Hydrothermally Grown MoS2 as an Efficient Electrode Material for the Fabrication of a Resorcinol Sensor
Abstract
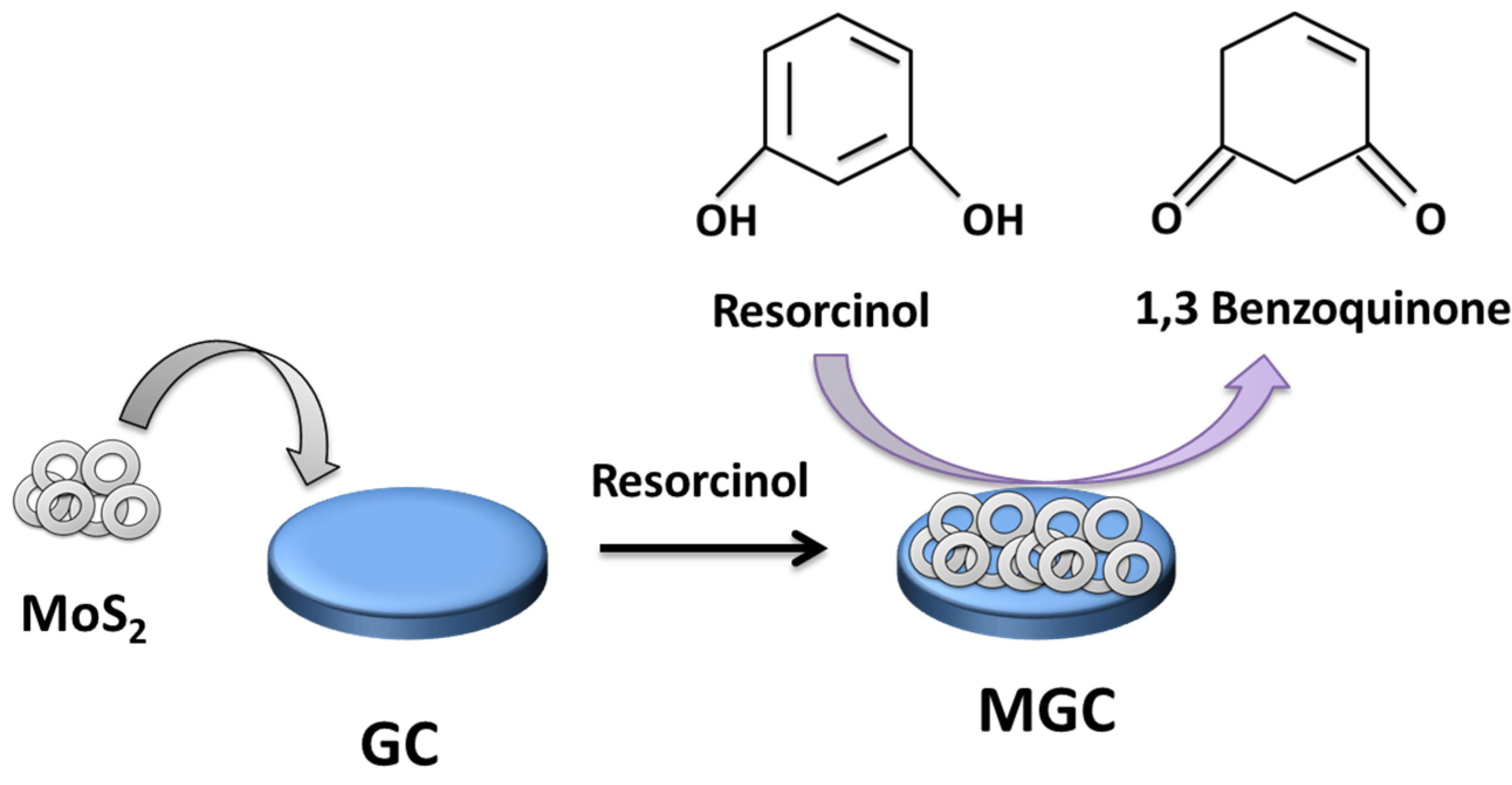
:1. Introduction
2. Experimental Section
Chemical
3. Results and Discussion
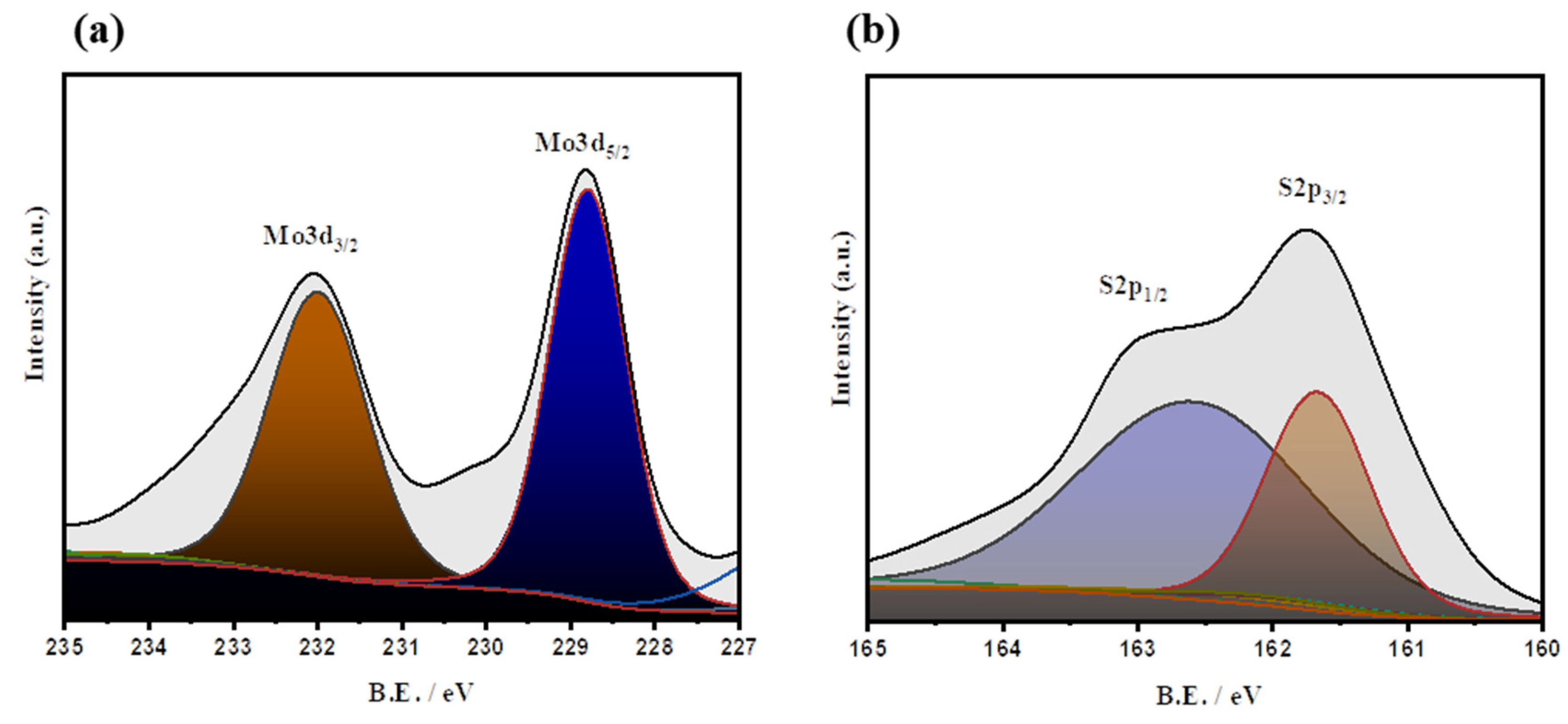
3.1. MoS2 Characterization
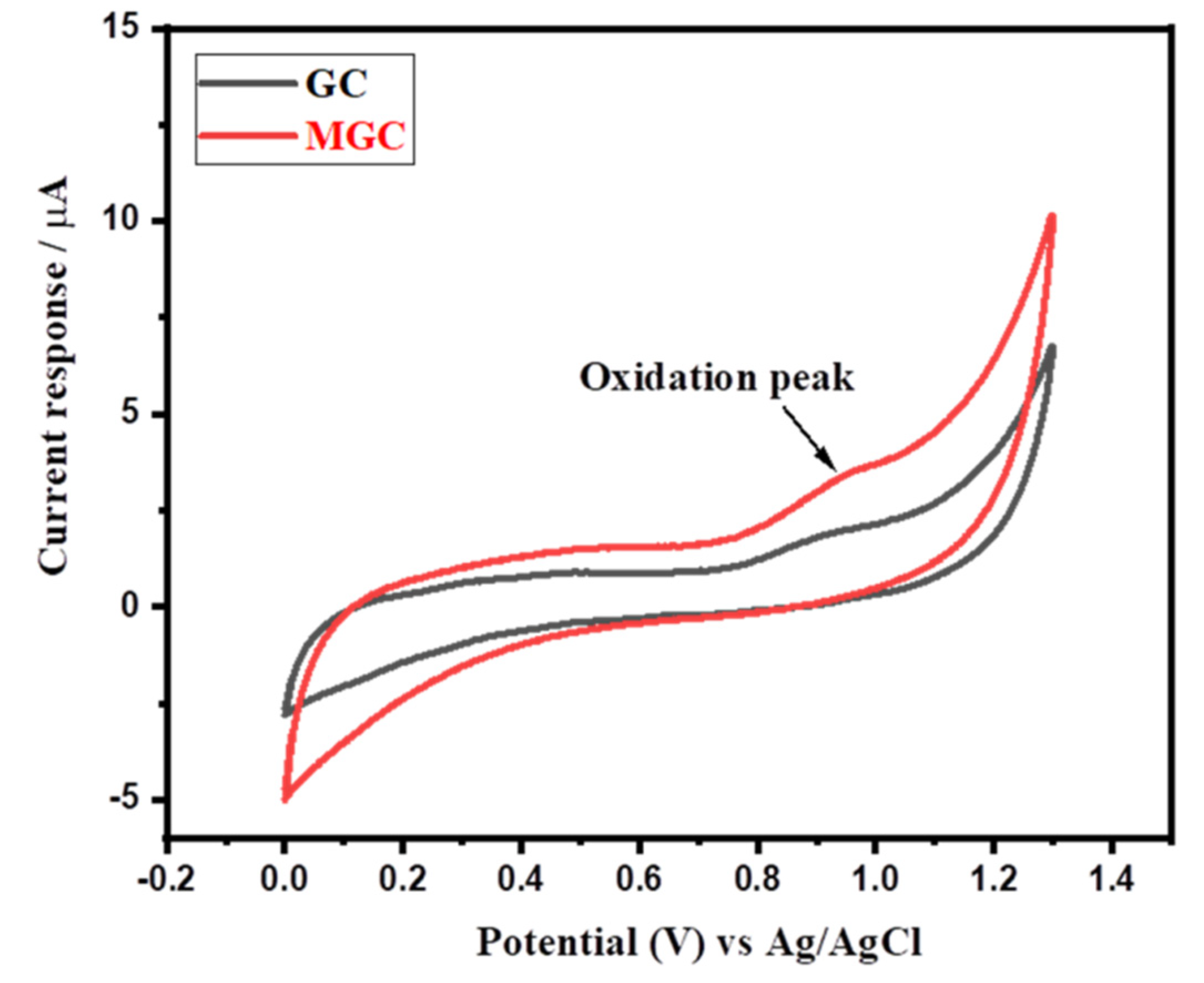
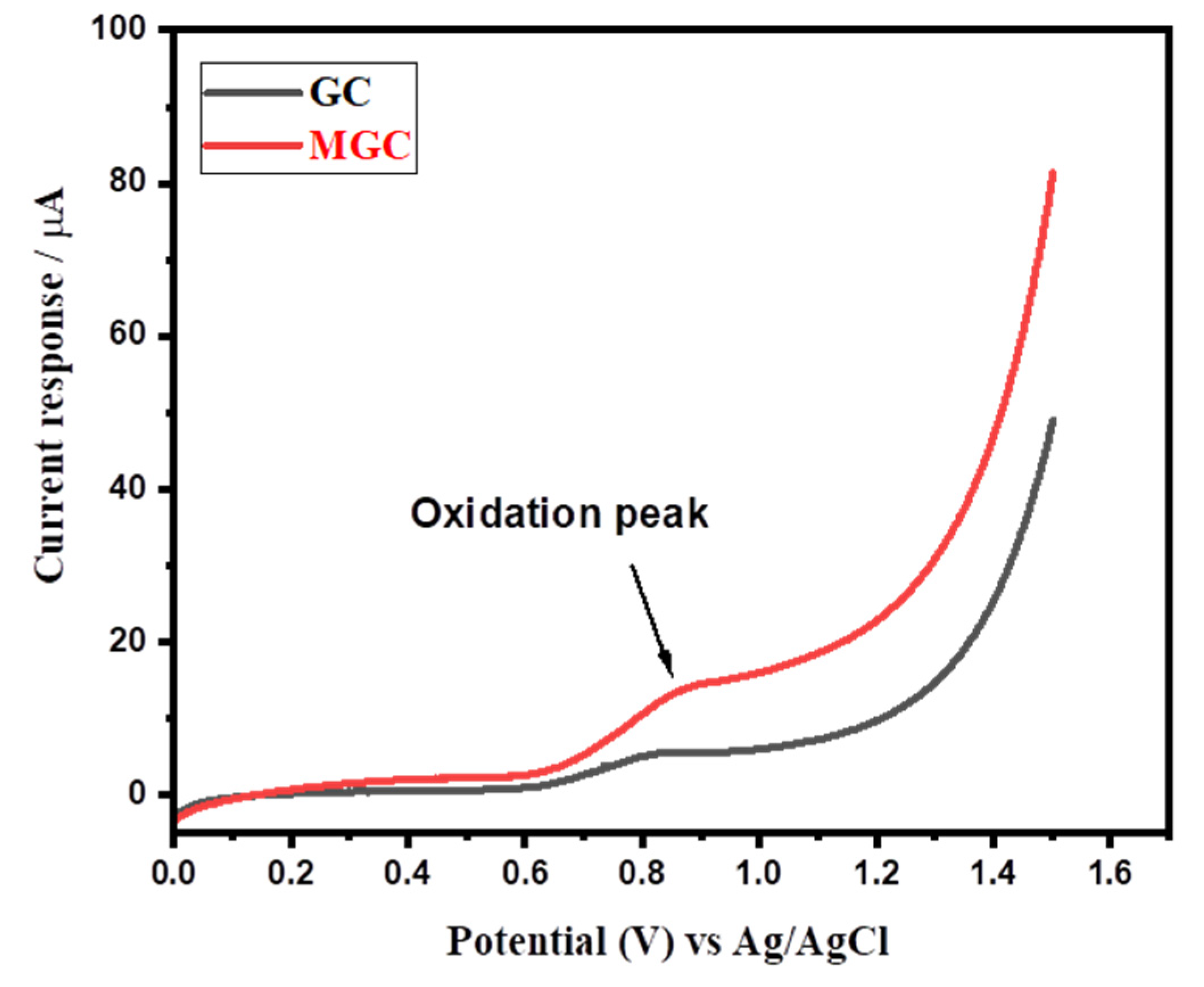
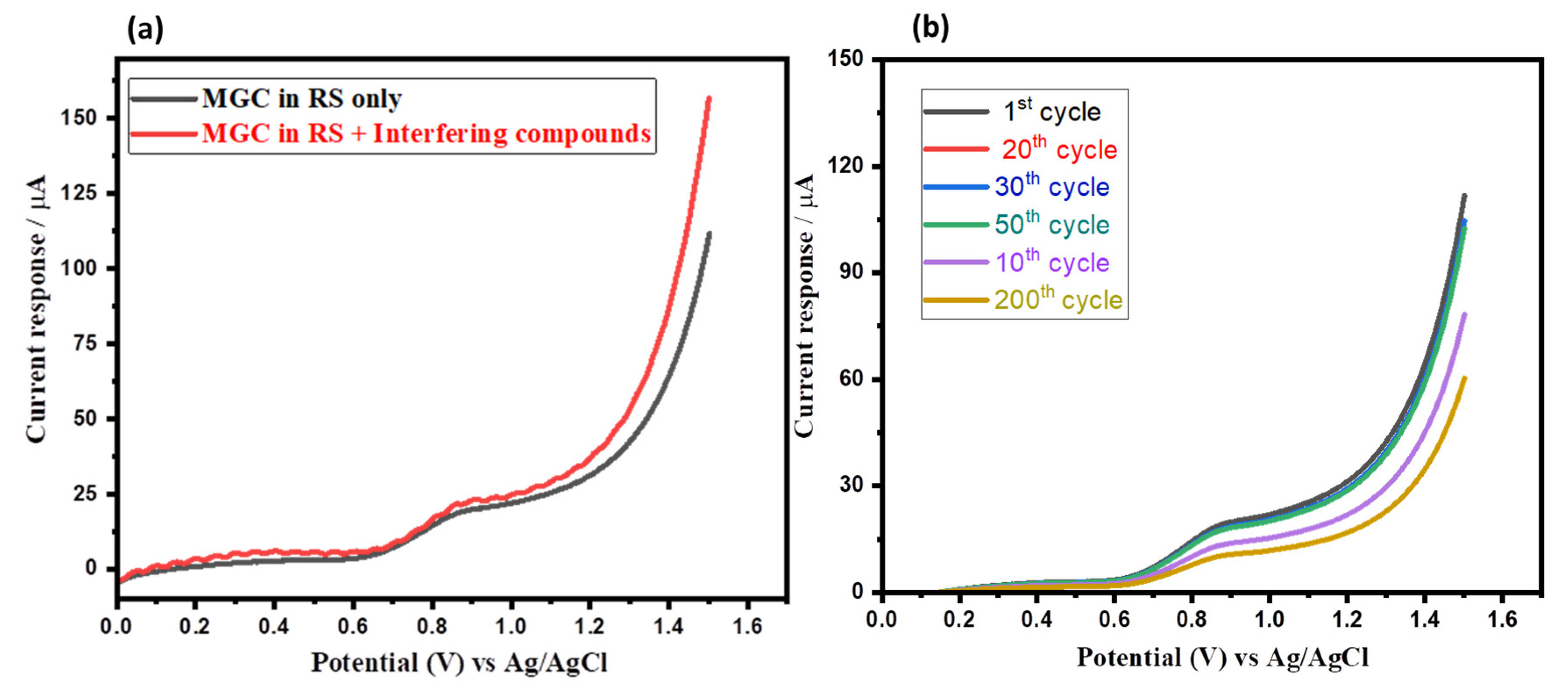
3.2. Electrochemical Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of Vertically-Ordered Mesoporous Silica Film on Electrochemically Pretreated Three-Dimensional Graphene Electrodes for Sensitive Detection of Methidazine in Urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Adhikari, B.-R.; Govindhan, M.; Chen, A. Carbon Nanomaterials Based Electrochemical Sensors/Biosensors for the Sensitive Detection of Pharmaceutical and Biological Compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Zhang, T.; Chen, P.; Yan, F.; Liu, J. Bipolar silica nanochannel array for dual-mode electrochemiluminescence and electrochemical immunosensing platform. Sens. Actuators B Chem. 2022, 368, 132086. [Google Scholar] [CrossRef]
- Hassan, K.M.; Hathoot, A.A.; Azzem, M.A. Simultaneous and selective electrochemical determination of hydroquinone, catechol and resorcinol at poly (1,5-diaminonaphthalene)/glassy carbon-modified electrode in different media. RSC Adv. 2018, 8, 6346–6355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Lee, S.E.; Kwon, K.; Oh, S.W.; Park, S.J.; Yu, E.; Kim, H.; Yang, S.; Park, J.Y.; Chung, W.