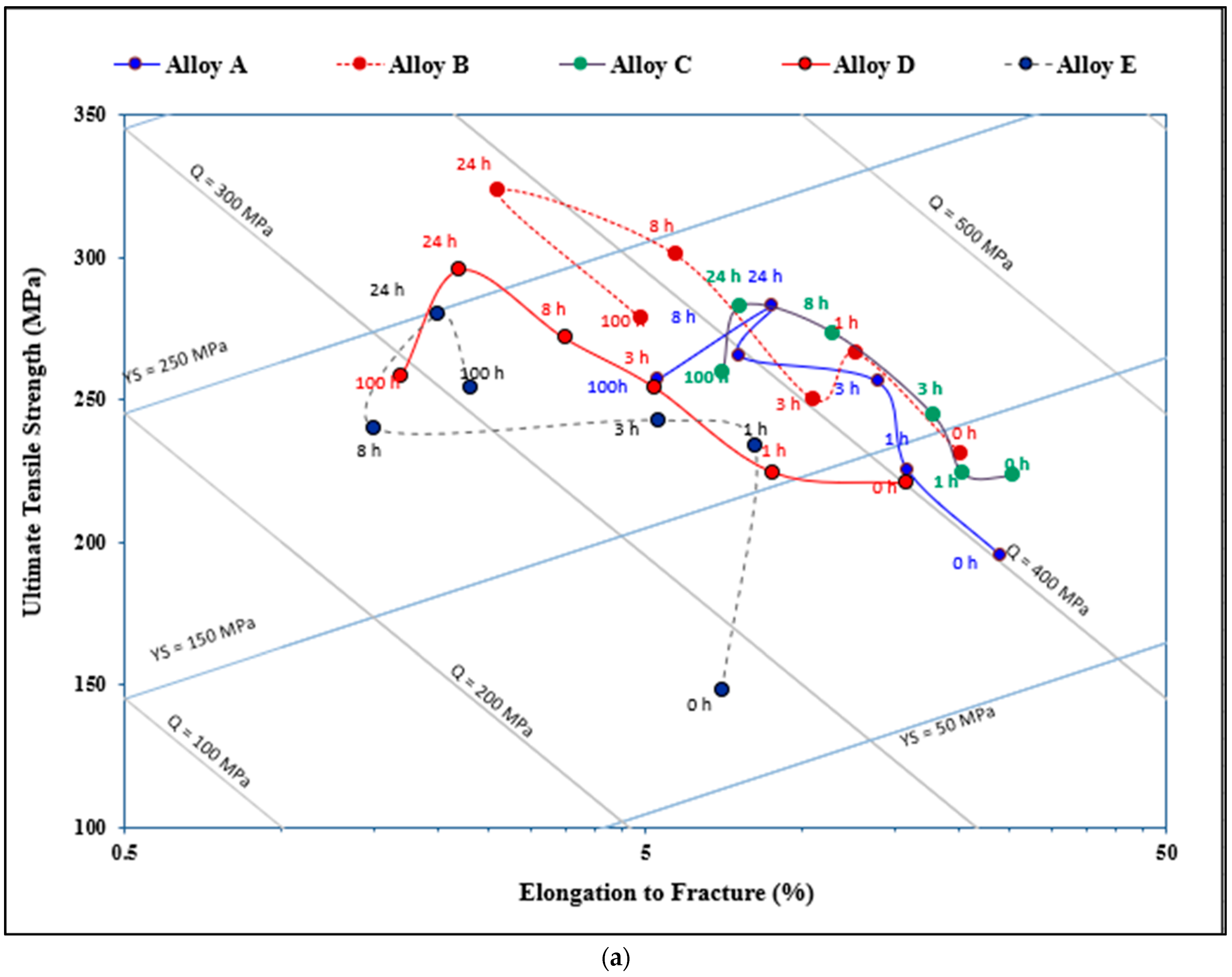
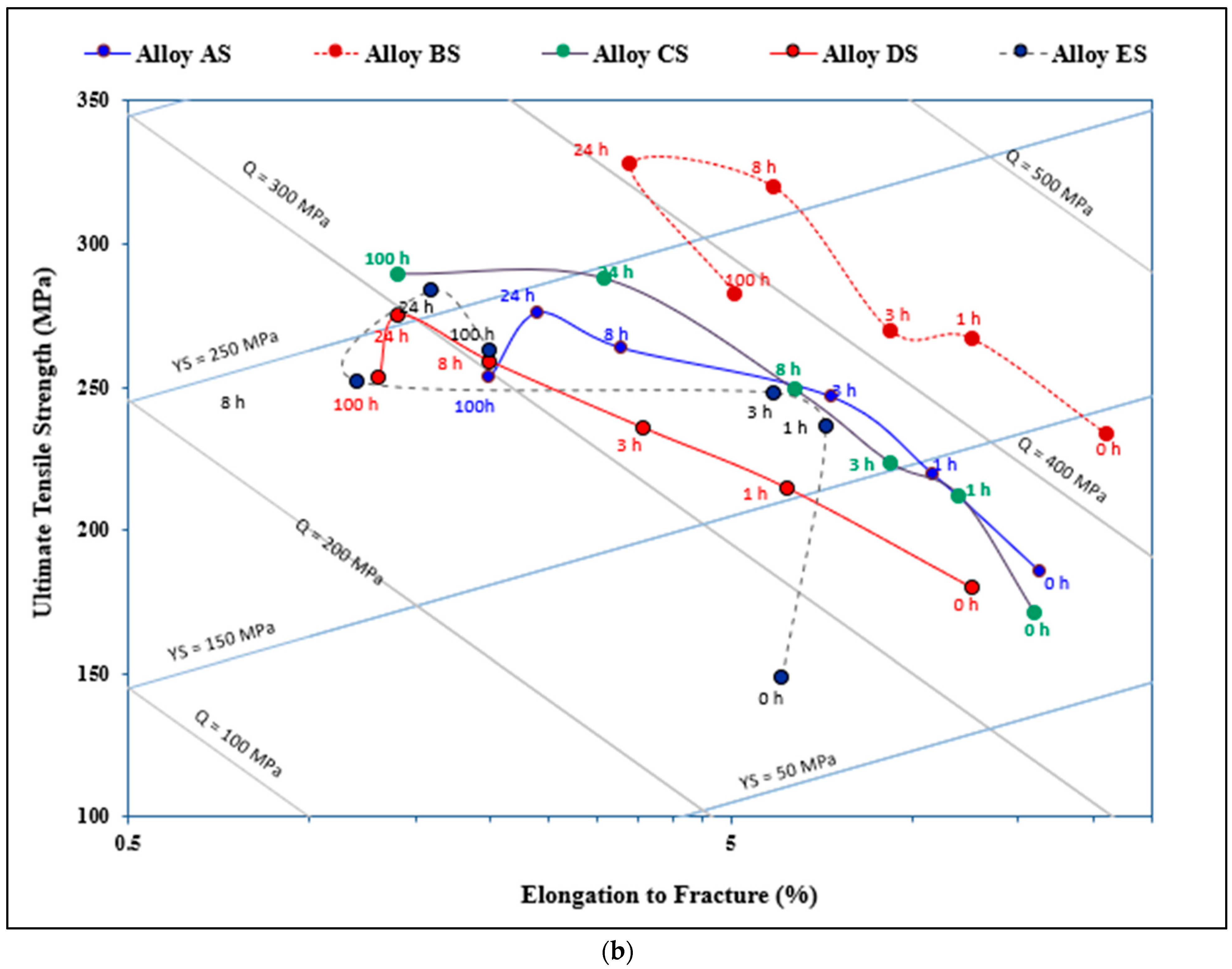
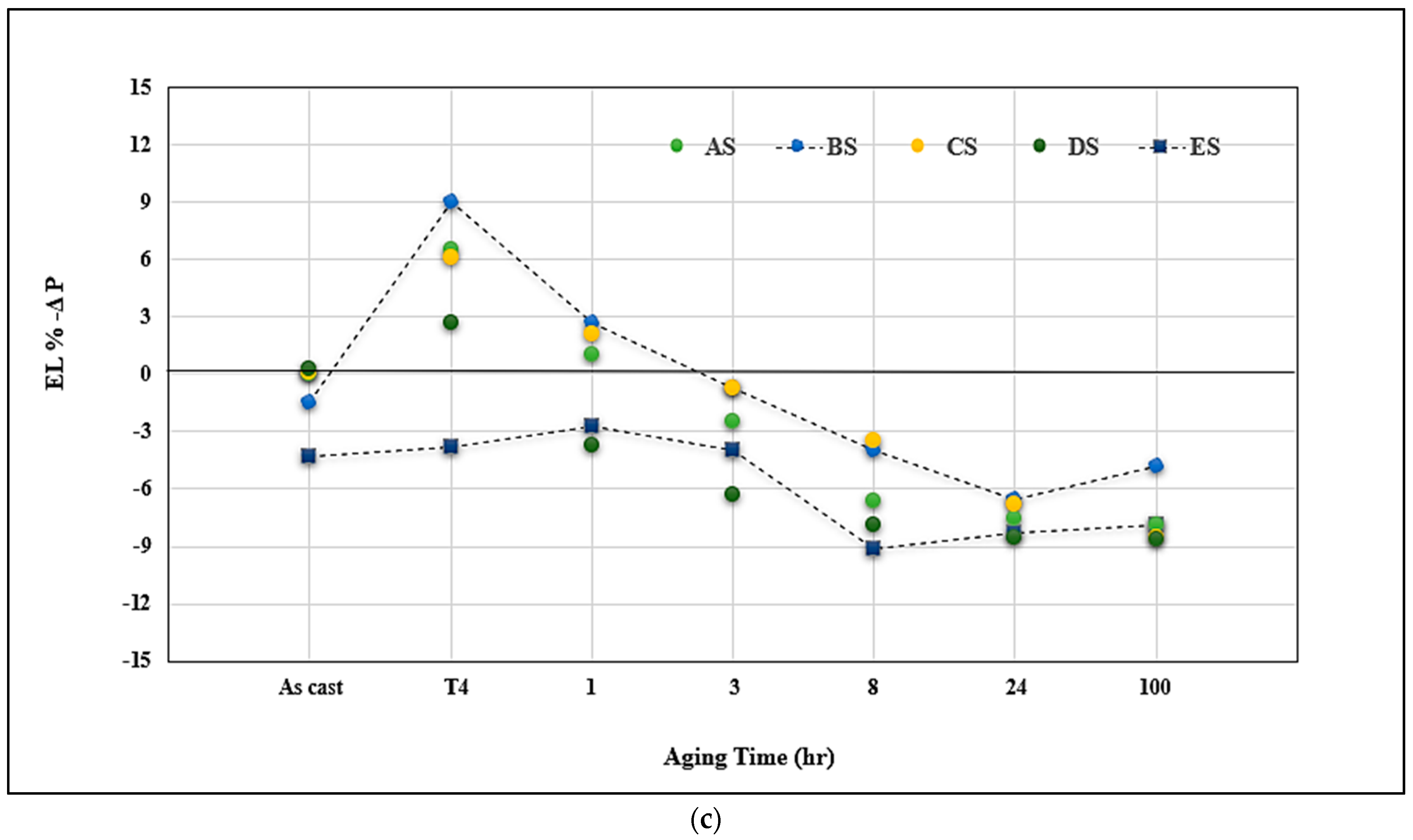
3.3. Q-Charts and ∆P
