Improved Mechanical Properties of Alumina Ceramics Using Plasma-Assisted Milling Technique
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Alumina Powder
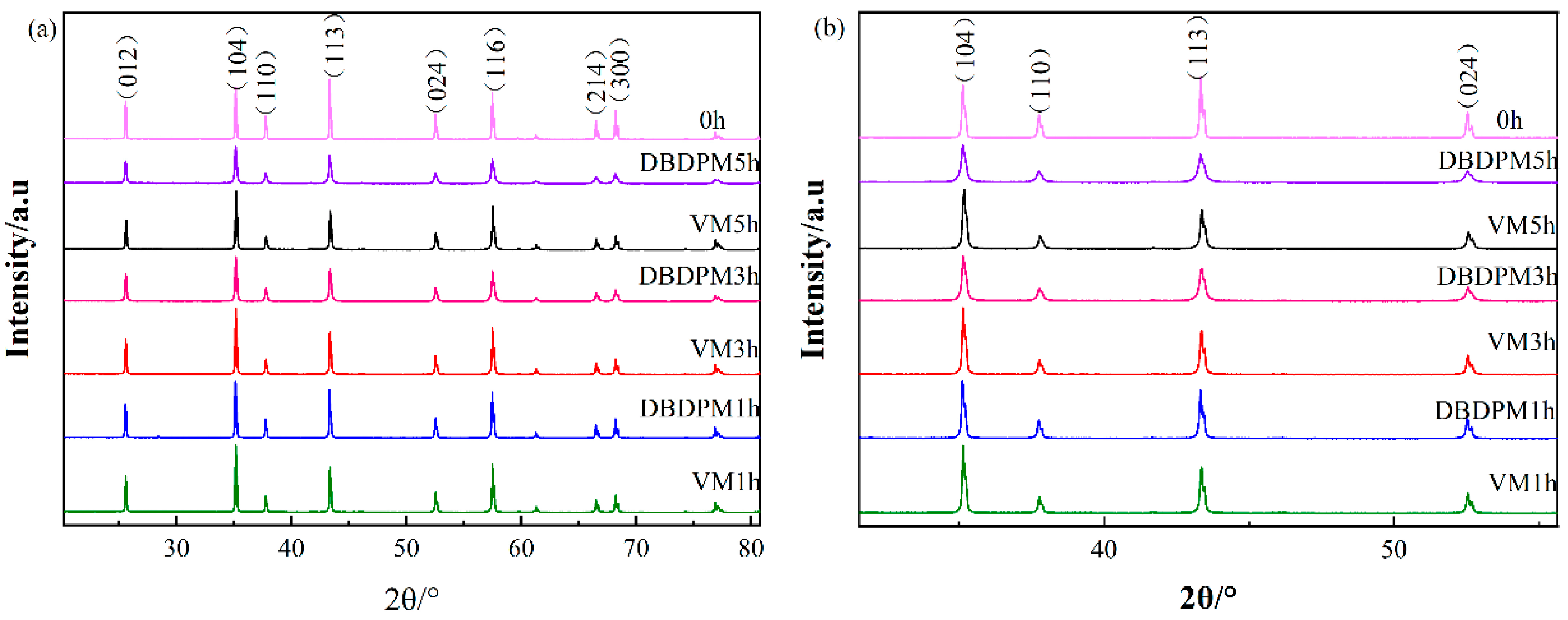
3.1.1. Phase Compositions
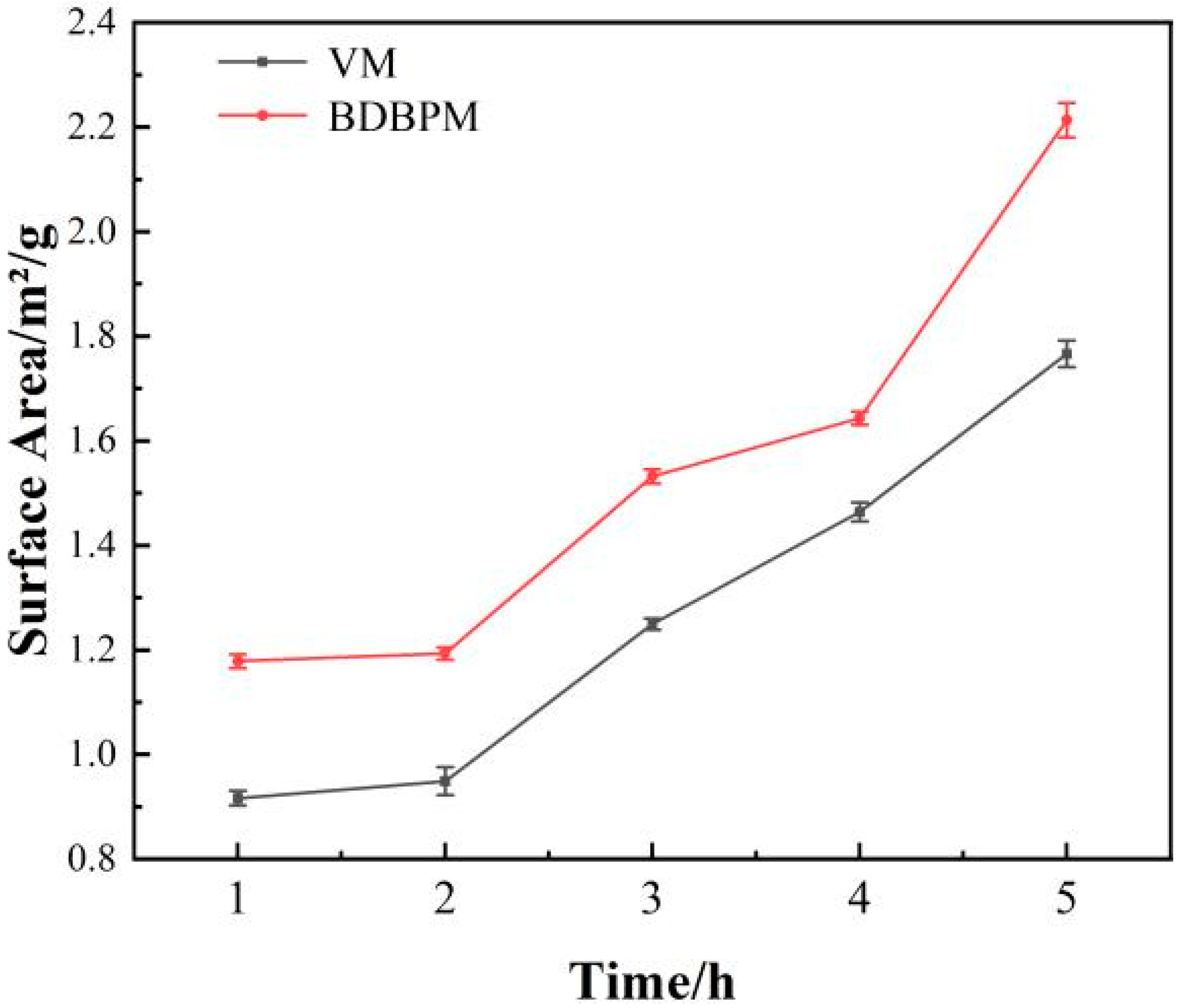
3.1.2. Particle Size
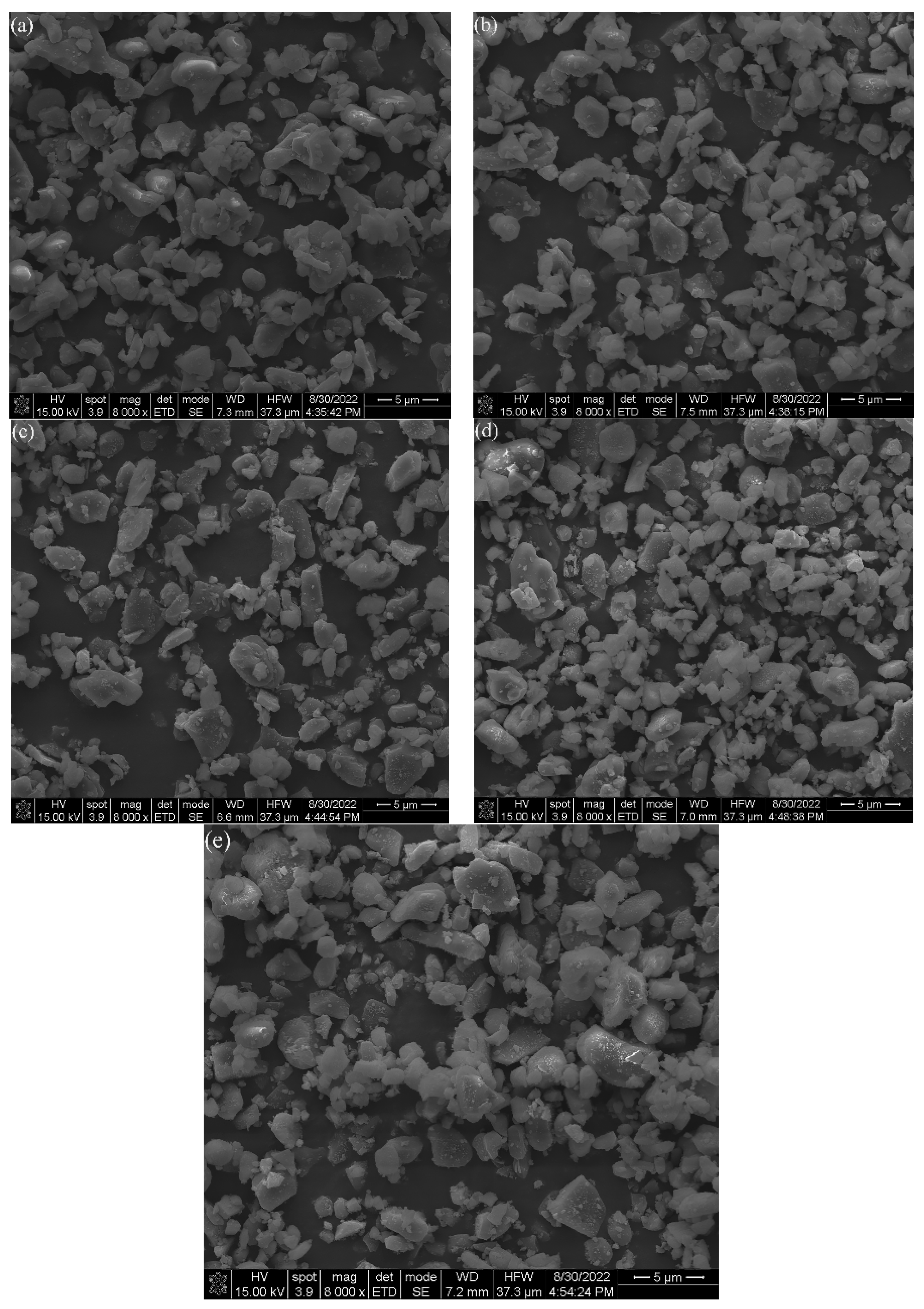