-J.; Cho, J.Y.; et al. Mechanisms of Resorcinol Antagonism of Benzo[a]pyrene-Induced Damage to Human Keratinocytes. Biomol. Ther. 2021, 29, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Subhani, Q.; Muhammad, N.; Huang, Z.; Asif, M.; Hussain, I.; Zahid, M.; Hairong, C.; Zhu, Y.; Guo, D. Simultaneous determination of acetamiprid and 6-chloronicotinic acid in environmental samples by using ion chromatography hyphenated to online photoinduced fluorescence detector. J. Sep. Sci. 2020, 43, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Baldisserotto, A.; Vicentini, C.B.; Mares, D.; Andreotti, E.; Vertuani, S.; Manfredini, S. Antidermatophytic Action of Resorcinol Derivatives: Ultrastructural Evidence of the Activity of Phenylethyl Resorcinol against Microsporum gypseum. Molecules 2016, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Manasa, G.; Bhakta, A.K.; Mekhalif, Z.; Mascarenhas, R.J. Voltammetric Study and Rapid Quantification of Resorcinol in Hair Dye and Biological Samples Using Ultrasensitive Maghemite/MWCNT Modified Carbon Paste Electrode. Electroanalysis 2019, 31, 1363–1372. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Tharat, B.; Hirunsit, P.; Suthirakun, S. Electrochemical oxidation of resorcinol: Mechanistic insights from experimental and computational studies. RSC Adv. 2020, 10, 28454–28463. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, L.A.; Liu, L.; Sathishkumar, P.; Nan, J.; Gu, F.L. Copper oxide and carbon nano-fragments modified glassy carbon electrode as selective electrochemical sensor for simultaneous determination of catechol and hydroquinone in real-life water samples. J. Electroanal. Chem. 2018, 815, 68–75. [Google Scholar] [CrossRef]
