Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanostructured Carbon Materials (NCMs)
2.2. Methods
3. Results and Discussion
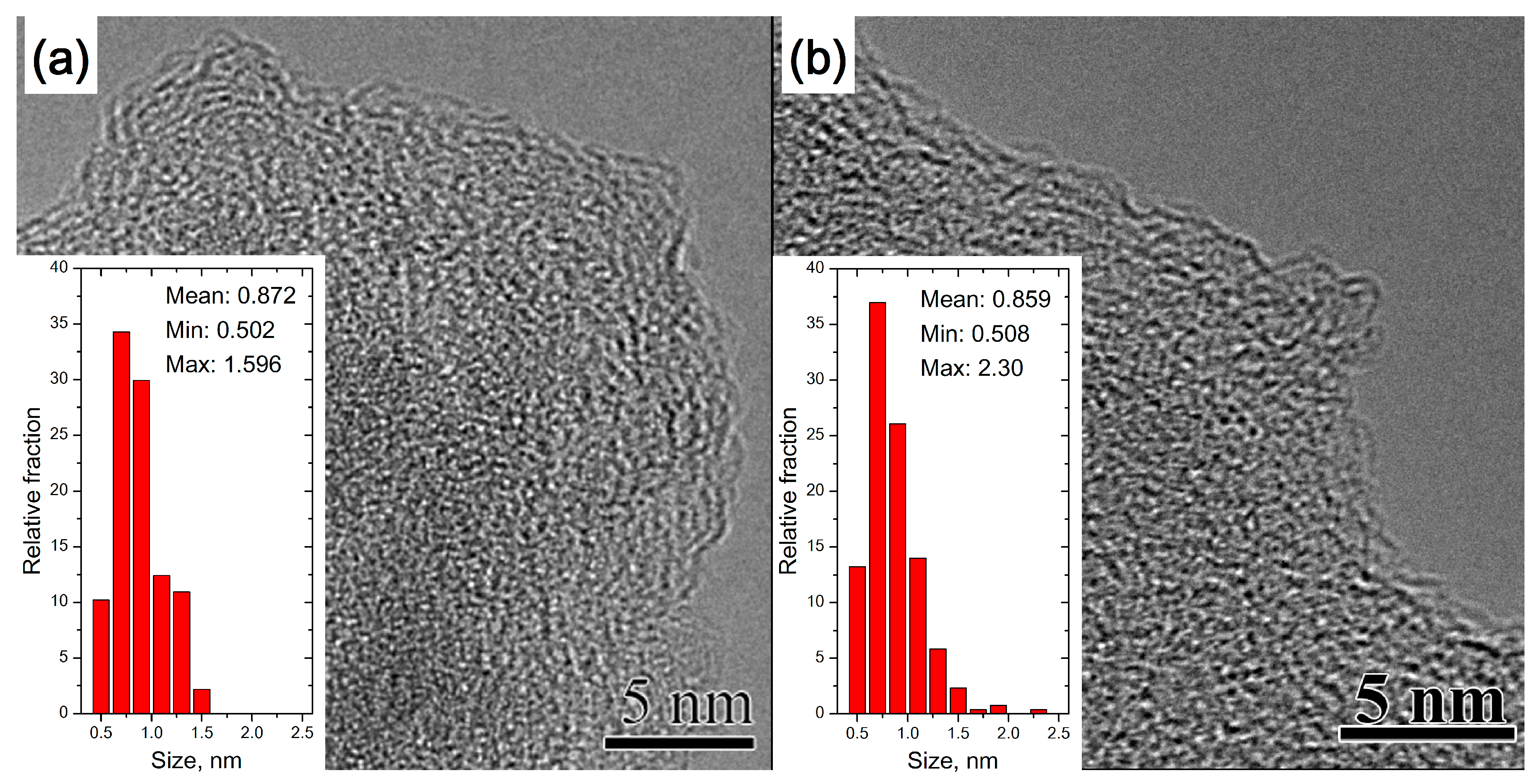
3.1. Amorphous Carbon
3.1.1. TEM of Heat-Treated Amorphous Carbon
3.1.2. XPS of Heat-Treated Amorphous Carbon
3.1.3. Raman of Heat-Treated Amorphous Carbon
3.2. Nanodiamond
3.2.1. TEM Characterization of ND
3.2.2. XRD Characterization of ND
3.2.3. Raman Characterization of ND
3.3. Carbon Black Printex-XE2-B
3.3.1. XRD Characterization of the Structure of Initial CB
3.3.2. TEM Comparative Study
3.3.3. Raman Spectroscopy Characterization
3.4. MWCNTs
3.4.1. TEM Characterization
3.4.2. Raman Spectroscopy Characterization
4. Conclusions
- The use of the 7-peak model for fitting the Raman spectra showed its practical applicability for studying complex systems such as amorphous carbon and made it possible to distinguish between two types of amorphous carbon, which are characterized by different modes in the Raman spectra—D3′ and D3″.