3.1.3. Micromorphology Evolution of Al2O3 Powder in Ball Milling Process
3.2. Alumina Ceramics
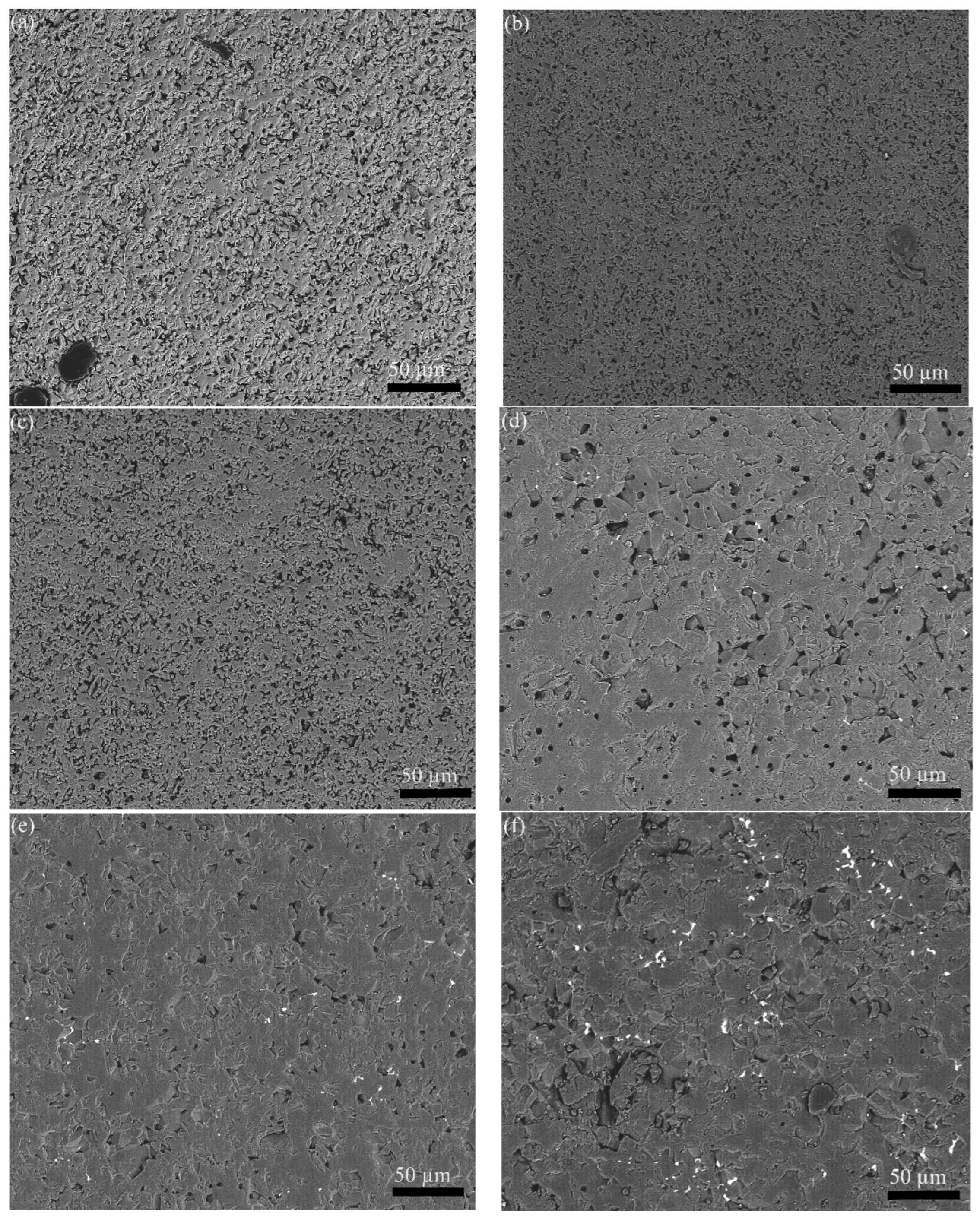
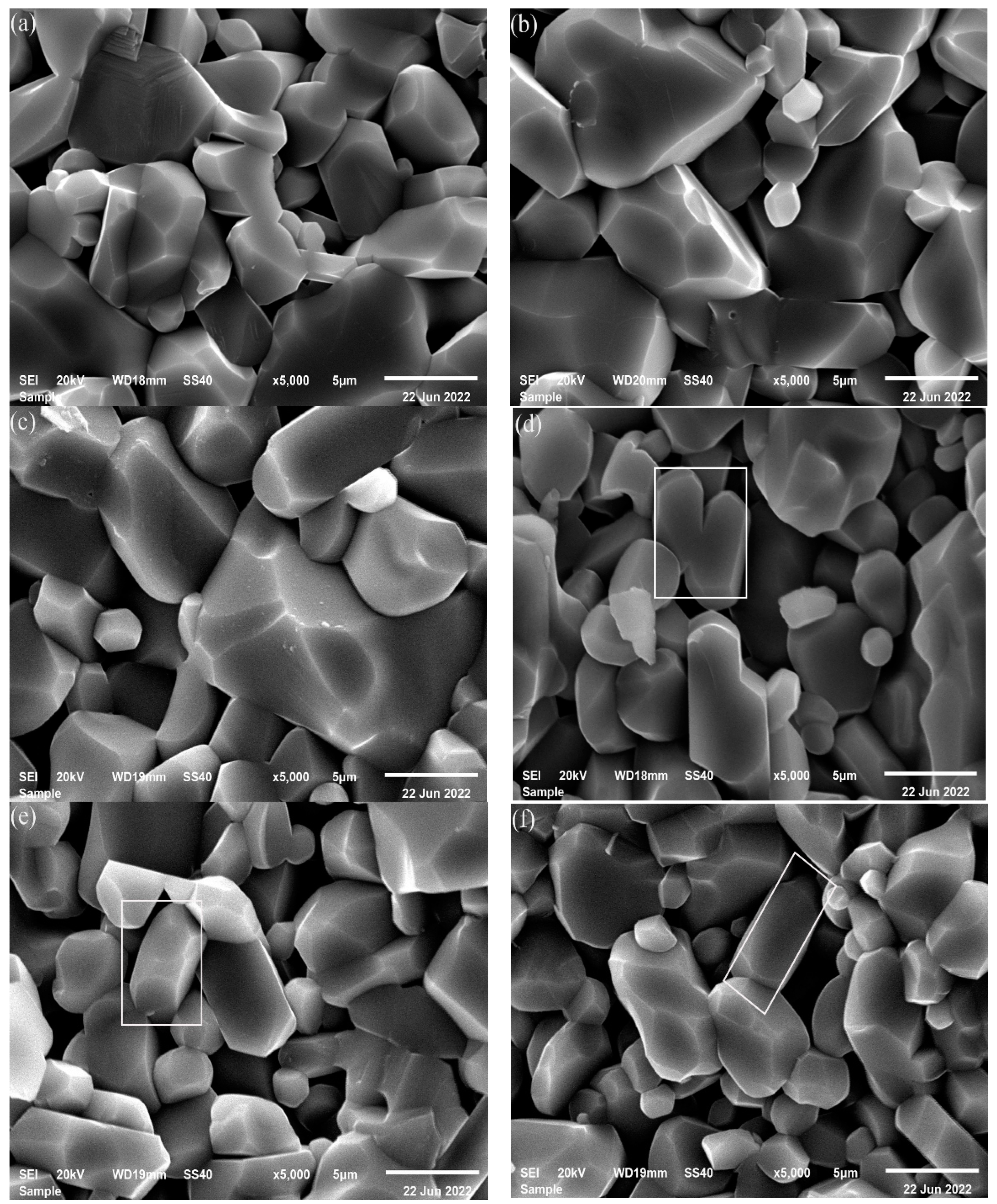