- Zargar, B.; Hatamie, A. Colorimetric determination of resorcinol based on localized surface plasmon resonance of silver nanoparticles. Analyst 2012, 137, 5334–5338. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.I.; Abass, S.M. Azo Coupling Reaction for indirect Spectrophotometric Determination of Furosemide using Resorcinol as a Reagent. IOP Conf. Series Mater. Sci. Eng. 2021, 1058, 012077. [Google Scholar] [CrossRef]
- Yang, H.; Zha, J.; Zhang, P.; Qin, Y.; Chen, T.; Ye, F. Fabrication of CeVO4 as nanozyme for facile colorimetric discrimination of hydroquinone from resorcinol and catechol. Sens. Actuators B Chem. 2017, 247, 469–478. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, Y.; Liang, W.Y.; Yang, X.P.; Jiang, W.D.; Liu, X.H.; Zhang, W. A facile and sensitive ratiometric fluorescence sensor for rapid visual monitoring of trace resorcinol. Sens. Actuators B Chem. 2021, 330, 129390. [Google Scholar] [CrossRef]
- Nsanzamahoro, S.; Zhang, Y.; Wang, W.-F.; Ding, Y.-Z.; Shi, Y.-P.; Yang, J.-L. Fluorescence “turn-on” of silicon-containing nanoparticles for the determination of resorcinol. Microchimica. Acta 2021, 188, 1–9. [Google Scholar] [CrossRef]
