Effect of In Situ Mg-Sialon on the Oxidation Behavior of Low-Carbon MgO-C Refractories
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of the MgO-C Refractories
2.3. Oxidation Tests
2.4. Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, X.M.; Ma, B.Y.; Liu, H.; Wang, Z.F.; Deng, C.J.; Liu, G.Q.; Yu, J.K. Designing Low–Carbon MgO–Al2O3–La2O3–C Refractories with Balanced Performance for Ladle Furnaces. J. Eur. Ceram. Soc. 2022, 42, 3986–3995. [Google Scholar] [CrossRef]
- Qi, X.; Luo, X.D.; Zhang, L.; Wang, S.Y.; Zhao, J.L. In situ Synthesis and Interfacial Bonding Mechanism of SiC in MgO–SiC–C Refractories. Int. J. Appl. Ceram. Technol. 2022, 19, 2723–2733. [Google Scholar] [CrossRef]
- Zhu, T.B.; Li, Y.W.; Sang, S.B.; Xie, Z.P. A New Approach to Fabricate MgO–C Refractories with High Thermal Shock Resistance by Adding Artificial Graphite. J. Eur. Ceram. Soc. 2018, 38, 2179–2185. [Google Scholar] [CrossRef]
- Li, Y.G.; Wang, J.K.; Duan, H.J.; Han, L.; Jia, Q.L.; Liu, X.Y.; Zhang, S.W.; Zhang, H.J. Catalytic Preparation of Carbon Nanotube/SiC Whisker Bonded Low Carbon MgO–C Refractories and Their High–Temperature Mechanical Properties. Ceram. Int. 2022, 48, 5546–5556. [Google Scholar] [CrossRef]
- Yu, C.; Zheng, Y.X.; Xing, G.C.; Ding, J.; Zhu, H.X.; Wang, Z.F.; Deng, C.J.; Di, J.H. A Novel Strategy for the Fabrication of AlN–AlON Refractories: Nitrogen Gas Pressure Sintering of Al4O4C. J. Am. Ceram. Soc. 2022, 105, 7111–7121. [Google Scholar] [CrossRef]
