Carbonation Behavior of Mortar Made from Treated Recycled Aggregates: Influence of Diammonium Phosphate
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Tests
- Water absorption
- Water absorption was determined by using the innovative thermogravimetric balance halogen light (TBHL) technique, based on ASTM C138 for fine NA [51], which has demonstrated absorption values with less dispersion and optimizing resources such as energy, time and material. TBHL starts from the saturation state of the RFA to find the surface saturation dry state (SSD) via a thermogravimetric process using an XM 60-HR halogen light balance with the uniform and soft heating of the sample up to a maximum temperature of 85 °C. Real-time reproducible results are achieved by coupling to a ABSORPTION INNOVATION version 1.0 with a signal communication device (TTL to RS 234 level conversion) [51,52].
- Optical Microscopy (OM)
- Optical microscopy analysis was carried out in order to determine the morphological characteristics of the shape, texture and gradation of the RFA. Micrographs were taken with a Nikon stereoscope, reference Eclipse LV100, objectives 2× up to 11.5×.
- X-ray diffraction (XRD)
- A mineralogical composition via XRD analysis was carried out with the aim of identifying the phases formed from the DAP treatment in both the RFA and the mortars made from treated RFA. A PANalytical X “Pert PRO MPD reference instrument was used, in a range of 2θ between 15–36°, with a step of 0.02°. A copper anode with Kα1 = 1.5406 Å was used. Peak positions and relative intensities were compared with the X’Pert Hihg Score Plus license software database.
- Thermogravimetric analysis (TGA)
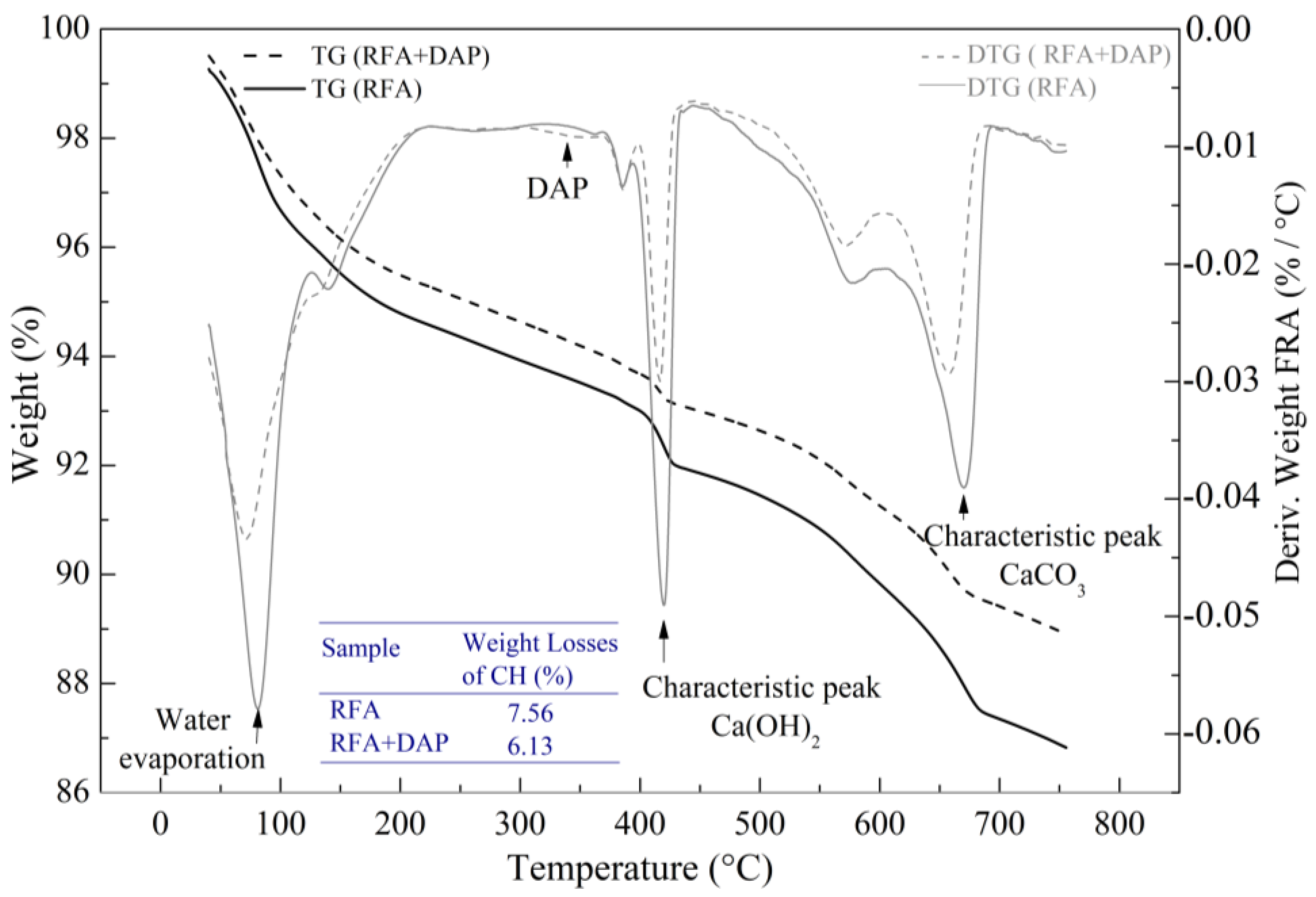
- TGA was used to characterize the thermal evolution of the dry precipitates identified with XRD in the case of RFA. The analysis was performed under a nitrogen atmosphere and continuous heating with a temperature range between ambient 30–380 °C at 10 °C/min and 380–800 °C at 5 °C/min to study the mass loss behavior of the precipitate. Peak positions and mass loss quantification was processed with the license-free software tool TRIOS V5 1.1.
- Scanning Electron Microscopy (SEM)
- The morphological characteristics and microstructure of the RFA powder products and mortars were characterized using a JEOL JSM 6490 LV high-vacuum scanning electron microscope for high-resolution images, using a secondary electron detector. The samples were fixed on a graphite tape and coated with gold (Au) by using the DENTON VACUUM Desk IV. An elemental analysis was performed using an X-ray Microprobe-EDX by INCA PentaFETx3 Oxford Instruments.
- Flow and pH of the mortar mix
- The behavior of the fresh mortar was evaluated using the Standard Test Method for Flow of Hydraulic Cement Mortar (ASTM C1437) and the pH value with a peachimeter to validate if the DAP has an effect on the pH value of the mix.
- Compressive strength
- The mechanical behavior of the mortars manufactured with the treated RFA was determined by using a Controls press, Model CT-0151/E. A measuring range between 0–150 KN and a loading rate of 1250 N/s was used.
- Accelerated carbonation
- The accelerated carbonation process was performed by using a continuous flow of CO2 (4%) and the gas flow rate (a mixture of pure CO2 and air) was controlled from 1.0 to 10 L/min. The exposure time was 28 days with a relative humidity and temperature of 60 ± 5% and 23 ± 5 °C, respectively.
- Carbonation depth
- According to RILEM guidelines, a pH change indicator was used to determine the depth of carbonation in the mortar by using a small amount of phenolphthalein at a 1% concentration, which was applied to the inside of the faces after cutting cubes perpendicular to the poured face [53]. Additionally, an image analysis was performed with the free ImageJ software version 1.44 to quantify the carbonation depth. A pH change indicator test was performed for comparative purposes, and to validate the results, XRD and SEM analysis were performed.