- Kumar, M.; Swamy, B.K.; Hu, B.; Wang, M.; Yasin, G.; Liang, B.; Madhuchandra, H.D.; Zhao, W. Electrochemical activation of copper oxide decorated graphene oxide modified carbon paste electrode surface for the simultaneous determination of hazardous Di-hydroxybenzene isomers. Microchem. J. 2021, 168, 106503. [Google Scholar] [CrossRef]
- Baig, N.; Waheed, A.; Sajid, M.; Khan, I.; Kawde, A.-N.; Sohail, M. Porous graphene-based electrodes: Advances in electrochemical sensing of environmental contaminants. Trends Environ. Anal. Chem. 2021, 30, e00120. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. Shape controlled synthesis of high surface area MgO microstructures for highly efficient congo red dye removal and peroxide sensor. J. Environ. Chem. Eng. 2019, 7, 103347. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Ansari, S.N.; Mobin, S.M. Construction of graphene oxide sheets based modified glassy carbon electrode (GO/GCE) for the highly sensitive detection of nitrobenzene. Mater. Res. Express 2018, 5, 075601. [Google Scholar] [CrossRef]
- Ahmad, K.; Kumar, P.; Mobin, S.M. Hydrothermally grown novel pyramids of the CaTiO3 perovskite as an efficient electrode modifier for sensing applications. Mater. Adv. 2020, 1, 2003–2009. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. Construction of polyanilne/ITO electrode for electrochemical sensor applications. Mater. Res. Express 2019, 6, 085508. [Google Scholar] [CrossRef]
- Ahmad, K.; Mobin, S.M. Design and fabrication of cost-effective and sensitive non-enzymatic hydrogen peroxide sensor using Co-doped δ-MnO2 flowers as electrode modifier. Anal. Bioanal. Chem. 2021, 413, 789–798. [Google Scholar] [CrossRef]
