3D Printing and Performance Study of Porous Artificial Bone Based on HA-ZrO2-PVA Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Composition
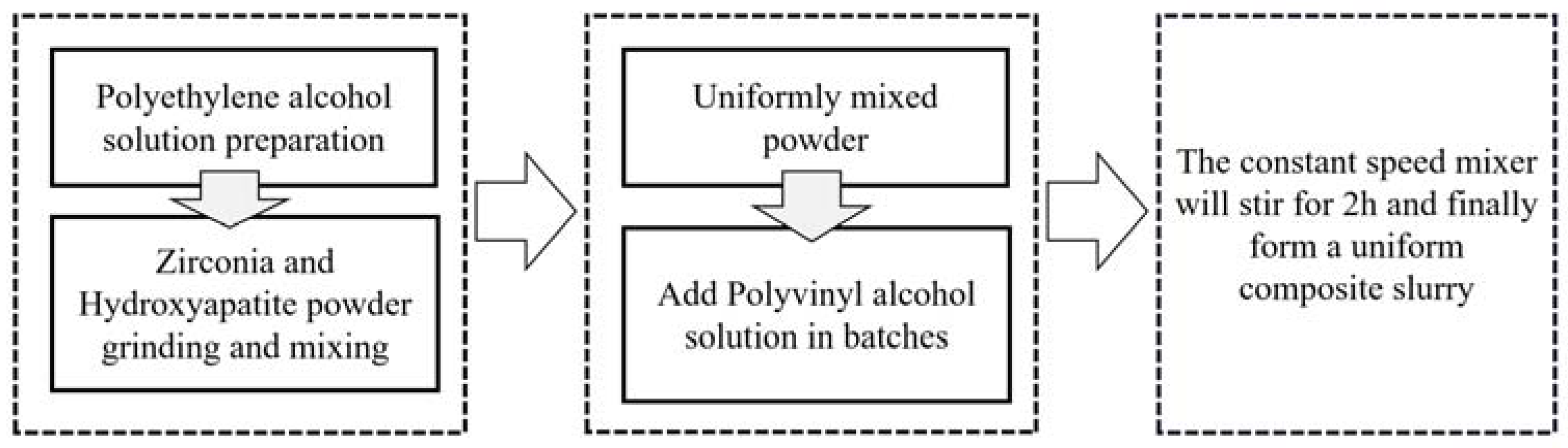
2.2. Material Composition
- (1)
- The material itself has the right viscosity to maintain its shape after extrusion.
- (2)
- There is a uniform mixing of materials, capable of stable extrusion.
- (3)
- The material itself is in a stable state and does not change its chemical or physical properties over time.
- (1)
- Add the appropriate amount of polyvinyl alcohol powder slowly to deionized water at room temperature several times under the stirring of a constant speed mixer to make it disperse evenly, and keep stirring for 30 min.
- (2)
- Put the beaker with polyvinyl alcohol which is well dispersed in a digital thermostat water bath with the temperature set at 75 °C. As the water temperature rises, continue to stir for 1~2 h until the polyvinyl alcohol is fully dissolved.
- (3)
- Leave the configured polyvinyl alcohol solution to defoam and then place it in a wide-mouth bottle in a reagent box for storage until use.
- (4)
- Weigh a proper amount of zirconia powder (AR, purchased from Aladdin company, China) and hydroxyapatite powder (AR, purchased from Aladdin company, China), respectively, and then place them in the planetary mixer for grinding and mixing for 1 h to make them disperse evenly. The particle size of hydroxyapatite powder is mainly 18–23 μm, while that of zirconia powder is mainly 17–22 μm.
- (5)
- Use a disposable dropper to suck a proper amount of polyvinyl alcohol solution from the jar and drop it into the beaker, and use magnetic stirrer (Ika, (Staufen, Germany)) to stir it evenly.
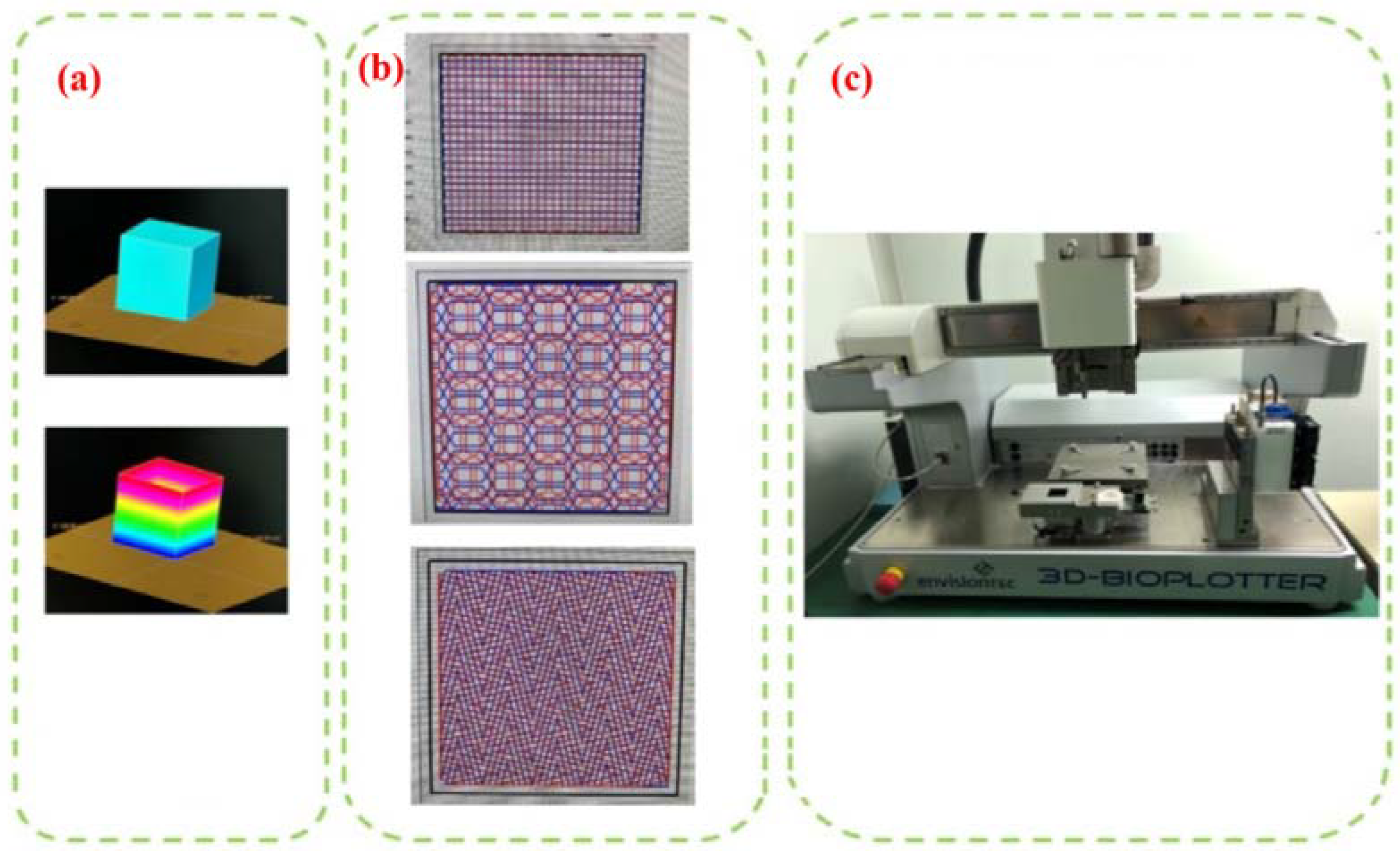
2.3. Printing Equipment and Test Equipment
- (1)
- Slice Processing
- (2)
- Internal Structure Selection
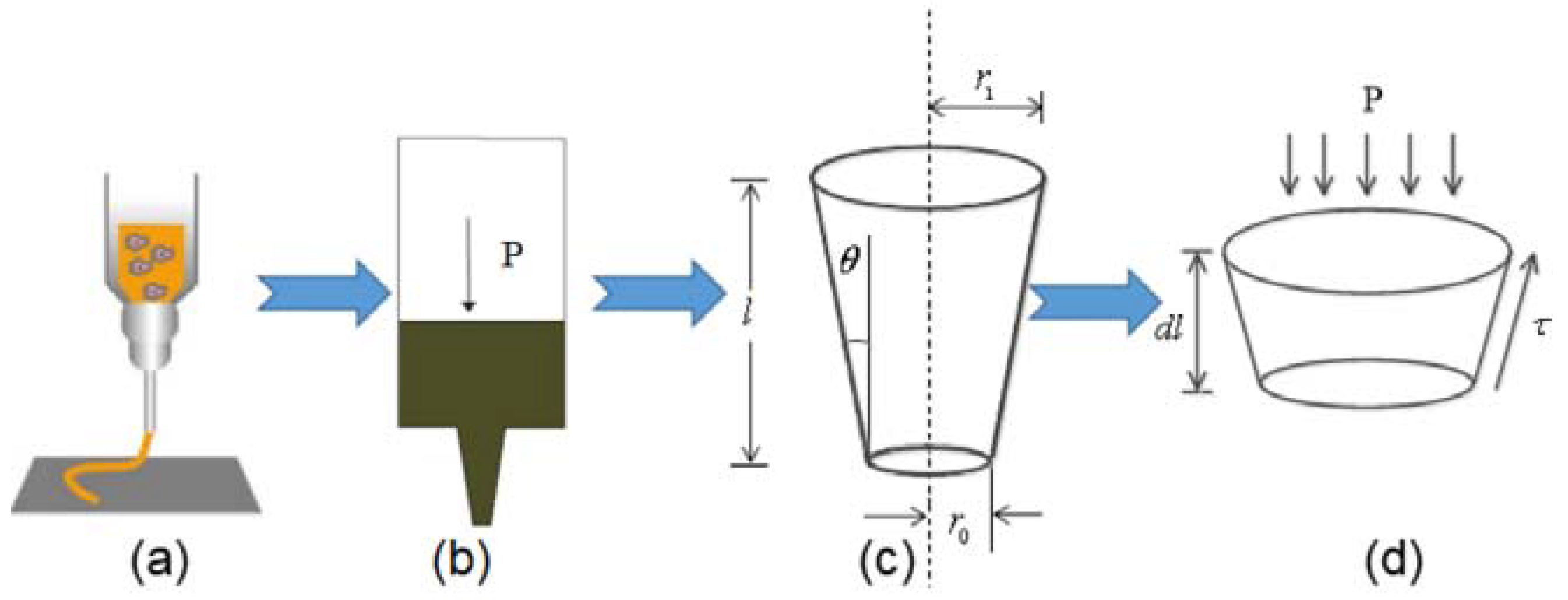
2.4. Optimization of Printing Parameters Based on Theoretical Modeling and Simulation of Composite Extrusion Process
2.4.1. Theoretical Model Construction of Composite Material Extrusion
2.4.2. Finite Element Simulation of Extrusion Process
- (1)
- The flow of the composite material in the silo is incompressible.
