Selective Recovery of Gold from Electronic Waste by New Efficient Type of Sorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characteristics of Resin
2.3. Characteristics of Extractant
2.4. Methods and Analysis
2.4.1. Preparation of Pure Resin
2.4.2. Preparation of the Impregnated Resin
Determination of Cyanex 272 Concentration in Purolite MN 202
FTIR Studies
Surface Area
2.4.3. Preparation of Metal Solutions
2.4.4. Adsorption Studies
Desorption Studies
2.4.5. SEM
3. Results and Discussion
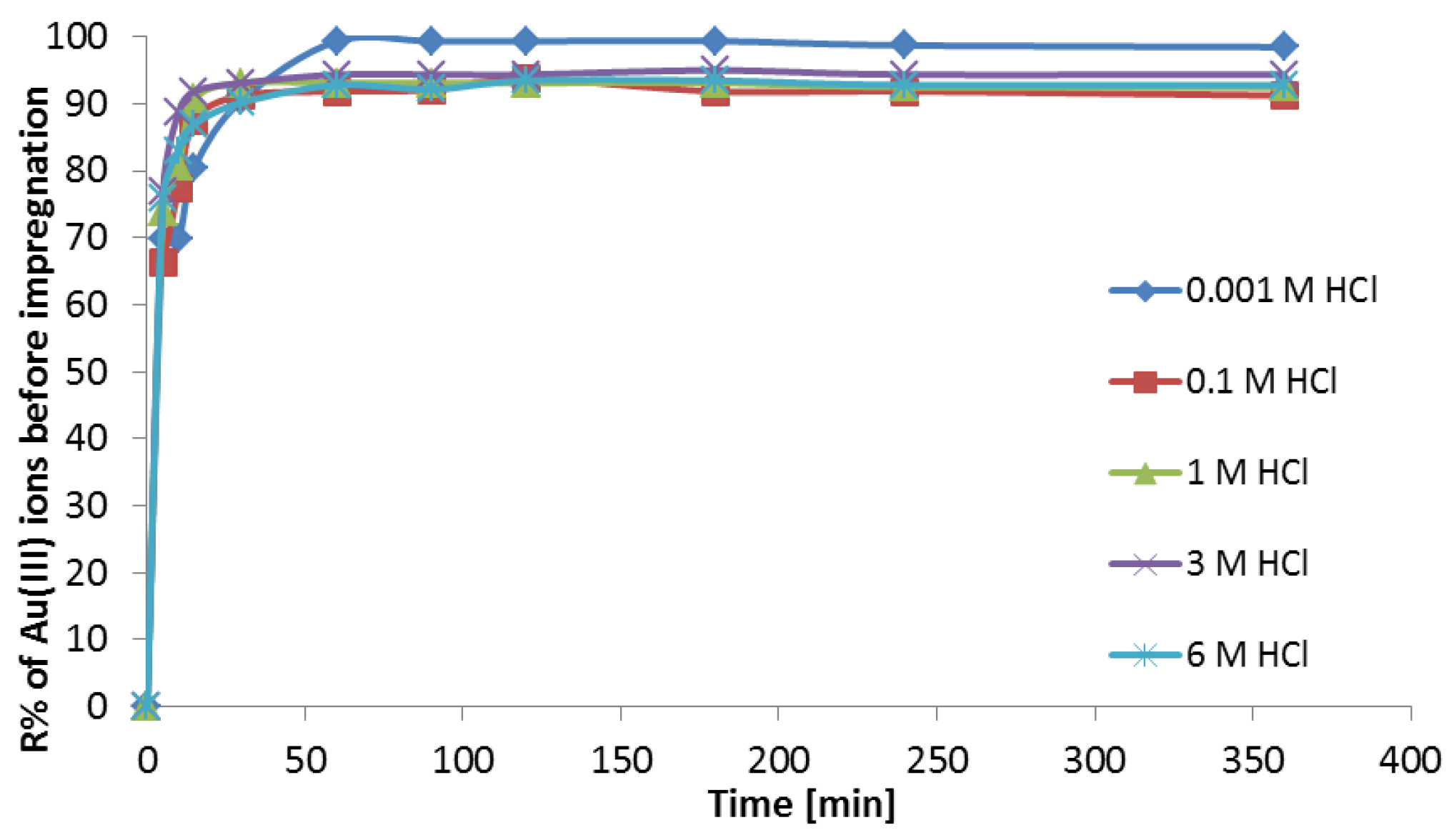
3.1. Sorption Studies of Au(III) on Purolite MN 202
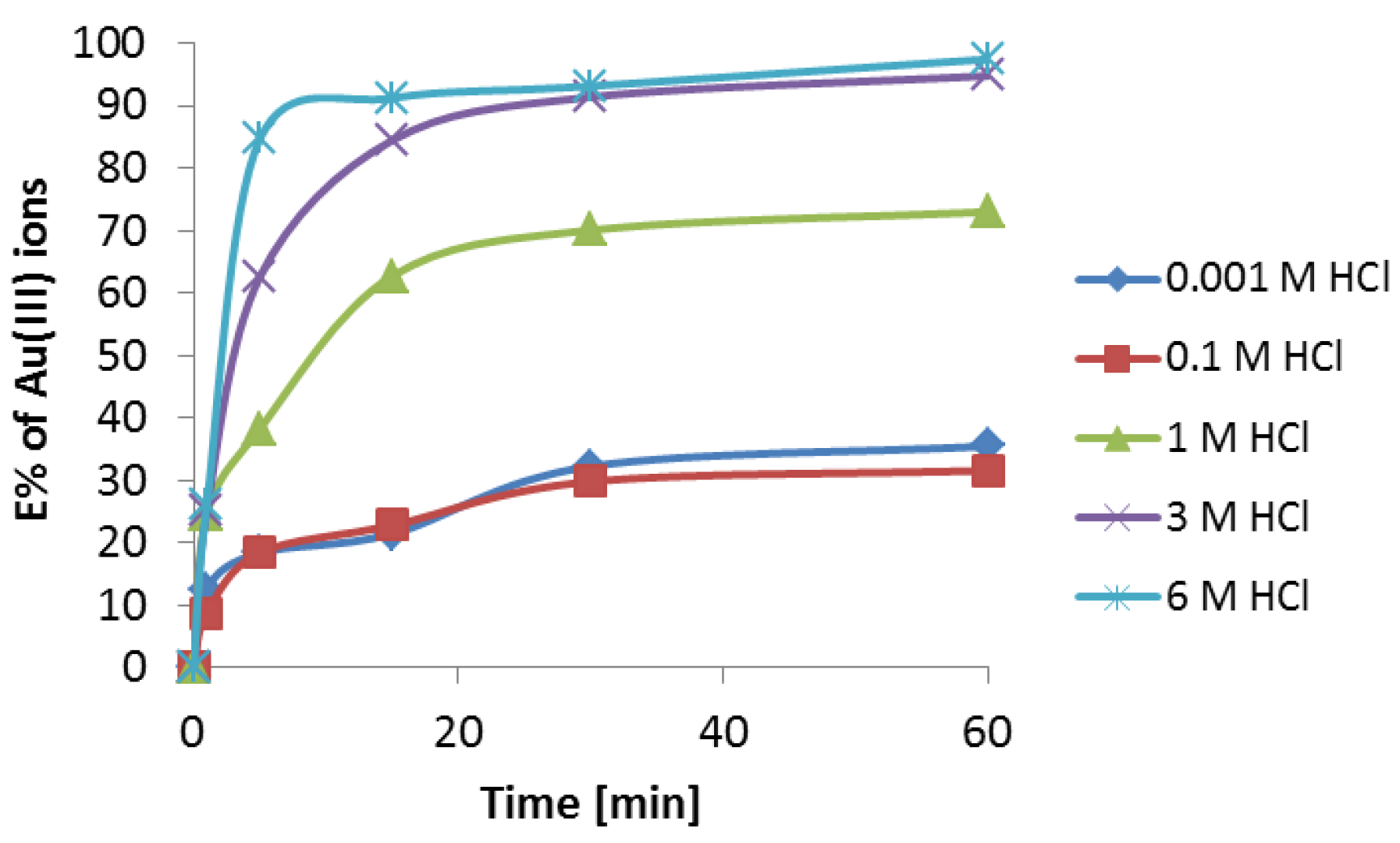
3.2. Extraction Studies of Au(III) Using Cyanex 272 in Toluene
3.3. Sorption Studies of Au(III) on Purolite MN 202 Impregnated with Cyanex 272
3.3.1. Determination of Cyanex 272 Concentration in Purolite MN 202
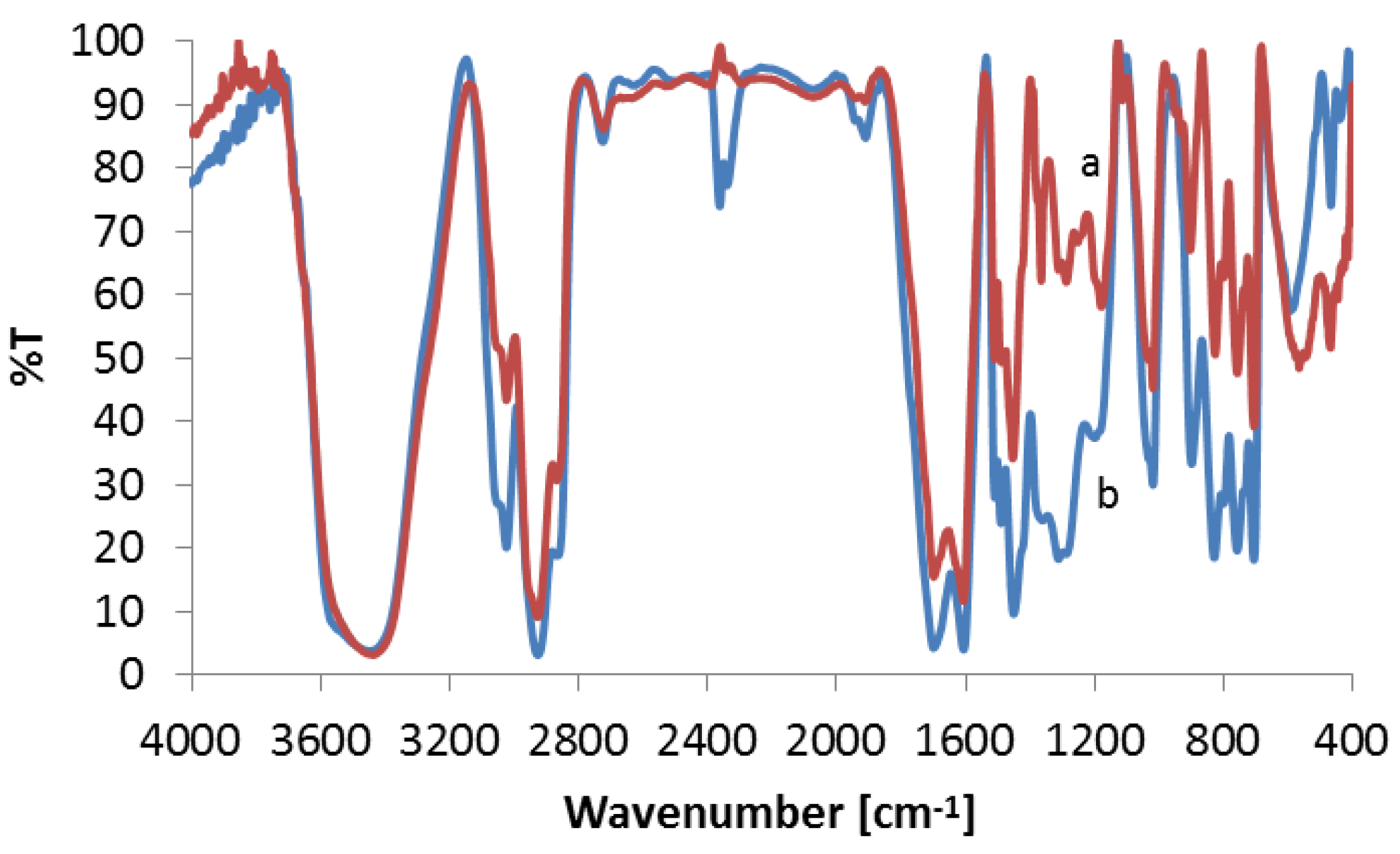
3.3.2. FTIR
3.3.3. Surface Area
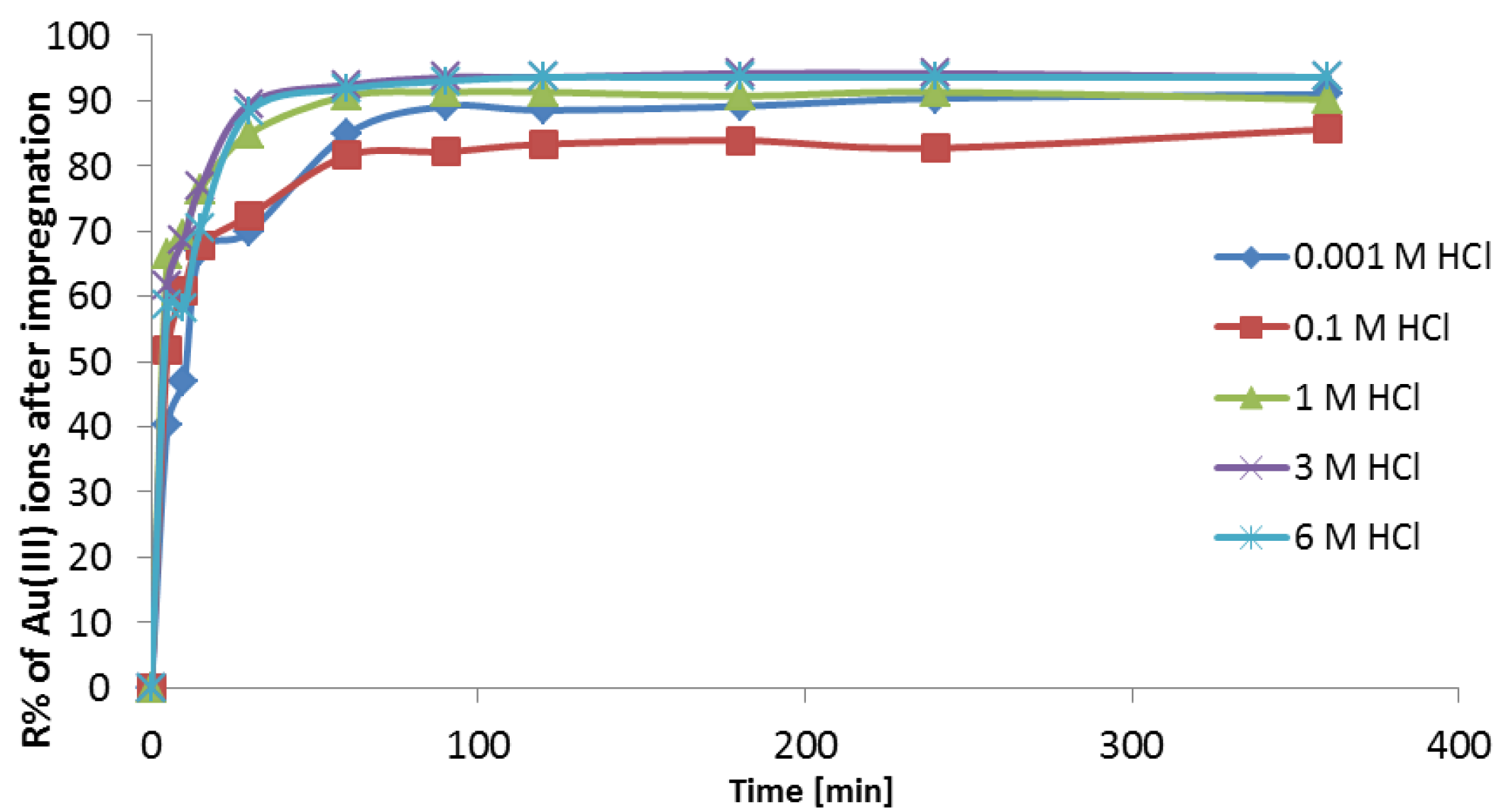
3.3.4. Adsorption Investigations
3.4. Kinetic Parameters
3.5. Adsorption Isotherms Models
3.6. SEM Studies
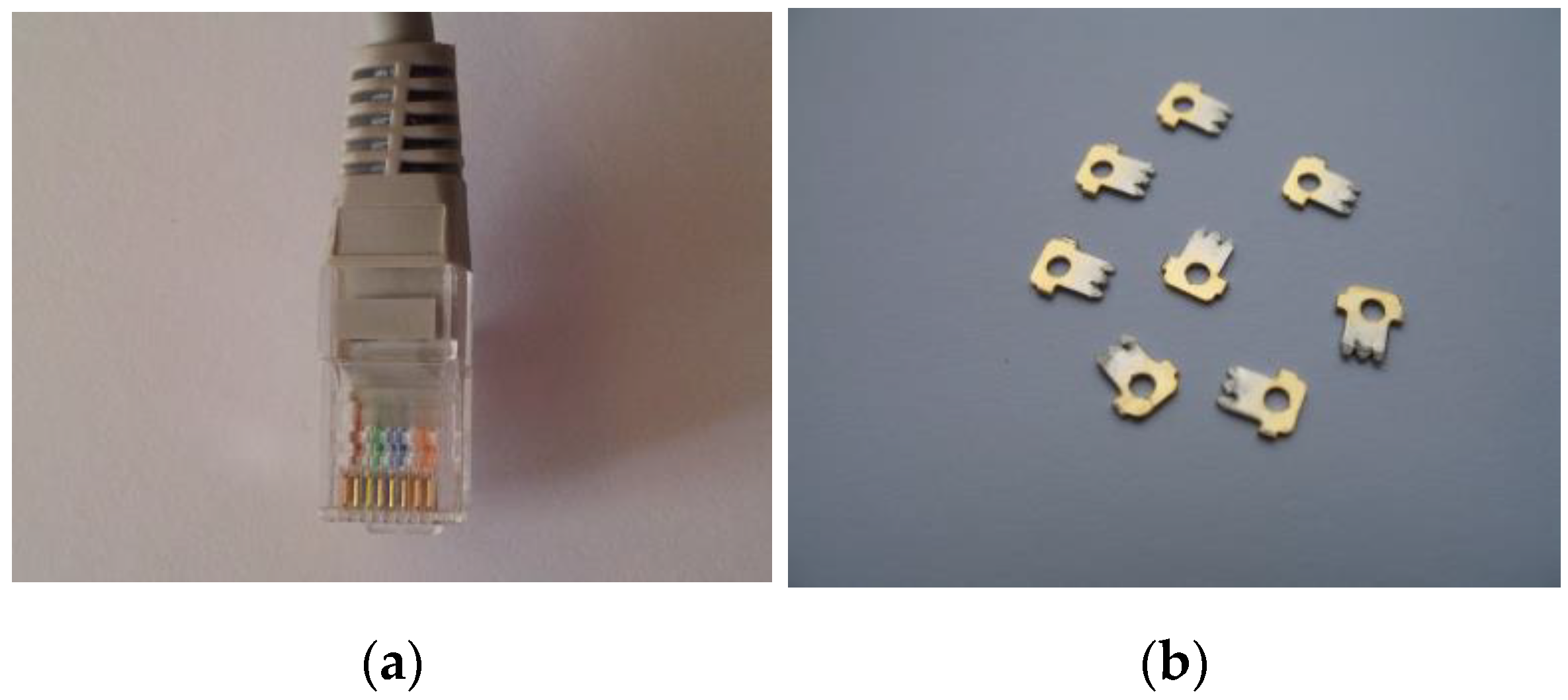
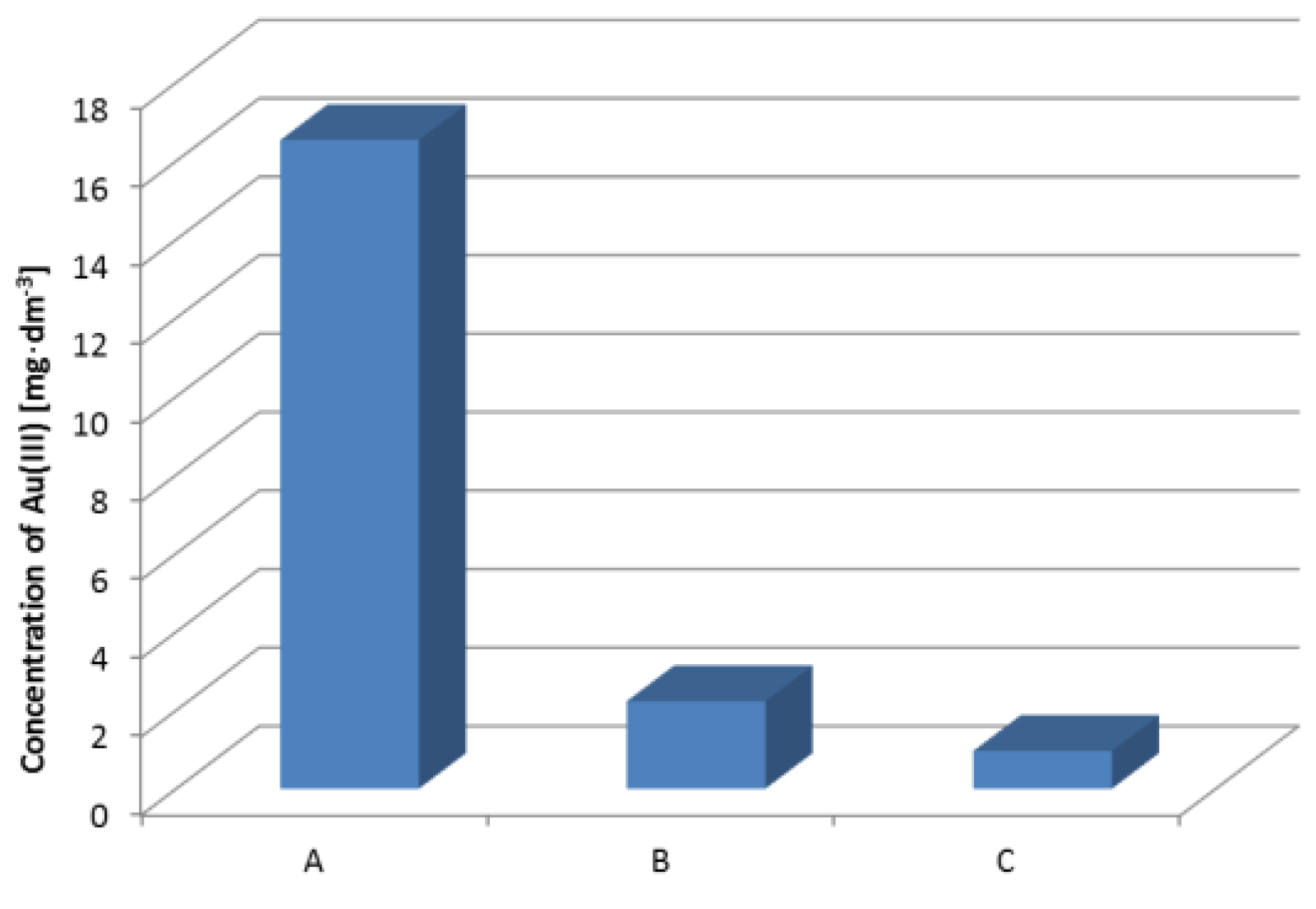