2.2. RFA Sourcing and Characterization
2.3. DAP Treatment
2.4. Mortar Mix Design
3. Results and Discussions
3.1. RFA Improvement with DAP Treatment
3.2. Durability Behavior of Mortars with Treated RFA
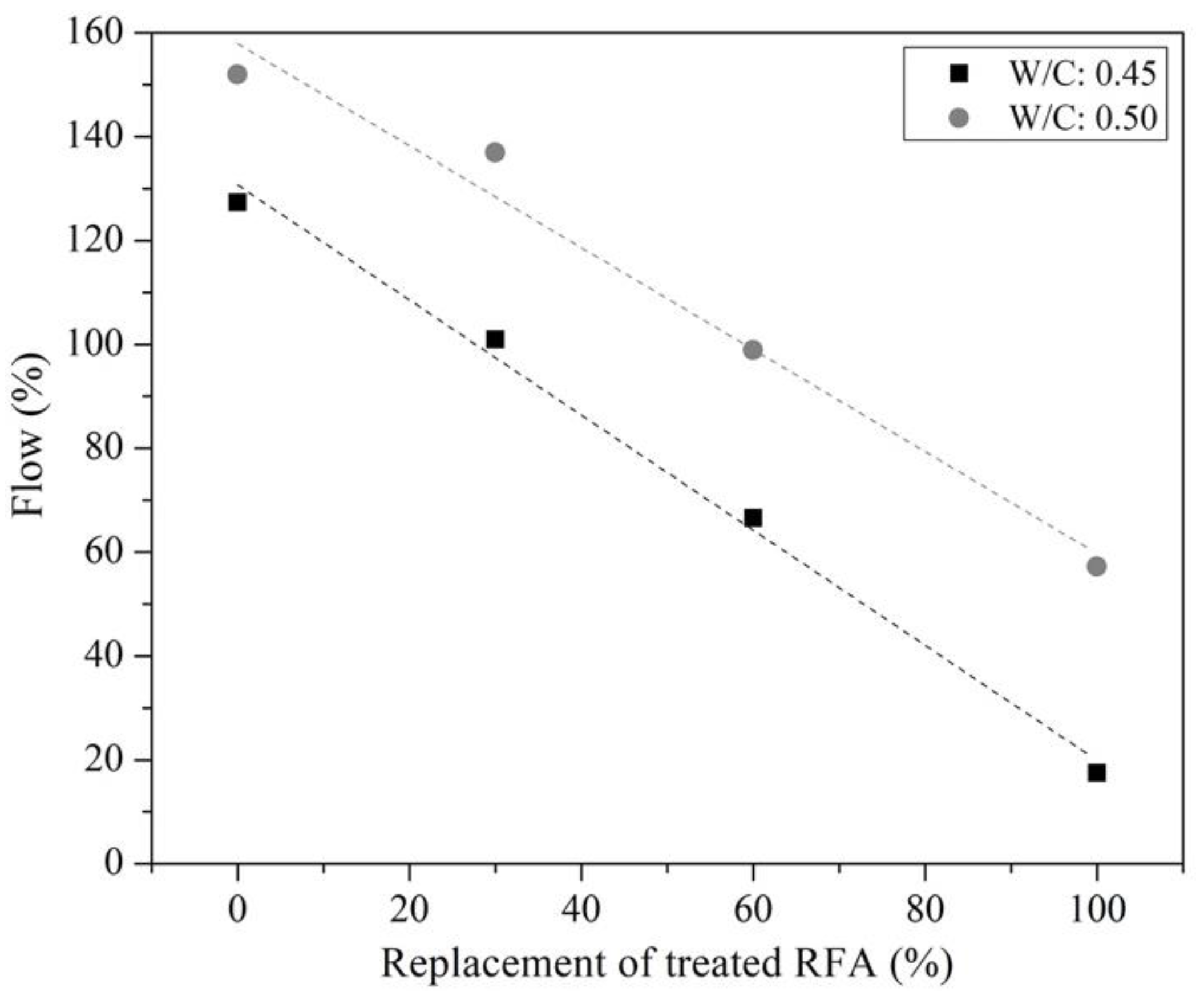
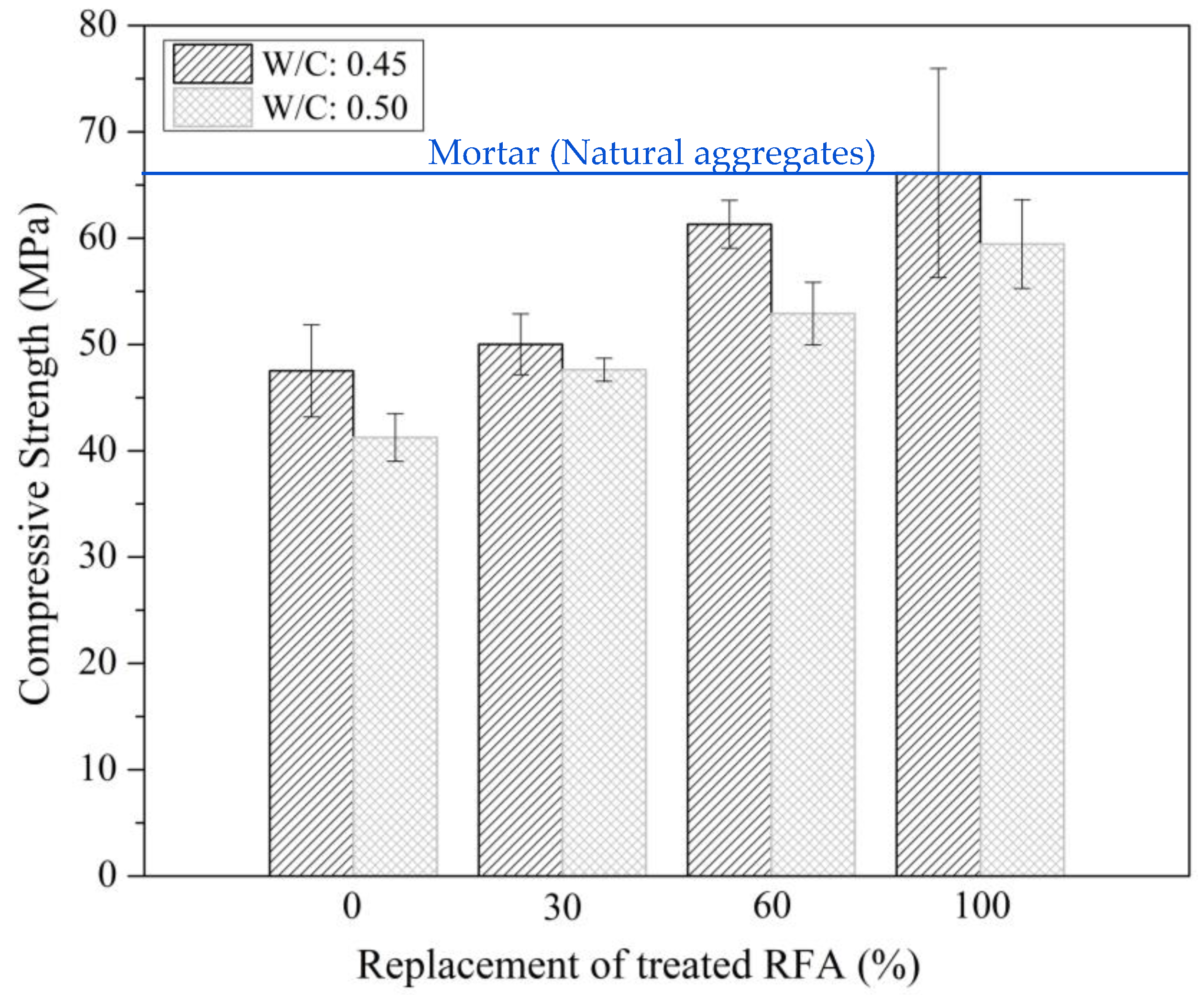
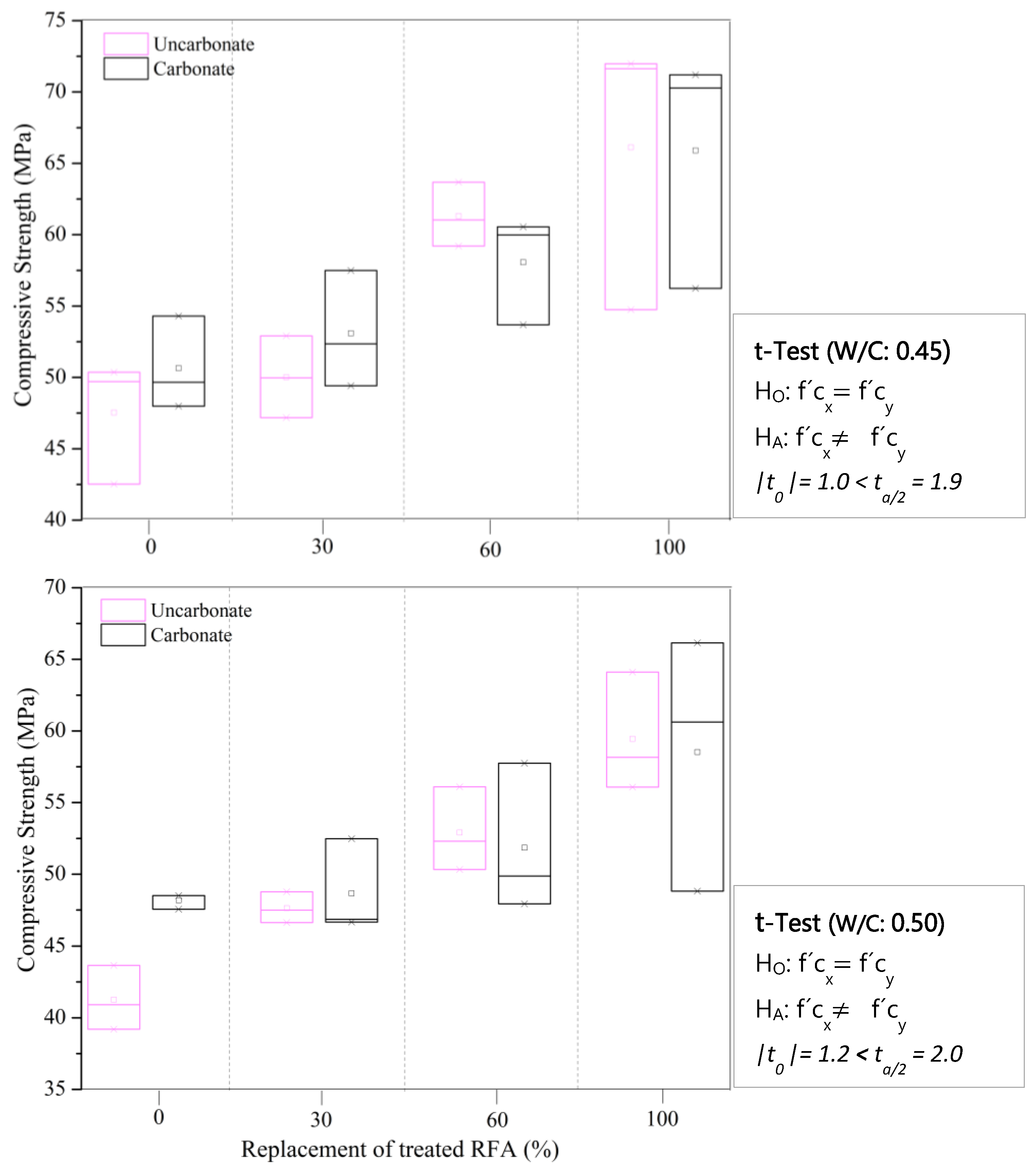
3.2.1. Flow, pH and Compressive Strength of Mortars
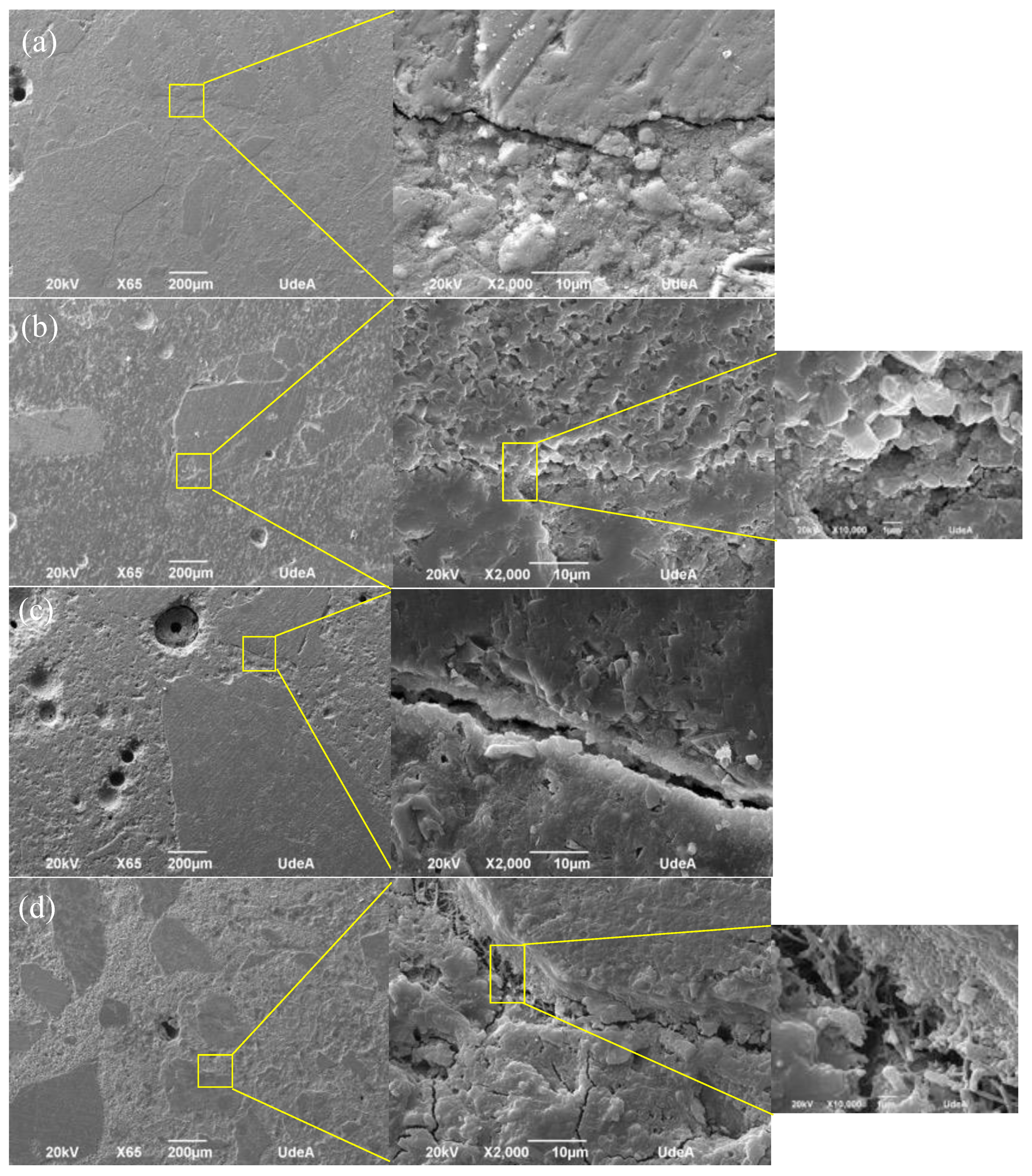
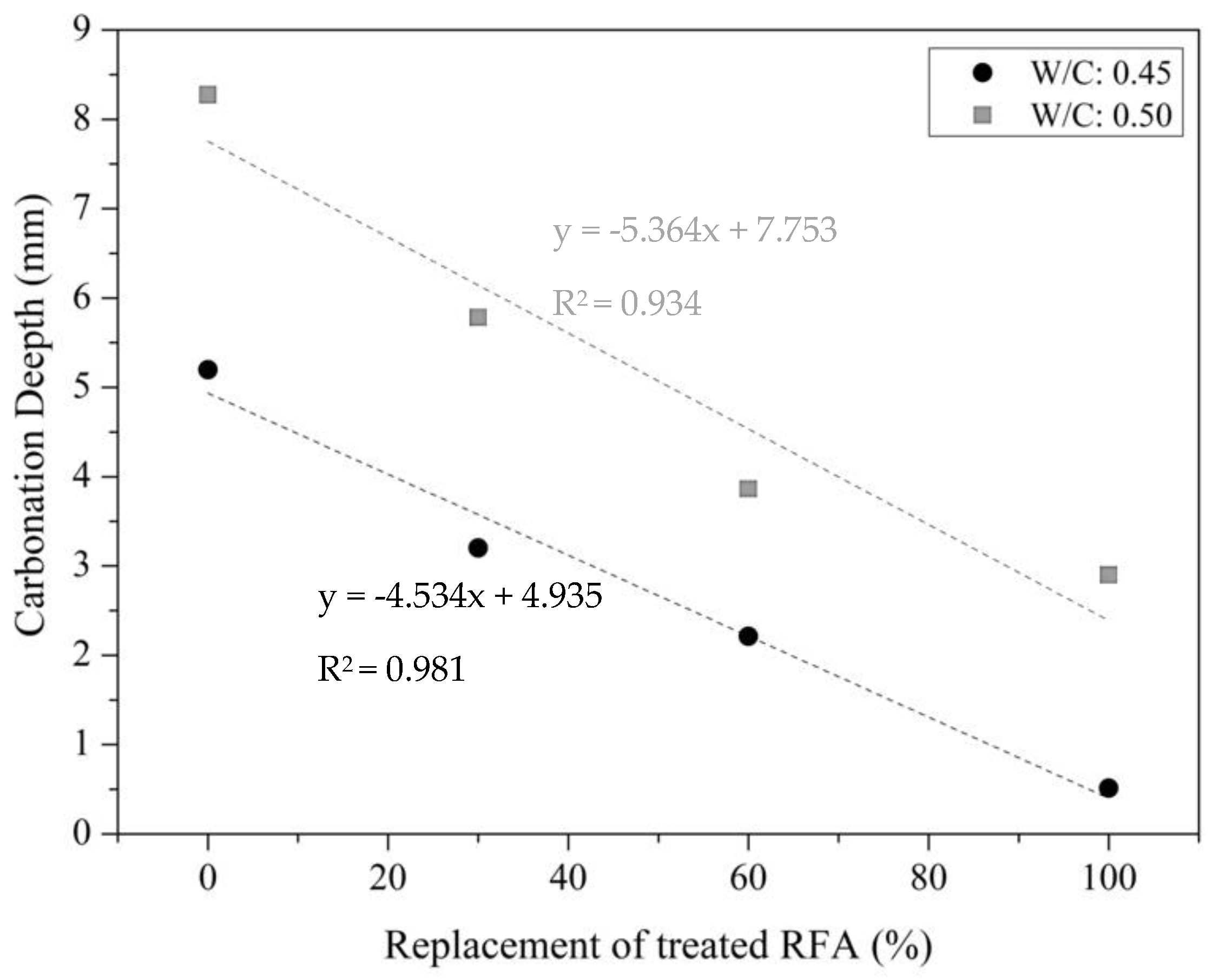
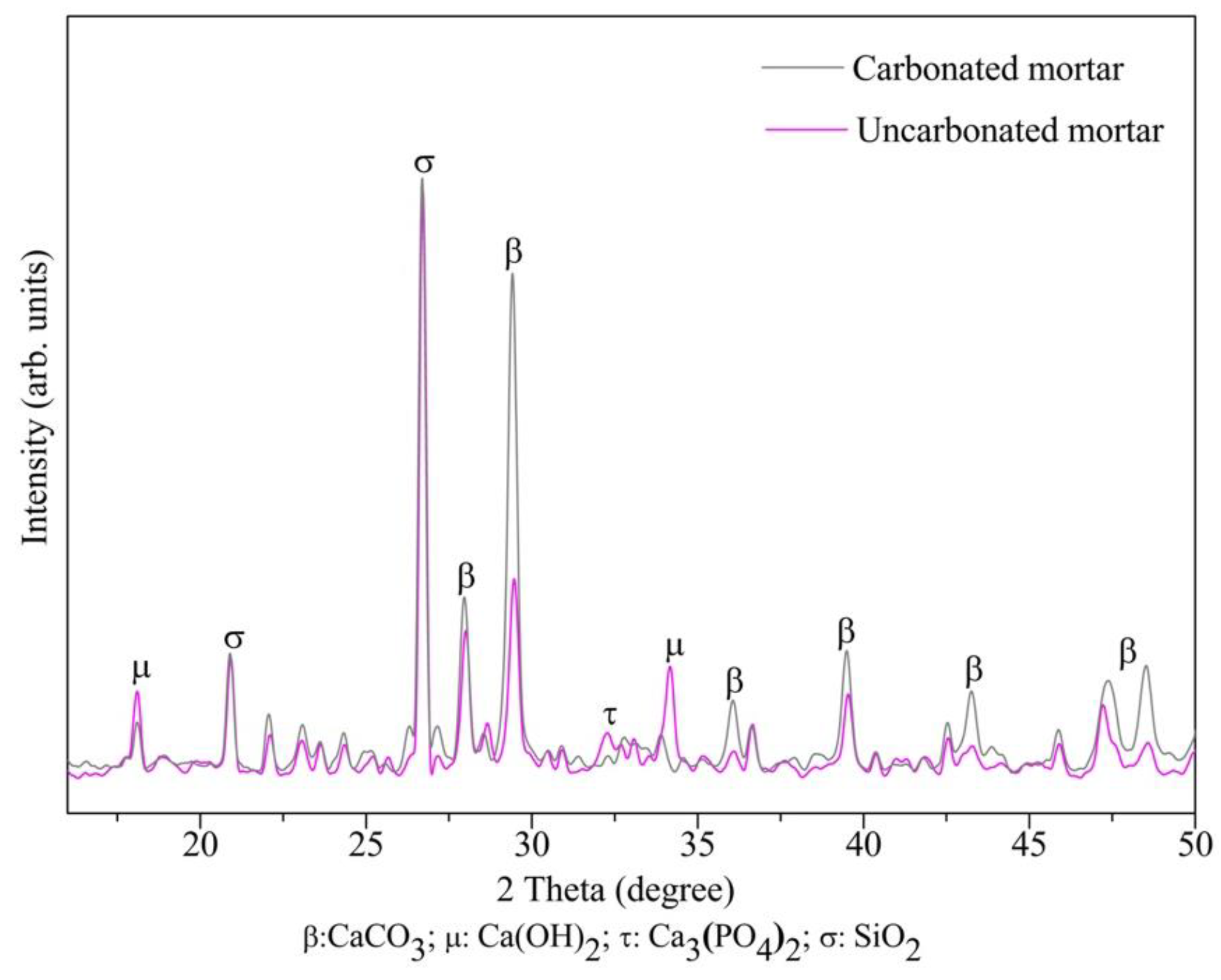
3.2.2. Carbonation Phenomenon of Mortar
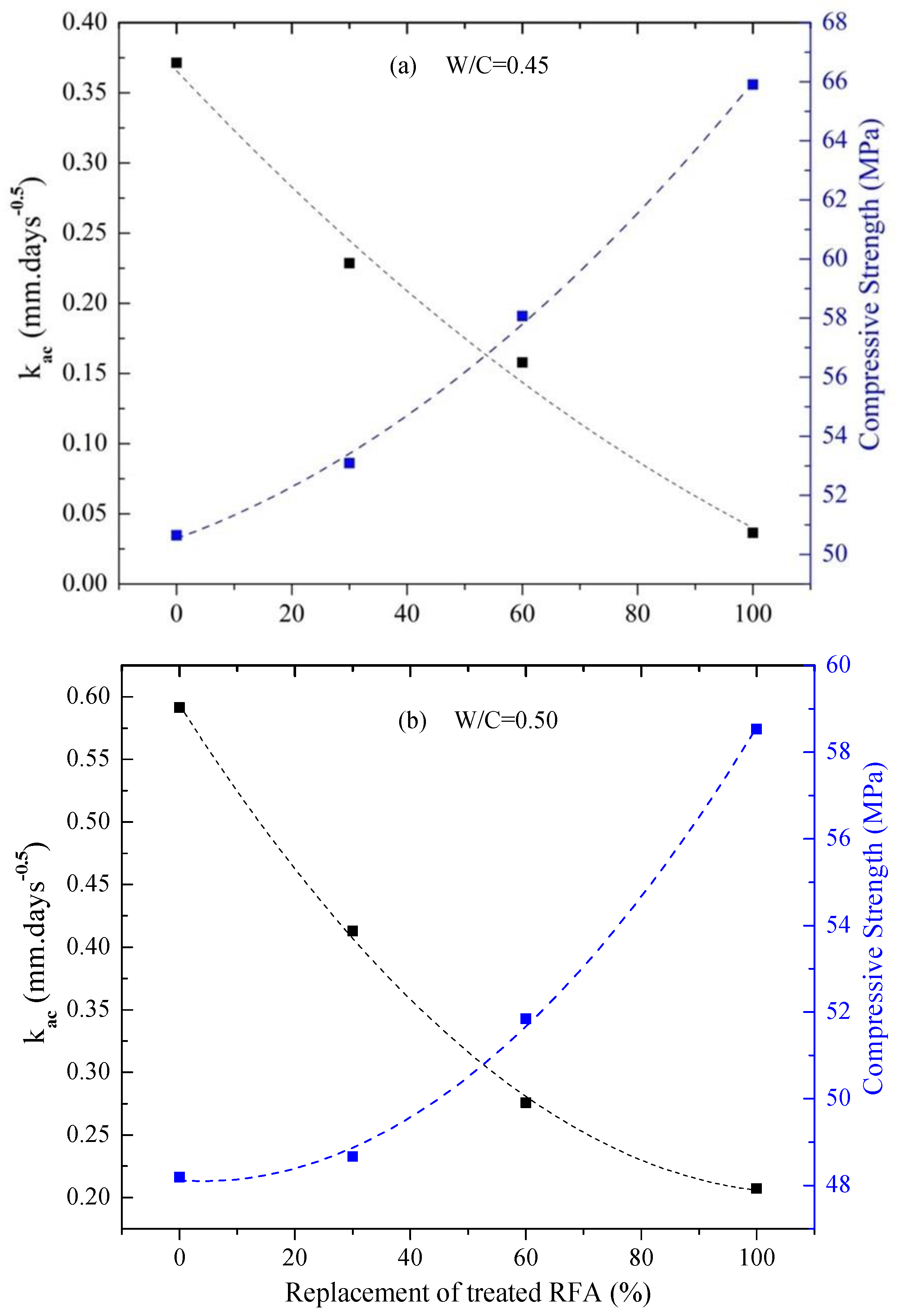
3.2.3. Correlation between Compressive Strength and Carbonation Phenomenon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monteiro, P.J.M.; Miller, S.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Ponnada, M.; Kameswari, P. Construction and Demolition Waste Management—A Review. Int. J. Adv. Sci. Technol. 2015, 84, 19–46. [Google Scholar] [CrossRef]
- Kabirifar, K.; Mojtahedi, M.; Wang, C.; Tam, V.W.Y. Construction and demolition waste management contributing factors coupled with reduce, reuse, and recycle strategies for effective waste management: A review. J. Clean. Prod. 2020, 263, 121265. [Google Scholar] [CrossRef]
- Wang, R.; Yu, N.; Li, Y. Methods for improving the microstructure of recycled concrete aggregate: A review. Constr. Build. Mater. 2020, 242, 118164. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Impacts of booming concrete production on water resources worldwide. Nat. Sustain. 2018, 1, 69–76. [Google Scholar] [CrossRef]
- Ray, S.; Haque, M.; Sakib, N.; Mita, A.F.; Rahman, M.M.; Tanmoy, B.B. Use of ceramic wastes as aggregates in concrete production: A review. J. Build. Eng. 2021, 43, 102567. [Google Scholar] [CrossRef]
- Behera, M.; Bhattacharyya, S.; Minocha, A.; Deoliya, R.; Maiti, S. Recycled aggregate from C & D waste & its use in concrete–A breakthrough towards sustainability in construction sector: A review. Constr. Build. Mater. 2014, 68, 501–516. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- de Brito Prado Vieira, L.; de Figueiredo, A.D.; John, V.M. Evaluation of the use of crushed returned concrete as recycled aggregate in ready-mix concrete plant. J. Build. Eng. 2020, 31, 101408. [Google Scholar] [CrossRef]