- (2)
- The influence of gravity is ignored.
- (3)
- The pressure drop caused by the non-pressure setting value is ignored.
3. Results and Discussion
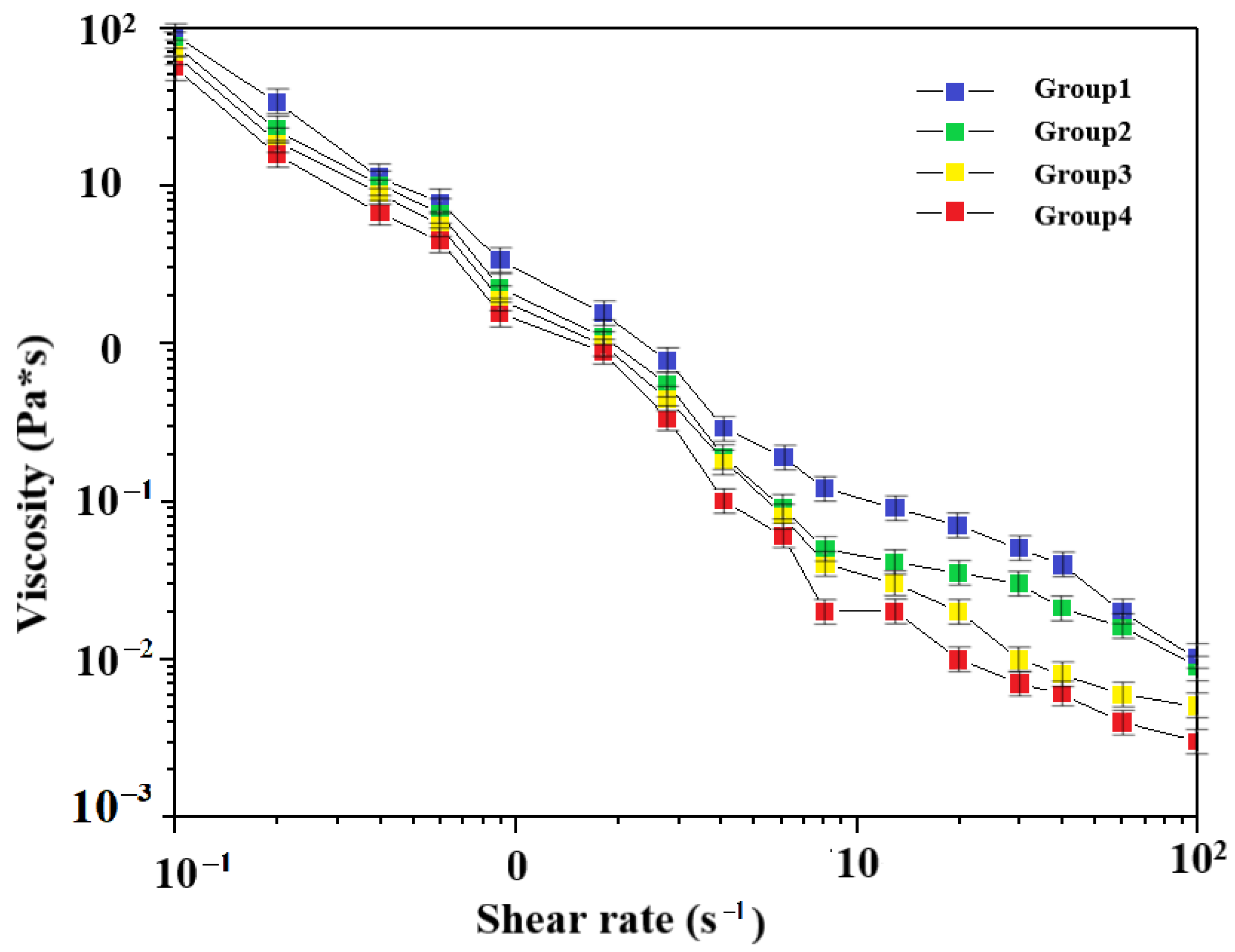
3.1. Viscosity Test of Different Slurries
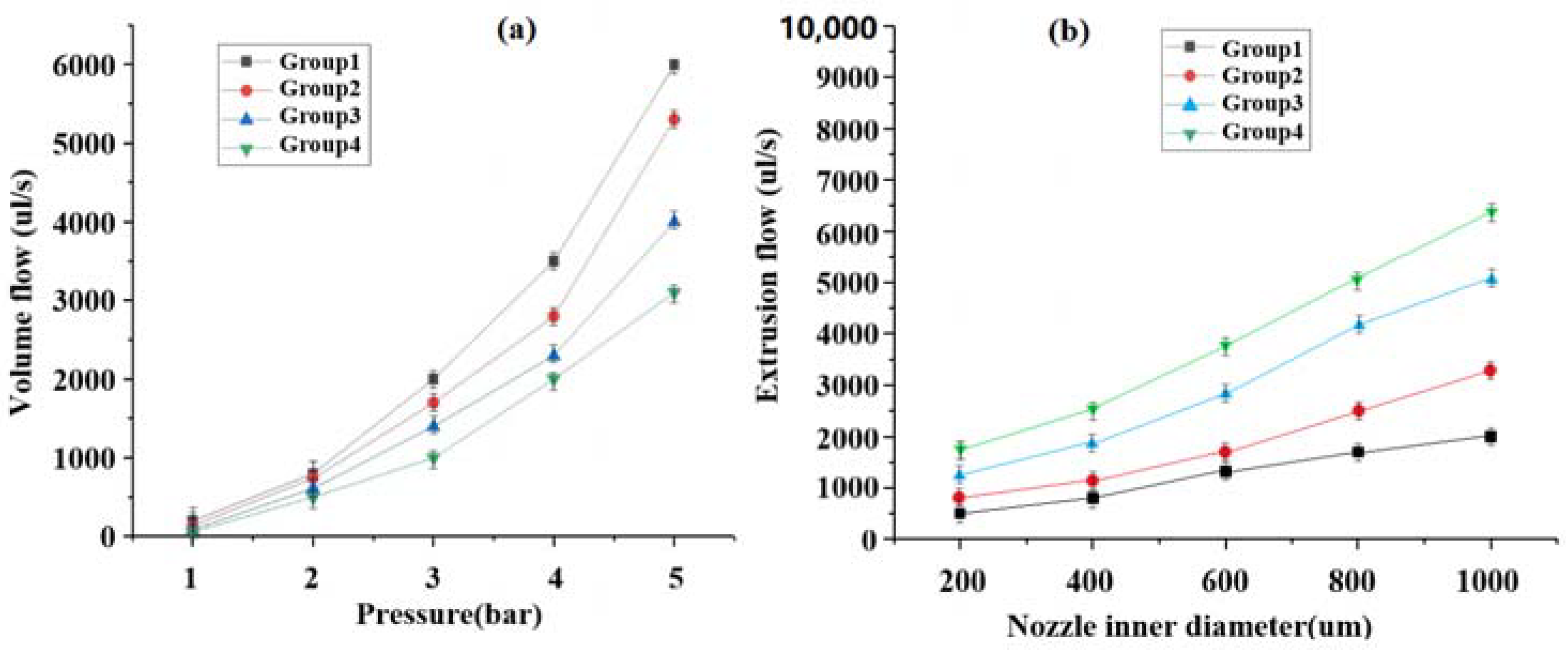
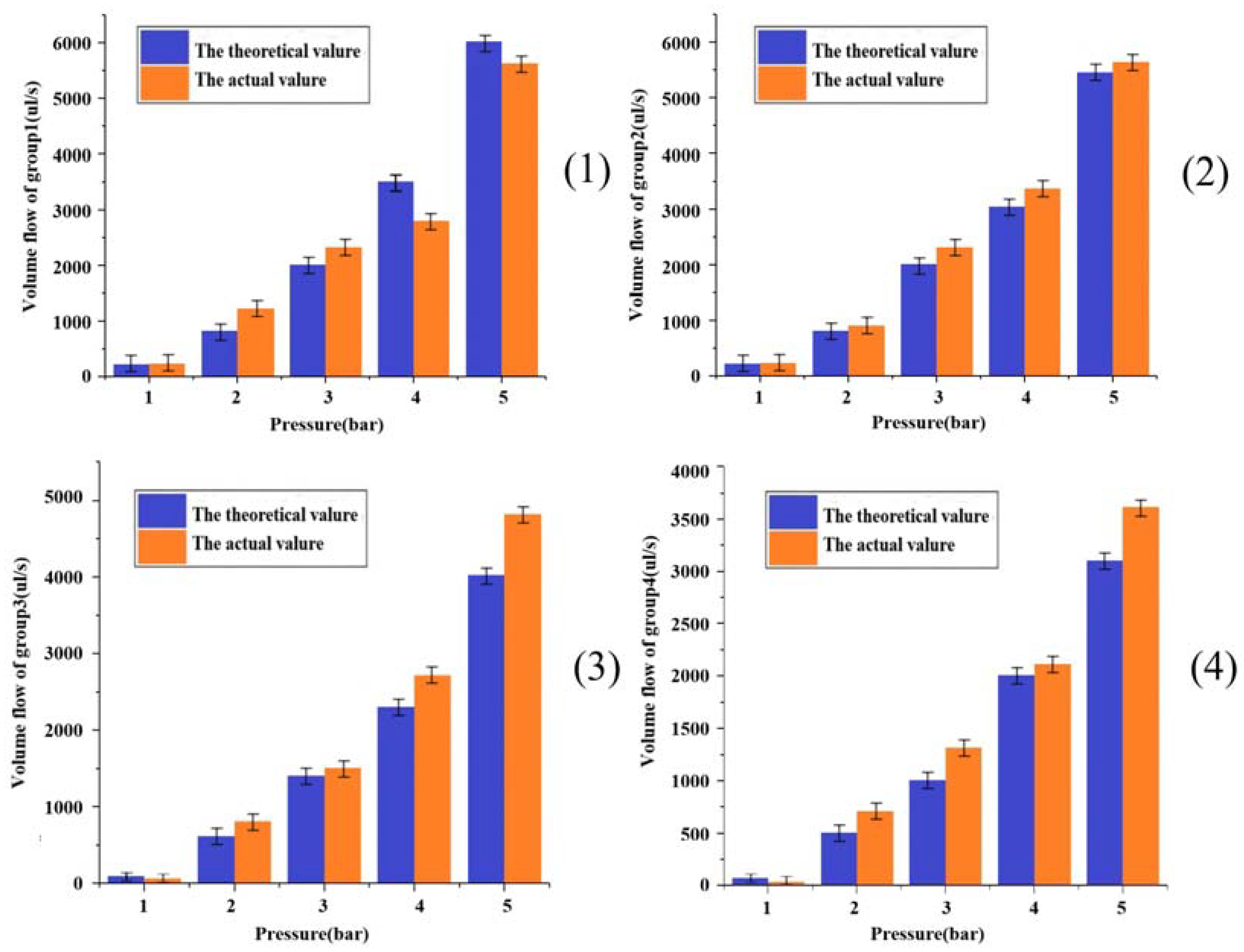
3.2. Printed Parameter Air Pressure and Flow Rate Relationship Verification
