Up-Converting K2Gd(PO4)(WO4):20%Yb3+,Ho3+ Phosphors for Temperature Sensing
Abstract
:1. Introduction
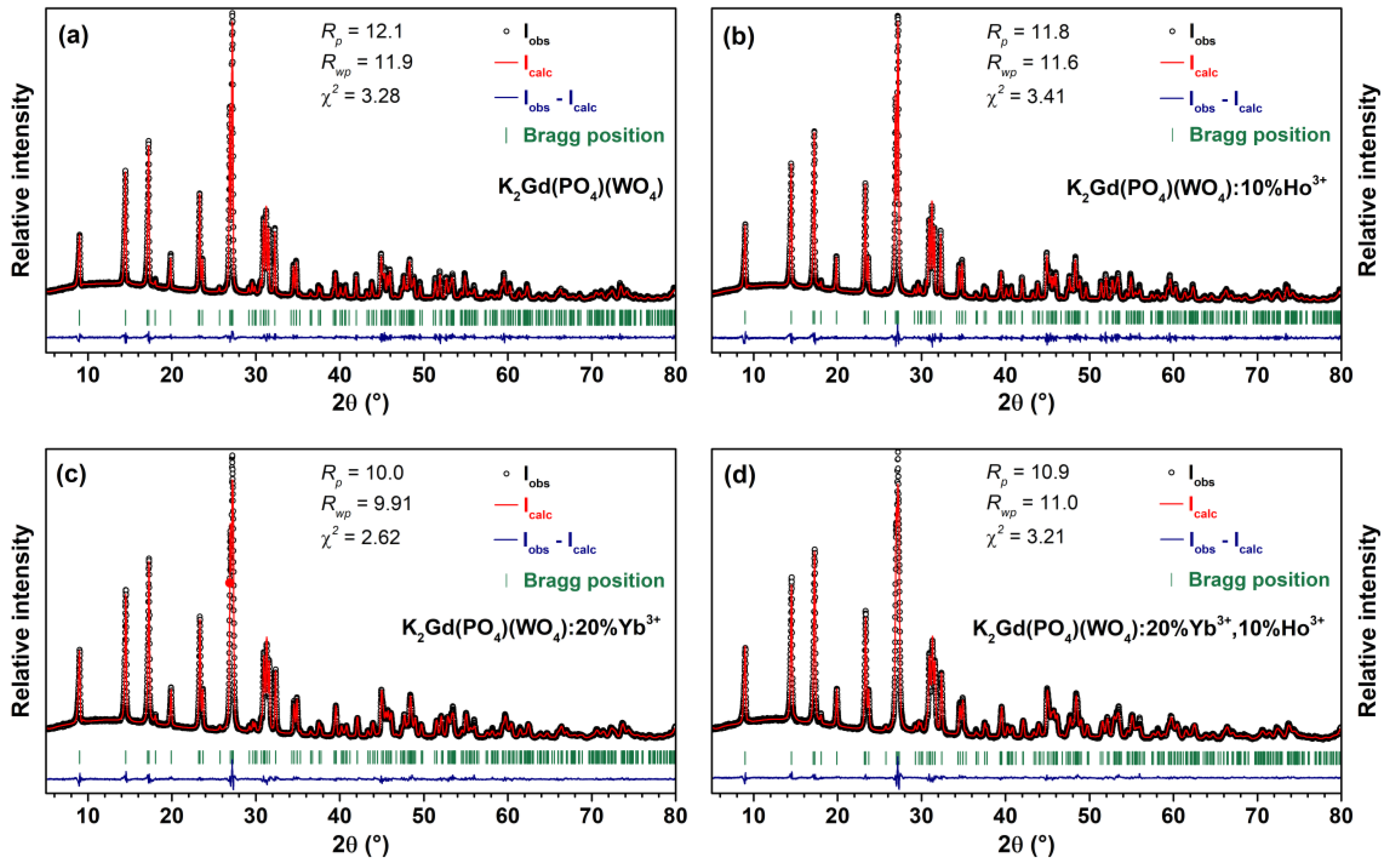
2. Materials and Methods
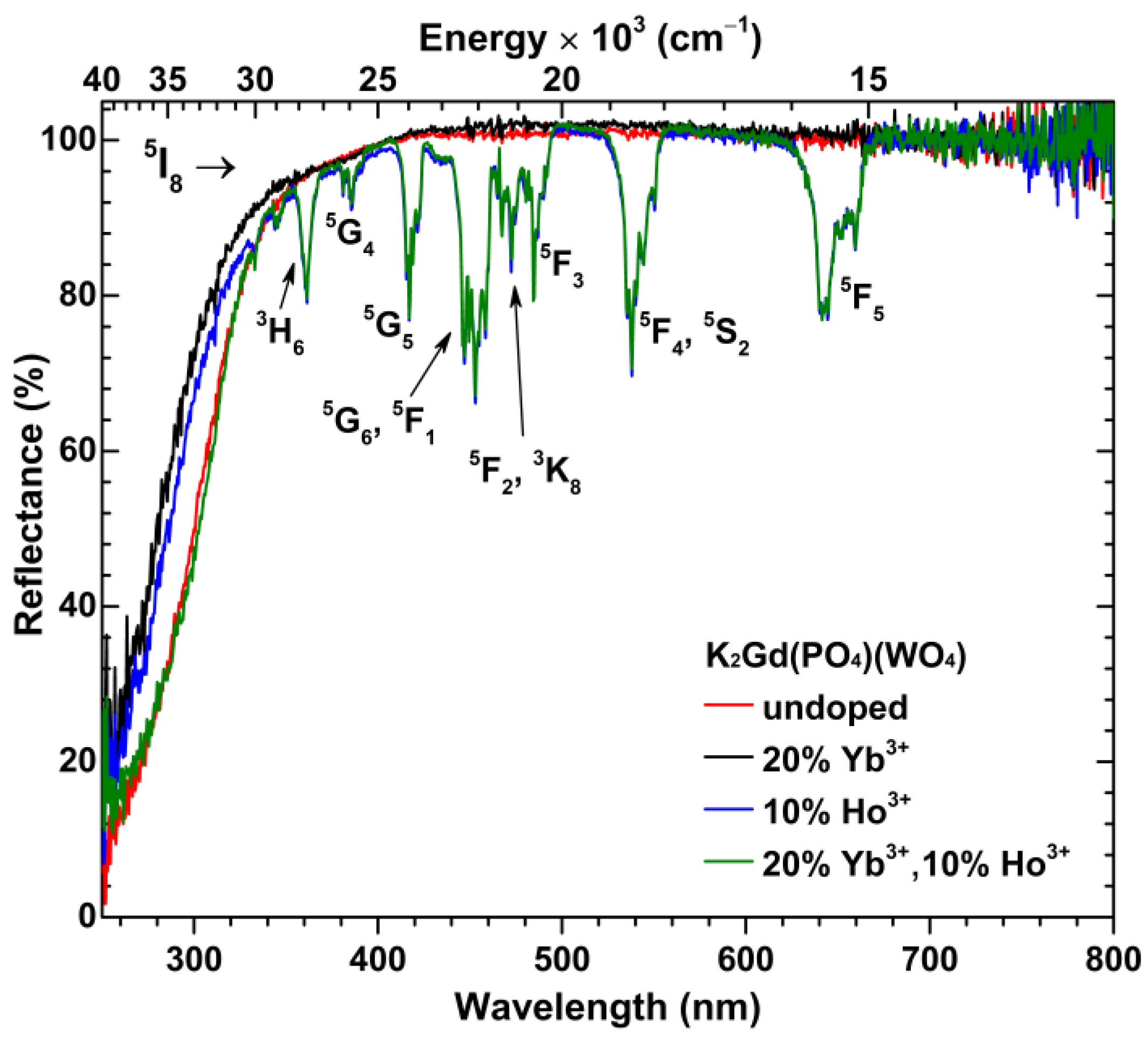
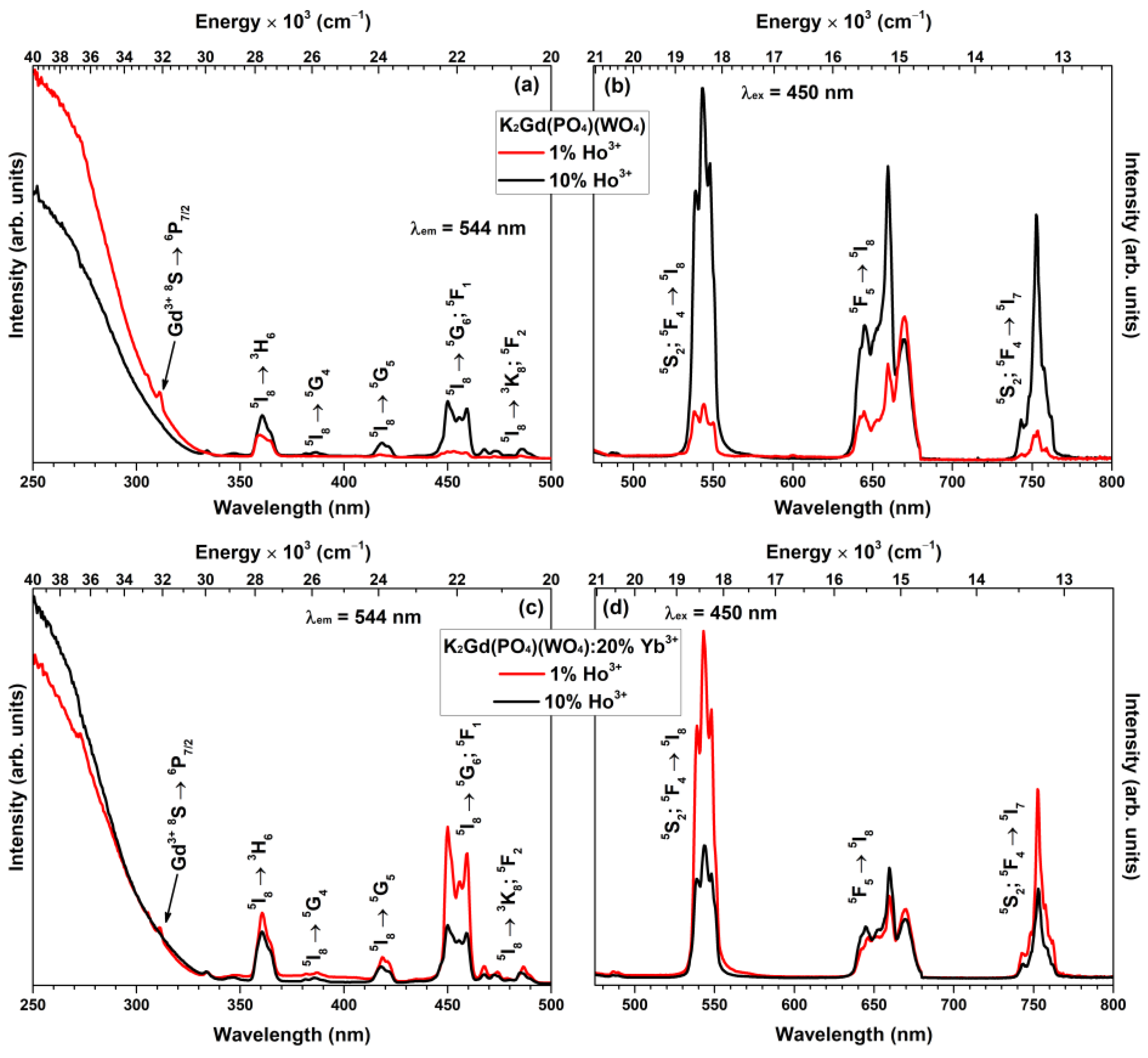
3. Results and Discussion
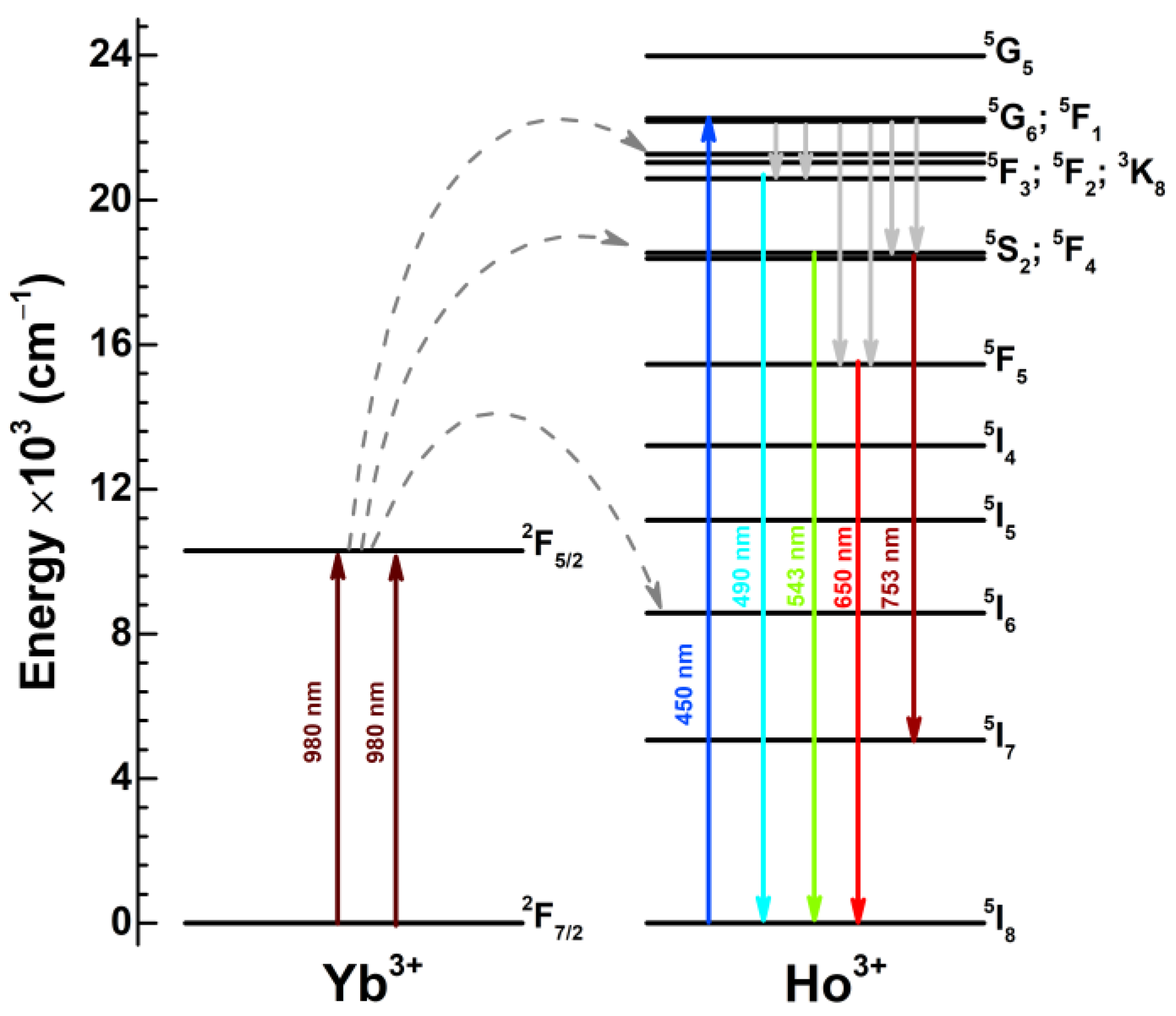
- I step
- 2F5/2(Yb3+) + 5I8(Ho3+) → 2F7/2(Yb3+) + 5I6(Ho3+)
- II step
- 2F5/2(Yb3+) + 5I6(Ho3+) → 2F7/2(Yb3+) + 5S2;5F4(Ho3+)
- III step
- 2F5/2(Yb3+) + 5I8(Ho3+) → 2F7/2(Yb3+) + 5F3(Ho3+)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, D.G.; Liu, Y.M.; Tang, J.X.; Zhao, F.F.; Chen, Z.W.; Zhang, T.T.; Zhang, X.Y.; Chang, N.; Wu, C.L.; Chen, D.W.; et al. Huge enhancement of upconversion luminescence by broadband dye sensitization of core/shell nanocrystals. Dalton. Trans. 2016, 45, 13392–13398. [Google Scholar] [CrossRef]
- Ramachari, D.; Esparza, D.; Lopez-Luke, T.; Romero, V.H.; Perez-Mayen, L.; De la Rosa, E.; Jayasankar, C.K. Synthesis of co-doped Yb3+-Er3+:ZrO2 upconversion nanoparticles and their applications in enhanced photovoltaic properties of quantum dot sensitized solar cells. J. Alloys Compd. 2017, 698, 433–441. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F.Y. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Raman, T.R.; Lakshmi, R.P.V.; Ratnakaram, Y.C. Effect of Ho3+ ion concentration on structure and spectroscopic properties of LiPbB5O9:Ho3+ phosphor. J. Mol. Struct. 2021, 1243, 130759. [Google Scholar] [CrossRef]