- Iftikhar, T.; Asif, M.; Aziz, A.; Ashraf, G.; Jun, S.; Li, G.; Liu, H. Topical advances in nanomaterials based electrochemical sensors for resorcinol detection. Trends Environ. Anal. Chem. 2021, 31, e00138. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Gong, T.; Pan, R.; Wang, H.; Guo, Z.; Zhang, H.; Fu, X. Recent advances in emerging Janus two-dimensional materials: From fundamental physics to device applications. J. Mater. Chem. A 2020, 8, 8813–8830. [Google Scholar] [CrossRef]
- Chen, E.; Xu, W.; Chen, J.; Warner, J. 2D layered noble metal dichalcogenides (Pt, Pd, Se, S) for electronics and energy applications. Mater. Today Adv. 2020, 7, 100076. [Google Scholar] [CrossRef]
- Villa-Manso, A.M.; Revenga-Parra, M.; Vera-Hidalgo, M.; Sulleiro, M.V.; Pérez, E.M.; Lorenzo, E.; Pariente, F. 2D MoS2 nanosheets and hematein complexes deposited on screen-printed graphene electrodes as an efficient electrocatalytic sensor for detecting hydrazine. Sens. Actuators B Chem. 2021, 345, 130385. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Yin, Z.; He, Q.; Li, H.; Zhang, Q.; Zhang, H. Optical Identification of Single- and Few-Layer MoS2 Sheets. Small 2012, 8, 682–686. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Saini, A.K.; Mobin, S.M. Small biomolecule sensors based on an innovative MoS2–rGO heterostructure modified electrode platform: A binder-free approach. Dalton Trans. 2017, 46, 15848–15858. [Google Scholar] [CrossRef]