- Le, H.-B.; Bui, Q.-B. Recycled aggregate concretes–A state-of-the-art from the microstructure to the structural performance. Constr. Build. Mater. 2020, 257, 119522. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.C.; Zhang, H.; Wang, Y. Durability of recycled aggregate concrete–A review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Silva, R.V.; De Brito, J.; Dhir, R.K. Properties and composition of recycled aggregates from construction and demolition waste suitable for concrete production. Constr. Build. Mater. 2014, 65, 201–217. [Google Scholar] [CrossRef]
- Kim, J. Influence of quality of recycled aggregates on the mechanical properties of recycled aggregate concretes: An overview. Constr. Build. Mater. 2022, 328, 127071. [Google Scholar] [CrossRef]
- Silva, R.; de Brito, J.; Dhir, R. The influence of the use of recycled aggregates on the compressive strength of concrete: A review. Eur. J. Environ. Civ. Eng. 2014, 19, 825–849. [Google Scholar] [CrossRef]
- Singh, R.; Nayak, D.; Pandey, A.; Kumar, R.; Kumar, V. Effects of recycled fine aggregates on properties of concrete containing natural or recycled coarse aggregates: A comparative study. J. Build. Eng. 2021, 45, 103442. [Google Scholar] [CrossRef]
- Cano, D.M.G.; Gómez, D.P.; Escobar, S.R.; Jaramillo, Y.P.A.; Correa, R.A.B.; Botero, J.C.O. Moisture correction in mixtures using innovative thermogravimetric technique to determine water absorption in different Colombian fine aggregates. Rev. EIA 2023, 20, 1–19. [Google Scholar]
- Yunusa, M.; Zhang, X.; Cui, P.; Tian, X. Durability of Recycled Concrete Aggregates Prepared with Mechanochemical and Thermal Treatment. Materials 2022, 15, 5792. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Minocha, A.K. Studies on thermo-chemical treatment of recycled concrete fine aggregates for use in concrete. J. Mater. Cycles Waste Manag. 2017, 20, 469–480. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Wan, H.; Cao, B. Rehydration reactivity of recycled mortar from concrete waste experienced to thermal treatment. Constr. Build. Mater. 2008, 22, 1723–1729. [Google Scholar] [CrossRef]
- Pandurangan, K.; Dayanithy, A.; Prakash, S.O. Influence of treatment methods on the bond strength of recycled aggregate concrete. Constr. Build. Mater. 2016, 120, 212–221. [Google Scholar] [CrossRef]
- Ogawa, H.; Nawa, T. Improving the Quality of Recycled Fine Aggregate by Selective Removal of Brittle Defects. J. Adv. Concr. Technol. 2012, 10, 395–410. [Google Scholar] [CrossRef]
- Purushothaman, R.; Amirthavalli, R.R.; Karan, L. Influence of Treatment Methods on the Strength and Performance Characteristics of Recycled Aggregate Concrete. J. Mater. Civ. Eng. 2015, 27, 04014168. [Google Scholar] [CrossRef]