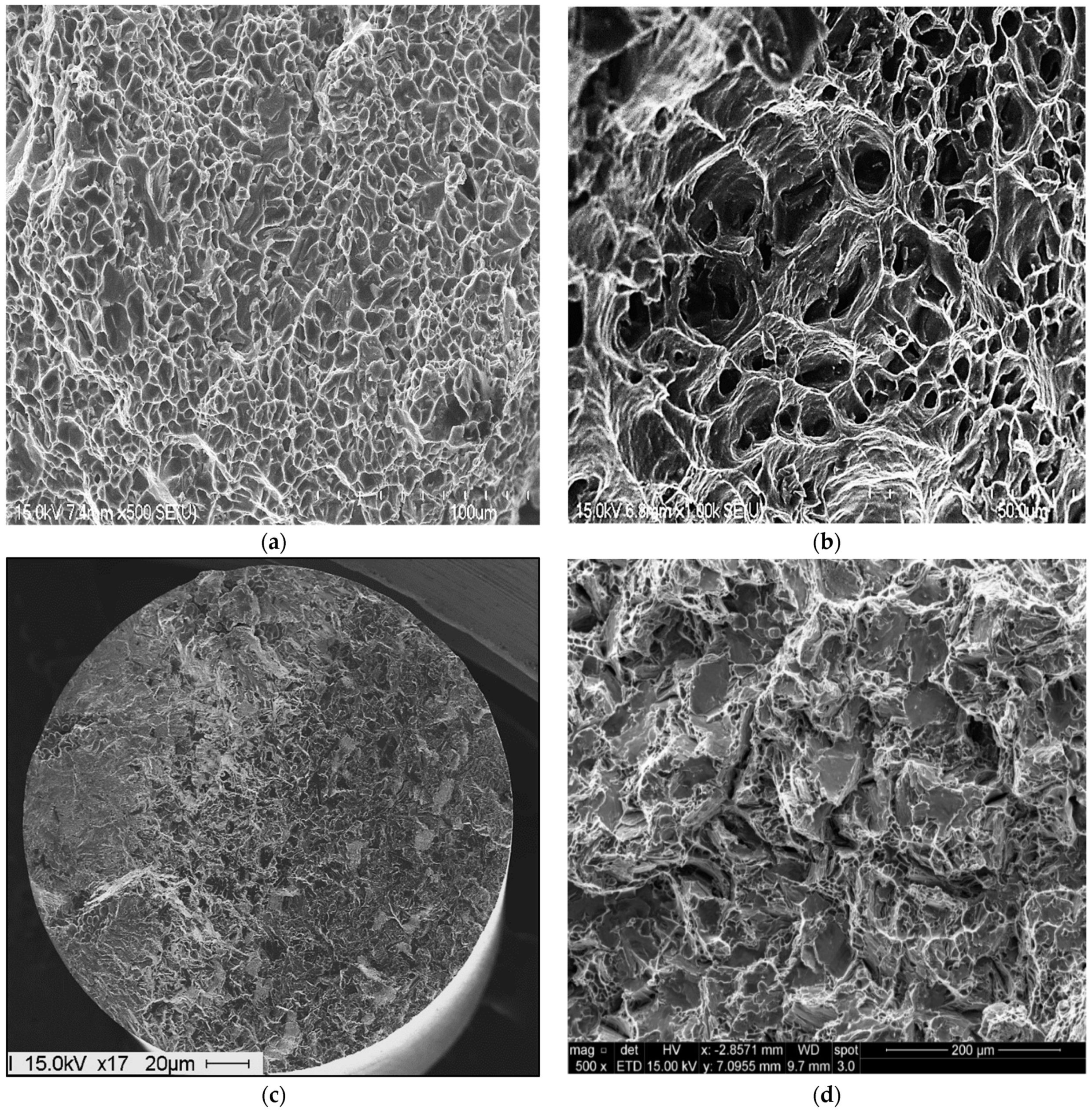
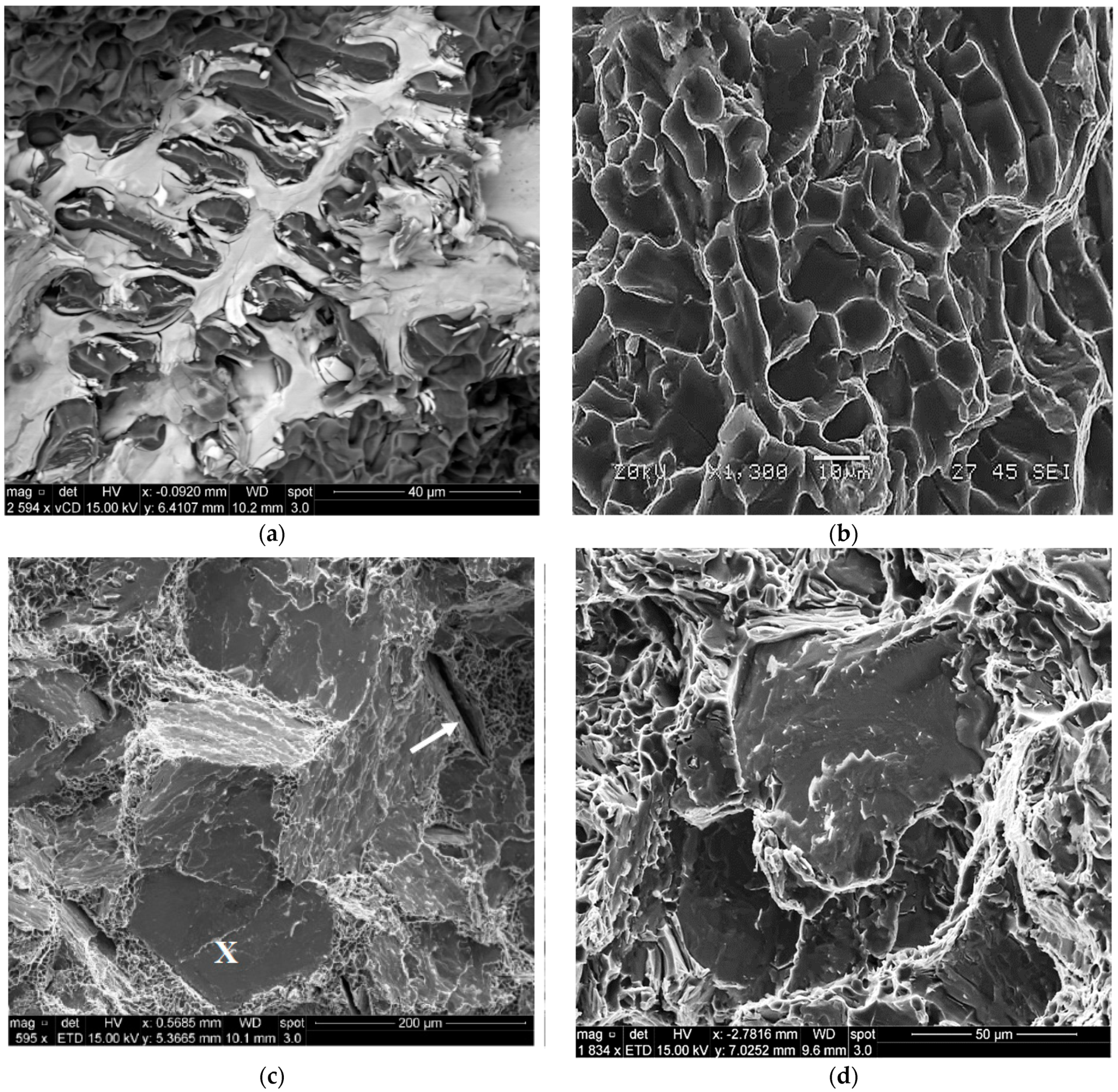
3.4. Fractography
4. Conclusions
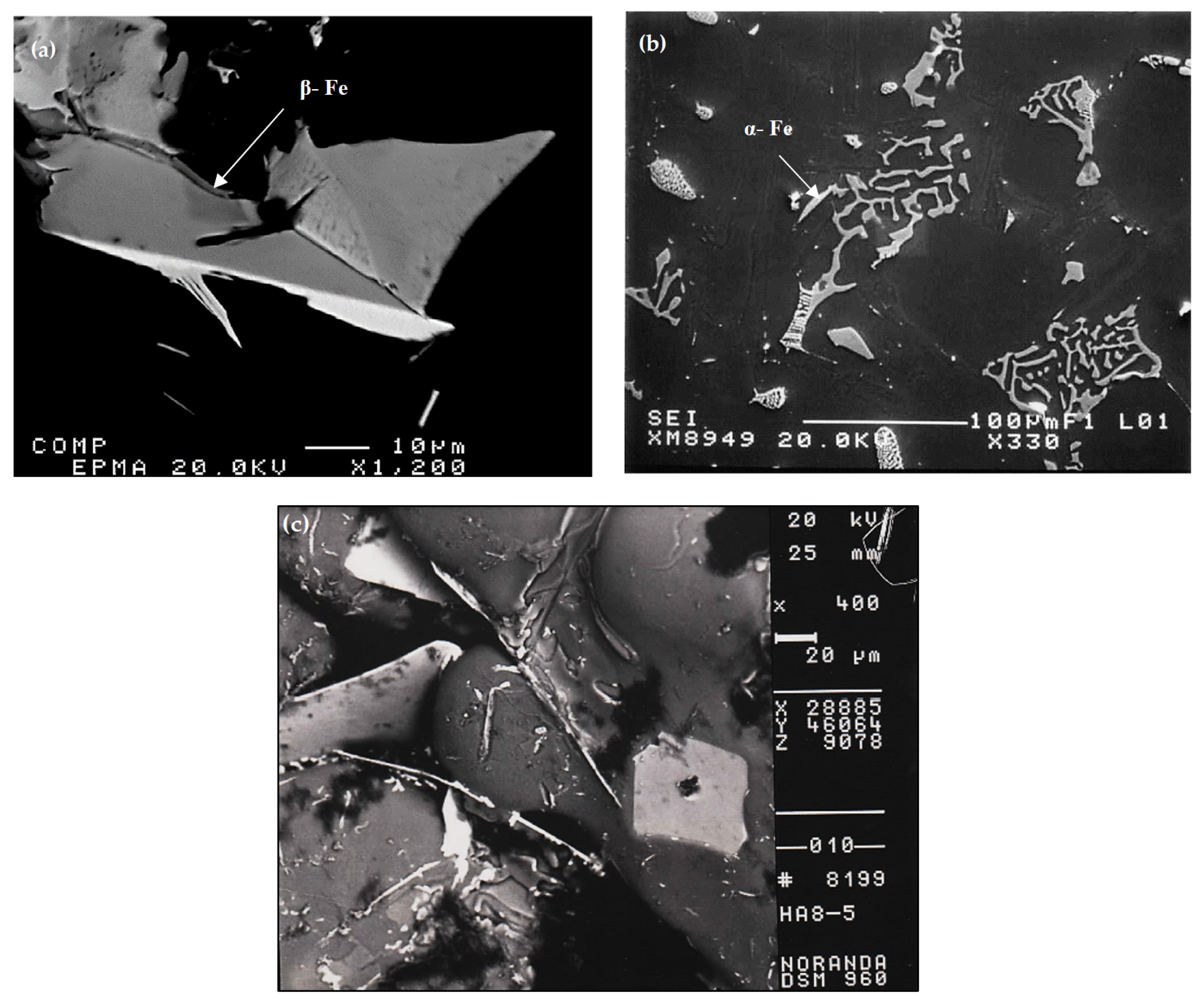