- Kondekar, N.P.; Boebinger, M.G.; Woods, E.V.; McDowell, M.T. In Situ XPS Investigation of Transformations at Crystallographically Oriented MoS2 Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 32394–32404. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Wang, L.; Liu, Y.J.; Gan, T.; Liu, Y.M.; Wang, L.L.; Fan, Y. Synthesis and electrochemical performances of layered tungsten sulfide-graphene nanocomposite as a sensing platform for catechol, resorcinol and hydroquinone. Electrochim. Acta 2013, 107, 379–387. [Google Scholar] [CrossRef]
- Li, Z.; Yue, Y.; Hao, Y.; Feng, S.; Zhou, X. A glassy carbon electrode modified with cerium phosphate nanotubes for the simultaneous determination of hydroquinone, catechol and resorcinol. Microchim. Acta 2018, 185, 215. [Google Scholar] [CrossRef]
- Chetankumar, K.; Swamy, B.K.; Sharma, S. Fabrication of voltammetric efficient sensor for catechol, hydroquinone and resorcinol at MgO modified pre-treated carbon paste electrode. Mater. Chem. Phys. 2020, 252, 123231. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Zhang, S.; Yang, L.; Liu, M.; Zhang, Y.; Yao, S. Ultrasensitive and simultaneous detection of hydroquinone, catechol and resorcinol based on the electrochemical co-reduction prepared Au-Pd nanoflower/reduced graphene oxide nanocomposite. Electrochim. Acta 2017, 231, 677–685. [Google Scholar] [CrossRef]
- Buleandra, M.; Rabinca, A.A.; Badea, I.A.; Balan, A.; Stamatin, I.; Mihailciuc, C.; Ciucu, A.A. Voltammetric determination of dihydroxybenzene isomers using a disposable pencil graphite electrode modified with cobalt-phthalocyanine. Microchim. Acta 2017, 184, 1481–1488. [Google Scholar] [CrossRef]