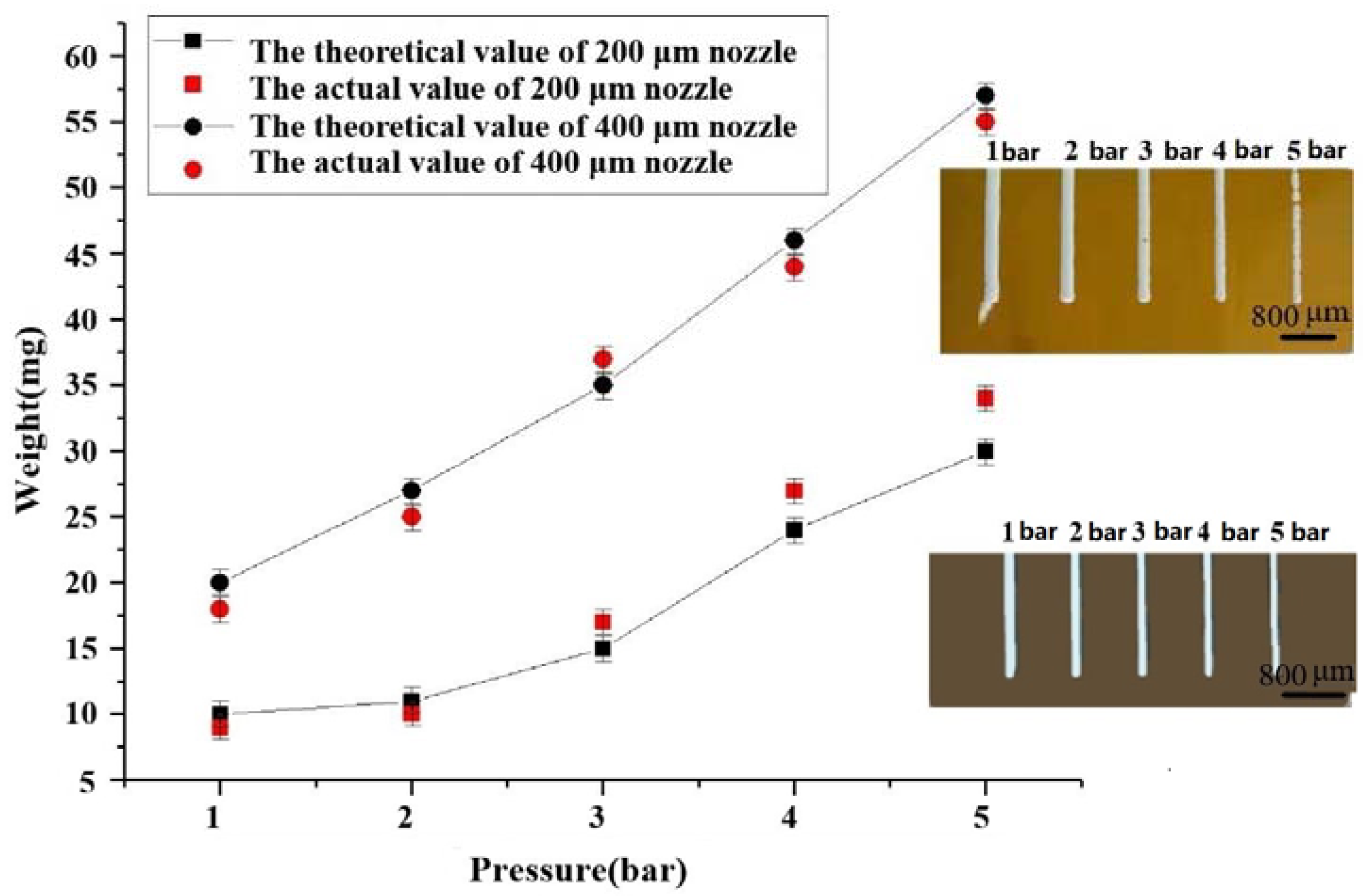
3.3. Effect of Internal Diameter Extrusion Pressure from Different Spray Heads on Extrusion Speed
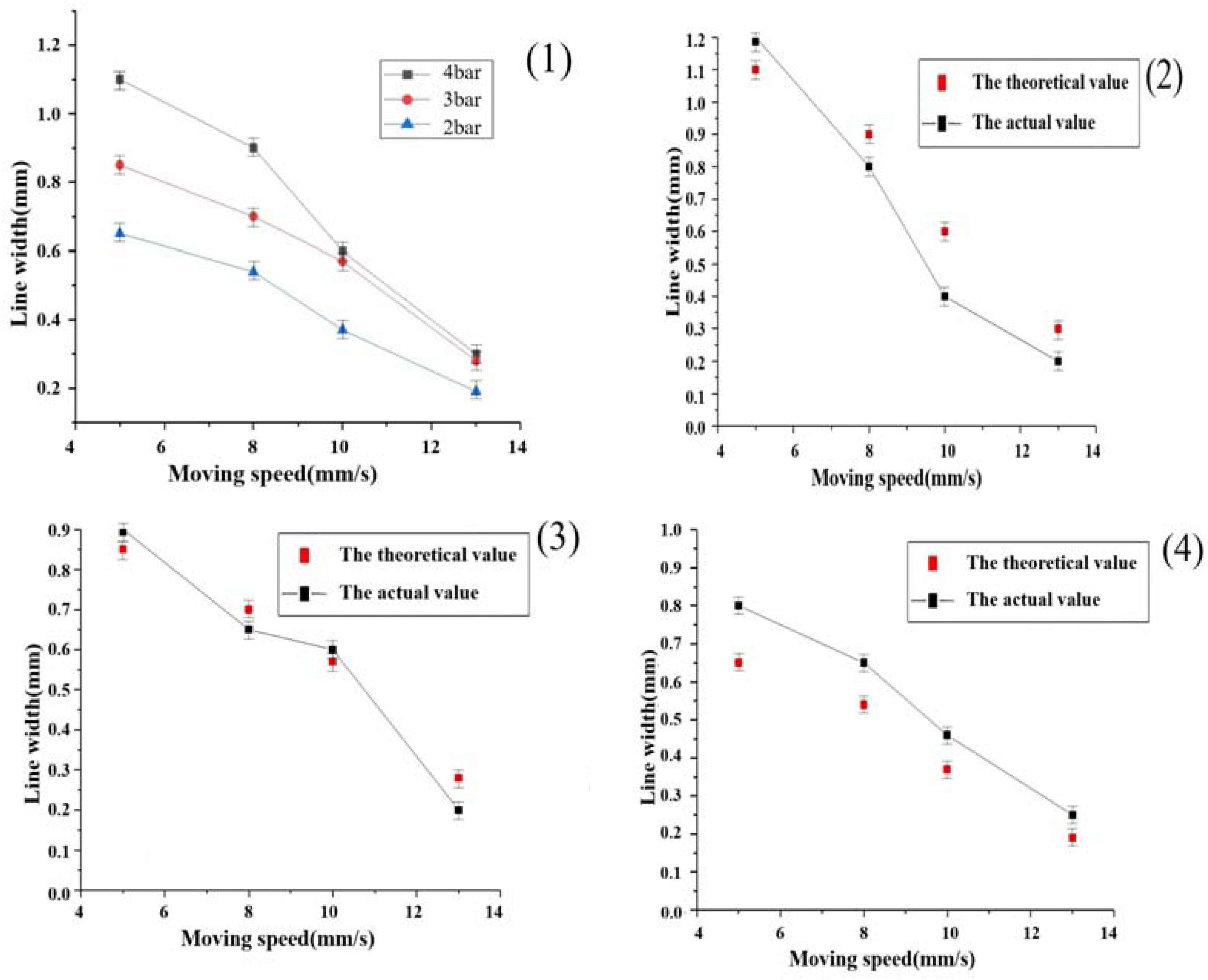
3.4. The Effect of Different Air Pressure Nozzle Shift Speeds on the Line Width
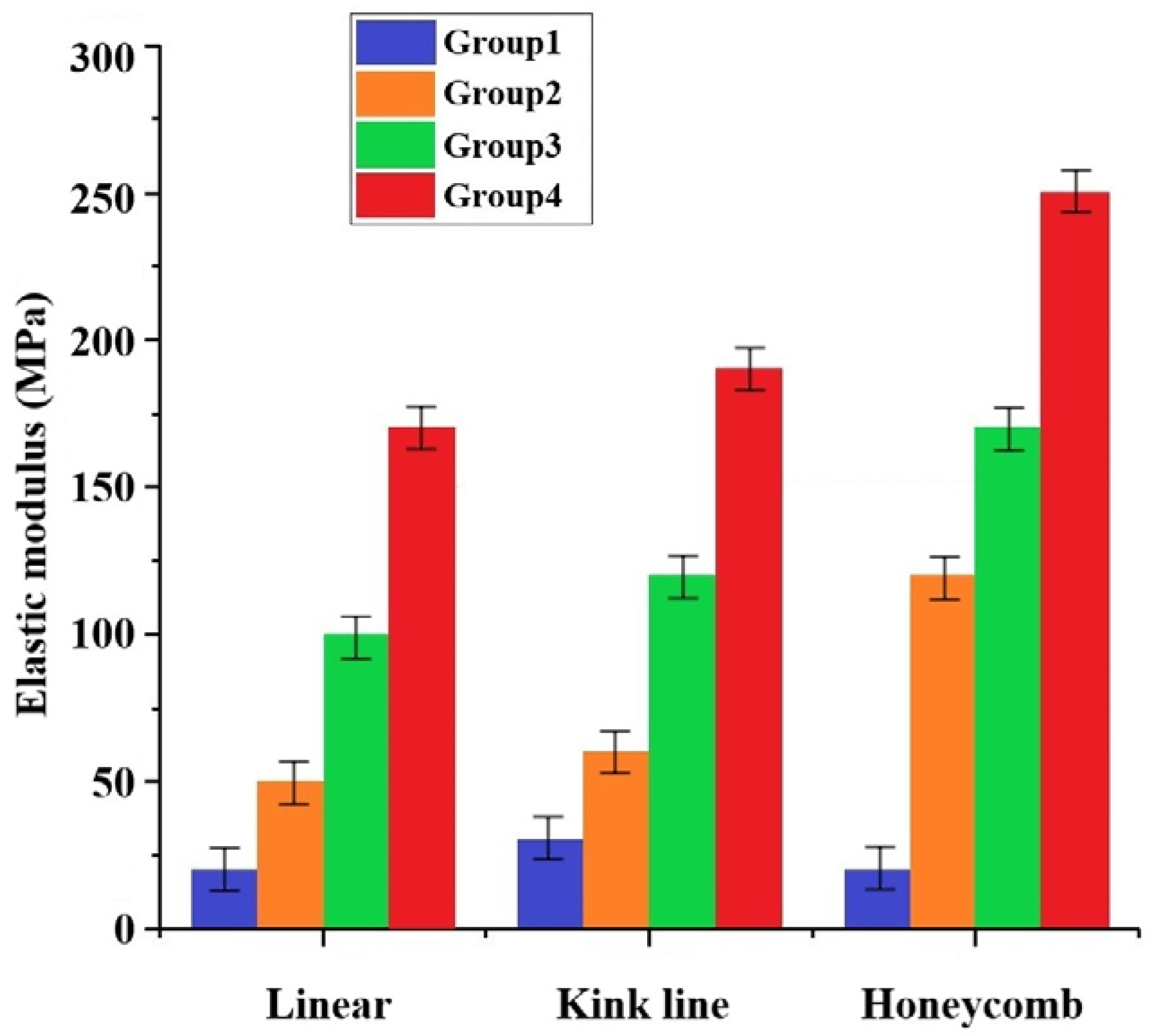
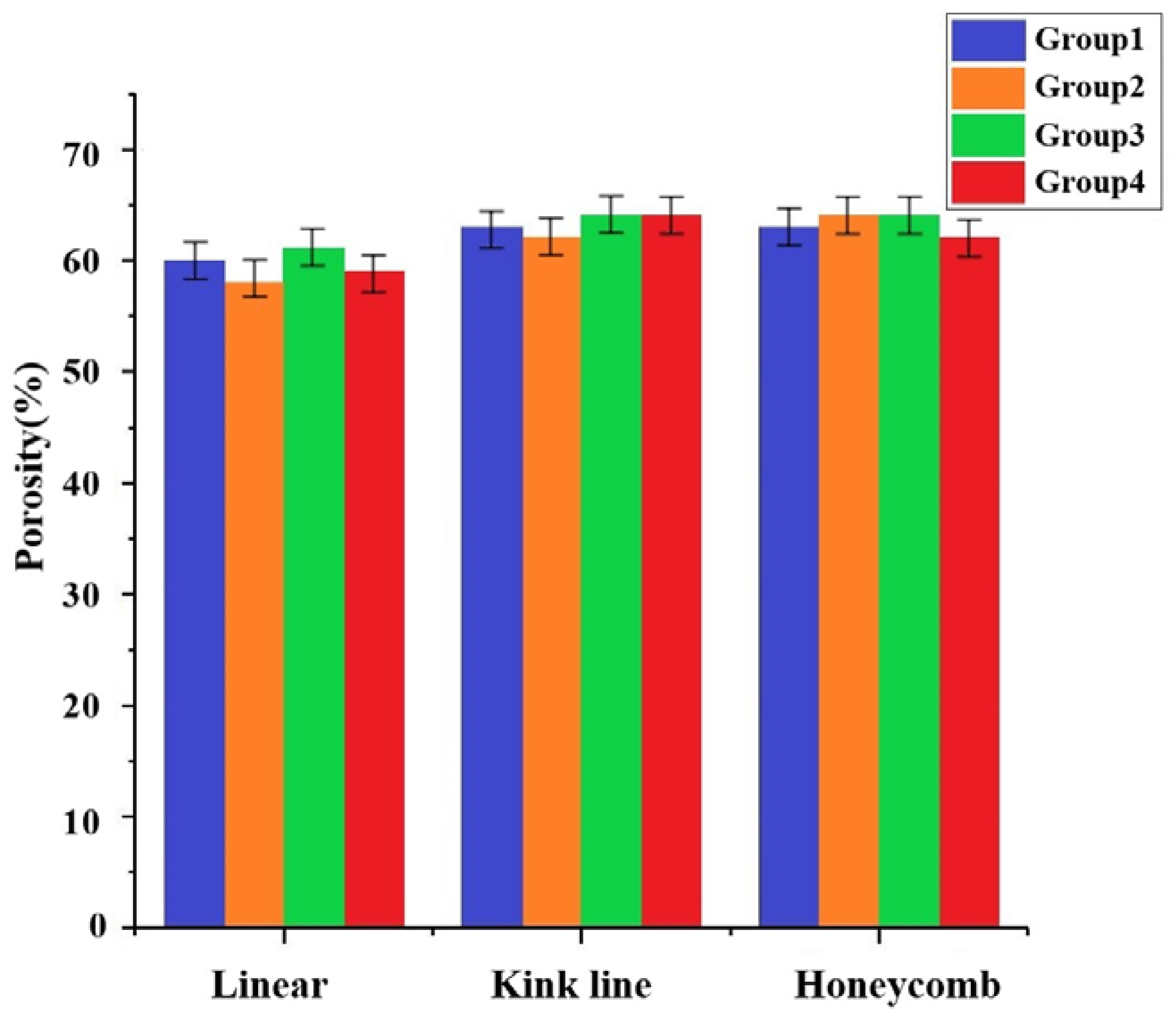