- In addition, the separation of the contributions of D3′ and D3″ amorphous carbon modes, as well as of D1′ and D1″ modes in the 7-peak model, allows for improving the accuracy of fitting the main modes G and D2 (D) and obtaining a satisfactory convergence between XPS and Raman spectroscopy.
- Our previous study on the optical properties of NDs made it possible to estimate the critical size of the graphene fragments as 1.7 nm; below it appears in the Raman spectra as the D3′ mode, and above it as the D3″ mode.
- The use of the 7-peak model for fitting the Raman spectra confirmed the obtained data on the presence of amorphous carbon by an independent TEM method in the heat-treated samples of CB Printex-XE2-B. For the heat-treated MWCNTs, the 7-peak fitting model made it possible to isolate the change in the contribution of D3′ and D3″ modes of amorphous carbon and to determine its correspondence to the structural elements (sp2 fragments on the surface, wall defects and nanotube bends).
- The approval of the 7-peak model for fitting the Raman spectra of various NCMs—AC, heated ND, CB Printex-XE2-B and MWCNT—also showed good agreement between the Raman data and data obtained with independent methods, such as TEM, XPS and XRD in the current work and the optical properties of the heat-treated NDs and simulation of the ND graphitization process in our previous works.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donnet, J.-B.; Bansal, R.C.; Wang, M.-J. Carbon Black: Science and Technology, 2nd ed.; Donnet, J.-B., Ed.; Dekker: New York, NY, USA, 1993; ISBN 978-0-8247-8975-6. [Google Scholar]
- Arnault, J.-C.; Gif-sur-Yvette; Eder, D. Synthesis and Applications of Nanocarbons, 1st ed.; Arnault, J., Eder, D., Eds.; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-42941-8. [Google Scholar]
- Yao, F.; Pham, D.T.; Lee, Y.H. Carbon-Based Materials for Lithium-Ion Batteries, Electrochemical Capacitors, and Their Hybrid Devices. ChemSusChem 2015, 8, 2284–2311. [Google Scholar] [CrossRef] [PubMed]
- Bezzon, V.D.N.; Montanheiro, T.L.A.; de Menezes, B.R.C.; Ribas, R.G.; Righetti, V.A.N.; Rodrigues, K.F.; Thim, G.P. Carbon Nanostructure-Based Sensors: A Brief Review on Recent Advances. Adv. Mater. Sci. Eng. 2019, 2019, 4293073. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.D.; Pathak, A.; Semwal, V. Carbon-Based Nanomaterials for Plasmonic Sensors: A Review. Sensors 2019, 19, 3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Hu, G.; He, H.; Yi, M.; Ge, Y.; Ran, L.; Peng, K. Effect of Amorphous Carbon on the Tensile Behavior of Polyacrylonitrile (PAN)-Based Carbon Fibers. J. Mater. Sci. 2019, 54, 8800–8813. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Hsu, H.-Y.; Yeh, J.-H.; Chen, W.-C.; Fu, Y.-C.; Chiu, H.-T. Synthesis and Characterization of Carbon Fiber-Reinforced Silicon Carbide Composites with an Interlayer of Amorphous Carbon Thin Film Prepared by Precursor Infiltration and Pyrolysis Processes. Adv. Eng. Mater. 2019, 21, 1800583. [Google Scholar] [CrossRef]
- Krasnikov, D.V.; Kuznetsov, V.L.; Romanenko, A.I.; Shmakov, A.N. Side Reaction in Catalytic CVD Growth of Carbon Nanotubes: Surface Pyrolysis of a Hydrocarbon Precursor with the Formation of Lateral Carbon Deposits. Carbon 2018, 139, 105–117. [Google Scholar] [CrossRef]