- Zhang, B.B.; Meng, J.J.; Mi, X.H.; Zhang, C.J.; Zhang, Z.L.; Zheng, H.R. Enhanced upconversion fluorescent probe of single NaYF4:Yb3+/Er3+/Zn2+ nanoparticles for copper ion detection. Rsc. Adv. 2018, 8, 37618–37622. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.M.; Ma, P.A.; Hou, Z.Y.; Cheng, Z.Y.; Li, C.X.; Lin, J. Current advances in lanthanide ion (Ln3+)-based upconversion nanomaterials for drug delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Q.; Qin, L.Y.; Li, H.J.; Wang, Y.X.; Zhang, R.; Shi, J.M.; Wu, J.H.; Dong, G.X.; Zhou, P. Application of lanthanide-doped upconversion nanoparticles for cancer treatment: A review. Nanomedicine 2021, 16, 2207–2242. [Google Scholar] [CrossRef]
- Pavani, K.; Kumar, J.S.; Srikanth, K.; Soares, M.J.; Pereira, E.; Neves, A.J.; Graca, M.P.F. Highly efficient upconversion of Er3+ in Yb3+ codoped non-cytotoxic strontium lanthanum aluminate phosphor for low temperature sensors. Sci. Rep. 2017, 7, 17646. [Google Scholar] [CrossRef] [Green Version]
- Solis, D.; De la Rosa, E.; Meza, O.; Diaz-Torres, L.A.; Salas, P.; Angeles-Chavez, C. Role of Yb3+ and Er3+ concentration on the tunability of green-yellow-red upconversion emission of codoped ZrO2:Yb3+-Er3+ nanocrystals. J. Appl. Phys. 2010, 108, 023103. [Google Scholar] [CrossRef]
- Li, K.; Van Deun, R. Mutual energy transfer luminescent properties in novel CsGd(MoO4)2:Yb3+,Er3+/Ho3+ phosphors for solid-state lighting and solar cells. Phys. Chem. Chem. Phys. 2019, 21, 4746–4754. [Google Scholar] [CrossRef]
- Mahata, M.K.; Koppe, T.; Kumar, K.; Hofsass, H.; Vetter, U. Upconversion photoluminescence of Ho3+-Yb3+ doped barium titanate nanocrystallites: Optical tools for structural phase detection and temperature probing. Sci. Rep. 2020, 10, 8775. [Google Scholar] [CrossRef]