- Ershadifar, H.; Akhond, M.; Absalan, G. Gold Nanoparticle Decorated Multiwall Carbon Nanotubes/Ionic Liquid Composite Film on Glassy Carbon Electrode for Sensitive and Simultaneous Electrochemical Determination of Dihydroxybenzene Isomers. IEEE Sens. J. 2017, 17, 5030–5037. [Google Scholar] [CrossRef]
- Ge, C.; Li, H.; Li, M.; Li, C.; Wu, X.; Yang, B. Synthesis of a ZnO nanorod/CVD graphene composite for simultaneous sensing of dihydroxybenzene isomers. Carbon 2015, 95, 1–9. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Hajisadeghian, E.; Golestaneh, M. Voltammetric determination of resorcinol on the surface of a glassy carbon electrode modified with multi-walled carbon nanotube. Arab. J. Chem. 2016, 9, S1563–S1568. [Google Scholar] [CrossRef] [Green Version]
- Yin, D.; Liu, J.; Bo, X.; Guo, L. Cobalt-iron selenides embedded in porous carbon nanofibers for simultaneous electrochemical detection of trace of hydroquinone, catechol and resorcinol. Anal. Chim. Acta 2019, 1093, 35–42. [Google Scholar] [CrossRef]
- Aragó, M.; Ariño, C.; Dago, À.; Díaz-Cruz, J.M.; Esteban, M. Simultaneous determination of hydroquinone, catechol and resorcinol by voltammetry using graphene screen-printed electrodes and partial least squares calibration. Talanta 2016, 160, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Bo, X.; Guo, L. Electrochemical preparation of porous graphene and its electrochemical application in the simultaneous determination of hydroquinone, catechol, and resorcinol. Sens. Actuators B Chem. 2015, 220, 919–926. [Google Scholar] [CrossRef]