- Wu, X.X.; Deng, C.J.; Di, J.H.; Ding, J.; Zhu, H.X.; Yu, C. Fabrication of Novel AlN–SiC–C Refractories by Nitrogen Gas–Pressure Sintering of Al4SiC4. J. Eur. Ceram. Soc. 2022, 42, 3634–3643. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, H.X.; Yuan, W.J.; Deng, C.J.; Cui, P.; Zhou, S.M. Synthesis of Monophase Al4O4C and the Effect of Al4O4C Addition to MgO-C Refractory. J. Alloys Compd. 2013, 579, 348–354. [Google Scholar] [CrossRef]
- Ding, D.; Chong, X.; Xiao, G.; Lv, L.; Lei, C.; Luo, J.; Zang, Y. Combustion Synthesis of B4C/Al2O3/C Composite Powders and Their Effects on Properties of Low Carbon MgO-C Refractories. Ceram. Int. 2019, 45, 16433–16441. [Google Scholar] [CrossRef]
- Yao, H.B.; Xing, X.M.; Wang, E.H.; Li, B.; Chen, J.H.; Sun, J.L.; Hou, X.M. Oxidation Behavior and Mechanism of Al4SiC4 in MgO-C-Al4SiC4 System. Coat. 2017, 7, 85. [Google Scholar] [CrossRef]
- Yu, C.; Dong, B.; Chen, Y.F.; Ma, B.Y.; Ding, J.; Deng, C.J.; Zhu, H.X.; Di, J.H. Enhanced Oxidation Resistance of Low–Carbon MgO–C Refractories with Ternary Carbides: A Review. J. Iron Steel Res. Int. 2022, 29, 1052–1062. [Google Scholar] [CrossRef]
- Xiong, S.X.; Kuang, J.L.; Zheng, Q.F.; Xiao, T.; Liu, W.X.; Wang, Q.; Jiang, P.; Cao, W.B. Effects of Si/Al, Na/Al, and H2O/Na2O Molar Ratios on Formaldehyde Barrier Properties of Inorganic Aluminosilicate Coatings. Int. J. Miner. Metall. Mater. 2021, 28, 1868–1874. [Google Scholar] [CrossRef]
- Bag, M.; Adak, S.; Sarkar, R. Study on Low Carbon Containing MgO–C Refractory: Use of Nano Carbon. Ceram. Int. 2012, 38, 2339–2346. [Google Scholar] [CrossRef]