- Lim, C.S.; Atuchin, V.V.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S. Incommensurately modulated structure and spectroscopic properties of CaGd2(MoO4)4:Ho3+/Yb3+ phosphors for up-conversion applications. J. Alloys Compd. 2017, 695, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Structural and Spectroscopic Effects of Li+ Substitution for Na+ in LixNa1−xCaGd0.5Ho0.05Yb0.45(MoO4)3 Scheelite-Type Upconversion Phosphors. Molecules 2021, 26, 7357. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.Y.; Liu, L.; Ma, B.; Li, B.X.; Zhao, X.D.; Tong, W.M.; Wang, X.F. beta-NaYF4: Yb,Tm: Upconversion properties by controlling the transition probabilities at the same energy level. Inorg. Chem. Front. 2016, 3, 1082–1090. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhou, S.S.; Jiang, G.C.; Wei, X.T.; Chen, Y.H.; Yin, M. Blue upconversion of Tm3+ using Yb3+ as energy transfer bridge under 1532 nm excitation in Er3+, Yb3+, Tm3+ tri-doped CaMoO4. J. Rare Earth 2015, 33, 475–479. [Google Scholar] [CrossRef]
- Yu, H.Q.; Jiang, P.P.; Chen, B.J.; Sun, J.S.; Cheng, L.H.; Li, X.P.; Zhang, J.S.; Xu, S. Electrospinning preparation and upconversion luminescence of Y2Ti2O7:Tm/Yb nanofibers. Appl. Phys. A-Mater. 2020, 126, 690. [Google Scholar] [CrossRef]
- Tessitore, G.; Mandl, G.A.; Brik, M.G.; Park, W.; Capobianco, J.A. Recent insights into upconverting nanoparticles: Spectroscopy, modeling, and routes to improved luminescence. Nanoscale 2019, 11, 12015–12029. [Google Scholar] [CrossRef]
- Wu, X.; Chen, G.Y.; Shen, J.; Li, Z.J.; Zhang, Y.W.; Han, G. Upconversion Nanoparticles: A Versatile Solution to Multiscale Biological Imaging. Bioconjugate Chem. 2015, 26, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Du, S.R.; Zheng, X.Y.; Lyu, G.M.; Sun, L.D.; Li, L.D.; Zhang, P.Z.; Zhang, C.; Yan, C.H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Huang, H.N.; Wang, T.; Zhou, H.F.; Huang, D.P.; Wu, Y.Q.; Zhou, G.J.; Hu, J.F.; Zhan, J. Luminescence, energy transfer, and up-conversion mechanisms of Yb3+ and Tb3+ co-doped LaNbO4. J. Alloys Compd. 2017, 702, 209–215. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Van Deun, R. Rare earth tungstate and molybdate compounds-from 0D to 3D architectures. Chem. Soc. Rev. 2013, 42, 8835–8848. [Google Scholar] [CrossRef]
- Su, J.Y.; Zhang, X.Y.; Li, X. Hydrothermal synthesis and green up-conversion luminescence of Yb3+ and Ho3+ co-doped SrGd2(WO4)2(MoO4)2 nanocrystal. Aip Adv. 2019, 9, 125246. [Google Scholar] [CrossRef] [Green Version]
- Atuchin, V.V.; Kesler, V.G.; Maklakova, N.Y.; Pokrovsky, L.D.; Sheglov, D.V. Core level spectroscopy and RHEED analysis of KGd0.95Nd0.05(WO4)2 surface. Eur. Phys. J. B 2006, 51, 293–300. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Galashov, E.N.; Kozhukhov, A.S.; Pokrovsky, L.D.; Shlegel, V.N. Epitaxial growth of ZnO nanocrystals at ZnWO4(0 1 0) cleaved surface. J. Cryst. Growth 2011, 318, 1147–1150. [Google Scholar] [CrossRef]
- Huang, X.Y.; Li, B.; Guo, H. Highly efficient Eu3+-activated K2Gd(WO4)(PO4) red-emitting phosphors with superior thermal stability for solid-state lighting. Ceram. Int. 2017, 43, 10566–10571. [Google Scholar] [CrossRef]
- Fan, G.; Tian, Z.C.; Wang, X.J.; Tang, S.L.; Chen, Y.N. High quantum efficiency red-emitting K2Gd(PO4)(WO4): Sm3+ phosphor: Preparation, characterization and photoluminescence properties. J. Mater. Sci.-Mater. Electron. 2018, 29, 17681–17688. [Google Scholar] [CrossRef]