3.7. Recovery of Gold from the Modular Connector RJ 45 (8P8C)
3.8. Limitation of Purolite MM 202 Impregnated with Cyanex 272
4. Desorption Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Constable, E.C. Chemistry of precious metals. Polyhedron 1998, 17, 397. [Google Scholar] [CrossRef]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Bigum, M.; Brogaard, L.; Christensen, T.H. Metal recovery from high-grade WEEE: A life cycle assessment. J. Hazard. Mater. 2012, 207–208, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)–co-hosted SCYCLE Programme: Bonn, Germany; International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Rotterdam, The Netherlands, 2020; ISBN 9789280891140. [Google Scholar]
- Parodi, A.; Vincent, T.; Pilsniak, M.; Trochimczuk, A.W.; Guibal, E. Palladium and platinum binding on an imidazol containing resin. Hydrometallurgy 2008, 92, 1–10. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Chong, M.N.; Herath, G.; Moyo, T.; Petersen, J. E-waste in the international context—A review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef]
- Negrea, A.; Ronka, S.; Ciopec, M.; Duteanu, N.; Negrea, P.; Mihailescu, M. Kinetics, thermodynamics and equilibrium studies for gold recovery from diluted waste solution. Materials 2021, 14, 5325. [Google Scholar] [CrossRef]
- Ronka, S.; Targońska, S. Gold(III) ions sorption on sulfur-containing polymeric sorbent based on 2,2‘-thiobisethanol dimethacrylate. Sep. Sci. Technol. 2020, 55, 2158–2169. [Google Scholar] [CrossRef]
- Msumange, D.A.; Yazici, E.Y.; Celep, O.; Deveci, H. A comparison of ion-exchange resins and activated carbon in recovering gold from cyanide leach solutions with low levels of copper. Bull. Miner. Res. Explor. 2022, 168, 35–41. [Google Scholar] [CrossRef]
- Guo, J.; Fan, X.; Li, Y.; Yu, S.; Zhang, Y.; Wang, L.; Ren, X. Mechanism of selective gold adsorption on ion-imprinted chitosan resin modified by thiourea. J. Hazard. Mater. 2021, 415, 125617. [Google Scholar] [CrossRef] [PubMed]
- Cortina, J.L.; Meinhardt, E.; Roijals, O.; Martí, V. Modification and preparation of polymeric adsorbents for precious-metal extraction in hydrometallurgical processes. React. Funct. Polym. 1998, 36, 149–165. [Google Scholar] [CrossRef]