- Atzenhofer, C.; Harmuth, H. Phase formation in MgO–C refractories with different antioxidants. J. Eur. Ceram. Soc. 2021, 41, 7330–7338. [Google Scholar] [CrossRef]
- Wang, X.; Deng, C.J.; Di, J.H.; Xing, G.C.; Ding, J.; Zhu, H.X.; Yu, C. Enhanced Oxidation Resistance of Low–carbon MgO–C Refractories with Al3BC3–Al Antioxidants: A Synergistic Effect. J. Am. Ceram. Soc. 2023. [Google Scholar] [CrossRef]
- Dong, B.; Deng, C.J.; Di, J.H.; Ding, J.; Zhu, Q.Y.; Liu, H.; Zhu, H.X.; Yu, C. Oxidation Behavior of Al3BC3 Powders at 800–1400 °C in Ambient Air. J. Mater. Res. Technol. 2023, 23, 670–679. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A.I. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MgAl2O4 Ceramics. Nanomater. 2021, 11, 3373. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ding, J.; Deng, C.J.; Zhu, H.X.; Peng, N. The Effects of Sintering Temperature on the Morphology and Physical Properties of in situ Si3N4 Bonded MgO–C Refractory. Ceram. Int. 2018, 44, 1104–1109. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, C.J.; Wang, X.; Ding, J.; Yu, C.; Zhu, H.X. Effect of Si Powder-Supported Catalyst on the Microstructure and Properties of Si3N4–MgO–C Refractories. Constr. Build. Mater. 2020, 240, 117964. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Ding, J.; Yu, C.; Deng, C.J.; Zhu, H.X. Influence of ceramic phase content and its morphology on mechanical properties of MgO–C refractories under high temperature nitriding. Ceram. Int. 2021, 47, 10603–10610. [Google Scholar] [CrossRef]