- Han, L.L.; Wang, Y.H.; Zhang, J.; Tao, Y. Visible quantum cutting via downconversion in a novel green-emitting K2Gd(WO4)(PO4):Tb3+ phosphor. Mater. Chem. Phys. 2014, 143, 476–479. [Google Scholar] [CrossRef]
- Guo, L.N.; Wang, Y.H.; Zhang, J.; Wang, Y.Z.; Dong, P.Y. Near-infrared quantum cutting in Ho3+, Yb3+-codoped BaGdF5 nanoparticles via first- and second-order energy transfers. Nanoscale Res. Lett. 2012, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Terebilenko, K.V.; Zatovsky, I.V.; Baumer, V.N.; Slobodyanik, N.S.; Shishkin, O.V. K2Ho(PO4)(WO4). Acta Crystallogr. Sect. E-Crystallogr. Commun. 2008, 64, i75. [Google Scholar] [CrossRef] [Green Version]
- Zatovsky, I.V.; Terebilenko, K.V.; Slobodyanik, N.S.; Baumer, V.N.; Shishkin, O.V. K2Bi(PO4)(WO4) with a layered anionic substructure. Acta Crystallogr. Sect. E-Crystallogr. Commun. 2006, 62, I193–I195. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Huang, X.Y.; Guo, H.; Li, B. Eu3+-activated Na2Gd(PO4)(MoO4): A novel high-brightness red-emitting phosphor with high color purity and quantum efficiency for white light-emitting diodes. J. Alloys Compd. 2017, 720, 29–38. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Ezerskyte, E.; Minderyte, A.; Stanionyte, S.; Juskenas, R.; Sakirzanovas, S.; Katelnikovas, A. Optical Properties of Red-Emitting Rb2Bi(PO4)(MoO4):Eu3+ Powders and Ceramics with High Quantum Efficiency for White LEDs. Materials 2019, 12, 3275. [Google Scholar] [CrossRef] [Green Version]
- Daub, M.; Lehner, A.J.; Hoppe, H.A. Synthesis, crystal structure and optical properties of Na2RE(PO4)(WO4) (RE = Y, Tb-Lu). Dalton. T 2012, 41, 12121–12128. [Google Scholar] [CrossRef]