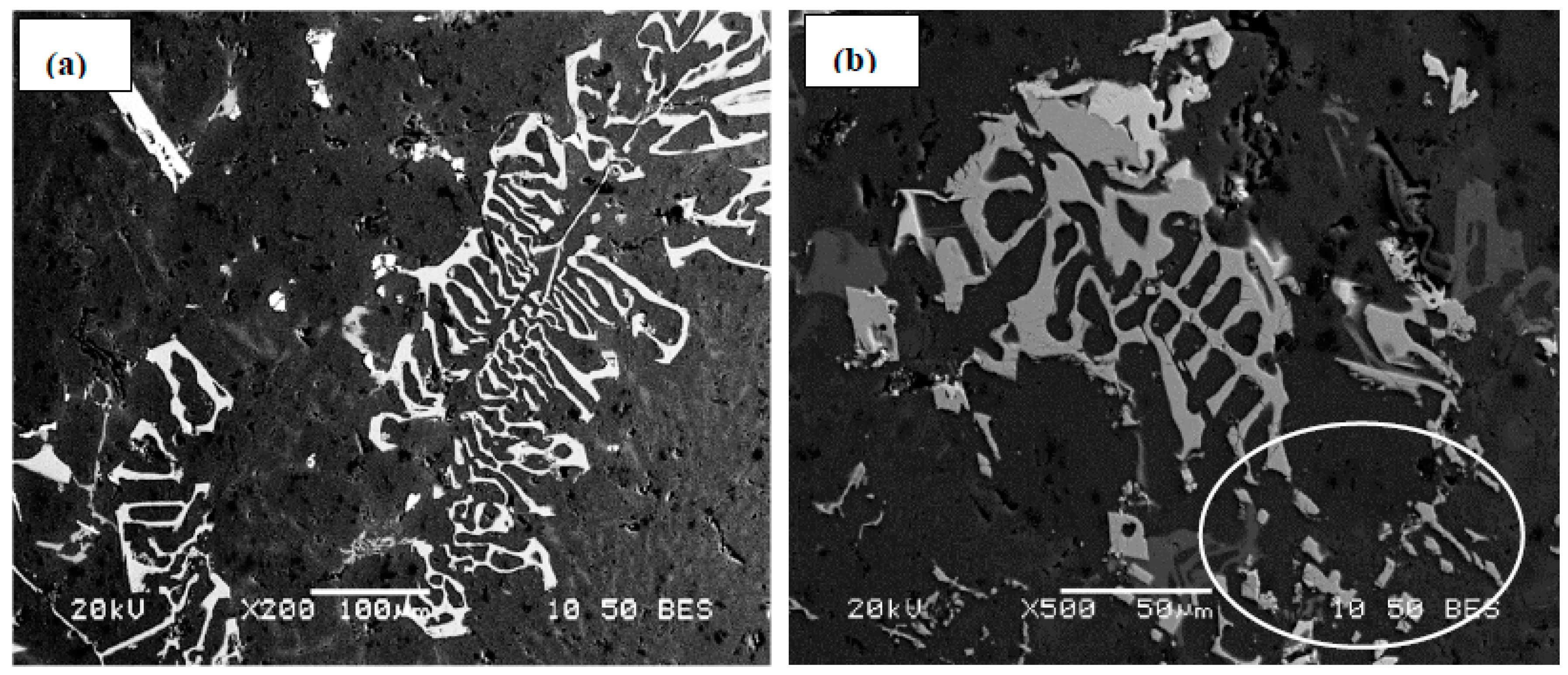
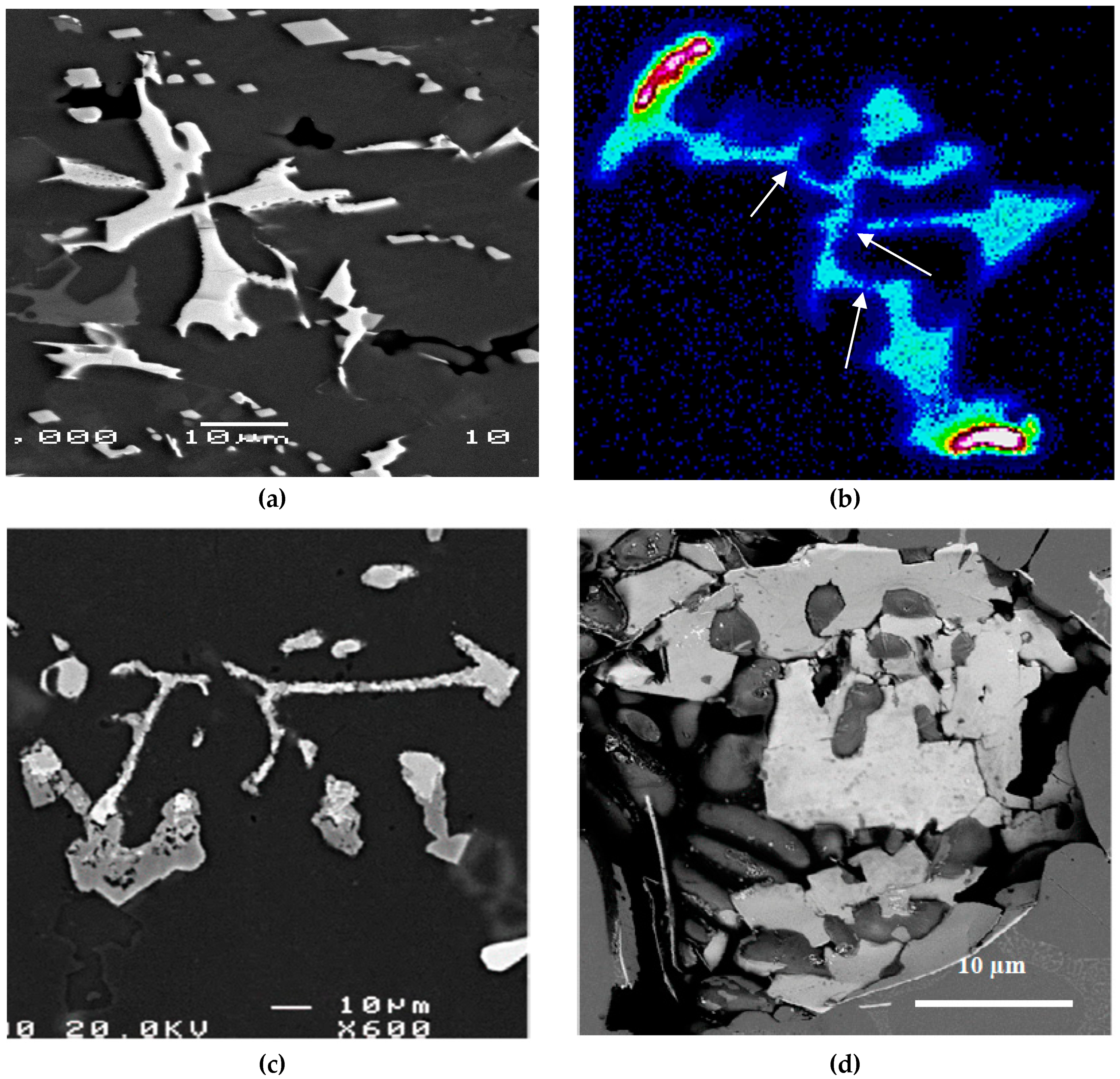
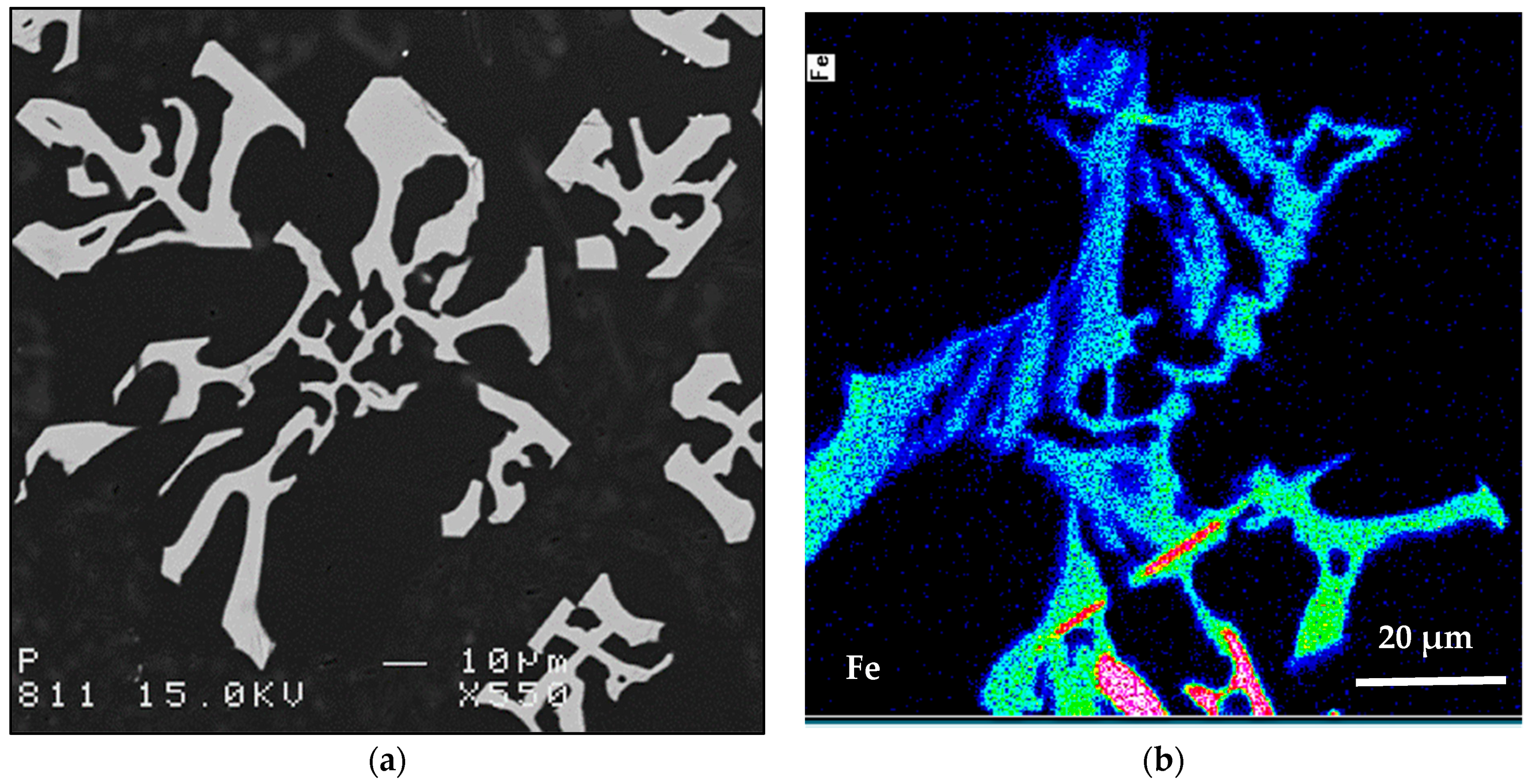
- The main parameter controlling the type of Fe-based intermetallic phases which will precipitate in 6061 alloys is the Si/Fe ratio in such a way that a ratio of 1 will produce mainly the β-Al5FeSi phase, while a ratio of 2 will mainly result in α-Al15(Fe, Mn)3Si2 particles. Increasing the solidification rate encourages the precipitation of α-Al15(Fe, Mn)3Si2.