3.5. Mechanical Properties Testing of Molded Brackets
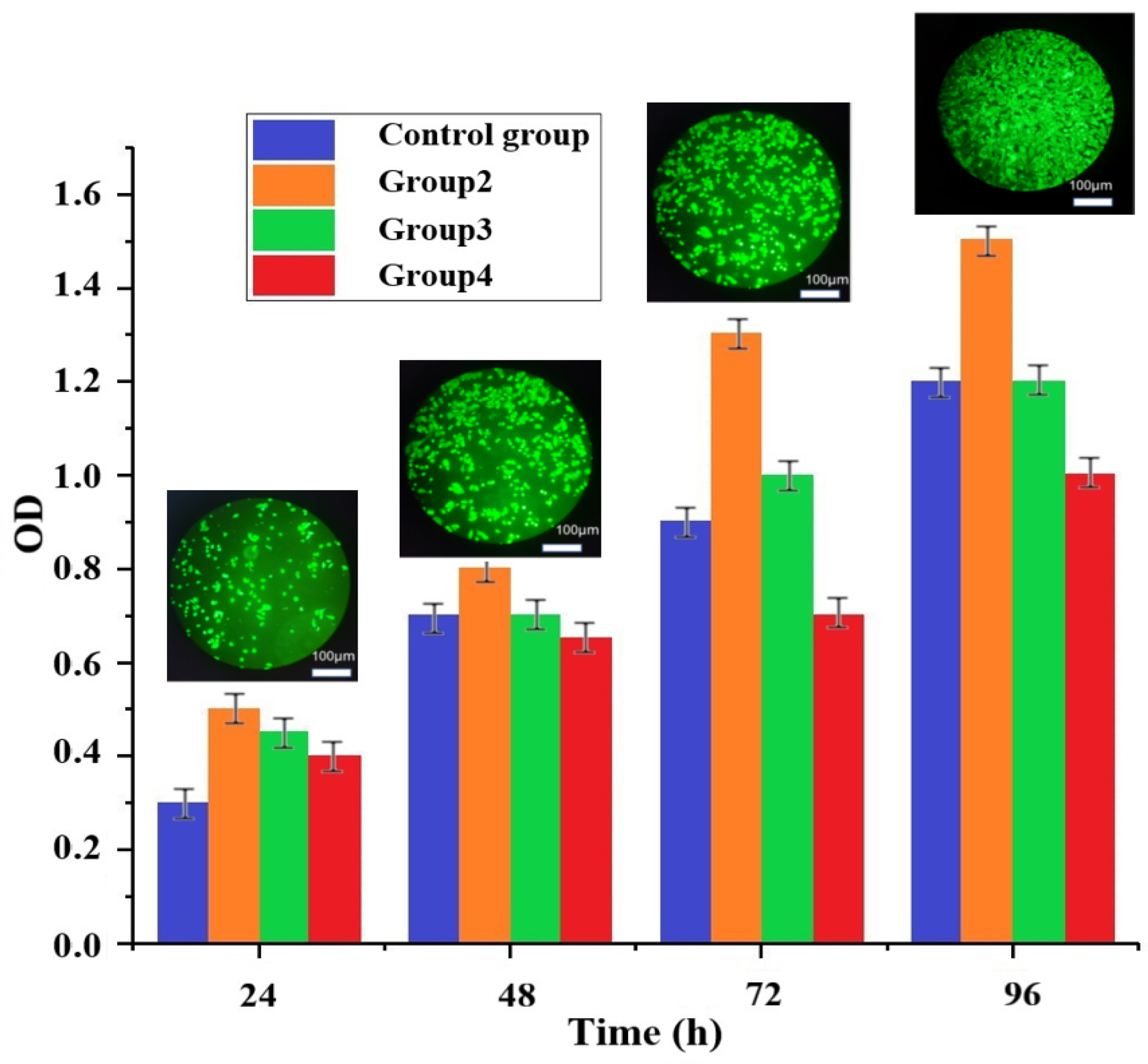
3.6. Cytotoxicity Evaluation of Composite Scaffolds
- (1)
- Stent pre-treatment
- (2)
- Preparation of extracts
- (3)
- Cell Processing
- (4)
- MTT (Methylthiazole) assay for cell activity
4. Conclusions
- The mechanical properties of the artificial bone made by different grouping ratio schemes under three structures were obtained. Depending on the percentage of ZrO2, compressive strengths of 5–17 MPa and elastic moduli of 52–174 MPa were obtained under the linear internal structure, and compressive strengths of 6–19 MPa and 12–25 MPa and elastic moduli of 64–196 MPa and 126–259 MPa were obtained by improving the internal structure to the folded and honeycomb types, respectively.