- Katz, A. Treatments for the Improvement of Recycled Aggregate. J. Mater. Civ. Eng. 2004, 16, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Tam, V.W.; Soomro, M.; Evangelista, A.C.J. Quality improvement of recycled concrete aggregate by removal of residual mortar: A comprehensive review of approaches adopted. Constr. Build. Mater. 2021, 288, 123066. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M.; Le, K.N. Removal of cement mortar remains from recycled aggregate using pre-soaking approaches. Resour. Conserv. Recycl. 2007, 50, 82–101. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Kim, B.; Kim, K.-S.; Kim, J.-M. Quality improvement of recycled aggregates using the acid treatment method and the strength characteristics of the resulting mortar. J. Mater. Cycles Waste Manag. 2016, 19, 968–976. [Google Scholar] [CrossRef]
- Kim, Y.; Hanif, A.; Kazmi, S.M.; Munir, M.J.; Park, C. Properties enhancement of recycled aggregate concrete through pretreatment of coarse aggregates–Comparative assessment of assorted techniques. J. Clean. Prod. 2018, 191, 339–349. [Google Scholar] [CrossRef]
- Kumar, G.S.; Saini, P.K.; Karade, S.R.; Minocha, A.K. Chemico-thermal treatment for quality enhancement of recycled concrete fine aggregates. J. Mater. Cycles Waste Manag. 2019, 21, 1197–1210. [Google Scholar] [CrossRef]
- Kou, S.-C.; Zhan, B.-J.; Poon, C.-S. Use of a CO2 curing step to improve the properties of concrete prepared with recycled aggregates. Cem. Concr. Compos. 2014, 45, 22–28. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.-S.; Xie, Z. Performance Enhancement of Recycled Concrete Aggregates through Carbonation. J. Mater. Civ. Eng. 2015, 27, 04015029. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Zeng, W.; Poon, C.S. Carbonation treatment of recycled concrete aggregate: Effect on transport properties and steel corrosion of recycled aggregate concrete. Cem. Concr. Compos. 2019, 104, 103360. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Cao, Z.; Guo, M.; Zheng, J. Effect of carbonated coarse recycled concrete aggregate on the properties and microstructure of recycled concrete. J. Clean. Prod. 2019, 233, 421–428. [Google Scholar] [CrossRef]
- Chinzorigt, G.; Lim, M.K.; Yu, M.; Lee, H.; Enkbold, O.; Choi, D. Strength, shrinkage and creep and durability aspects of concrete including CO2 treated recycled fine aggregate. Cem. Concr. Res. 2020, 136, 106062. [Google Scholar] [CrossRef]