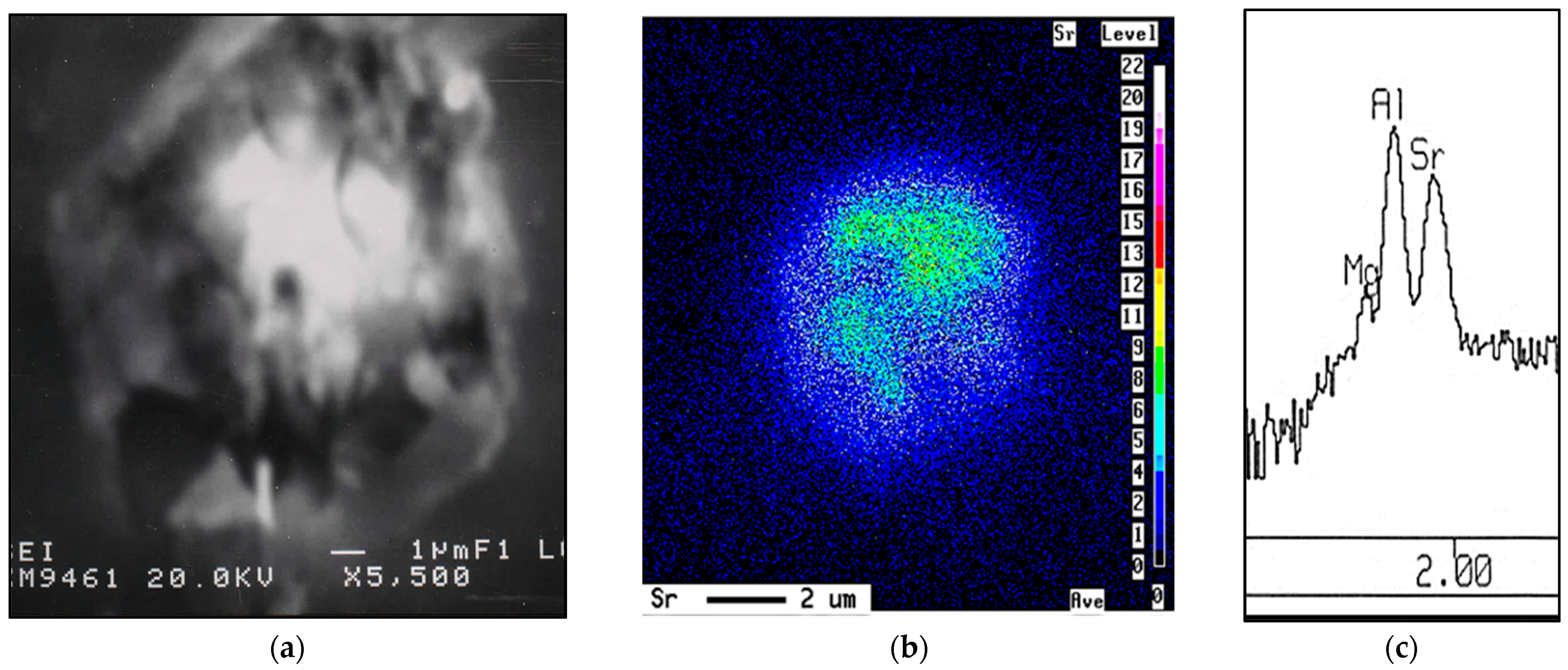
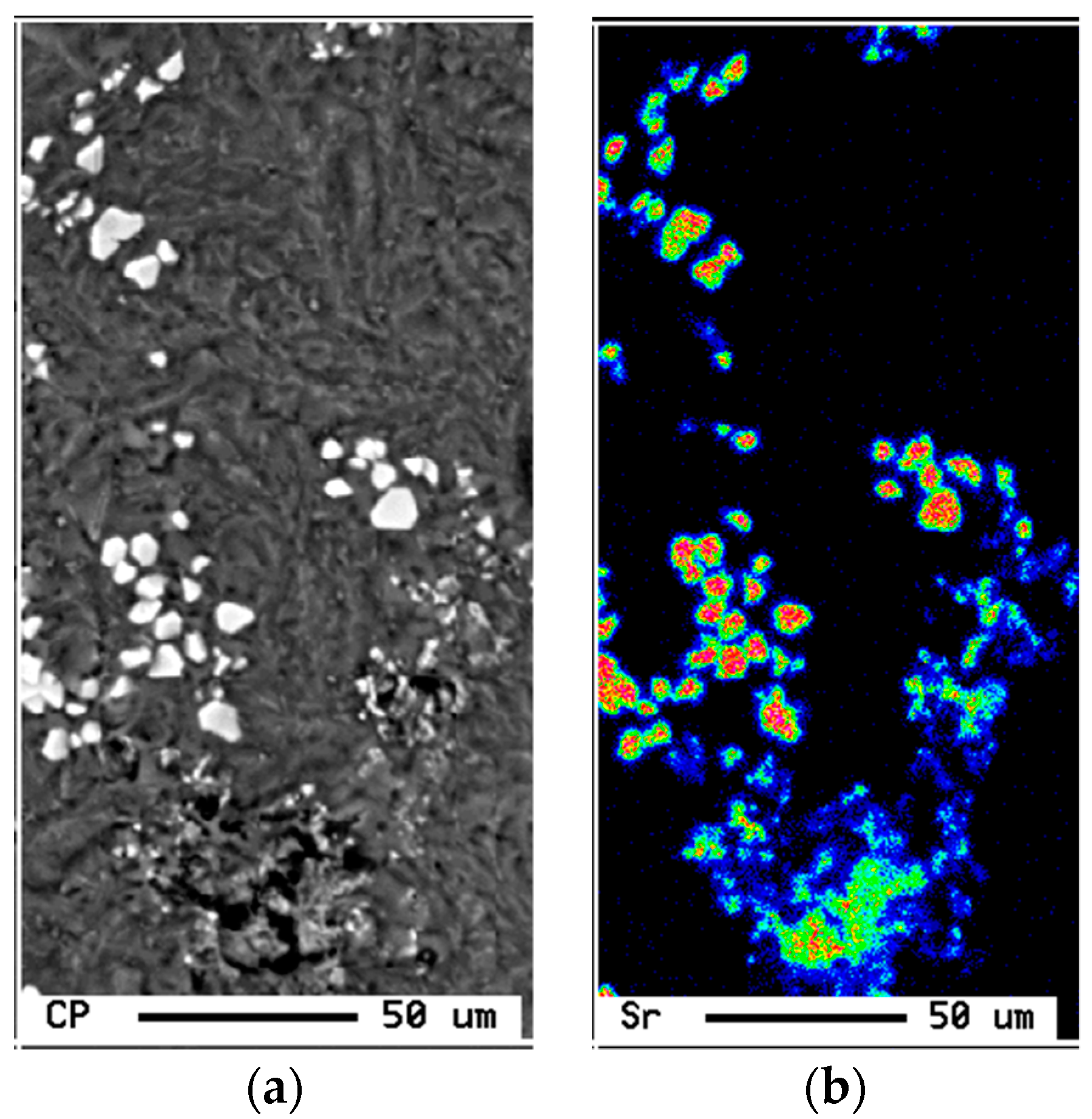
- Although 6061 alloy does not contain a sufficient amount of Si to form the Al-Si eutectic, the addition of Sr in the order of 200 ppm leads to fragmentation of Fe-based intermetallics, particularly during solution heat treatment.
- Addition of Be in amounts of 200–400 ppm results in:
- (a)
- fragmentation of α-Al15(Fe, Mn)3Si2 phase particles during solidification;
- (b)
- precipitation of a new compound, α-Al8Fe2SiBe in the form of Chinese script, characterized by its spongy surfaces instead of the smooth surfaces of the α-Al15(Fe, Mn)3Si2 phase;
- (c)
- possibility of porosity formation when Be is added in excess of 400 ppm.
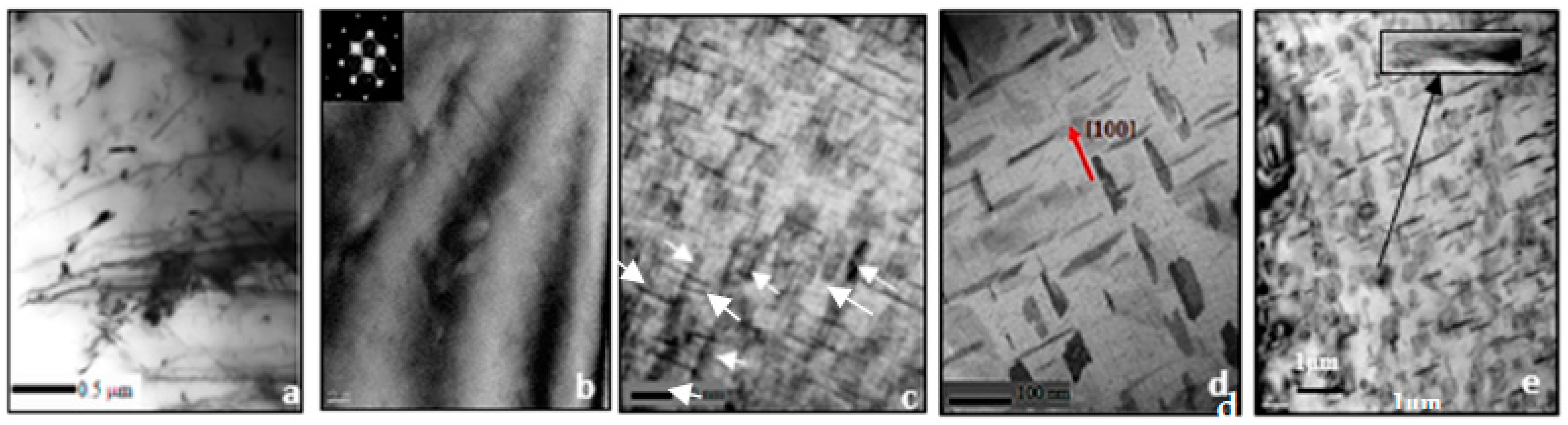
- Increasing the Si from 0.8% (in the base alloy) to 1.5% increases the volume fraction of the precipitated Mg2Si phase particles coupled with a marked improvement in the alloy strength. In all cases, the UTS curve reveals two peaks corresponding to the formation of GP1 and GP2 zones.