- Krasnikov, D.V.; Bokova-Sirosh, S.N.; Tsendsuren, T.-O.; Romanenko, A.I.; Obraztsova, E.D.; Volodin, V.A.; Kuznetsov, V.L. Influence of the Growth Temperature on the Defective Structure of the Multi-Walled Carbon Nanotubes. Phys. Status Solidi B 2018, 255, 1700255. [Google Scholar] [CrossRef] [Green Version]
- Moseenkov, S.I.; Kuznetsov, V.L.; Kolesov, B.A.; Zavorin, A.V.; Serkova, A.N.; Zolotarev, N.A. Design of Effective Surface Contacts on Polymer Composites Modified with Multiwalled Carbon Nanotubes. Express Polym. Lett. 2021, 15, 826–838. [Google Scholar] [CrossRef]
- Franklin, R.E. The Interpretation of Diffuse X-Ray Diagrams of Carbon. Acta Crystallogr. 1950, 3, 107–121. [Google Scholar] [CrossRef]
- Ergun, S.E.; Tiensuu, V.H. Interpretation of the Intensities of X-Rays Scattered by Coals. Fuel 1959, 38, 64–78. [Google Scholar]
- Lee, S.-M.; Lee, S.-H.; Roh, J.-S. Analysis of Activation Process of Carbon Black Based on Structural Parameters Obtained by XRD Analysis. Crystals 2021, 11, 153. [Google Scholar] [CrossRef]
- Kasatochkin, V.I.; Kaverov, A.T. The Kinetics and Mechanism of Homogeneous Graphitization of Carbon. Dokl. Akad. Nauk. SSSR 1957, 117, 837–840. [Google Scholar]
- Knight, D.S.; White, W.B. Characterization of Diamond Films by Raman Spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Resonant Raman Spectroscopy of Disordered, Amorphous, and Diamondlike Carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Jorio, A.; Pimenta, M.A.; Filho, A.G.S.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing Carbon Nanotube Samples with Resonance Raman Scattering. New J. Phys. 2003, 5, 139. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman Spectroscopy of Graphite. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2004, 362, 2271–2288. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General Equation for the Determination of the Crystallite Size La of Nanographite by Raman Spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying Disorder in Graphite-Based Systems by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman Spectroscopy of Graphene and Carbon Nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.C.; Hernandez, E. Electronic, Thermal and Mechanical Properties of Carbon Nanotubes. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, C.; Tommasini, M.; Zerbi, G. Raman Spectroscopy of Polyconjugated Molecules and Materials: Confinement Effect in One and Two Dimensions. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2004, 362, 2425–2459. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Jorio, A.; Souza Filho, A.G.; Dresselhaus, G.; Dresselhaus, M.S.; Pimenta, M.A. Probing Phonon Dispersion Relations of Graphite by Double Resonance Raman Scattering. Phys. Rev. Lett. 2001, 88, 027401. [Google Scholar] [CrossRef] [PubMed]
- Perebeinos, V.; Tersoff, J.; Avouris, P. Effect of Exciton-Phonon Coupling in the Calculated Optical Absorption of Carbon Nanotubes. Phys. Rev. Lett. 2005, 94, 027402. [Google Scholar] [CrossRef] [Green Version]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman Microspectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman Spectroscopy of Carbon Materials: Structural Basis of Observed Spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman Spectroscopic Characterization of Some Commercially Available Carbon Black Materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Dippel, B.; Heintzenberg, J. Soot Characterization in Atmospheric Particles from Different Sources by NIR FT Raman Spectroscopy. J. Aerosol Sci. 1999, 30, S907–S908. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman Microprobe Studies on Carbon Materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Dippel, B.; Jander, H.; Heintzenberg, J. NIR FT Raman Spectroscopic Study of Flame Soot. Phys. Chem. Chem. Phys. 1999, 1, 4707–4712. [Google Scholar] [CrossRef]