- Russo, N.; Lollini, F. Effect of carbonated recycled coarse aggregates on the mechanical and durability properties of concrete. J. Build. Eng. 2022, 51, 104290. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Arakha, M.; Sarkar, P.; Jha, S. Enhancement of properties of recycled coarse aggregate concrete using bacteria. Int. J. Smart Nano Mater. 2016, 7, 22–38. [Google Scholar] [CrossRef]
- Wu, C.-R.; Zhu, Y.-G.; Zhang, X.-T.; Kou, S.-C. Improving the properties of recycled concrete aggregate with bio-deposition approach. Cem. Concr. Compos. 2018, 94, 248–254. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Xu, P.; Wang, X.; Kou, S. Properties of Concrete Prepared with Recycled Aggregates Treated by Bio-Deposition Adding Oxygen Release Compound. Materials 2019, 12, 2147. [Google Scholar] [CrossRef] [Green Version]
- Bedoya, M.A.; Tobón, J.I. Incidence of recycled aggregates and ternary cements on the compressive strength and durability of ecological mortars. Case Stud. Constr. Mater. 2022, 17, e01192. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Ye, Z.-M.; Vernerey, F.; Xi, Y. Development of Processing Methods to Improve Strength of Concrete with 100% Recycled Coarse Aggregate. J. Mater. Civ. Eng. 2015, 27, 04014163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhao, Y.; Meng, T.; Shah, S.P. Surface Treatment on Recycled Coarse Aggregates with Nanomaterials. J. Mater. Civ. Eng. 2016, 28, 04015094. [Google Scholar] [CrossRef]
- Sasanipour, H.; Aslani, F. Durability assessment of concrete containing surface pretreated coarse recycled concrete aggregates. Constr. Build. Mater. 2020, 264, 120203. [Google Scholar] [CrossRef]
- Shaban, W.M.; Elbaz, K.; Yang, J.; Thomas, B.S.; Shen, X.; Li, L.; Du, Y.; Xie, J.; Li, L. Effect of pozzolan slurries on recycled aggregate concrete: Mechanical and durability performance. Constr. Build. Mater. 2021, 276, 121940. [Google Scholar] [CrossRef]
- Spaeth, V.; Tegguer, A.D. Improvement of recycled concrete aggregate properties by polymer treatments. Int. J. Sustain. Built Environ. 2013, 2, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, J.; Qian, X.; Fang, Y.; Chen, P.; Tuinukuafe, A. Tea stain-inspired treatment for fine recycled concrete aggregates. Constr. Build. Mater. 2020, 262, 120027. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Ren, J.; Han, N.; Xing, F. A novel treatment method for recycled aggregate and the mechanical properties of recycled aggregate concrete. J. Mater. Res. Technol. 2021, 10, 1389–1401. [Google Scholar] [CrossRef]