3.2.1. Microstructure
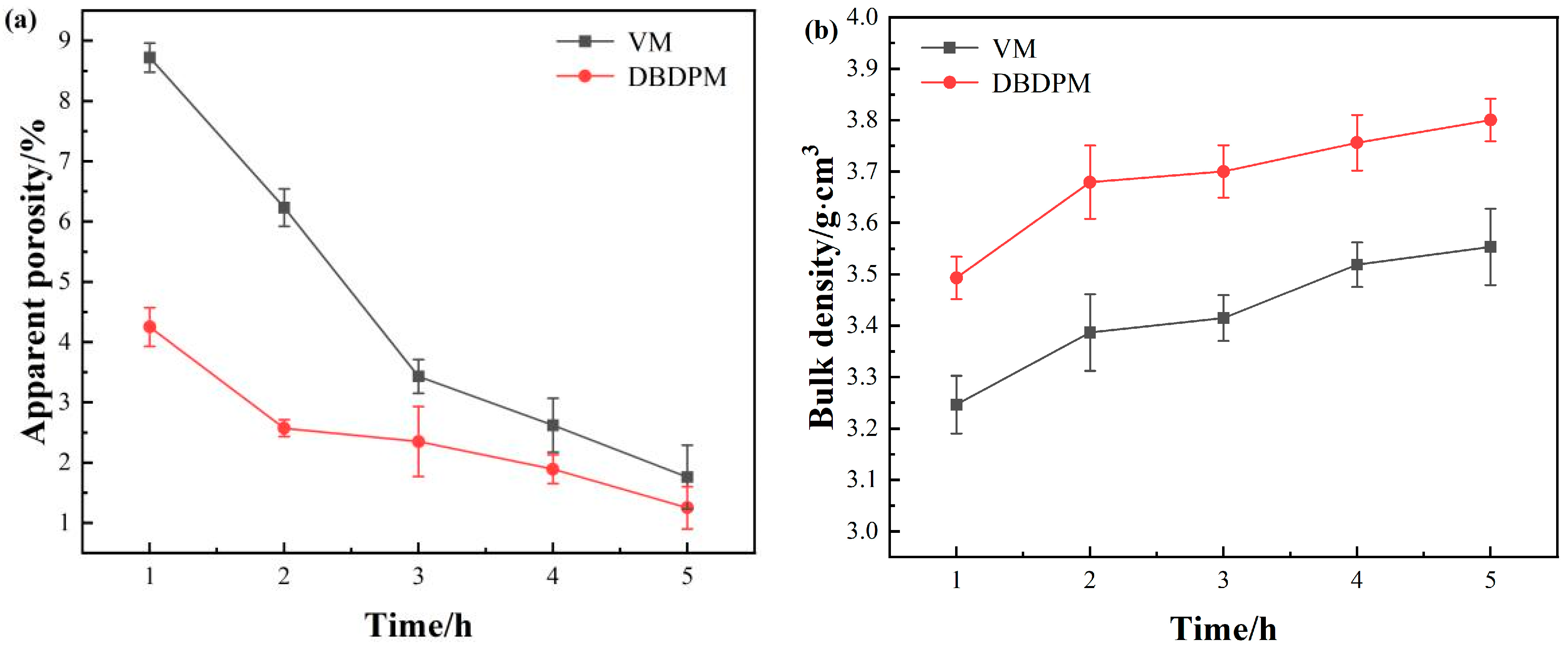
3.2.2. Sintering Properties
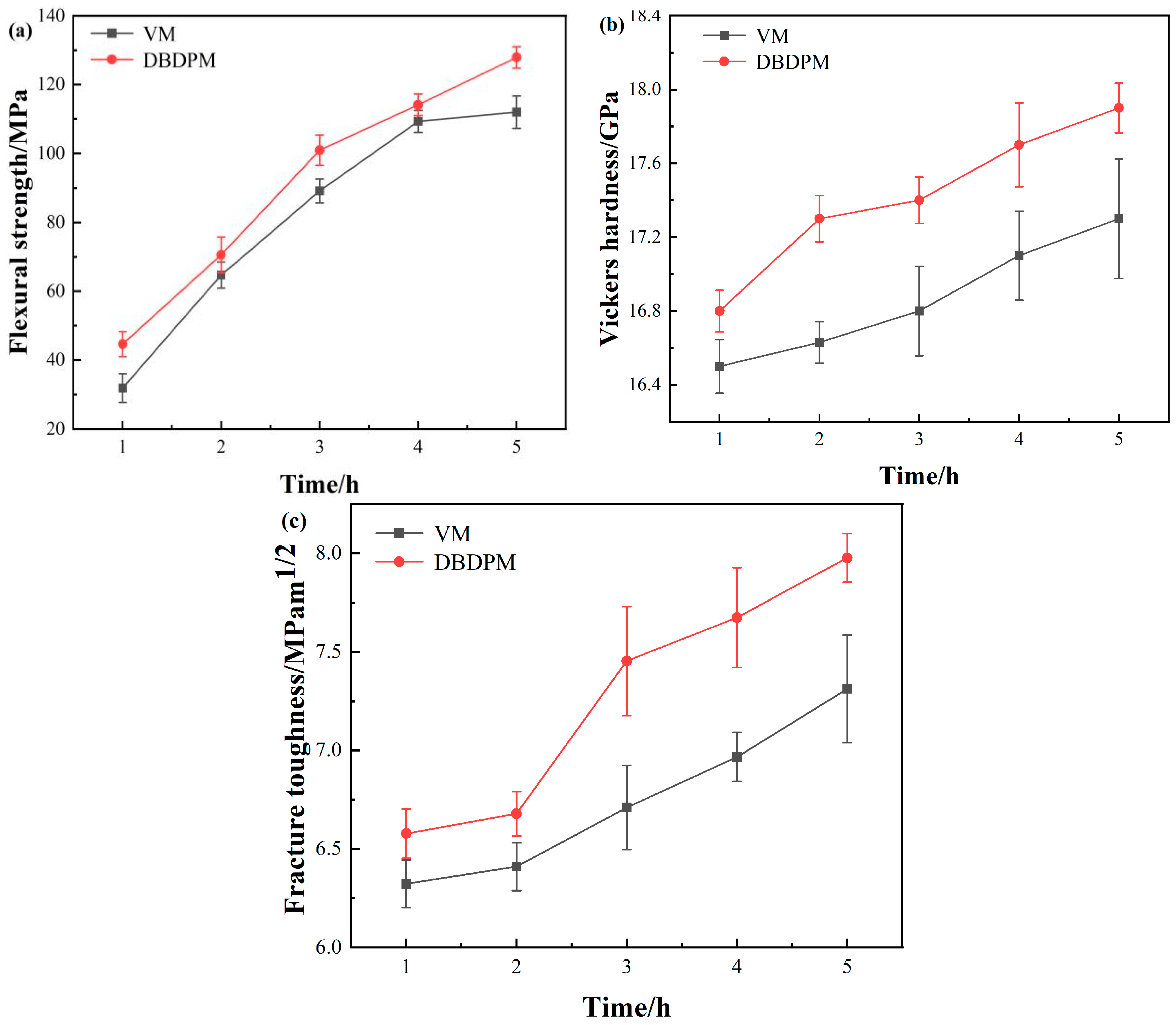
3.2.3. Mechanical Properties