- Al-Jishi, R.; Dresselhaus, G. Lattice-Dynamical Model for Graphite. Phys. Rev. B 1982, 26, 4514–4522. [Google Scholar] [CrossRef]
- Kouketsu, Y.; Mizukami, T.; Mori, H.; Endo, S.; Aoya, M.; Hara, H.; Nakamura, D.; Wallis, S. A New Approach to Develop the R Aman Carbonaceous Material Geothermometer for Low-grade Metamorphism Using Peak Width. Isl. Arc 2014, 23, 33–50. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.K.; Kim, N.-H.; Kim, S.; Lee, Y.-N. Raman Spectroscopy Detects Amorphous Carbon in an Enigmatic Egg from the Upper Cretaceous Wido Volcanics of South Korea. Front. Earth Sci. 2020, 7, 349. [Google Scholar] [CrossRef]
- Brubaker, Z.E.; Langford, J.J.; Kapsimalis, R.J.; Niedziela, J.L. Quantitative Analysis of Raman Spectral Parameters for Carbon Fibers: Practical Considerations and Connection to Mechanical Properties. J. Mater. Sci. 2021, 56, 15087–15121. [Google Scholar] [CrossRef]
- Sheka, E.F.; Golubev, Y.A.; Popova, N.A. Graphene Domain Signature of Raman Spectra of Sp2 Amorphous Carbons. Nanomaterials 2020, 10, 2021. [Google Scholar] [CrossRef]
- Sheka, E.F.; Popova, V.A. Virtual Spectrometer for Radicals Vibrations. 1. Polyacenes and Fullerenes. arXiv 2020, arXiv:2008.08645. [Google Scholar]
- Sheka, E.F.; Popova, N.A. Virtual Spectrometer for Stable Radicals Vibrations. 2. Graphene Molecules. arXiv 2020, arXiv:2008.09158. [Google Scholar]
- Börrnert, F.; Avdoshenko, S.M.; Bachmatiuk, A.; Ibrahim, I.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Amorphous Carbon under 80 KV Electron Irradiation: A Means to Make or Break Graphene. Adv. Mater. 2012, 24, 5630–5635. [Google Scholar] [CrossRef]
- Aizawa, T.; Iwamura, E. Nano-Graphitization in Amorphous Carbon Films via Electron Beam Irradiation and the Iron Implantation. MRS Proc. 2011, 960, 1204. [Google Scholar] [CrossRef]
- Warner, J.H.; Schäffel, F.; Zhong, G.; Rümmeli, M.H.; Büchner, B.; Robertson, J.; Briggs, G.A.D. Investigating the Diameter-Dependent Stability of Single-Walled Carbon Nanotubes. ACS Nano 2009, 3, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shyam Kumar, C.N.; Korvink, J.G.; Kübel, C. Evolution of Glassy Carbon Microstructure: In Situ Transmission Electron Microscopy of the Pyrolysis Process. Sci. Rep. 2018, 8, 16282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larionova, I.; Kuznetsov, V.; Frolov, A.; Shenderova, O.; Moseenkov, S.; Mazov, I. Properties of Individual Fractions of Detonation Nanodiamond. Diam. Relat. Mater. 2006, 15, 1804–1808. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Bokova-Sirosh, S.N.; Moseenkov, S.I.; Ishchenko, A.V.; Krasnikov, D.V.; Kazakova, M.A.; Romanenko, A.I.; Tkachev, E.N.; Obraztsova, E.D. Raman Spectra for Characterization of Defective CVD Multi-Walled Carbon Nanotubes: Raman Spectra for Characterization of Defective CVD MWCNTs. Phys. Status Solidi B 2014, 251, 2444–2450. [Google Scholar] [CrossRef]