- Gómez-Cano, D.; Arias, Y.; Bernal-Correa, R.; Tobón, J.I. Effect of enhancement treatments applied to recycled concrete aggregates on concrete durability: A review. Mater. Construcción. 2023, 73. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Xu, Y.; Cui, L.; Qian, X.; Chen, P.; Fang, Y. Consolidating recycled concrete aggregates using phosphate solution. Constr. Build. Mater. 2019, 200, 703–712. [Google Scholar] [CrossRef]
- Thiery, M.; Dangla, P.; Belin, P.; Habert, G.; Roussel, N. Carbonation kinetics of a bed of recycled concrete aggregates: A laboratory study on model materials. Cem. Concr. Res. 2013, 46, 50–65. [Google Scholar] [CrossRef]
- You, X.; Hu, X.; He, P.; Liu, J.; Shi, C. A review on the modelling of carbonation of hardened and fresh cement-based materials. Cem. Concr. Compos. 2021, 125, 104315. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Durability of recycled aggregate concrete prepared with carbonated recycled concrete aggregates. Cem. Concr. Compos. 2017, 84, 214–221. [Google Scholar] [CrossRef]
- Arias, Y.P.; Payá, J.; Ochoa, J.C. Halogen light thermogravimetric technique for determining the retained water in fine aggregates used for concrete mixing design. J. Therm. Anal. Calorim. 2015, 123, 127–134. [Google Scholar] [CrossRef]
- Gómez-Cano, D.; Ochoa-Botero, J.C.; Correa, R.B.; Arias, Y.P. Mechanical Behavior of Recycled Mortars Manufactured from Moisture Correction Using the Halogen Light Thermogravimetric Balance as an Alternative to the Traditional ASTM C 128 Method. Int. J. Struct. Constr. Eng. 2022, 16, 164–168. [Google Scholar]
- RILEM. CPC-18 measurement of hardened concrete carbonation depth. Mater Struct 1988, 21, 453–455. [Google Scholar] [CrossRef]
- Leó, B.; Jansen, J.A. Thin Calcium Phosphate Coatings for Medical Implants; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–326. [Google Scholar]
- Ramachandran, V.S.; Paroli, R.; Beaudoin, J.; Delgado, A.H. 1-Thermoanalytical Techniques. In Handbook of Thermal Analysis of Construction Materials; Ramachandran, V., Paroli, R., Beaudoin, J., Delgado, A.H., Eds.; William Andrew Publishing: Norwich, NY, USA, 2002; pp. 1–34. [Google Scholar]
- Cepuritis, R.; Jacobsen, S.; Smeplass, S.; Mørtsell, E.; Wigum, B.J.; Ng, S. Influence of crushed aggregate fines with micro-proportioned particle size distributions on rheology of cement paste. Cem. Concr. Compos. 2017, 80, 64–79. [Google Scholar] [CrossRef]
- Yilmaz, A.; Dehri, I.; Erbil, M. Effects of ammonium chloride salt added to mixing water on concrete and reinforced concrete subject to atmospheric corrosion. Cem. Concr. Res. 2002, 32, 91–95. [Google Scholar] [CrossRef]