- Ma, B.Y.; Gao, Z.; Su, C.; Ren, X.M.; Li, G.Q.; Zhu, Q. Recycling of Coal Ash for Production of Dense β-Sialon/ZrN/ZrON-Based Ceramics without Sintering Aids via Pressureless Sintering. Int. J. Appl. Ceram. Technol. 2020, 17, 175–183. [Google Scholar] [CrossRef]
- Janse van Vuuren, A.; Mutali, A.; Ibrayeva, A.; Sohatsky, A.; Skuratov, V.; Akilbekov, A.; Dauletbekova, A.; Zdorovets, M. High-Energy Heavy Ion Tracks in Nanocrystalline Silicon Nitride. Cryst. 2022, 12, 1410. [Google Scholar] [CrossRef]
- El-Amir, A.A.M.; El-Maddah, A.A.; Ewais, E.M.M.; El-Sheikh, S.M.; Bayoumi, I.M.I.; Ahmed, Y.M.Z. Sialon from Synthesis to Applications: An Overview. J. Asian Ceram. Soc. 2021, 9, 1390–1418. [Google Scholar] [CrossRef]
- Zhou, Y.; Vleugels, J.; Laoui, T.; Ratchev, P.; Van Der Biest, O. Preparation and Properties of X-sialon. J. Mater. Sci. 1995, 30, 4584–4590. [Google Scholar] [CrossRef]
- Plachký, T.; Křesťan, J.; Korenko, M.; Medri, V.; Lenčéš, Z.; Šajgalík, P. Corrosion and Oxidation Behaviour of β-SiAlON Ceramics via Different Processing Route. J. Ceram. Soc. Jpn. 2009, 117, 482–488. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Khan, M.; Ahmed, B.A.; Al Ghanim, A.; Patel, F.; Ehsan, M.A.; Ali, S.; Laoui, T.; Ali, S. Synthesis and Characterization of Alkaline Earth and Rare Earth Doped Sialon Ceramics by Spark Plasma Sintering. Int. J. Refract. Met. Hard Mater. 2021, 97, 105500. [Google Scholar] [CrossRef]