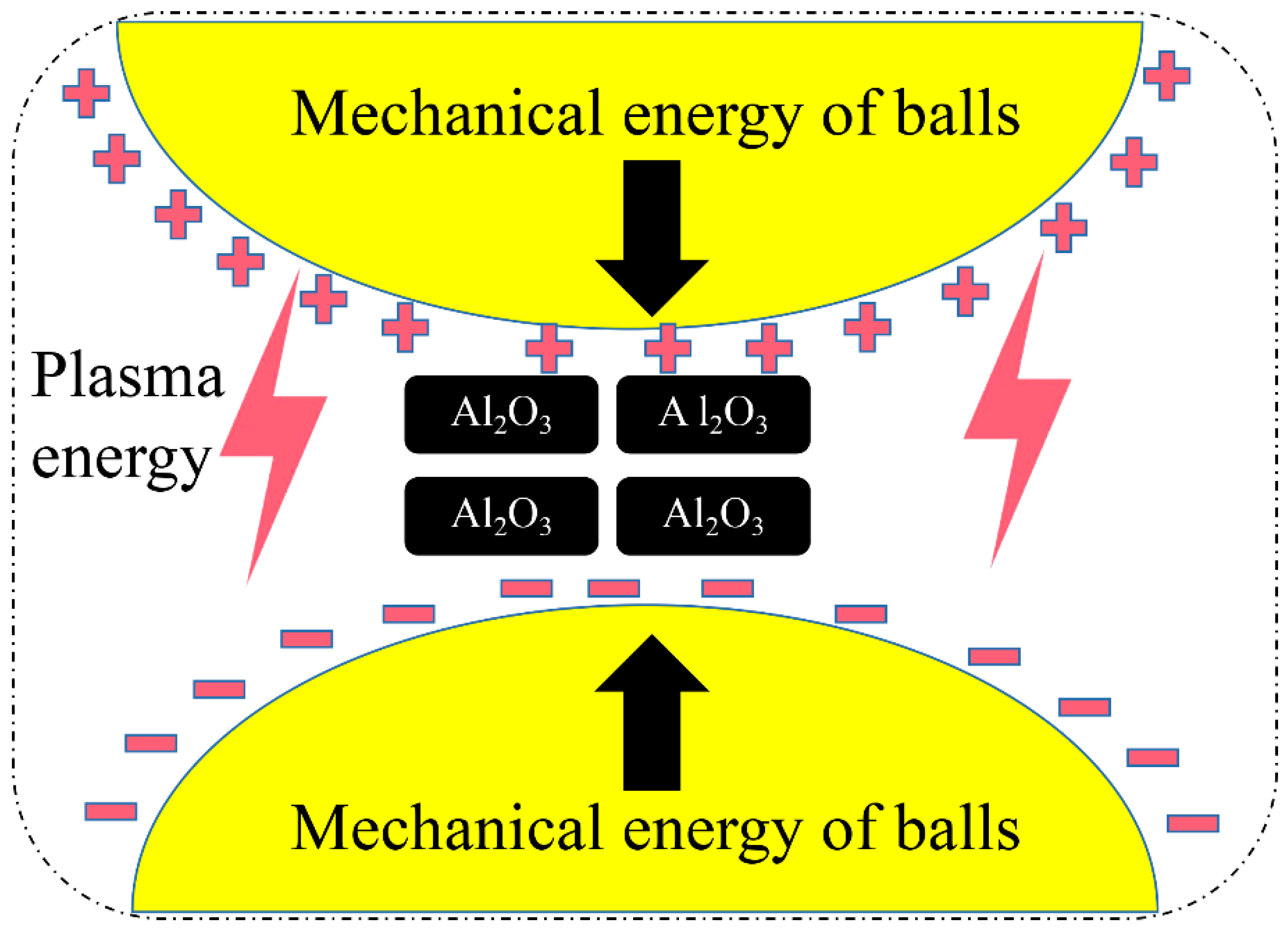
3.3. DBDPM Activation Mechanism
4. Conclusions
- (1)
- Compared with VM, the grain size of Al2O3 powder obtained with DBDPM is smaller, and the lattice distortion is larger. The lattice distortion degree of DBDPM-milled Al2O3 powder shows an obvious increasing trend with the extension of assisted ball milling time.