- The combined addition of Si and Sr to the base alloy resulted in the best quality among the five studied alloys, whereas the addition of high Be (400 ppm) or Be + Sr produced an unpredictable behavior of the alloy quality.
- Alloys containing high Si (without or with Sr) revealed the highest contribution to the strength of the base alloy with continuous increase up to the peak-aging condition. In contrast, high Be-containing alloys revealed the lowest contribution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abd El-Hameed, A.M.; Abdel-Aziz, Y.A. Aluminium Alloys in Space Applications: A Short Report. J. Adv. Res. Sci. Eng. Technol. 2021, 22, 1–7. Available online: www.akademiabaru.com/araset.html (accessed on 22 December 2022).
- Balaji, P.; Arun, R.; JegathPriyan, D.; Madhan Ram, I.; Manikandan, E. Comparative study of Al 6061 alloy with Al 6061–magnesium oxide (MgO) composite. Int. J. Sci. Eng. Res. 2015, 6, 408. [Google Scholar]
- David, J.R. Aluminum and Aluminum Alloys. In Alloying: Understanding the Basics; ASM International: Materials Park, OH, USA, 2001; pp. 35–416. [Google Scholar] [CrossRef]
- Axter, S.E. AIAA 2003-7879. Evolution of an Integrated Approach to Material Development. In Proceedings of the 44th AIAA/ASME/ASCE/AHS/ASC Structures, Structural Dynamics and Materials Conference, Norfolk, VA, USA, 7–10 April 2003. [Google Scholar]
- Siddiqui, R.A.; Abdullah, H.A.; Al-Belushi, K.R. Influence of Aging Parameters on the Mechanical Properties of 6063 Aluminum Alloy. J. Mater. Process. Technol. 2000, 102, 234–240. [Google Scholar] [CrossRef]
- Mrówka-Nowotnik, G.; Sieniawski, J. Influence of Heat Treatment on the Microstructure and Mechanical Properties of 6005 and 6082 Aluminium Alloys. In Proceedings of the 5th International Scientific Conference Achievements in Mechanical & Materials Engineering, Gliwice-Wisla, Poland, 4–6 December 2005; pp. 447–450. [Google Scholar]
- Mrówka-Nowotnik, G. Influence of Chemical Composition Variation and Heat Treatment on Microstructure and Mechanical Properties of 6xxx Alloys. Arch. Mater. Sci. Eng. 2010, 46, 98–107. [Google Scholar]
- Ludwig, T.H.; Schaffer, P.L.; Arnberg, L. Influence of Some Trace Elements on Solidification Path and Microstructure of Al- Si Foundry Alloys. Metall. Mater. Trans. A 2013, 44, 3783–3796. [Google Scholar] [CrossRef]
- Pitchayyapillai, G.; Seenikannan, P.; Balasundar, P.; Narayanasamy, P. Effect of Nano-Silver on Microstructure, Mechanical and Tribological Properties of Cast 6061 Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 2137–2145. [Google Scholar] [CrossRef]
- Hussein, S.G.; Al-Shammari, M.A.; Takhakh, A.M.; Al-Waily, M. Effect of Heat Treatment on Mechanical and Vibration Properties for 6061 and 2024 Aluminum Alloys. J. Mech. Eng. Res. 2020, 43, 48–66. [Google Scholar]
- Mohamed, I.F.; Lee, S.; Edalati, K.; Horita, Z.; Hirosawa, S.; Matsuda, K.; Terada, D. Aging Behavior of Al 6061 Alloy Processed by High-Pressure Torsion and Subsequent Aging. Metall. Mater. Trans. A 2015, 46, 2664–2673. [Google Scholar] [CrossRef]
- Nam, S.W.; Lee, D.H. The Effect of Mn on the Mechanical Behavior of Al Alloys. Met. Mater. 2000, 6, 13–16. [Google Scholar] [CrossRef]
- Wang, H.Q.; Sun, W.L.; Xing, Y.Q. Microstructure Analysis on 6061 Aluminum Alloy after Casting and Diffuses Annealing Process. Phys. Procedia 2013, 50, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, H.; Gokhale, A.M.; Graham, S.; Horstemeyer, M.F. Void Growth in 6061-Aluminum Alloy Under Triaxial Stress State. Mater. Sci. Eng. A 2003, 341, 35–42. [Google Scholar] [CrossRef]
- Ryen, Ø.; Holmedal, B.; Nijs, O.; Nes, E.; Sjölander, E.; Ekström, H.-E. Strengthening mechanisms in solid solution aluminum alloys. Metall. Mater. Trans. A 2006, 37, 1999–2006. [Google Scholar] [CrossRef]
- Farshidi, M.H.; Kazeminezhad, M.; Miyamoto, H. On the Natural Aging Behavior of Aluminum 6061 Alloy After Severe Plastic Deformation. Mater. Sci. Eng. A 2013, 580, 202–208. [Google Scholar] [CrossRef]
- Rana, R.S.; Rajesh, P.; Das, S. Reviews on the Influences of Alloying elements on the Microstructure and Mechanical Properties of Aluminum, Alloys and Aluminum Alloy Composites. Int. J. Sci. Res. Publ. 2012, 2. Available online: www.ijsrp.org (accessed on 22 December 2022).