- Tian, X.K.; Su, K.; Ouyang, D.Z.; Gao, J.X.; Jia, Q.L.; Liu, X.H. Effect of Impurities of Fe2O3 and TiO2 in Bauxite on Oxidation Kinetics of β-SiAlON Powders. Corros. Sci. 2022, 203, 110374. [Google Scholar] [CrossRef]
- Kirm, M.; Lushchik, A.; Lushchik, C.; Vielhauer, S.; Zimmerer, G. Luminescence of Pure and Doped Al2O3 and MgO Single Crystals under Inner-shell Excitation. J. Lumin. 2003, 102, 307–312. [Google Scholar] [CrossRef]
- Seeman, V.; Feldbach, E.; Kärner, T.; Maaroos, A.; Mironova-Ulmane, N.; Popov, A.I.; Shablonin, E.; Vasil’chenko, E.; Lushchik, A. Fast-neutron-induced and as-Grown Structural Defects in Magnesium Aluminate Spinel Crystals with Different Stoichiometry. Opt. Mater. 2019, 91, 42–49. [Google Scholar] [CrossRef]
- Baubekova, G.; Akilbekov, A.; Feldbach, E.; Grants, R.; Manika, I.; Popov, A.I.; Schwartz, K.; Vasil’chenko, E.; Zdorovets, M.; Lushchik, A. Accumulation of Radiation Defects and Modification of Micromechanical Properties under MgO Crystal Irradiation with Swift 132Xe Ions. Nucl. Instrum. Methods Phys. Res. Sect. B 2020, 463, 50–54. [Google Scholar] [CrossRef]
- Camps, I.; Mariscal, A.; Serna, R. Preparation and Broadband White Emission of Eu-doped Thin Films Based on SiAlON. J. Lumin. 2017, 191, 97–101. [Google Scholar] [CrossRef]
- Yang, J.Z.; Huang, Z.H.; Fang, M.H.; Hu, X.Z.; Liu, Y.G.; Sun, H.R. Reaction Sintered Fe–Sialon Ceramic Composite: Processing, Characterization and High Temperature Erosion Wear Behavior. J. Asian Ceram. Soc. 2013, 1, 163–169. [Google Scholar] [CrossRef]