- Kabay, N.; Arda, M.; Trochimczuk, A.; Streat, M. Removal of chromate by solvent impregnated resins (SIRs) stabilized by coating and chemical crosslinking. I. Batch-mode sorption studies. React. Funct. Polym. 2004, 59, 9–14. [Google Scholar] [CrossRef]
- Kabay, N.; Arda, M.; Trochimczuk, A.; Streat, M. Removal of chromate by solvent impregnated resins (SIRs) stabilized by coating and chemical crosslinking. II. Column-mode sorption/elution studies. React. Funct. Polym. 2004, 59, 15–22. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Xu, Z.; Liang, J.; Zhu, Z. Study on the extraction and separation of zinc, cobalt, and nickel using ionquest 801, cyanex 272, and their mixtures. Metals 2021, 11, 401. [Google Scholar] [CrossRef]
- Saleh, M.I.; Bari, M.F.; Saad, B. Solvent extraction of lanthanum(III) from acidic nitrate-acetato medium by Cyanex 272 in toluene. Hydrometallurgy 2002, 63, 75–84. [Google Scholar] [CrossRef]
- Dogmane, S.D.; Singh, R.K.; Bajpai, D.D.; Mathur, J.N. Extraction of U ( VI ) by cyanex-272. J. Radioanal. Nucl. Chem. 2002, 253, 477–482. [Google Scholar] [CrossRef]