- Elsharkawi, E.A.; Ibrahim, M.F.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Understanding the Effect of Be Addition on The Microstructure and Tensile Properties of Al–Si–Mg Cast Alloys. Int. J. Met. 2021, 16, 1777–1795. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Alkahtani, S.A.; Abuhasel, K.A.; Samuel, F.H. Microstructural Characterization of Beryllium Treated Al-Si Alloys. Adv. Mater. Sci. Eng. 2015, 2015, 673025. [Google Scholar] [CrossRef] [Green Version]
- Grant, N.J. Rapid Solidification of Metallic Particulates. JOM 1983, 35, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, W.; Samuel, F.H.; Gruzleski, J.E. Inclusion Additions to Molten Aluminum Alloys Using the Gas Injection Technique: Process and Analysis. In Proceedings of the 109th Metalcasting Congress, St. Louis, MO, USA, 16–19 April 2005; Volume 113, pp. 207–224. [Google Scholar]
- Khalifa, W.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Metallographic Observations of β-AlFeSi phase and Its Role in Porosity Formation in Al-7%Si Alloys. Int. J. Cast Met. Res. 2006, 19, 156–166. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y. Effects of beryllium in Al–Si–Mg–Ti cast alloy. Mater. Sci. Eng. A 2000, 280, 124–127. [Google Scholar] [CrossRef]
- Elsharkawi, E.A. Effects of Metallurgical Parameters on the Decomposition of the π-AlFeMgSi Phase in Al-Si-Mg Alloys and Its Influence on the Mechanical Properties. Ph.D. Thesis, Université Du Québec À Chicoutimi, Chicoutimi, QC, Canada, 2011. [Google Scholar]
- Andrade, N.R.; Gruzleski, J.E.; Samuel, F.H.; Valtierra, S.; Doty, H.W. Age-Hardening Precipitates in Cast 319 Aluminum Alloys. In International Symposium on Aluminium: From Raw Materials to Applications, Proceedings of the 45th Annual Conference of Metallurgists of CIM, Montreal, QC, Canada, 1–4 October 2006; The Metallurgy and Materials Society of The Canadian Institute of Mining, Metallurgy and Petroleum (MetSoc of CIM): Montreal, QC, Canada, 2006; pp. 104–114. [Google Scholar]
- Tavitas-Medrano, F.J.; Gruzleski, J.E.; Samuel, F.H.; Valtierra, S.; Doty, H.W. Conventional Versus Non-Conventional Aging Heat Treatments of 319-Type Alloys in Relation to Their Mechanical Properties. In Proceedings of the 45th Annual Conference of Metallurgists of CIM, Montreal, QC, Canada; 2006; pp. 185–200. [Google Scholar]
- Guan, R.G.; Tie, D. A Review on Grain Refinement of Aluminum Alloys: Progresses, Challenges and Prospects. Acta Metall. Sin. 2017, 30, 409–432. [Google Scholar] [CrossRef] [Green Version]
- Rathi, S.K.; Sharma, A.; Sabatino, M.D. Grain Refinement of Al-Si Alloys: Scientific and Industrial Aspects. J. Mod. Thermodyn. Mech. Syst. 2019, 1, 7–15. [Google Scholar]
- Ramli, R.; Talari, M.K.; Omar Arawi, A.Z. Microstructure and mechanical properties of Al-Si Cast Alloy Grain Refined with Ti-B-Sr-Sc-Mg. In Proceedings of the 2011 IEEE Colloquium on Humanities, Science and Engineering, Penang, Malaysia, 5–6 December 2011; pp. 692–695. [Google Scholar] [CrossRef]
- Nadendla, H.B.; Nowak, M.; Bolzoni, L. Grain refiner for Al-Si alloys. In Light Metals 2013; Springer: Cham, Switzerland, 2016; pp. 1009–1012. [Google Scholar] [CrossRef]
- Dong, X.; Ji, S. Grain Refinement of Al–Si–Mg Cast Alloys by Al3Ti3B Master Alloy. In Light Metals 2018, TMS 2018; The Minerals, Metals & Materials Series; Martin, O., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; pp. 107–138. [Google Scholar] [CrossRef]





| Alloy | Alloying Elements (wt%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | B | Be | Sr | V | Ti | Al | |
| A | 0.81 | 0.38 | 0.003 | 0.004 | 0.844 | 0.003 | <0.005 | 0.013 | 0.003 | 0 | 0.0009 | 0.004 | 0.018 | Bal. |
| AS | 0.83 | 0.38 | 0.003 | 0.004 | 0.853 | 0.003 | <0.005 | 0.013 | 0.003 | 0 | 0.0144 | 0.004 | 0.017 | Bal. |
| B | 1.49 | 0.35 | 0.003 | 0.006 | 0.682 | 0.002 | <0.005 | 0.013 | 0.003 | 0 | 0.0014 | 0.004 | 0.018 | Bal. |
| BS | 1.49 | 0.334 | 0.002 | 0.004 | 0.859 | 0.002 | <0.006 | 0.013 | 0.003 | 0 | 0.0163 | 0.004 | 0.019 | Bal. |
| C | 0.82 | 0.34 | 0.002 | 0.198 | 0.806 | 0.003 | <0.005 | 0.013 | 0.003 | 0 | 0.0002 | 0.004 | 0.017 | Bal. |
| CS | 0.88 | 0.34 | 0.002 | 0.217 | 0.838 | 0.004 | 0.005 | 0.013 | 0.003 | 0 | 0.0206 | 0.005 | 0.015 | Bal. |
| D | 0.84 | 0.35 | 0.005 | 0.006 | 0.817 | 0.006 | 0.006 | 0.014 | 0.003 | 0.019 | 0.0038 | 0.007 | 0.016 | Bal. |
| DS | 0.84 | 0.34 | 0.003 | 0.005 | 0.806 | 0.004 | <0.005 | 0.014 | 0.003 | 0.018 | 0.0147 | 0.007 | 0.019 | Bal. |
| E | 0.88 | 0.39 | 0.002 | 0.004 | 0.883 | 0.001 | <0.005 | 0.013 | 0.004 | 0.040 | 0.0005 | 0.005 | 0.016 | Bal. |
| ES | 0.87 | 0.39 | 0.003 | 0.005 | 0.597 | 0.009 | 0.006 | 0.013 | 0.003 | 0.040 | 0.0182 | 0.005 | 0.017 | Bal. |
| Stage | Heat Treatment | Phases |
|---|---|---|
| I | None | Mostly precipitated as equilibrium CuAl2 phases |
| II | Solution treatment and rapid cooling | All Cu in solution Most Mg phases in solution |
| III | Natural aging at room temperature | Segregation into GP I zones (coherent) |
| IV | Age hardening at 180 C | Dissolution of GP I zones Segregation into GP II zones (coherent) Precipitation of S’phase (CuAl2Mg) |
| Further age hardening at 180 C | Increased diffusion into GP II zones and precipitation as θ phase (partially coherent) Precipitation of S′ phase (CuAl2Mg) Precipitation of β″ phase Precipitation of traces of Si | |
| V | Overaging resulting from treatment time too long | Precipitated as θ phase, the equilibrium phase (incoherent) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, A.M.; Elsharkawi, E.A.; Abdelaziz, M.H.; Samuel, E.; Samuel, F.H. Effect of Si, Mn, Be and Sr Addition on the Tensile Properties of 6061 Type Alloys: Role of Aging Treatment. Materials 2023, 16, 1110. https://doi.org/10.3390/ma16031110
Samuel AM, Elsharkawi EA, Abdelaziz MH, Samuel E, Samuel FH. Effect of Si, Mn, Be and Sr Addition on the Tensile Properties of 6061 Type Alloys: Role of Aging Treatment. Materials. 2023; 16(3):1110. https://doi.org/10.3390/ma16031110
Chicago/Turabian StyleSamuel, Agnes M., Ehab A. Elsharkawi, Mohamed H. Abdelaziz, Ehab Samuel, and Fawzy H. Samuel. 2023. "Effect of Si, Mn, Be and Sr Addition on the Tensile Properties of 6061 Type Alloys: Role of Aging Treatment" Materials 16, no. 3: 1110. https://doi.org/10.3390/ma16031110