- (2)
- In DBDPM, the thermal effect produced by plasma and the high-energy particle bombardment effect greatly promote the lattice distortion of Al2O3, which is an important reason for activating the Al2O3 reaction powder. Therefore, compared with the common vibratory milling technique, the fabricated alumina ceramics exhibited improved sintering and mechanical properties when using alumina powders produced via the DBDPM method as the starting materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, C.; Tang, H.; Guo, P.; Ren, R. Study on mechanism of chip formation of grinding plasma-sprayed alumina ceramic coating. Ceram. Int. 2017, 43, 2697–2704. [Google Scholar] [CrossRef]
- Schwentenwein, M.; Homa, J. Additive manufacturing of dense alumina ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Jiusti, J.; Kammer, E.H.; Neckel, L.; L´oh, N.J.; Trindade, W.; Silva, A.O.; Montedo, O.R.K.; Noni, A.D. Ballistic performance of Al2O3 mosaic armors with gap-filling materials. Ceram. Int. 2017, 43, 2697–2704. [Google Scholar] [CrossRef]
- Fu, L.P.; Gu, H.Z.; Huang, A.; Ni, H.W. Correlations among processing parameters and porosity of a lightweight alumina. Ceram. Int. 2018, 44, 14076–14081. [Google Scholar] [CrossRef]
- Fu, L.P.; Gu, H.Z.; Huang, A.; Zou, Y.S.; Zhang, M.J. Fabrication of lightweight alumina with nanoscale intracrystalline pores. J. Am. Ceram. Soc. 2018, 103, 2262–2271. [Google Scholar] [CrossRef]
- Ighodaro, O.L.; Okoli, O.I. Fracture toughness enhancement for alumina systems: A review. Int. J. Appl. Ceram. Technol. 2008, 5, 313–323. [Google Scholar] [CrossRef]
- Fu, L.P.; Gu, H.Z.; Huang, A.; Or, S.W.; Zou, Y.; Zou, Y.S.; Zhang, M.J. Design, fabrication and properties of lightweight wear lining refractories: A review. J. Eur. Ceram. Soc. 2022, 42, 744–763. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, B.Y.; Hu, C.B.; Liu, G.Q.; Li, H.X.; Su, C.; Ren, X.M.; Yu, J.Y.; Zhang, Y.R.; Yu, J.K. Preparation and properties of porous SiC-Al2O3 ceramics using coal ash. Int. J. Appl. Ceram. Technol. 2019, 16, 23–31. [Google Scholar] [CrossRef]
- Ren, X.M.; Ma, B.Y.; Zhang, Y.R.; Li, D.X.; Zhu, Q.; Yuan, L.; Yu, J.K.; Liu, G.Q.; Li, H.X. Effects of sintering temperature and V2O5 additive on the properties of SiC-Al2O3 ceramic foams. J. Alloys Compd. 2018, 732, 716–724. [Google Scholar] [CrossRef]
- Paul, F.; Becher, F. Microstructural design of toughened ceramics. J. Am. Ceram. Soc. 1991, 74, 255–269. [Google Scholar] [CrossRef]
- Zhang, D.L. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004, 49, 537–560. [Google Scholar] [CrossRef]
- Ander, B.; Coulet, M.V.; Coulet, P.H.; Coulet, B.; Denoyel, R. High-energy ball milling to enhance the reactivity of aluminum nanopowders. Mater. Lett. 2013, 110, 108–110. [Google Scholar] [CrossRef]
- Xu, D.; Xu, D.M.; Jiao, L.; Yuan, H.M.; Zhao, G.P.; Cheng, X.N. Effects of high-energy ball milling oxide-doped and varistor ceramic powder on ZnO varistor T. Nonferr. Metal. Soc. 2012, 22, 1423–1431. [Google Scholar] [CrossRef]