- Zhang, X.G.; Chen, M.Y.; Zhang, J.L.; Qin, X.Z.; Gong, M.L. Photoluminescence studies of high-efficient red-emitting K2Y(WO4)(PO4):Eu3+ phosphor for NUV LED. Mater. Res. Bull. 2016, 73, 219–225. [Google Scholar] [CrossRef]
- Zatovsky, I.V.; Terebilenko, K.V.; Slobodyanik, N.S.; Baumer, V.N.; Shishkin, O.V. Synthesis, characterization and crystal structure of K2Bi(PO4)(MoO4). J. Solid. State Chem. 2006, 179, 3550–3555. [Google Scholar] [CrossRef]
- Han, B.; Liu, B.K.; Zhang, J.; Shi, H.Z. Luminescence properties of novel Ba2MgWO6:Eu3+ and g-C3N4/Ba2MgWO6:Eu3+ phosphors. Optik 2017, 131, 764–768. [Google Scholar] [CrossRef]
- Foldvari, I.; Baraldi, A.; Capelletti, R.; Magnani, N.; Sosa, R.; Munoz, A.; Kappers, L.A.; Watterich, A. Optical absorption and luminescence of Ho3+ ions in Bi2TeO5 single crystal. Opt. Mater. 2007, 29, 688–696. [Google Scholar] [CrossRef]
- Kaczkan, M.; Malinowski, M. Optical Transitions and Excited State Absorption Cross Sections of SrLaGaO4 Doped with Ho3+ Ions. Materials 2021, 14, 3831. [Google Scholar] [CrossRef]
- Li, Y.C.; Chang, Y.H.; Chang, Y.S.; Lin, Y.J.; Laing, C.H. Luminescence and energy transfer properties of Gd3+ and Tb3+ in LaAlGe2O7. J. Phys. Chem. C 2007, 111, 10682–10688. [Google Scholar] [CrossRef]
- Wang, J.; Bu, Y.Y.; Wang, X.F.; Seo, H.J. A novel optical thermometry based on the energy transfer from charge transfer band to Eu3+-Dy3+ ions. Sci. Rep. 2017, 7, 6023. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.X.; Ye, Y.F.; Liu, H.H.; Guo, X.F.; Tang, H.X.; Yin, W.Z.; Gao, Y.P. Hydrothermal synthesis of NaLa(WO4)2: Eu3+ octahedrons and tunable luminescence by changing Eu3+ concentration and excitation wavelength. J. Mater. Sci.-Mater. Electron. 2017, 28, 1301–1306. [Google Scholar] [CrossRef]