- Chah, S.; Kim, J.S.; Yi, J. Separation of zinc ions from aqueous solutions using modified silica impregnated with CYANEX 272. Sep. Sci. Technol. 2002, 37, 701–716. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, G.; Li, D. EXTRACTION AND SEPARATION OF HEAVY RARE EARTH(III) WITH EXTRACTION RESIN CONTAINING DI(2,4,4-TRIMETHYL PENTYL) PHOSPHINIC ACID (CYANEX 272). Solvent Extr. Ion Exch. 1998, 16, 813–828. [Google Scholar] [CrossRef]
- Bokhove, J. Solvent Impregnated Resins for Selective Cyanopyridine Trace Recovery from Aqueous Streams. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2013. Volume 1, ISBN 9789461085009. [Google Scholar]
- Hubicki, Z.; Wójcik, G. Studies of removal of platinum(IV) ion microquantities from the model solutions of aluminium, copper, iron, nickel and zinc chloride macroquantities on the anion exchanger Duolite S 37. J. Hazard. Mater. 2006, 136, 770–775. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Saoudi, F.; Chiha, M.; Naffrechoux, E. Sorption of malachite green by a novel sorbent, dead leaves of plane tree: Equilibrium and kinetic modeling. Chem. Eng. J. 2008, 143, 73–84. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Gurung, M.; Adhikari, B.B.; Gao, X.; Alam, S.; Inoue, K. Sustainability in the metallurgical industry: Chemically modified cellulose for selective biosorption of gold from mixtures of base metals in chloride media. Ind. Eng. Chem. Res. 2014, 53, 8565–8576. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Liang, K.H. Adsorption of gold(III) onto chitosan and N-carboxymethyl chitosan: Equilibrium studies. Ind. Chem. Eng. Res. 1999, 38, 1411–1414. [Google Scholar] [CrossRef]
- Medina, D.; Anderson, C.G. A review of the cyanidation treatment of copper-gold ores and concentrates. Metals 2020, 10, 897. [Google Scholar] [CrossRef]




| Pseudo Second Order | Ion | HCl Concentration [M] | Kinetic Parameters | ||||
|---|---|---|---|---|---|---|---|
| R2 | q2 (mg·g−1) | k2 (g·(mg·min)−1) | h (mg·(g·min)−1) | SD (Standard Deviation) | |||
| Before impregnation | |||||||
| Type 1 | Au(III) | 0.001 | 0.99980 | 9.96 | 0.0512 | 5.08 | 0.18 |
| 0.1 | 0.99986 | 9.18 | 0.3072 | 25.88 | 0.16 | ||
| 1 | 0.99995 | 9.28 | 0.3569 | 30.71 | 0.09 | ||
| 3 | 0.99998 | 9.46 | 0.2378 | 21.29 | 0.06 | ||