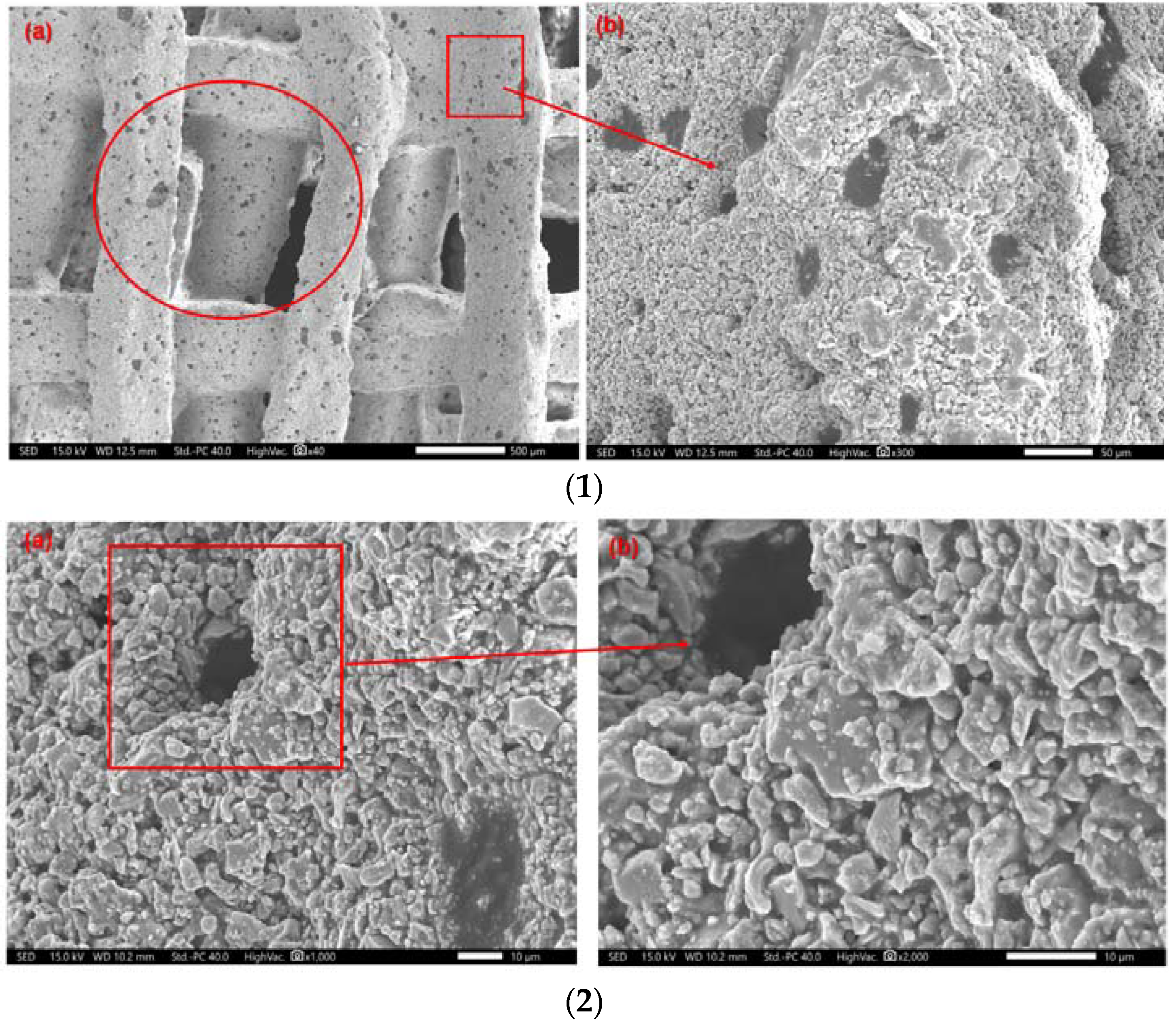
- In vitro degradation experiments found that the surface of the scaffold can be a deposited apatite layer. The mechanical strength decreases with decomposition with the generation of an apatite layer and increase.
- Experiments on the cytotoxicity of artificial bone scaffolds were conducted for three groups of rationing schemes, and the cytotoxicity of group 2 and group 3 showed a grade of 0, and group 4 showed a grade of 1. As the number of days of culture increases, the cells survive better and better. Because the porosity of the cartilage scaffold is high, and there are many holes on its micro surface, the existence of these holes provides more growth space for the growth of chondrocytes, which can better facilitate the material transfer between cells. High porosity also increases the surface area of the scaffold, providing more space for cell growth and proliferation. After 96 h, the four groups of cells have grown to more than 100%, which shows that the biomaterial has good biocompatibility.
- The observed results showed that the cells grew in good condition on the scaffold surface.
- (1)
- The sintering temperature and sintering process have an influence on the molding of artificial bone. In this study, the only sintering experiment was conducted at 1080 °C, so the influence of temperature on the performance of the sintered scaffold will be considered in the future work.
- (2)
- There are few test items for biocompatibility. Due to the limitation of the actual conditions and equipment that available in our laboratory, not all test items can be carried out in our laboratory, and only three main test items can be evaluated. We could cooperate with hospital or medical research institutes to develop composite scaffolds for animal experiments to verify the biocompatibility of the composite material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, F.; Wang, T.; Yang, Y. Hydroxyapatite composite scaffold for bone regeneration via rapid prototyping technique: A review. Rapid Prototyp. J. 2022, 28, 585–605. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Suresh, S.; Ratheesh, V.; Wong, R.; Gupta, M. Biocompatibility of Metal Matrix Composites Used for Biomedical Applications. Encycl. Mater. Compos. 2021, 1, 474–501. [Google Scholar]
- Rutkūnas, V.; Gedrimienė, A.; Auškalnis, L.; Admakin, O.; Mangano, F. Accuracy of Fixed Implant-Supported Dental Prostheses Additively Manufactured by Metal, Ceramic, or Polymer: A Systematic Review. J. Prosthodont. 2022, 31, 70–87. [Google Scholar] [CrossRef]
- Alharbi, D.; Ahmed, Z. Morphometric study of the knee joint in Saudi Arabian population based on magnetic resonance imaging scan. J. Anat. Soc. India 2021, 70, 3. [Google Scholar]
- Shah, R.R.; Mohammed, S.; Saifuddin, A.; Taylor, B.A. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur. Spine J. 2003, 12, 378–385. [Google Scholar] [CrossRef]
- Mitra, J.; Tripathi, G.; Sharma, A.; Basu, B. Scaffolds for bone tissue engineering: Role of surface patterning on osteoblast response. RSC Adv. 2013, 3, 11073–11094. [Google Scholar] [CrossRef]
- Lee, C.; Darling, C.L.; Fried, D. Polarization-sensitive optical coherence tomographic imaging of artificial demineralization on exposed surfaces of tooth roots. Dent. Mater. 2009, 25, 721–728. [Google Scholar] [CrossRef]
- Ly, M.; Spinelli, S.; Hays, S.; Zhu, D. 3D Printing of Ceramic Biomaterials. Eng. Regen. 2022, 3, 41–52. [Google Scholar] [CrossRef]
- Ratnatunga, C.P.; Edwards, M.-B.; Dore, C.J.; Taylor, K.M. Tricuspid valve replacement: UK heart valve registry mid-term results comparing mechanical and biological prostheses. Ann. Thorac. Surg. 1998, 66, 1940–1947. [Google Scholar] [CrossRef]
- De Luca, S.; Verdoliva, V.; Kargozar, S.; Baino, F. Bioactive Glass-Ceramic Scaffolds Coated with Hyaluronic Acid–Fatty Acid Conjugates: A Feasibility Study. J. Funct. Biomater. 2023, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, M.; Taira, M.; Sawada, T.; Hachinohe, Y.; Hatakeyama, W.; Takafuji, K.; Tekemoto, S.; Kondo, H. Preparation of Collagen/Hydroxyapatite Composites Using the Alternate Immersion Method and Evaluation of the Cranial Bone-Forming Capability of Composites Complexed with Acidic Gelatin and b-FGF. Materials 2022, 15, 8802. [Google Scholar] [CrossRef] [PubMed]