- Malinowski, M.; Kaczkan, M.; Wnuk, A.; Szuflinska, M. Emission from the high lying excited states of Ho3+ ions in YAP and YAG. J. Lumin. 2004, 106, 269–279. [Google Scholar] [CrossRef]
- Malinowski, M.; Piramidowicz, R.; Frukacz, Z.; Chadeyron, G.; Mahiou, R.; Joubert, M.F. Spectroscopy and upconversion processes in YAlO3:Ho3+ crystals. Opt. Mater. 1999, 12, 409–423. [Google Scholar] [CrossRef]
- Wang, X.; Bu, Y.; Xiao, S.; Yang, X.; Ding, J. Upconversion in Ho3+-doped YbF3 particle prepared by coprecipitation method. Appl. Phys. B-Lasers O 2008, 93, 801–807. [Google Scholar] [CrossRef]
- Qian, H.Y.; Zhang, T.Q.; Jiang, X.L.; Wang, H.H.; Yang, W.L.; Li, C. Visible photon avalanche up-conversion of Yb3+ and Ho3+ doped NaBi(WO4)2 phosphors under excitation at 980 nm. J. Mater. Sci.-Mater. Electron. 2022, 33, 22718–22727. [Google Scholar] [CrossRef]
- Kochanowicz, M.; Zmojda, J.; Miluski, P.; Pisarska, J.; Pisarski, W.A.; Dorosz, D. NIR to visible upconversion in double-clad optical fiber co-doped with Yb3+/Ho3+. Opt Mater. Express 2015, 5, 1505–1510. [Google Scholar] [CrossRef]
- Liu, W.G.; Wang, X.J.; Zhu, Q.; Li, X.D.; Sun, X.D.; Li, J.G. Upconversion luminescence and favorable temperature sensing performance of eulytite-type Sr3Y(PO4)3:Yb3+/Ln3+ phosphors (Ln=Ho, Er, Tm). Sci. Technol. Adv. Mat. 2019, 20, 949–963. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.Q.; An, S.S.; Zhuang, C.; Sun, D.; Li, X.W.; Zhang, J. Yb3+-concentration-dependent upconversion luminescence of Ho3+-Yb3+ codoped La9.31(Si1.04O4)6O2 for optical thermometer. J. Lumin. 2022, 250, 119073. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, V.; Kroon, R.E.; Swart, H.C. Photon upconversion in Ho3+-Yb3+ embedded tungsten tellurite glass. J. Lumin. 2017, 192, 757–760. [Google Scholar] [CrossRef]