- Xiong, Y.; Fu, Z.Y.; Wang, H.; Wang, Y.C.; Zhang, J.Y.; Zhang, Q.J. Microstructure and Properties of Translucent Mg–sialon Ceramics Prepared by Spark Plasma Sintering. Mater. Sci. Eng. A 2008, 488, 475–481. [Google Scholar] [CrossRef]
- Rouxel, T.; Dély, N.; Sangleboeuf, J.C.; Deriano, S.; LeFloch, M.; Beuneu, B.; Hampshire, S. Structure–Property Correlations in Y–Ca–Mg–Sialon Glasses: Physical and Mechanical Properties. J. Am. Ceram. Soc. 2005, 88, 889–896. [Google Scholar] [CrossRef]
- Yang, Z.F.; Shang, Q.L.; Shen, X.Y.; Zhang, L.Q.; Gao, J.S.; Wang, H. Effect of Composition on Phase Assemblage, Microstructure, Mechanical and Optical Properties of Mg-Doped Sialon. J. Eur. Ceram. Soc. 2017, 37, 91–98. [Google Scholar] [CrossRef]
- Yu, C.; Yuan, W.J.; Deng, C.J.; Zhu, H.X.; Li, J. Synthesis of Hexagonal Plate-Like Al4SiC4 from Calcined Bauxite, Silica and Carbon black. Powder Technol. 2013, 247, 76–80. [Google Scholar] [CrossRef]
- Chandra, K.S.; Sarkar, D. Oxidation Resistance, Residual Strength, and Microstructural Evolution in Al2O3–MgO–C Refractory Composites with YAG Nanopowder. J. Eur. Ceram. Soc. 2021, 41, 3782–3797. [Google Scholar] [CrossRef]
- Chen, J.F.; Li, N.; Hubálková, J.; Aneziris, C.G. Elucidating the Role of Ti3AlC2 in Low Carbon MgO–C Refractories: Antioxidant or Alternative Carbon Source? J. Eur. Ceram. Soc. 2018, 38, 3387–3394. [Google Scholar] [CrossRef]