- Bystrova, A.V.; Dekhtyar, Y.D.; Popov, A.I.; Coutinho, J.; Bystrov, V.S. Modified Hydroxyapatite Structure and Properties: Modeling and Synchrotron Data Analysis of Modified Hydroxyapatite Structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.-J. The Influence of X-ray Radiation on the Mineral/Organic Matrix Interaction of Bone Tissue: An FT-IR Microscopic Investigation. Int. J. Artif. Organs 2005, 28, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ryu, J.; Jung, S.; Park, H.; Oh, H.; Kook, M. Effect of Hydroxyapatite Nanoparticles and Nitrogen Plasma Treatment on Osteoblast Biological Behaviors of 3D-Printed HDPE Scaffold for Bone Tissue Regeneration Applications. Materials 2022, 15, 827. [Google Scholar] [CrossRef]
- Graupner, N.; Herrmann, A.S.; Müssig, J. Natural and man-made cellulose fibre-reinforced poly(lactic acid) (PLA) composites: An overview about mechanical characteristics and application areas. Compos. Part A 2009, 40, 810–821. [Google Scholar] [CrossRef]
- Suradi, S.S.; Yunus, R.M.; Beg, M.D.H.; Rivai, M.; Yusof, Z.A.M. Oil Palm Bio-Fiber Reinforced Thermoplastic Composites-Effects of Matrix Modification on Mechanical and Thermal Properties. J. Appl. Sci. 2010, 10, 3271–3276. [Google Scholar] [CrossRef]
- Vishwas, M.; Basavaraj, C.; Vinyas, M. Experimental Investigation using Taguchi Method to Optimize Process Parameters of Fused Deposition Modeling for ABS and Nylon Materials. Mater. Today Proc. 2018, 5, 7106–7114. [Google Scholar] [CrossRef]
- Salama, A.; Kamel, B.M.; Osman, T.A.; Rashad, R.M. Investigation of mechanical properties of UHMWPE composites reinforced with HAPþTiO2 fabricated by solvent dispersing technique. J. Mater. Res. Technol. 2022, 11, 4330–4344. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Liu, S.; Dong, X.; Li, Y.; Zhang, H.; Yang, Z.; Su, Q.; Huang, W.; Zheng, W.; et al. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110054. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Nakonieczny, D.S.; Kern, F.; Dufner, L.; Dubiel, A.; Antonowicz, M.; Matus, K. Effect of Calcination Temperature on the Phase Composition, Morphology, and Thermal Properties of ZrO2 and Al2O3 Modified with APTES (3-aminopropyltriethoxysilane). Materials 2021, 14, 6651. [Google Scholar] [CrossRef]
- Ferreira, C.; Santiago, A.; Vasconcelos, R.; Paiva, D.; Pirih, F.; Araújo, A.; Motta, F.; Bomio, M. Study of microstructural, mechanical, and biomedical properties of zirconia/hydroxyapatite ceramic composites. Ceram. Int. 2022, 48, 12376–12386. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Slíva, A.; Paszenda, Z.; Hundáková, M.; Kratošová, G.; Holešová, S.; Majewska, J.; Kałużyński, P.; Sathish, S.K.; Martynková, G.S. Simple Approach to Medical Grade Alumina and Zirconia Ceramics Surface Alteration via Acid Etching Treatment. Crystals 2021, 11, 1232. [Google Scholar] [CrossRef]
- Almutairi, B.; Binhasan, M.; Shabib, S.; Al-Qahtani, A.S.; Tulbah, H.I.; Al-Aali, K.A.; Vohra, F.; Abduljabbar, T. Adhesive bond integrity of silanized zirconia nanoparticles in polymeric resin dentin bonding agent: An FTIR, SEM, TEM and Micro-tensile experiment. Int. J. Adhes. Adhes. 2022, 114, 103069. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Martynková, G.S.; Hundáková, M.; Kratošová, G.; Holešová, S.; Kupková, J.; Pazourková, L.; Majewska, J. Alkali-Treated Alumina and Zirconia Powders Decorated with Hydroxyapatite for Prospective Biomedical Applications. Materials 2022, 15, 1390. [Google Scholar] [CrossRef]
- Okuda, K.; Tai, H.; Tanabe, K.; Suzuki, H.; Sato, T.; Kawase, T.; Saito, Y.; Wolff, L.F.; Yoshiex, H. Platelet-Rich Plasma Combined With a Porous Hydroxyapatite Graft for the Treatment of Intrabony Periodontal Defects in Humans: A Comparative Controlled Clinical Study. J. Periodontol. 2005, 76, 890–898. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, Y.; Huang, L.; Liu, J.; Lu, H. Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics. BMC Musculoskelet. Disord. 2014, 15, 114. [Google Scholar] [CrossRef]
- Matthys, F.; Meirhaeghe, J.V.; Pattyn, C. Fracture risk during extraction of well-fixed extended cementless stems: Porous versus hydroxyapatite coated. Acta Orthop. Belg. 2021, 87, 41–45. [Google Scholar] [CrossRef]
- Fadli, A.; Widiyanti, P.; Noviana, D.; Prabowo, A.; Mulyadi, A.; Deska. Porous hydroxyapatite scaffold produced using Musa paradisiaca template and its in vitro bioactivity. J. Aust. Ceram. Soc. 2022, 58, 357–366. [Google Scholar] [CrossRef]
- Atyabi, F.; Bakhshandeh, H.; Soleimani, M.; Hosseini, S.S.; Hashemi, H.; Shabani, I.; Shafiee, A.; Nejad, A.H.B.; Erfan, M.; Dinarvand, R. Poly (ε-caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial cornea. Int. J. Nanomed. 2011, 6, 1509–1515. [Google Scholar] [CrossRef]