- Lupei, A.; Lupei, V.; Ikesue, A.; Gheorghe, C.; Hau, S. Nd → Yb energy transfer in (Nd, Yb):Y2O3 transparent ceramics. Opt. Mater. 2010, 32, 1333–1336. [Google Scholar] [CrossRef]
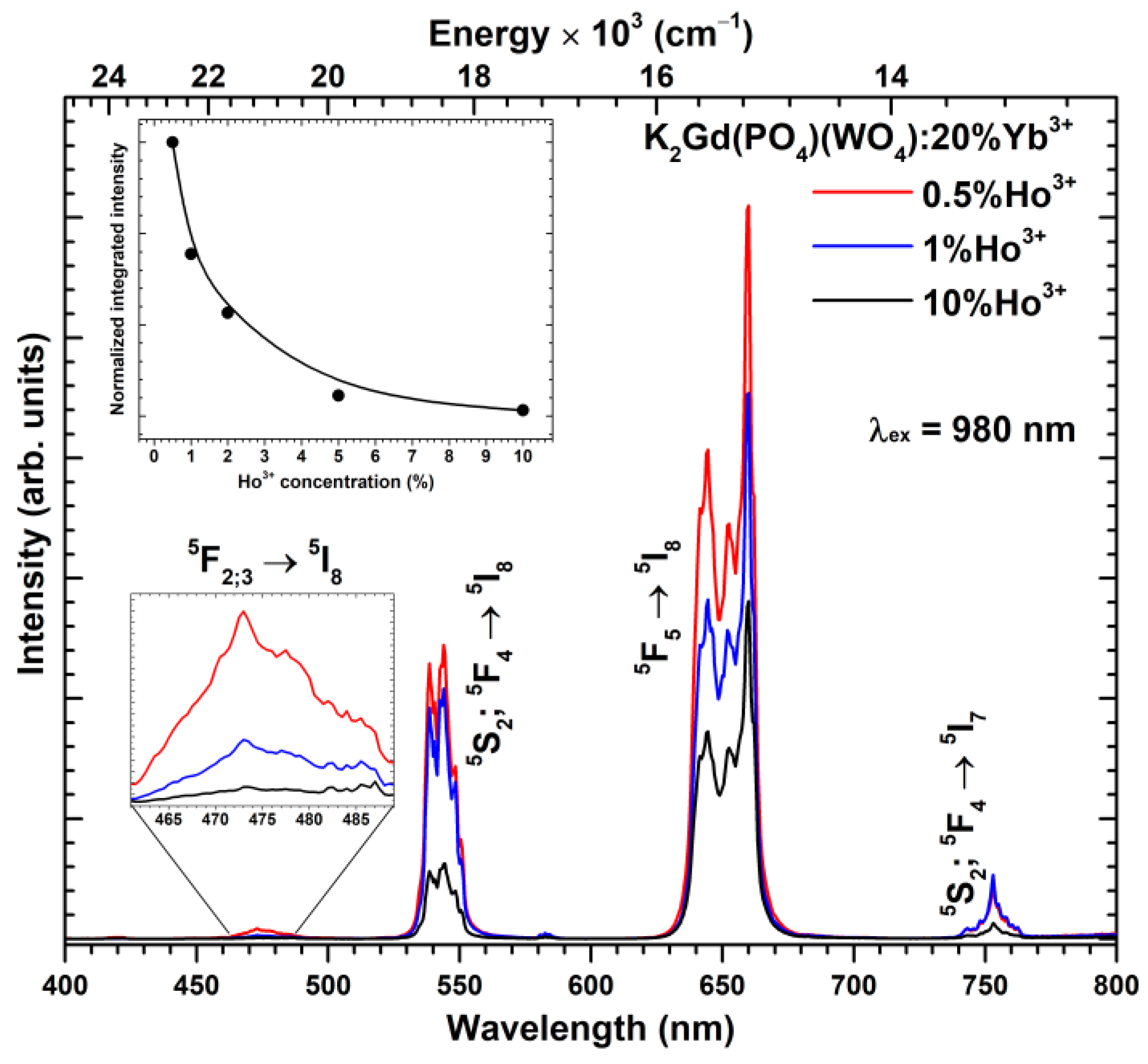

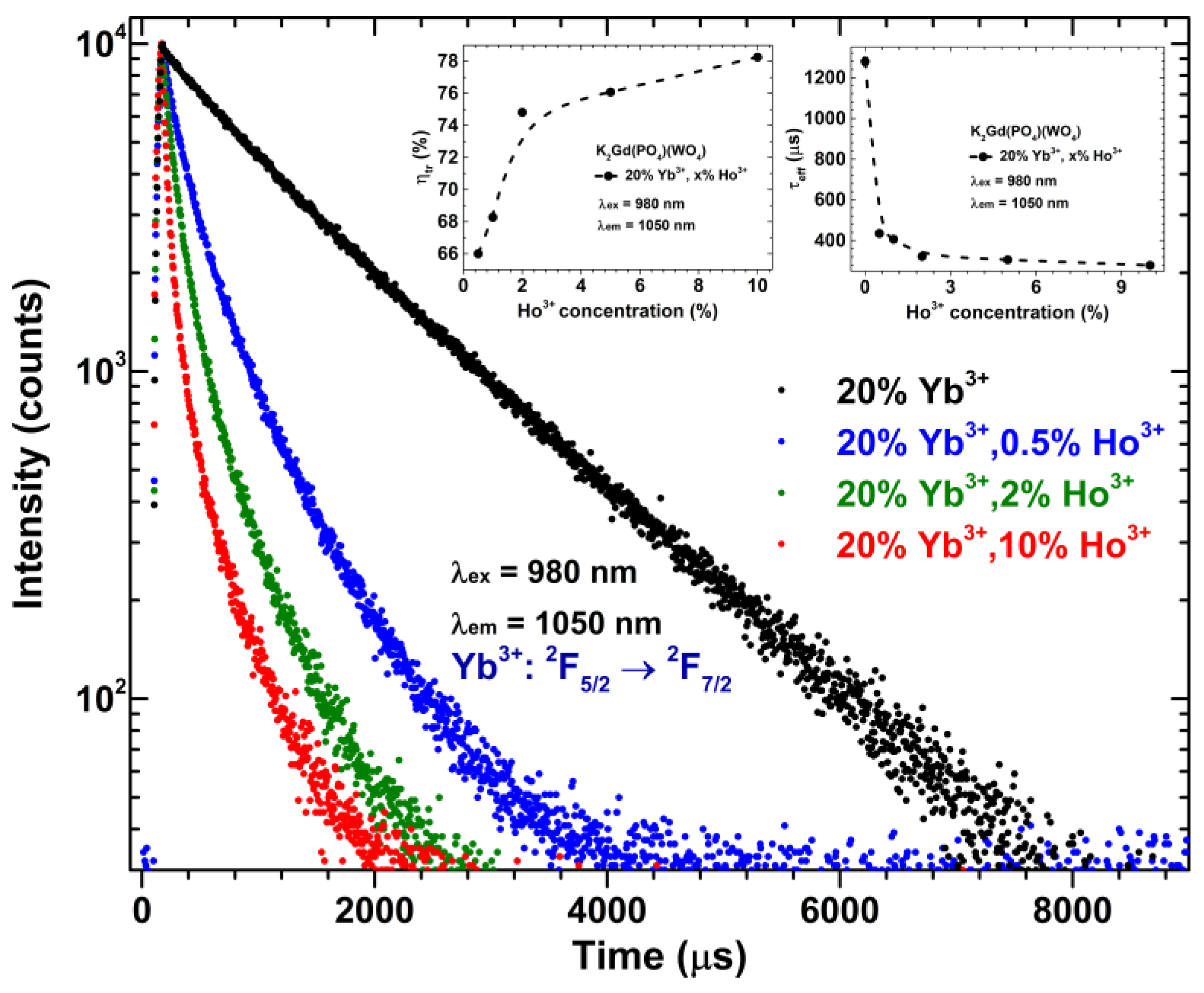
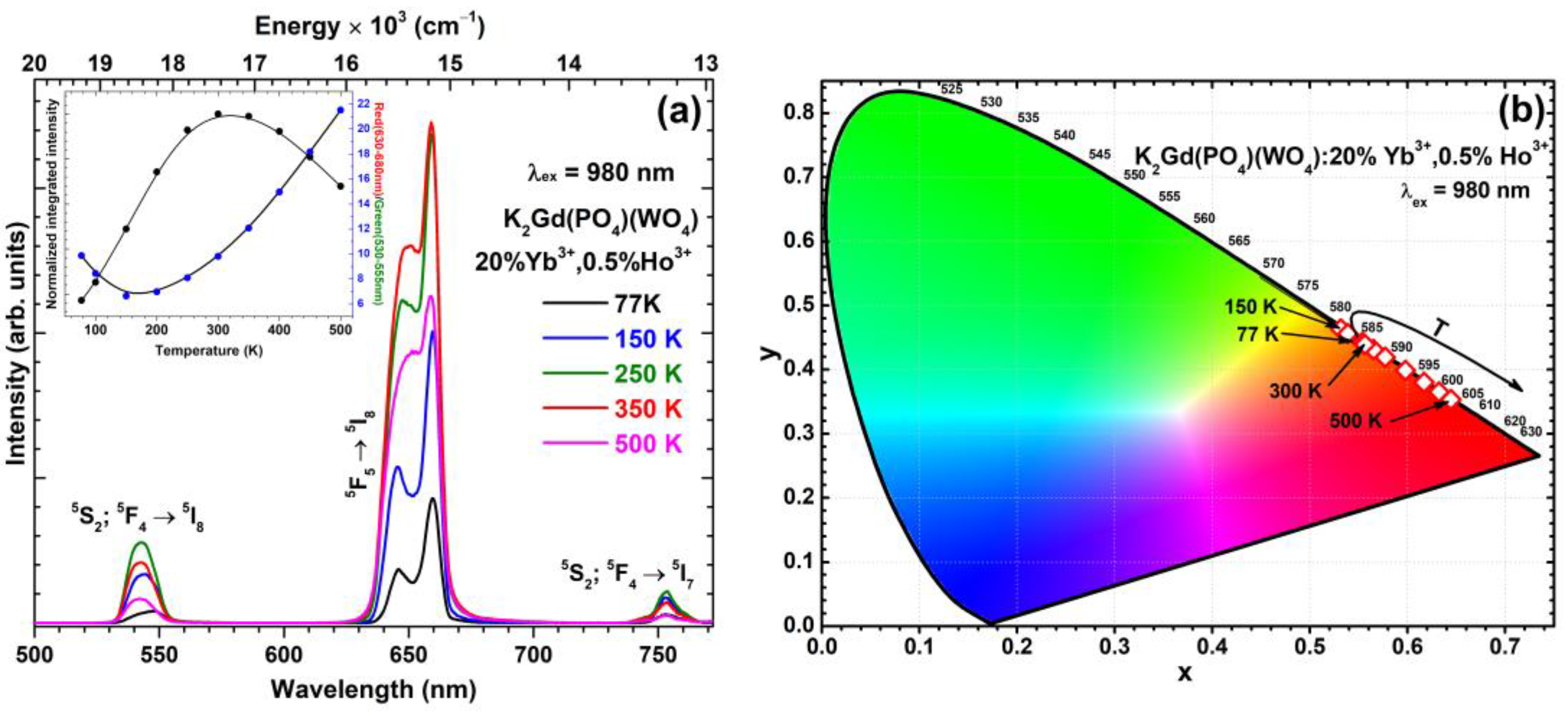
| Upconverting Material | UC PL Lifetime (μs) | Ref. |
| BaGdF5:20%Yb3+,1%Ho3+ | 131 | [28] |
| Sr3Y(PO4)3:10%Yb3+,2%Ho3+ | 261 | [48] |
| cubic BaTiO3:3%Yb3+,0.2%Ho3+ | 155 | [11] |
| tetragonal BaTiO3:3%Yb3+,0.2%Ho3+ | 125 | [11] |
| La9.31(Si1.04O4)6O2:20%Yb3+,1%Ho3+ | 18 | [49] |
| TeO2–WO3 glass | 33 | [50] |
| K2Gd(PO4)(WO4):20%Yb3+,0.5%Ho3+ | 191 | This work |
| K2Gd(PO4)(WO4):20%Yb3+,10%Ho3+ | 83 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorjevaite, J.; Katelnikovas, A. Up-Converting K2Gd(PO4)(WO4):20%Yb3+,Ho3+ Phosphors for Temperature Sensing. Materials 2023, 16, 917. https://doi.org/10.3390/ma16030917
Grigorjevaite J, Katelnikovas A. Up-Converting K2Gd(PO4)(WO4):20%Yb3+,Ho3+ Phosphors for Temperature Sensing. Materials. 2023; 16(3):917. https://doi.org/10.3390/ma16030917
Chicago/Turabian StyleGrigorjevaite, Julija, and Arturas Katelnikovas. 2023. "Up-Converting K2Gd(PO4)(WO4):20%Yb3+,Ho3+ Phosphors for Temperature Sensing" Materials 16, no. 3: 917. https://doi.org/10.3390/ma16030917
APA StyleGrigorjevaite, J., & Katelnikovas, A. (2023). Up-Converting K2Gd(PO4)(WO4):20%Yb3+,Ho3+ Phosphors for Temperature Sensing. Materials, 16(3), 917. https://doi.org/10.3390/ma16030917