| 6 | 0.99997 | 9.32 | 0.1457 | 12.65 | 0.08 | ||
| After impregnation | |||||||
| Type 1 | Au(III) | 0.001 | 0.99974 | 9.96 | 0.0088 | 0.88 | 0.77 |
| 0.1 | 0.99970 | 8.59 | 0.0271 | 2.00 | 0.25 | ||
| 1 | 0.99970 | 9.12 | 0.0766 | 6.36 | 0.18 | ||
| 3 | 0.99989 | 9.47 | 0.0488 | 4.37 | 0.14 | ||
| 6 | 0.99980 | 9.49 | 0.0324 | 2.92 | 0.18 | ||
| Sorbent | HCl Concentration [M] | Langmuir Isotherm Parameters | Freundlich Isotherm Parameters | ||||
|---|---|---|---|---|---|---|---|
| q0, mg·g−1 | b, dm3·mg−1 | R2 | n | KF, dm3·g−1 | R2 | ||
| Purolite MN 202 before impregnation | 0.001 | 162.97 | 0.0588 | 0.922 | 1.338 | 9.997 | 0.935 |
| 0.1 | 140.25 | 0.0429 | 0.992 | 1.451 | 7.437 | 0.961 | |
| 1 | 53.41 | 0.0152 | 0.996 | 2.355 | 3.725 | 0.978 | |
| 3 | 56.38 | 0.0228 | 0.997 | 2.528 | 5.116 | 0.970 | |
| 6 | 58.30 | 0.0145 | 0.997 | 2.214 | 3.524 | 0.975 | |
| Purolite MN 202 after impregnation | 0.001 | 259.45 | 0.0292 | 0.983 | 1.177 | 7.937 | 0.992 |
| 0.1 | 187.03 | 0.0296 | 0.999 | 1.290 | 6.501 | 0.990 | |
| 1 | 156.32 | 0.0051 | 0.995 | 1.304 | 1.444 | 0.995 | |
| 3 | 167.55 | 0.0057 | 0.997 | 1.284 | 1.609 | 0.994 | |