- Gougar, M.L.D.; Scheetz, B.; Roy, D.M. Ettringite and C-S-H Portland cement phases for waste ion immobilization: A review. Waste Manag. 1996, 16, 295–303. [Google Scholar] [CrossRef]
- Chen, C.; Liu, R.; Zhu, P.; Liu, H.; Wang, X. Carbonization Durability of Two Generations of Recycled Coarse Aggregate Concrete with Effect of Chloride Ion Corrosion. Sustainability 2020, 12, 10544. [Google Scholar] [CrossRef]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Carbonation of concrete made with high amount of fly ash and recycled concrete aggregates for utilization of CO2. J. CO2 Util. 2018, 29, 12–19. [Google Scholar] [CrossRef]
- de Brito, J.; Ferreira, J.; Pacheco, J.; Soares, D.; Guerreiro, M. Structural, material, mechanical and durability properties and behaviour of recycled aggregates concrete. J. Build. Eng. 2016, 6, 6792. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.; Wang, L.; Yao, Y. Numerical investigations on the influence of ITZ and its width on the carbonation depth of concrete with stress damage. Cem. Concr. Compos. 2022, 132, 104630. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Criado, M.; Fajardo, S.; La Iglesia, A.; Bastidas, J.M. Corrosion inhibition mechanism of phosphates for early-age reinforced mortar in the presence of chlorides. Cem. Concr. Compos. 2015, 61, 637–643. [Google Scholar] [CrossRef]
- Bastidas, J.M.; La Iglesia, V.; Criado, M.; Fajardo, S.; La Iglesia, A. A prediction study of hydroxyapatite entrapment ability in concrete. Constr. Build. Mater. 2010, 24, 2646–2649. [Google Scholar] [CrossRef]
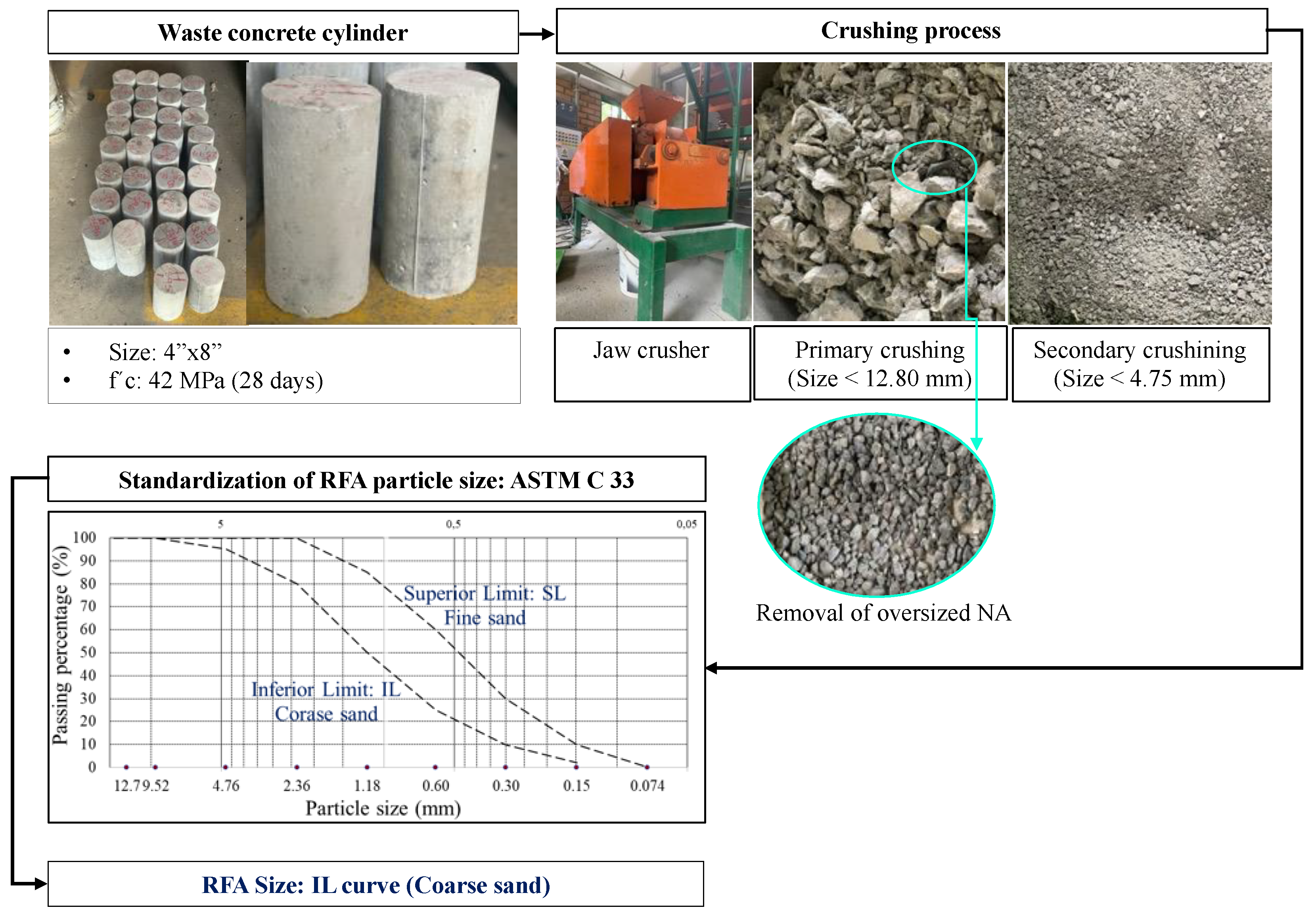



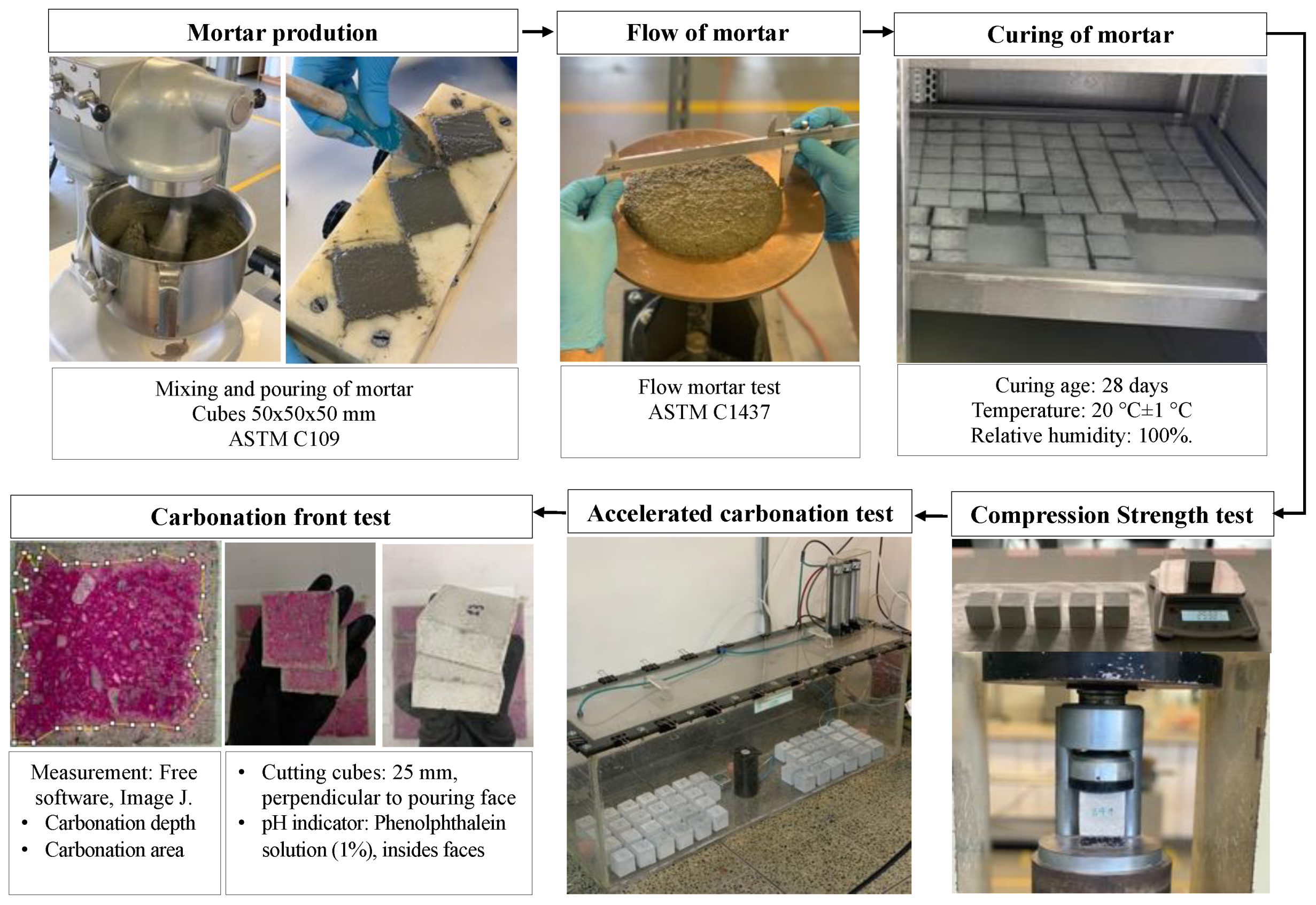


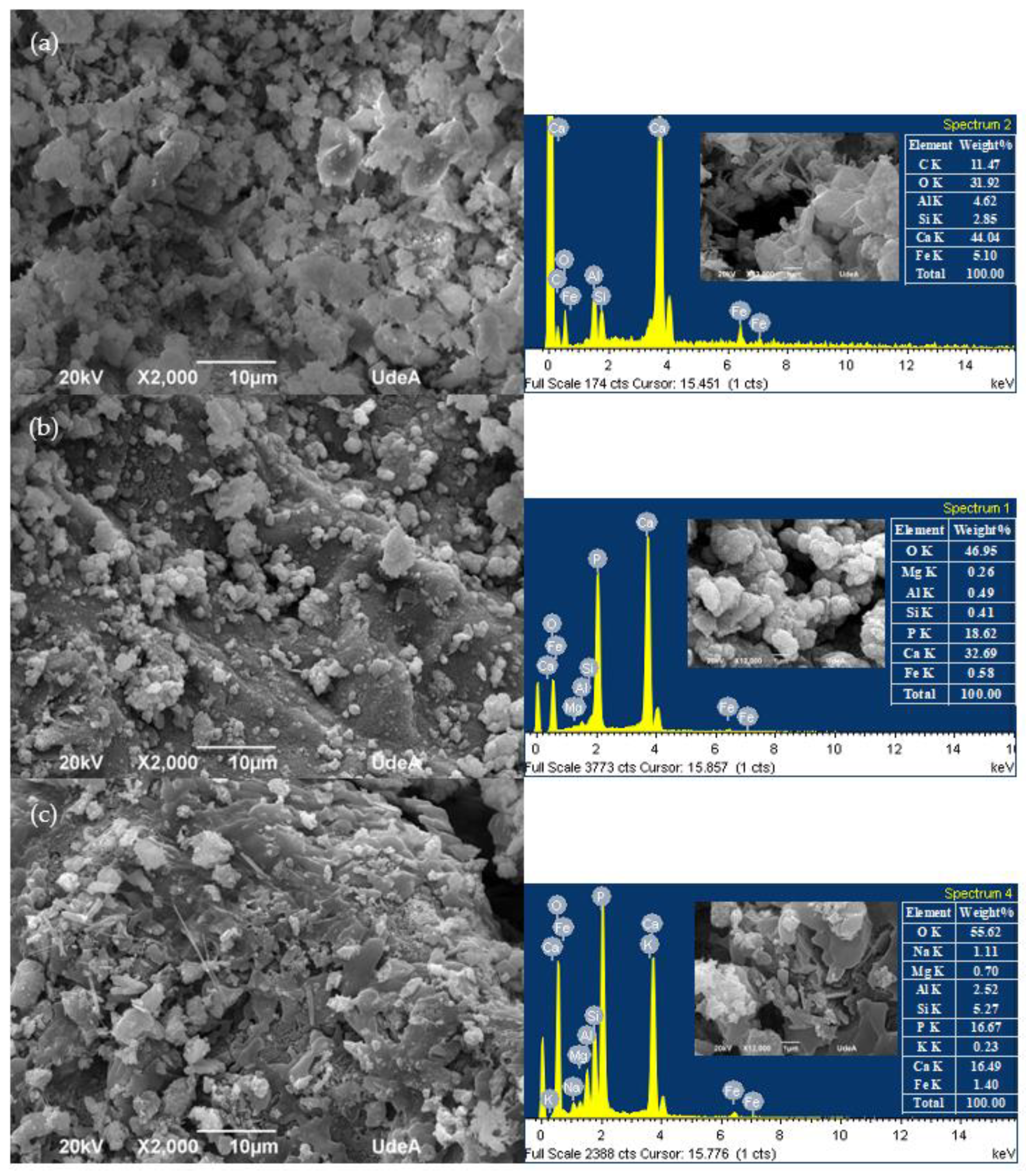


| Property | RFA | NA | Standard |
|---|---|---|---|
| Fineness | 3.10 | 2.80 | ASTM C 33 |
| Bulk density (kg/m3) | 1225 | 1615 | ASTM C 128 and TBHL |
| Water absorption (%) | 4.78 | 1.25 |
| Replacement of Treated RFA (%) | Water Absorption (TBHL Test) | |
|---|---|---|
| 0% | 0%-Treated RFA + 100%-Untreated RFA | 4.78 |
| 30% | 30%-Treated RFA + 70%-Untreated RFA | 4.32 |
| 60% | 60%-Treated RFA + 40%-Untreated RFA | 3.86 |
| 100% | 100%-Treated RFA + 0%-Untreated RFA | 3.24 |
| Mortar Type | w/c | Mix Proportions | |||||
|---|---|---|---|---|---|---|---|
| Cement (g) | Treated RFA (g) | Untreated RFA (g) | Water (g) | Total Water (g) | Additional Water | ||
| 0%/0.45 | 0.45 | 600 | 0 | 1200 | 270 | 327.29 | 18% |