- Usoltseva, A.; Kuznetsov, V.; Rudina, N.; Moroz, E.; Haluska, M.; Roth, S. Influence of Catalysts’ Activation on Their Activity and Selectivity in Carbon Nanotubes Synthesis. Phys. Status Solidi B 2007, 244, 3920–3924. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corp: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Doniach, S.; Sunjic, M. Many-Electron Singularity in X-ray Photoemission and X-ray Line Spectra from Metals. J. Phys. C Solid State Phys. 1970, 3, 285. [Google Scholar] [CrossRef]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Butenko, Y.V.; Kuznetsov, V.L.; Paukshtis, E.A.; Stadnichenko, A.I.; Mazov, I.N.; Moseenkov, S.I.; Boronin, A.I.; Kosheev, S.V. The Thermal Stability of Nanodiamond Surface Groups and Onset of Nanodiamond Graphitization. Fuller. Nanotub. Carbon Nanostructures 2006, 14, 557–564. [Google Scholar] [CrossRef]
- Vautard, F.; Ozcan, S.; Paulauskas, F.; Spruiell, J.E.; Meyer, H.; Lance, M.J. Influence of the Carbon Fiber Surface Microstructure on the Surface Chemistry Generated by a Thermo-Chemical Surface Treatment. Appl. Surf. Sci. 2012, 261, 473–480. [Google Scholar] [CrossRef]
- Couzi, M.; Bruneel, J.-L.; Talaga, D.; Bokobza, L. A Multi Wavelength Raman Scattering Study of Defective Graphitic Carbon Materials: The First Order Raman Spectra Revisited. Carbon 2016, 107, 388–394. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Butenko, Y.V. Chapter 7—Diamond Phase Transitions at Nanoscale. In Ultananocrystalline Diamond, 2nd ed.; Shenderova, O.A., Gruen, D.M., Eds.; William Andrew Publishing: Oxford, UK, 2012; pp. 181–244. ISBN 978-1-4377-3465-2. [Google Scholar]
- Moseenkov, S.I.; Kuznetsov, V.L.; Ishchenko, A.V. Change in Sizes of Carbon Aggregates and Primary Particles of the Onion-like Carbon Synthesized by High-Temperature Annealing of Nanodiamond. Russ. Chem. Bull. 2014, 63, 599–604. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Moseenkov, S.I.; Elumeeva, K.V.; Larina, T.V.; Anufrienko, V.F.; Romanenko, A.I.; Anikeeva, O.B.; Tkachev, E.N. Comparative Study of Reflectance Properties of Nanodiamonds, Onion-like Carbon and Multiwalled Carbon Nanotubes. Phys. Status Solidi B 2011, 248, 2572–2576. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Franklin, R.E.; Randall, J.T. Crystallite Growth in Graphitizing and Non-Graphitizing Carbons. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1951, 209, 196–218. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. https://doi.org/10.3390/ma16031112
Moseenkov SI, Kuznetsov VL, Zolotarev NA, Kolesov BA, Prosvirin IP, Ishchenko AV, Zavorin AV. Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials. 2023; 16(3):1112. https://doi.org/10.3390/ma16031112
Chicago/Turabian StyleMoseenkov, S. I., V. L. Kuznetsov, N. A. Zolotarev, B. A. Kolesov, I. P. Prosvirin, A. V. Ishchenko, and A. V. Zavorin. 2023. "Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD)" Materials 16, no. 3: 1112. https://doi.org/10.3390/ma16031112
APA StyleMoseenkov, S. I., Kuznetsov, V. L., Zolotarev, N. A., Kolesov, B. A., Prosvirin, I. P., Ishchenko, A. V., & Zavorin, A. V. (2023). Investigation of Amorphous Carbon in Nanostructured Carbon Materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials, 16(3), 1112. https://doi.org/10.3390/ma16031112