| 6 | 172.33 | 0.0059 | 0.996 | 1.281 | 1.702 | 0.995 | |
| Metal Ions/Concentration, mg·dm−3 | |||||
|---|---|---|---|---|---|
| Au3+ | Ni2+ | Co2+ | Fe3+ | Cu2+ | Zn2+ |
| 16.53 | 349.6 | 0.45 | 7.4 | 57,400 | 42.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, G.; Górska-Parat, M.; Hubicki, Z.; Zinkowska, K. Selective Recovery of Gold from Electronic Waste by New Efficient Type of Sorbent. Materials 2023, 16, 924. https://doi.org/10.3390/ma16030924
Wójcik G, Górska-Parat M, Hubicki Z, Zinkowska K. Selective Recovery of Gold from Electronic Waste by New Efficient Type of Sorbent. Materials. 2023; 16(3):924. https://doi.org/10.3390/ma16030924
Chicago/Turabian StyleWójcik, Grzegorz, Magdalena Górska-Parat, Zbigniew Hubicki, and Karolina Zinkowska. 2023. "Selective Recovery of Gold from Electronic Waste by New Efficient Type of Sorbent" Materials 16, no. 3: 924. https://doi.org/10.3390/ma16030924
APA StyleWójcik, G., Górska-Parat, M., Hubicki, Z., & Zinkowska, K. (2023). Selective Recovery of Gold from Electronic Waste by New Efficient Type of Sorbent. Materials, 16(3), 924. https://doi.org/10.3390/ma16030924