| 0%/0.50 | 0.50 | 0 | 1200 | 300 | 357.29 | 16% | |
| 30%/0.45 | 0.45 | 360 | 840 | 270 | 321.78 | 16% | |
| 30%/0.50 | 0.50 | 360 | 840 | 300 | 351.78 | 15% | |
| 60%/0.45 | 0.45 | 720 | 480 | 270 | 316.27 | 15% | |
| 60%/0.50 | 0.50 | 720 | 480 | 300 | 346.27 | 13% | |
| 100%/0.45 | 0.45 | 1200 | 0 | 270 | 308.92 | 13% | |
| 100%/0.50 | 0.50 | 1200 | 0 | 300 | 338.92 | 11% | |
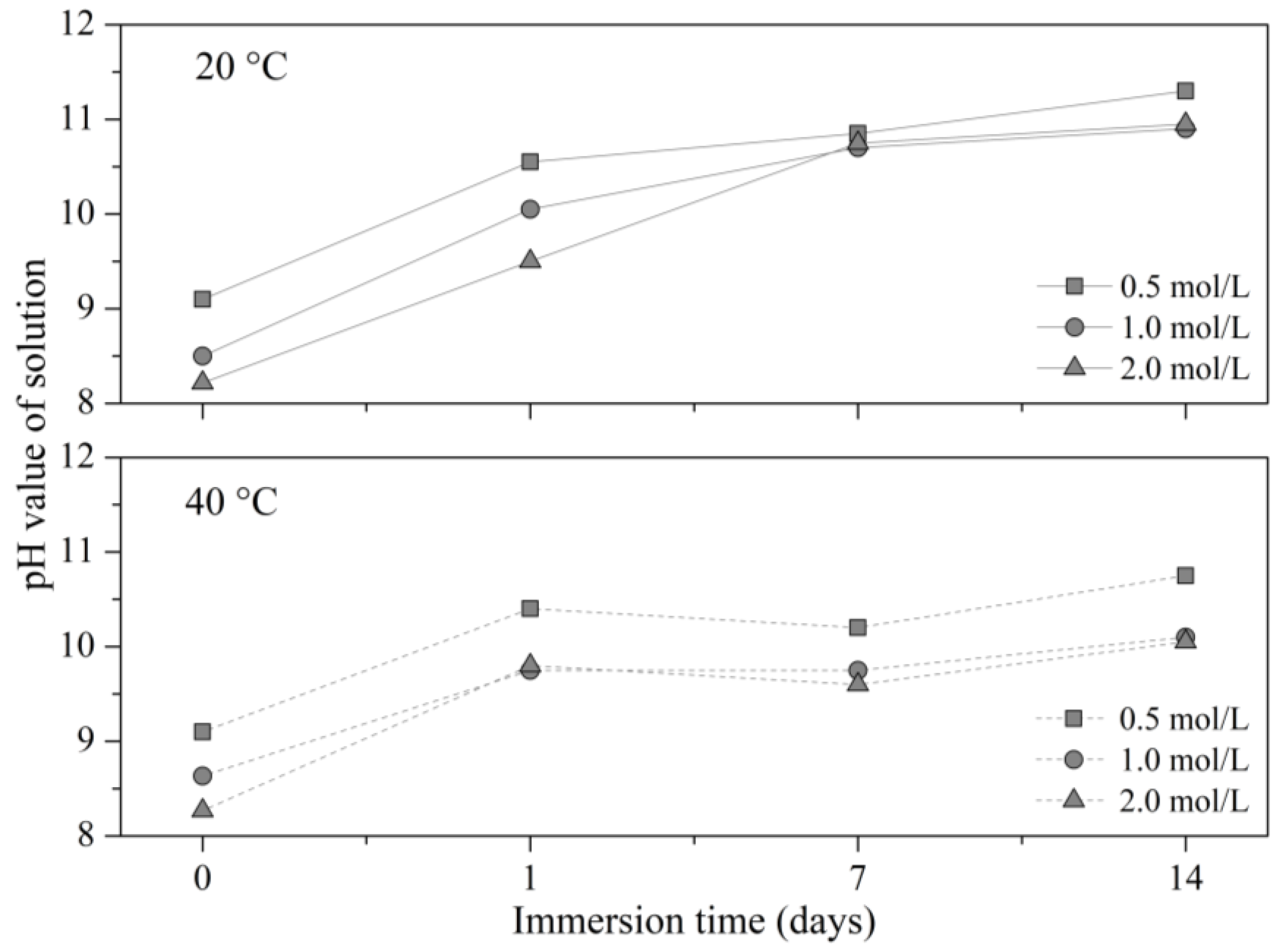
| Immersion Time (Days) | Temperature (°C) | 0.5 mol/L | 1 mol/L | 2 mol/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Value | σ | Reduction | Value | σ | Reduction | Value | σ | Reduction | ||
| 1 | 20 | 3.73 | 0.06 | 21.9% | 3.97 | 0.17 | 16.9% | 4.05 | 0.11 | 15.1% |
| 7 | 3.24 | 0.15 | 32.1% | 3.28 | 0.03 | 31.3% | 3.71 | 0.06 | 22.3% | |
| 14 | 3.24 | 0.08 | 32.1% | 4.03 | 0.04 | 15.6% | 4.21 | 0.05 | 11.7% | |
| 1 | 40 | 3.94 | 0.05 | 17.4% | 4.06 | 0.18 | 15.0% | 4.03 | 0.12 | 15.5% |
| 7 | 3.44 | 0.00 | 28.0% | 3.39 | 0.00 | 28.9% | 3.82 | 0.08 | 20.0% | |
| 14 | 3.51 | 0.02 | 26.5% | 3.96 | 0.16 | 17.0% | 4.40 | 0.11 | 7.9% | |
| Replacement of Treated RFA | pH of Mix | |||
|---|---|---|---|---|
| w/c: 0.45 | w/c: 0.50 | |||
| 0% | 12.00 | 0.23 | 12.10 | 0.05 |
| 30% | 11.79 | 0.24 | 11.75 | 0.15 |
| 60% | 11.38 | 0.04 | 11.50 | 0.16 |
| 100% | 11.37 | 0.02 | 11.26 | 0.17 |
| Treated RFA (%) | t (Years) | |
|---|---|---|
| W/C = 0.50 | W/C = 0.45 | |
| 0 | 13 | 32 |
| 30 | 26 | 84 |
| 60 | 58 | 176 |
| 100 | 102 | 438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Cano, D.; Arias-Jaramillo, Y.P.; Bernal-Correa, R.; Tobón, J.I. Carbonation Behavior of Mortar Made from Treated Recycled Aggregates: Influence of Diammonium Phosphate. Materials 2023, 16, 980. https://doi.org/10.3390/ma16030980
Gómez-Cano D, Arias-Jaramillo YP, Bernal-Correa R, Tobón JI. Carbonation Behavior of Mortar Made from Treated Recycled Aggregates: Influence of Diammonium Phosphate. Materials. 2023; 16(3):980. https://doi.org/10.3390/ma16030980
Chicago/Turabian StyleGómez-Cano, Diana, Yhan P. Arias-Jaramillo, Roberto Bernal-Correa, and Jorge I. Tobón. 2023. "Carbonation Behavior of Mortar Made from Treated Recycled Aggregates: Influence of Diammonium Phosphate" Materials 16, no. 3: 980. https://doi.org/10.3390/ma16030980
APA StyleGómez-Cano, D., Arias-Jaramillo, Y. P., Bernal-Correa, R., & Tobón, J. I. (2023). Carbonation Behavior of Mortar Made from Treated Recycled Aggregates: Influence of Diammonium Phosphate. Materials, 16(3), 980. https://doi.org/10.3390/ma16030980