- Xing, G.C.; Wan, H.; Deng, C.J.; Di, J.; Ding, J.H.; Ma, B.Y.; Wang, Z.F.; Zhu, H.X.; Yu, C. Thermal Stability and Selective Nitridation of Cr2AlC in Nitrogen at Elevated Temperatures. Ceram. Int. 2022, 48, 33151–33159. [Google Scholar] [CrossRef]
- Butt, D.P.; Albert, D.; Taylor, T.N. Kinetics of Thermal Oxidation of Silicon Nitride Powders. J. Am. Ceram. Soc. 1996, 79, 2809–2814. [Google Scholar] [CrossRef]
- Jia, Q.L.; Zhang, H.J.; Li, S.P.; Jia, X.L. Effect of Particle Size on Oxidation of Silicon Carbide Powders. Ceram. Int. 2007, 33, 309–313. [Google Scholar]
- Gao, S.; Xu, L.; Chen, M.; Wang, N. Effect of Fe Addition on the Microstructure and Oxidation Behavior of MgO–C Refractory. Mater. Chem. Phys. 2019, 238, 121935. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yu, J.K.; Yang, X.; Jin, E.D.; Yuan, L. Oxidation Resistance and Wetting Behavior of MgO–C Refractories: Effect of Carbon Content. Materials 2018, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Tsuchinari, A.; Hokii, T. Application of Fractal Geometry to Refractory. Key Eng. Mater. 1995, 108, 485–490. [Google Scholar] [CrossRef]
- Song, Y.F.; Deng, C.J.; Zhu, H.X.; Ding, J.; Yu, C.; Fan, G.D.; Leng, G.H. Correlation Between Porosity Characteristics and Thermal Conductivity of Forsterite Porous Materials Based on Grey System Theory. J. Chin. Ceram. Soc. 2018, 46, 449–454. (In Chinese) [Google Scholar]
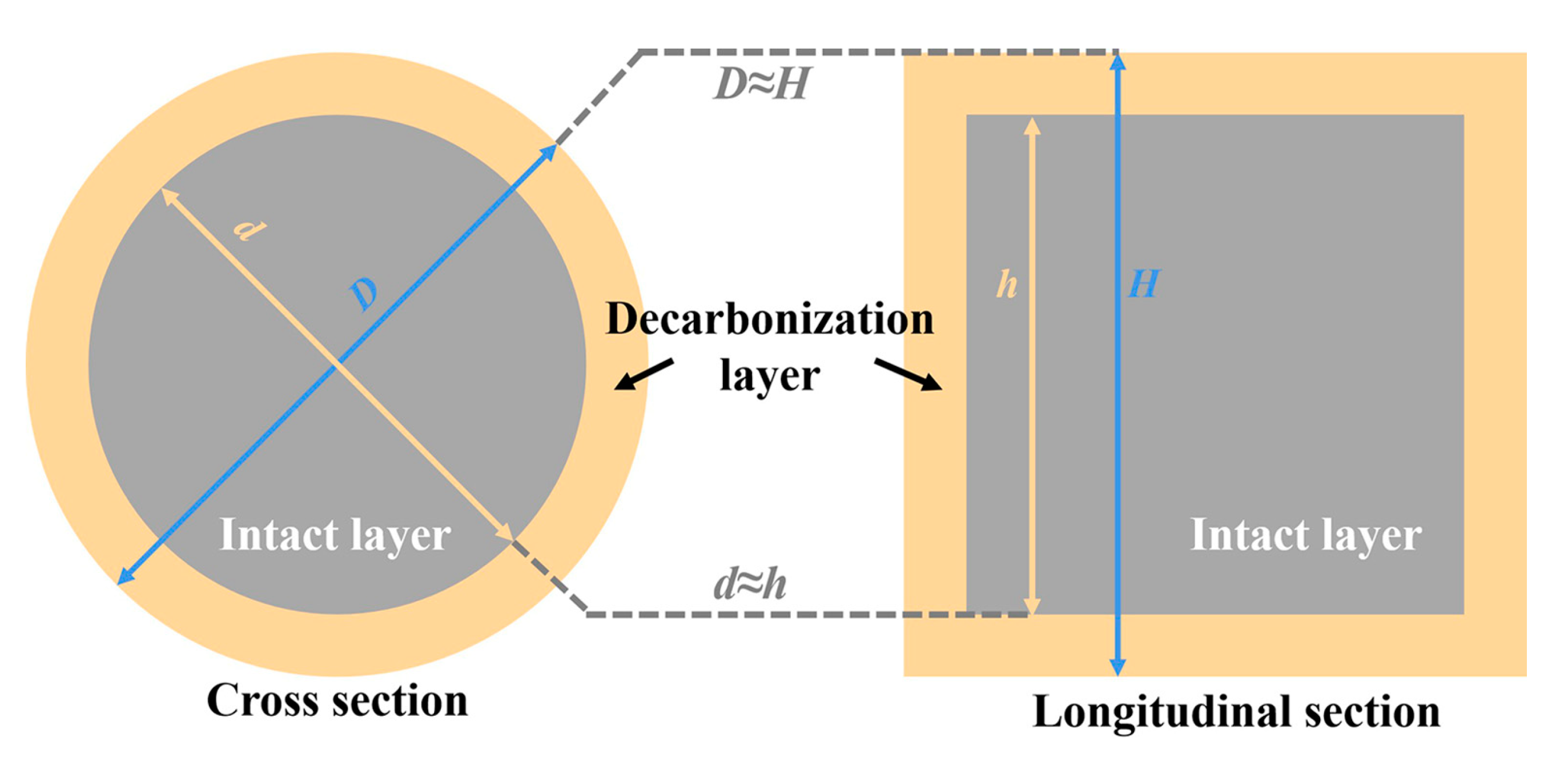
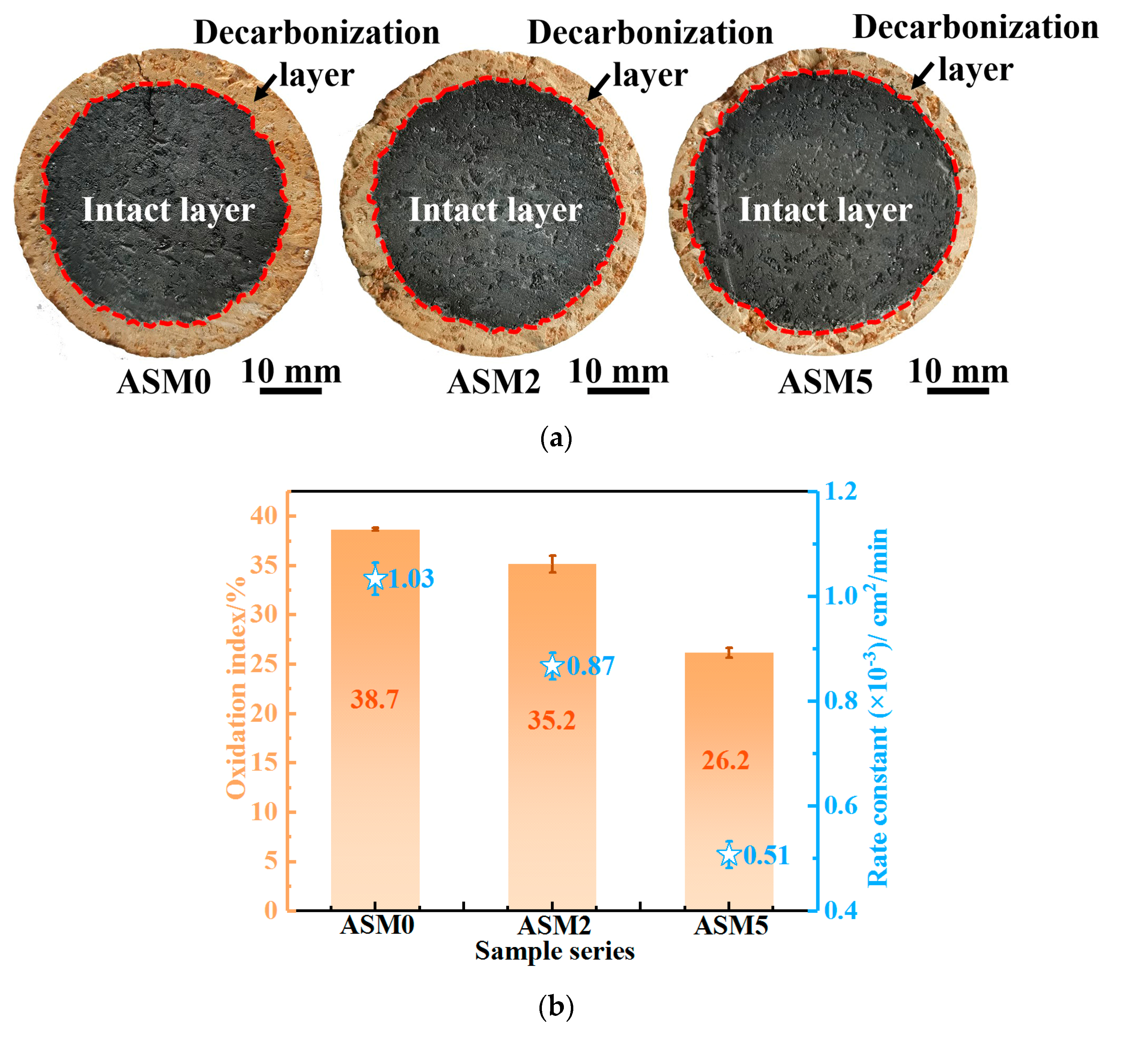


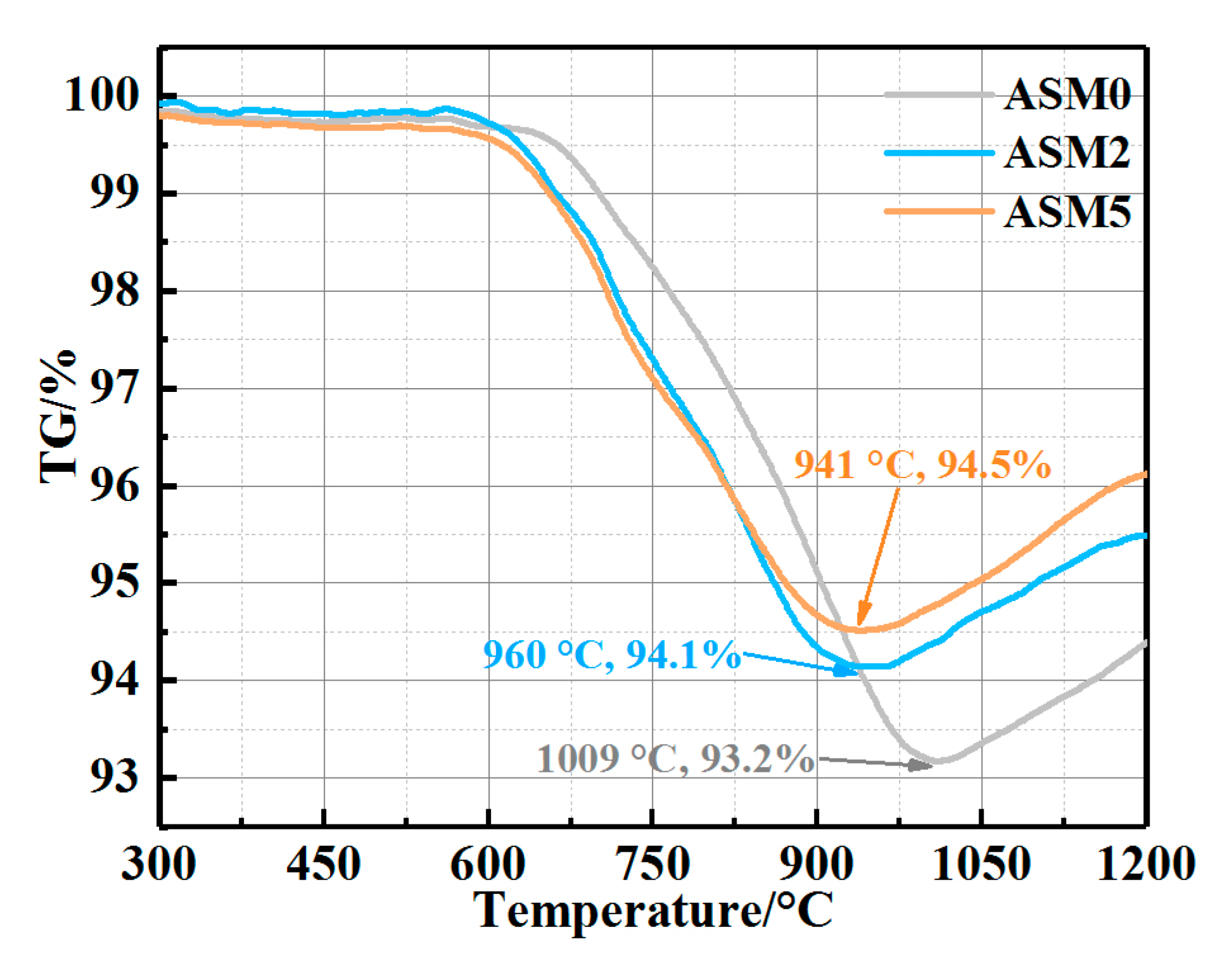