- Siahmed, F.; Faghi, L. Synthesis and characterization of polymer nanocomposites containing Fe-40 at.% Si powder particles prepared by high energy ball mill. J. Nano 2014, 9, 2965–2973. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Chen, D.; Wei, F.H.; Chen, N.N.; Liang, Z.; Luo, Y. Removal of congo red dye from aqueous solution with nickel-based metal-organic framework/graphene oxide composites prepared by ultrasonic wave-assisted ball milling. Ultrason Sonochem. 2017, 39, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Li, X.D.; Chang, Y.; Zhu, M.G.; Li, W.; Qi, M. Anisotropic Sm2Co17 nano-flakes produced by surfactant and magnetic field assisted high energy ball milling. J. Rare Earth 2013, 31, 366–369. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.C.; Wang, D.Z. Direct synthesis of AlN nano powder by dielectric barrier discharge plasma assisted high-energy ball milling. J. Mater. Sci. 2016, 27, 8518–8523. [Google Scholar] [CrossRef]
- Sun, W.; Hu, R.Z.; Liu, H.; Zeng, M.Q.; Yang, L.C.; Wang, H.H.; Zhu, M. Embedding nano-silicon in graphene nanosheets by plasma assisted milling for high capacity anode materials in lithium ion batteries. J. Power Sources 2014, 68, 610–618. [Google Scholar] [CrossRef]
- Dai, L.Y.; Lin, S.F.; Chen, J.F.; Zeng, M.Q.; Zhu, M. A new method of synthesizing of ultrafine vanadium carbide by dielectric barrier discharge plasma assisted milling. Int. J. Refract. Met. H 2012, 30, 48–50. [Google Scholar] [CrossRef]
- Nie, D.X.; Wang, W.C.; Yang, D.Z.; Shi, H.C.; Huo, Y.; Dai, L.Y. Optical study of diffuse bi-directional nanosecond pulsed dielectric barrier discharge in nitrogen. Spectrochim. Acta. A 2011, 79, 1896–1903. [Google Scholar] [CrossRef] [PubMed]



| Al2O3 | SiO2 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | ZrO2 |
|---|---|---|---|---|---|---|---|---|
| 99.59 | 0.052 | 0.033 | 0.013 | 0.087 | 0.011 | 0.16 | 0.015 | 0.001 |
| 1 h | 2 h | 3 h | |
|---|---|---|---|
| VM | 0.15419 | 0.15863 | 0.16603 |
| DBDP | 0.15458 | 0.18687 | 0.20884 |
| Material | a (Å) | b (Å) | c (Å) | α = β (◦) | γ(◦) | Crystal System | Space Group |
|---|---|---|---|---|---|---|---|
| Al2O3 | 4.7581 | 4.7581 | 4.1815 | 90 | 120 | Hexagonal | R-3C |
| D10 | D50 | D90 | ||||
|---|---|---|---|---|---|---|
| VM | DBDPM | VM | DBDPM | VM | DBDPM | |
| 1 h | 1.01 | 0.93 | 3.58 | 2.13 | 5.80 | 5.23 |
| 2 h | 0.91 | 0.91 | 3.47 | 1.88 | 5.43 | 4.95 |
| 3 h | 0.90 | 0.88 | 3.18 | 1.78 | 5.15 | 4.67 |
| 4 h | 0.85 | 0.83 | 2.92 | 1.68 | 4.83 | 4.16 |
| 5 h | 0.82 | 0.79 | 2.78 | 1.43 | 4.53 | 3.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Fu, L.; Gu, H.; Huang, A.; Yang, S.; Lv, R. Improved Mechanical Properties of Alumina Ceramics Using Plasma-Assisted Milling Technique. Materials 2023, 16, 1128. https://doi.org/10.3390/ma16031128
Tang S, Fu L, Gu H, Huang A, Yang S, Lv R. Improved Mechanical Properties of Alumina Ceramics Using Plasma-Assisted Milling Technique. Materials. 2023; 16(3):1128. https://doi.org/10.3390/ma16031128
Chicago/Turabian StyleTang, Shaopeng, Lvping Fu, Huazhi Gu, Ao Huang, Shuang Yang, and Renxiang Lv. 2023. "Improved Mechanical Properties of Alumina Ceramics Using Plasma-Assisted Milling Technique" Materials 16, no. 3: 1128. https://doi.org/10.3390/ma16031128
APA StyleTang, S., Fu, L., Gu, H., Huang, A., Yang, S., & Lv, R. (2023). Improved Mechanical Properties of Alumina Ceramics Using Plasma-Assisted Milling Technique. Materials, 16(3), 1128. https://doi.org/10.3390/ma16031128