- Friedrich, B.K.; Ford, T.D.; Glaspell, A.W.; Choo, K. Experimental study of the hydrodynamic and heat transfer of air-assistant circular water jet impinging a flat circular disk. Int. J. Heat Mass Transf. 2017, 106, 804–809. [Google Scholar] [CrossRef]
- Belšak, G.; Bajt, S.; Šarler, B. Numerical Study of the Micro-Jet Formation in Double Flow Focusing Nozzle Geometry Using Different Water-Alcohol Solutions. Materials 2021, 14, 3614. [Google Scholar] [CrossRef]
- Kang, S. Characteristics of flow over two circular cylinders in a side-by-side arrangement at low Reynolds numbers. Phys. Fluids 2003, 15, 2486–2498. [Google Scholar] [CrossRef]
- Wu, S.; Qin, G.; Cao, J. Deformation, Failure, and Acoustic Emission Characteristics under Different Lithological Confining Pressures. Materials 2022, 15, 4257. [Google Scholar] [CrossRef]
- Solonenko, A.P.; Shevchenko, A.E.; Rozdestvensky, A.A.; Dzyuba, G.G. Investigation of the process and products of calcination of composite granules based on hydroxyapatite, wollastonite and gelatine. J. Phys. Conf. Ser. 2022, 2182, 012081. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Kang, Y.; Sun, L. Design and analysis of the mechanical properties of controllable porous scaffolds for bone tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 236, 748–760. [Google Scholar] [CrossRef]
- Chauvette, J.-F.; Brzeski, D.; Hia, I.L.; Farahani, R.D.; Piccirelli, N.; Therriault, D. High-speed multinozzle additive manufacturing and extrusion modeling of large-scale microscaffold networks. Addit. Manuf. 2021, 47, 102294. [Google Scholar] [CrossRef]
- Mohseni-Vadeghani, E.; Karimi-Soflou, R.; Khorshidi, S.; Karkhaneh, A. Fabrication of oxygen and calcium releasing microcarriers with different internal structures for bone tissue engineering: Solid filled versus hollow microparticles. Colloids Surf. B Biointerfaces 2021, 197, 111376. [Google Scholar] [CrossRef]
- Kamboj, N.; Kazantseva, J.; Rahmani, R.; Rodríguez, M.A.; Hussainova, I. Selective laser sintered bio-inspired silicon-wollastonite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111223. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; Garcia-Gonzalez, C.A. 3D-Printed, Dual Crosslinked and Sterile Aerogel Scaffolds for Bone Tissue Engineering. Polymers 2022, 14, 1211. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A.I. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MgAl2O4 Ceramics. Nanomaterials 2021, 11, 3373. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Sun, Y.; Lv, H.; Lu, M.; Huangfu, H.; Li, Y.; Zhang, Y.; Wang, D.; Wang, L.; Zhou, Y. 3D printing of MXene composite hydrogel scaffolds for photothermal antibacterial activity and bone regeneration in infected bone defect models. Nanoscale 2022, 14, 8112–8129. [Google Scholar] [CrossRef] [PubMed]

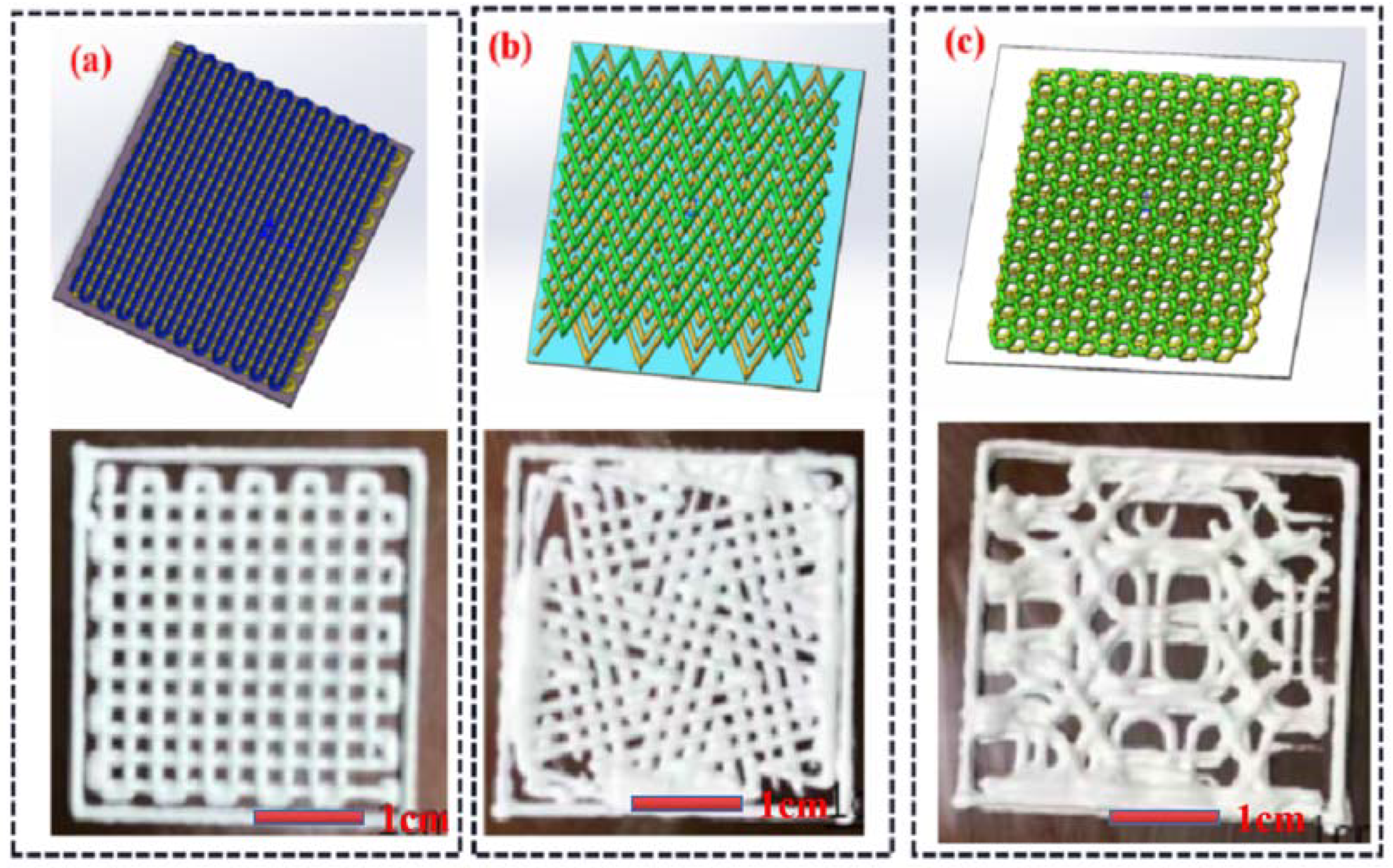

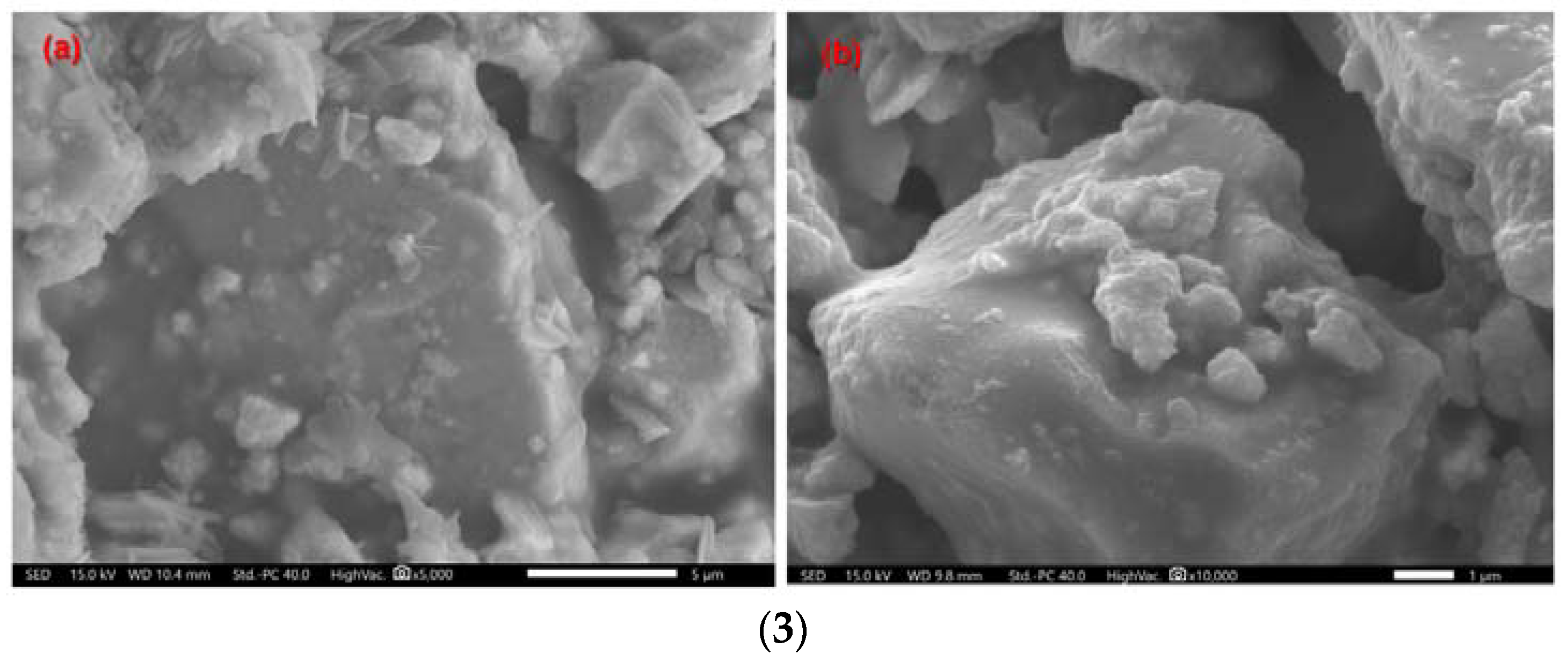
| Number | HA (g) | ZrO2 (g) | PVA (g) |
|---|---|---|---|
| 1 | 14.00 | 0.00 | 4.00 |
| 2 | 12.60 | 1.40 | 4.00 |
| 3 | 11.20 | 2.80 | 4.00 |
| 4 | 9.80 | 4.20 | 4.00 |
| Group Name | Print Height (mm) | Nozzle Size (μm) | Movement (mm/s) | Pressure (bar) |
|---|---|---|---|---|
| 1 | 20 | 400 | 10 | 1 |
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 2 | 20 | 400 | 10 | 1 |
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 3 | 20 | 400 | 10 | 1 |
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 4 | 20 | 400 | 10 | 1 |
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bie, H.; Chen, H.; Shan, L.; Tan, C.Y.; Al-Furjan, M.S.H.; Ramesh, S.; Gong, Y.; Liu, Y.F.; Zhou, R.G.; Yang, W.; et al. 3D Printing and Performance Study of Porous Artificial Bone Based on HA-ZrO2-PVA Composites. Materials 2023, 16, 1107. https://doi.org/10.3390/ma16031107
Bie H, Chen H, Shan L, Tan CY, Al-Furjan MSH, Ramesh S, Gong Y, Liu YF, Zhou RG, Yang W, et al. 3D Printing and Performance Study of Porous Artificial Bone Based on HA-ZrO2-PVA Composites. Materials. 2023; 16(3):1107. https://doi.org/10.3390/ma16031107
Chicago/Turabian StyleBie, Hongling, Honghao Chen, Lijun Shan, C. Y. Tan, M. S. H. Al-Furjan, S. Ramesh, Youping Gong, Y. F. Liu, R. G. Zhou, Weibo Yang, and et al. 2023. "3D Printing and Performance Study of Porous Artificial Bone Based on HA-ZrO2-PVA Composites" Materials 16, no. 3: 1107. https://doi.org/10.3390/ma16031107
APA StyleBie, H., Chen, H., Shan, L., Tan, C. Y., Al-Furjan, M. S. H., Ramesh, S., Gong, Y., Liu, Y. F., Zhou, R. G., Yang, W., & Wang, H. (2023). 3D Printing and Performance Study of Porous Artificial Bone Based on HA-ZrO2-PVA Composites. Materials, 16(3), 1107. https://doi.org/10.3390/ma16031107