- Ameen, S.; Kim, E.-B.; Akhtar, M.S.; Shin, H.S. Electrochemical detection of resorcinol chemical using unique cabbage like ZnO nanostructures. Mater. Lett. 2017, 209, 571–575. [Google Scholar] [CrossRef]



| Sensing Material | Detection Limit (µM) | Sensitivity (µA/µMcm2) | References |
|---|---|---|---|
| WS2-Gr/GCE | 0.1 | - | [32] |
| CePO4/GCE | 0.14 | - | [33] |
| MgO/MPCPE | 0.25 | - | [34] |
| Au-Pd NF/rGO/GCE | 0.7 | - | [35] |
| CoPC/PGE | 0.72 | - | [36] |
| MWCNTs-Au/GCE | 0.8 | - | [37] |
| ZnO/Gr/GCE | 1 | - | [38] |
| MWCNTs/GCE | 1.1 | - | [39] |
| 3D-CoFeSe4-PCF/GCE | 1.36 | - | [40] |
| GSPE | 2.4 | - | [41] |
| Porous rGO/GCE | 2.62 | - | [42] |
| C-ZnO NSs | 5.89 | 1.98 | [43] |
| MGC | 0.13 | 0.79 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaeedi, H.; Alsalme, A. Hydrothermally Grown MoS2 as an Efficient Electrode Material for the Fabrication of a Resorcinol Sensor. Materials 2023, 16, 1180. https://doi.org/10.3390/ma16031180
Alsaeedi H, Alsalme A. Hydrothermally Grown MoS2 as an Efficient Electrode Material for the Fabrication of a Resorcinol Sensor. Materials. 2023; 16(3):1180. https://doi.org/10.3390/ma16031180
Chicago/Turabian StyleAlsaeedi, Huda, and Ali Alsalme. 2023. "Hydrothermally Grown MoS2 as an Efficient Electrode Material for the Fabrication of a Resorcinol Sensor" Materials 16, no. 3: 1180. https://doi.org/10.3390/ma16031180