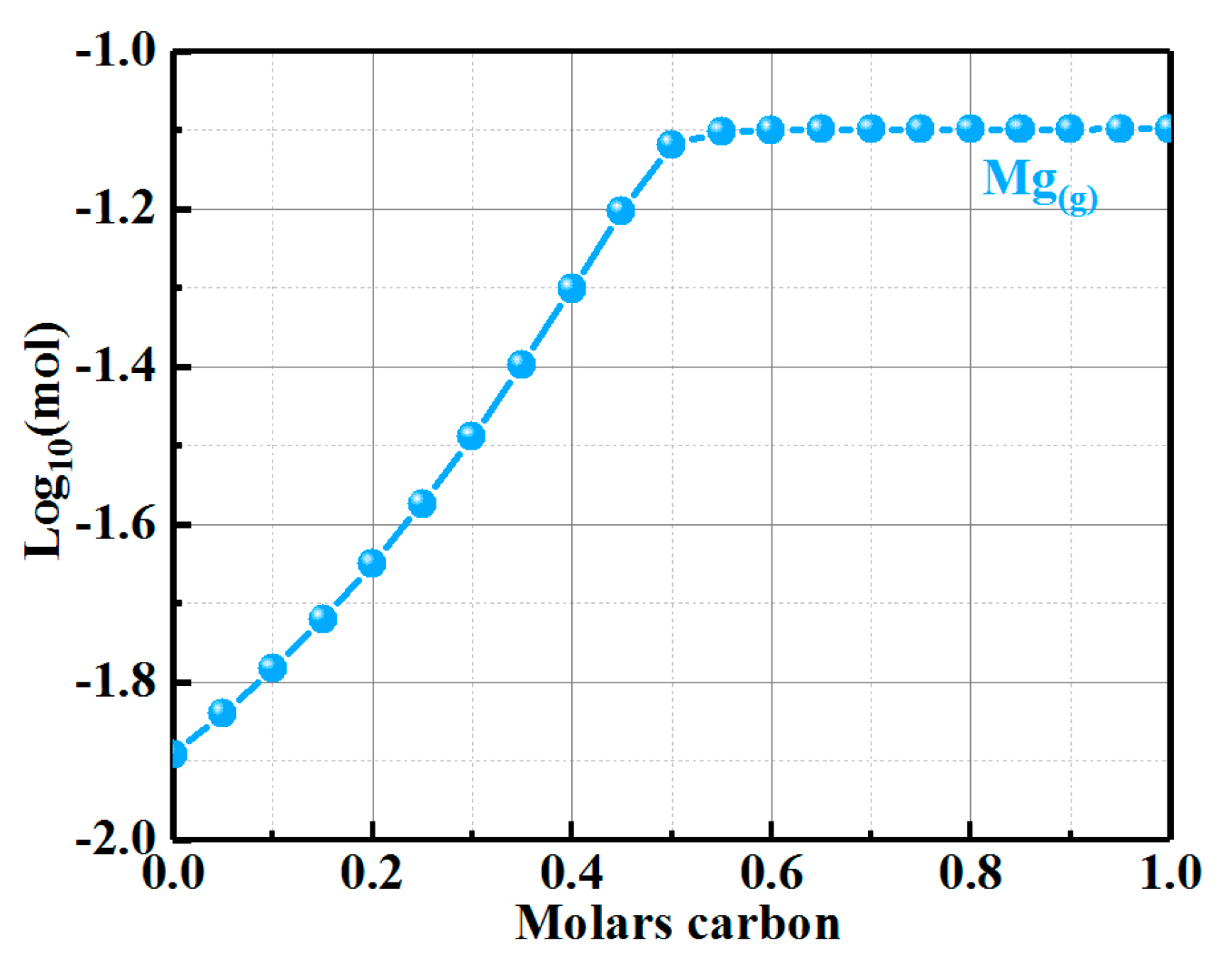
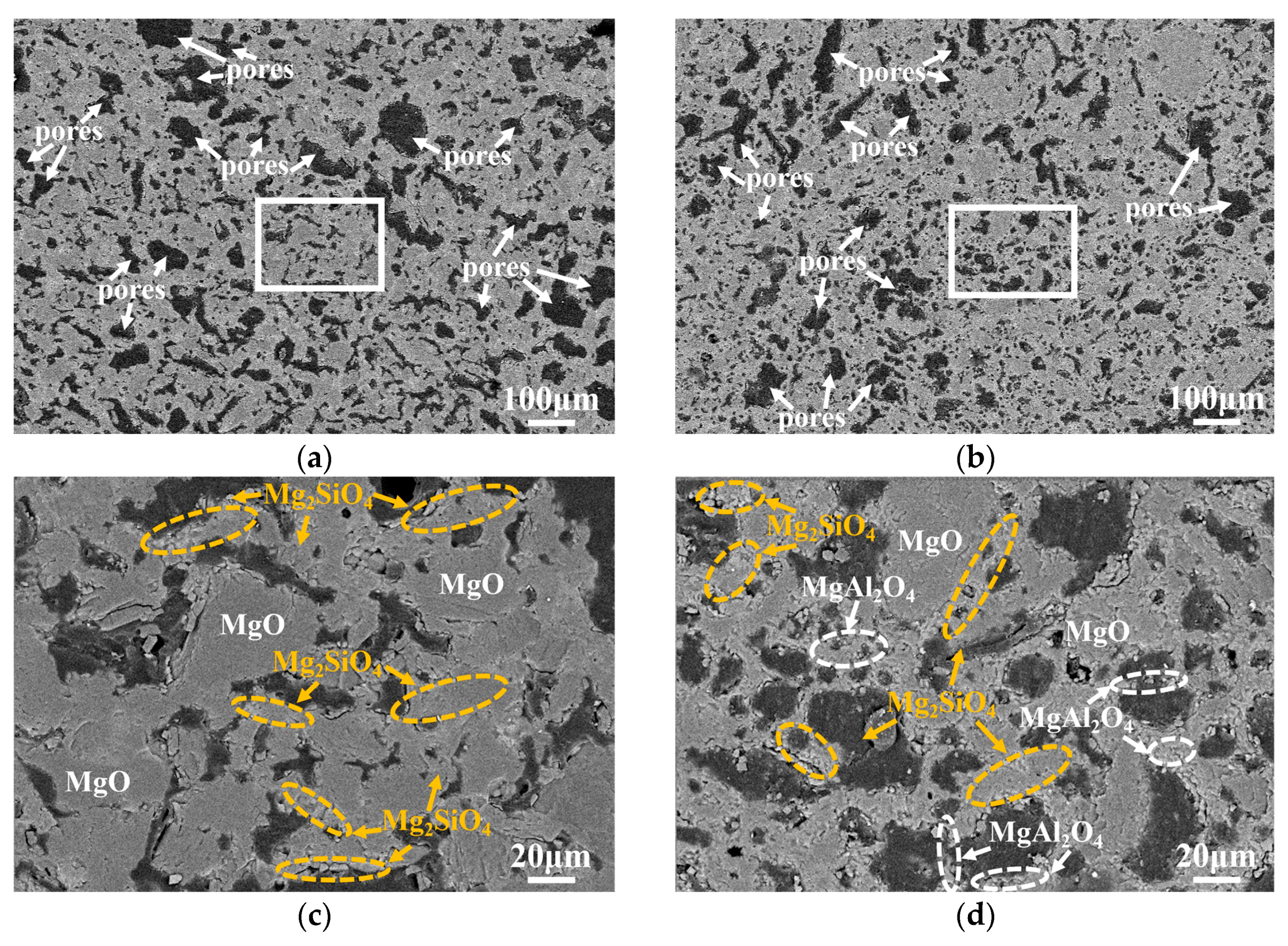
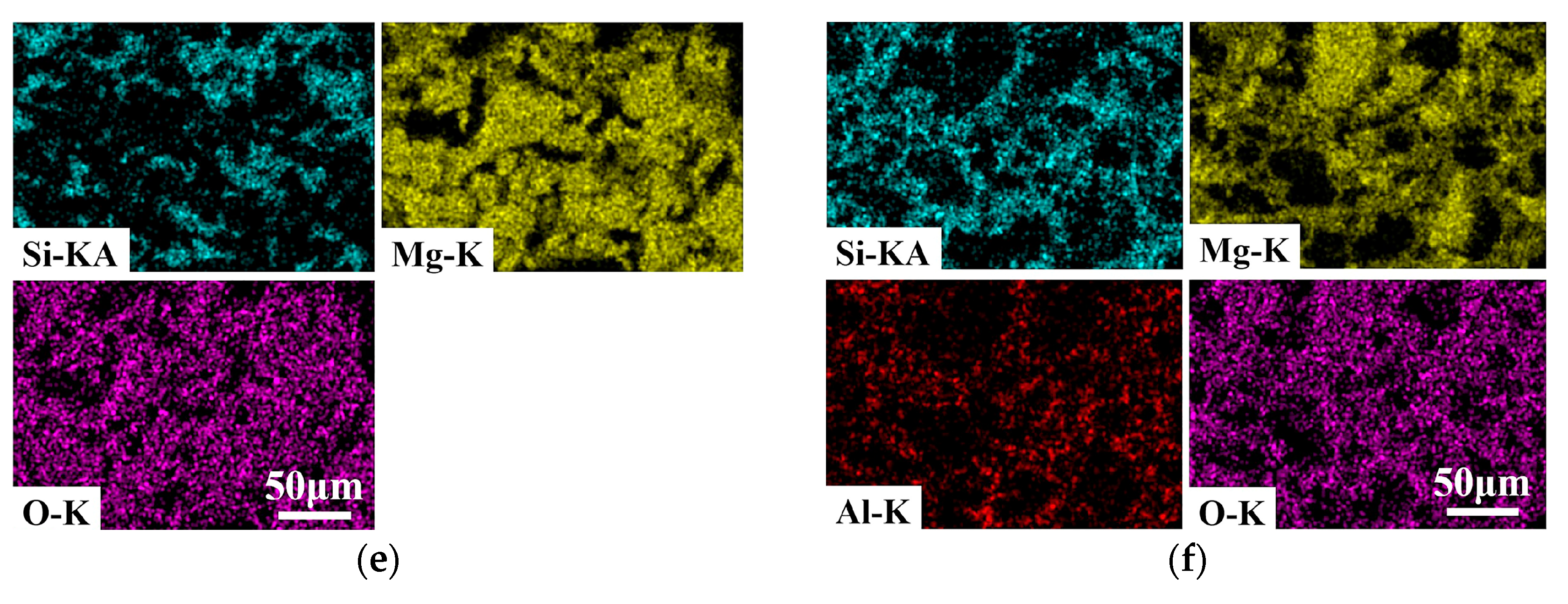

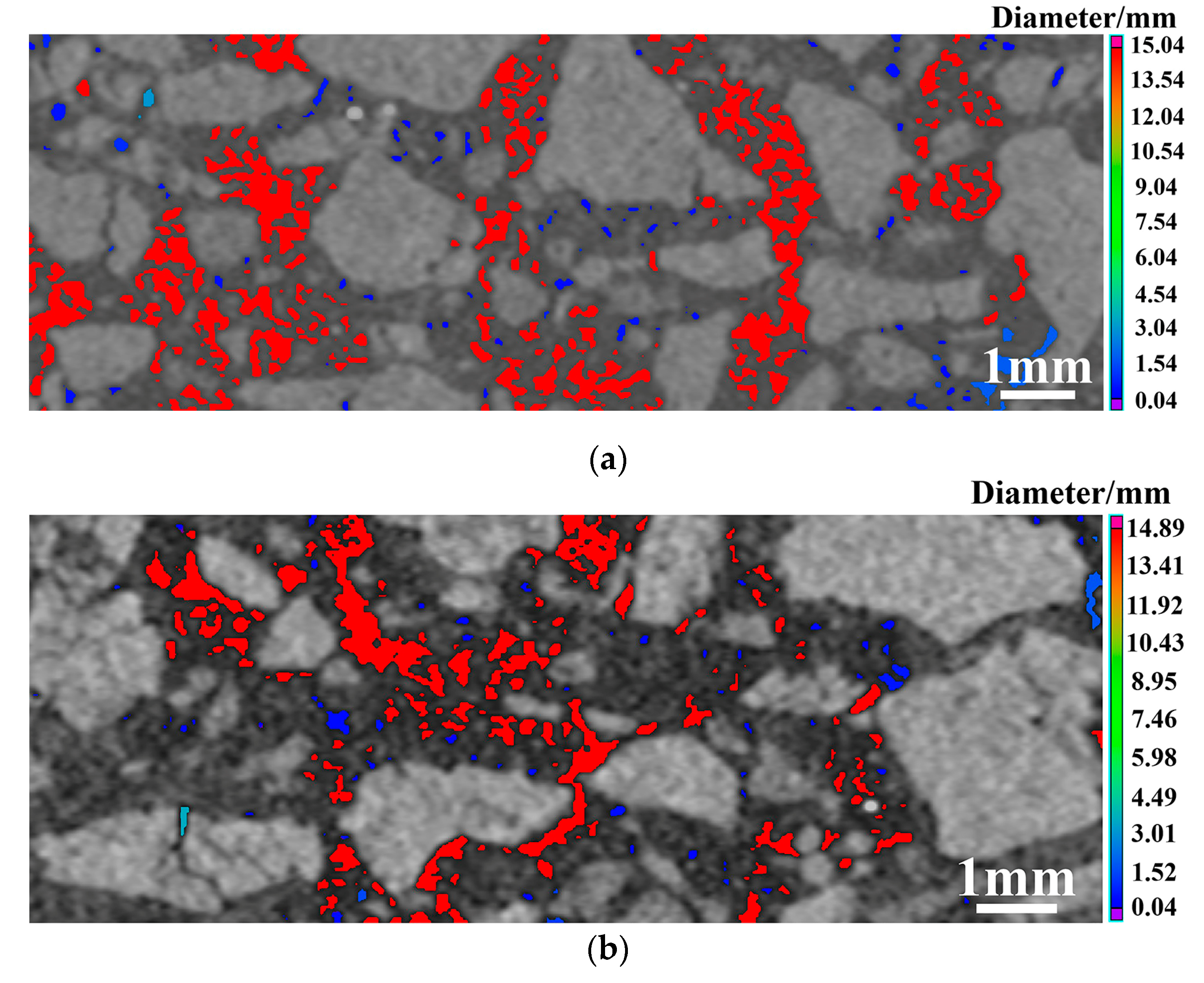
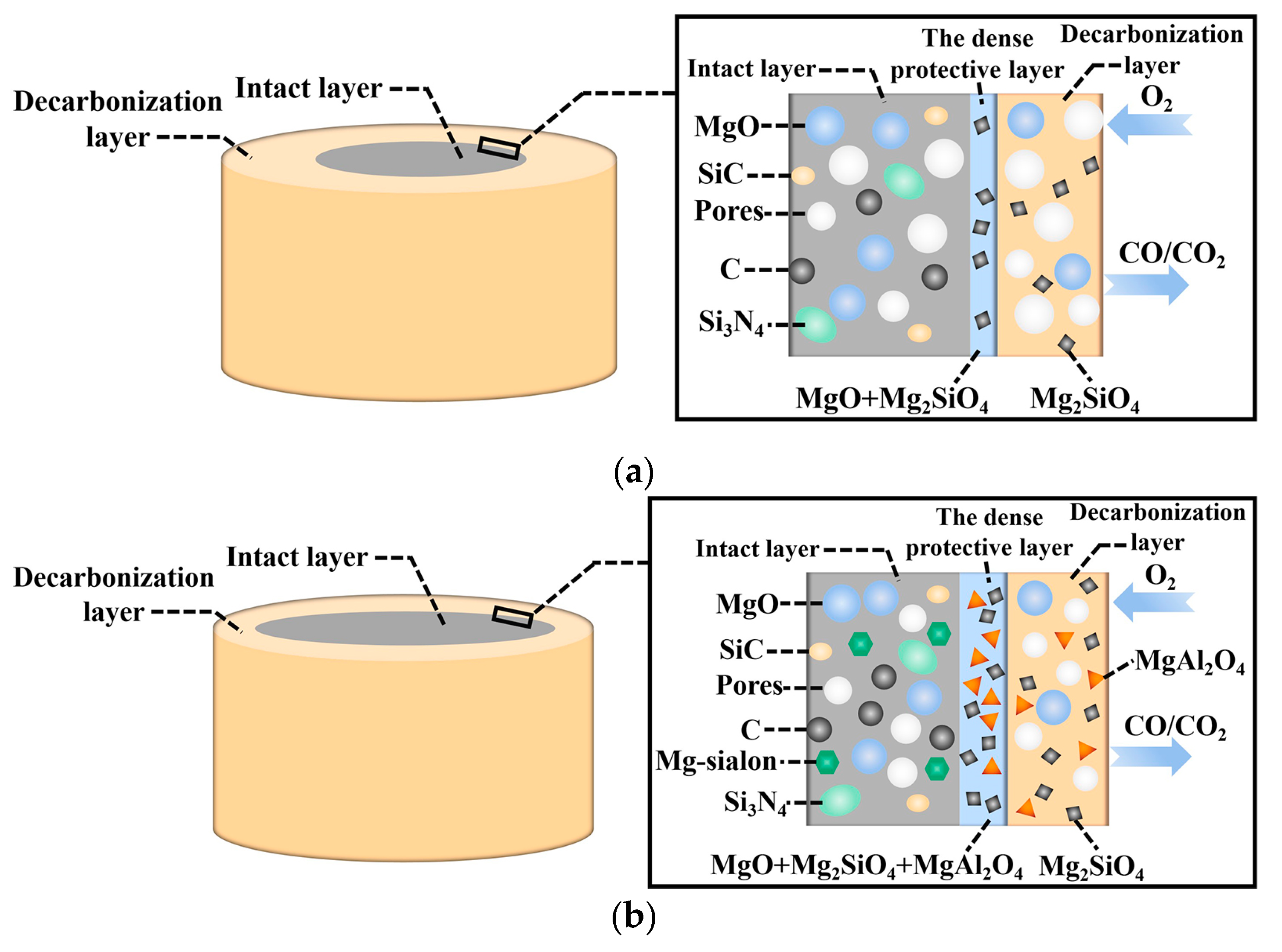
| Composition | ASM0 | ASM2 | ASM5 |
|---|---|---|---|
| Fused magnesia particles | 67 | 67 | 67 |
| Fused magnesia powder | 26 | 26 | 26 |
| Flake graphite | 4 | 4 | 4 |
| Silicon powder | 3 | 3 | 3 |
| Resin powder | +1 | +1 | +1 |
| Phenolic resin | +4 | +4 | +4 |
| Al4SiC4 powder | +0 | +2 | +5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Yu, C.; Xing, G.; Di, J.; Ding, J.; Zhu, Q.; Zhu, H.; Deng, C. Effect of In Situ Mg-Sialon on the Oxidation Behavior of Low-Carbon MgO-C Refractories. Materials 2023, 16, 1892. https://doi.org/10.3390/ma16051892
Dong B, Yu C, Xing G, Di J, Ding J, Zhu Q, Zhu H, Deng C. Effect of In Situ Mg-Sialon on the Oxidation Behavior of Low-Carbon MgO-C Refractories. Materials. 2023; 16(5):1892. https://doi.org/10.3390/ma16051892
Chicago/Turabian StyleDong, Bo, Chao Yu, Guangchao Xing, Jinghui Di, Jun Ding, Qingyou Zhu, Hongxi Zhu, and Chengji Deng. 2023. "Effect of In Situ Mg-Sialon on the Oxidation Behavior of Low-Carbon MgO-C Refractories" Materials 16, no. 5: 1892. https://doi.org/10.3390/ma16051892
APA StyleDong, B., Yu, C., Xing, G., Di, J., Ding, J., Zhu, Q., Zhu, H., & Deng, C. (2023). Effect of In Situ Mg-Sialon on the Oxidation Behavior of Low-Carbon MgO-C Refractories. Materials, 16(5), 1892. https://doi.org/10.3390/ma16051892






