Removal of Sodium from Vanadium Tailings by Calcification Roasting in Reducing Atmosphere
Abstract
1. Introduction
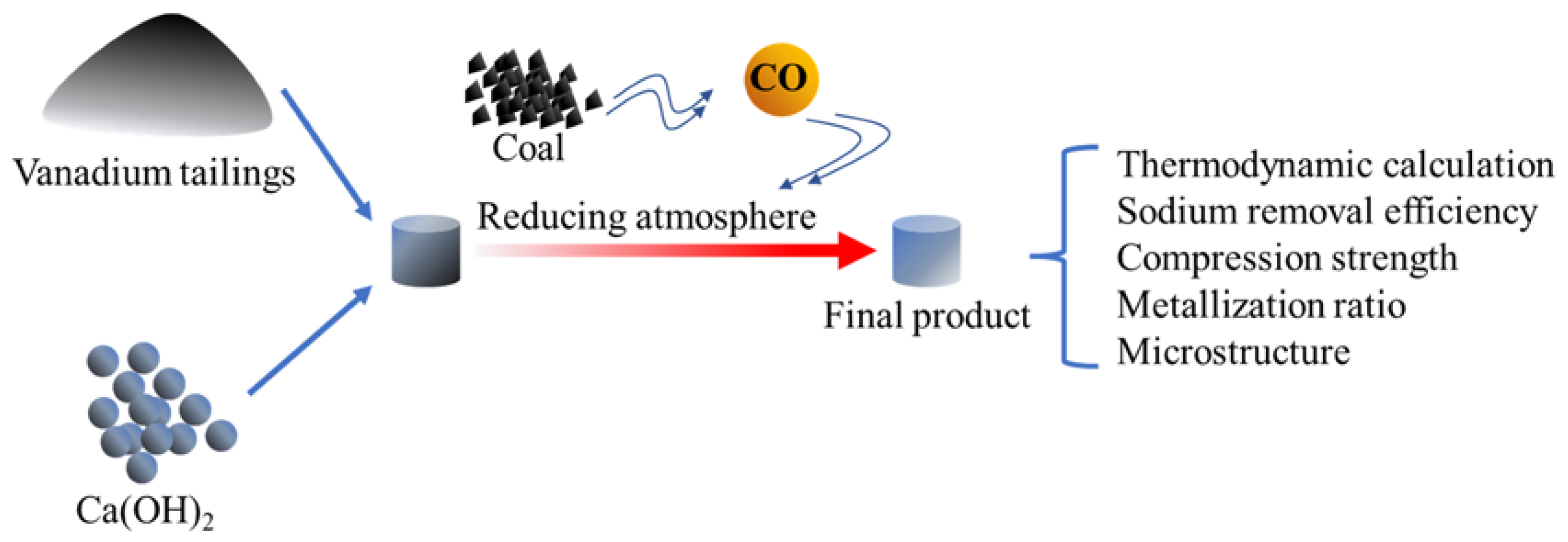
2. Experimental
2.1. Materials
2.2. Experimental Methods
2.3. Analysis and Characterization
2.4. Sodium Removal Efficiency
3. Results and Discussion
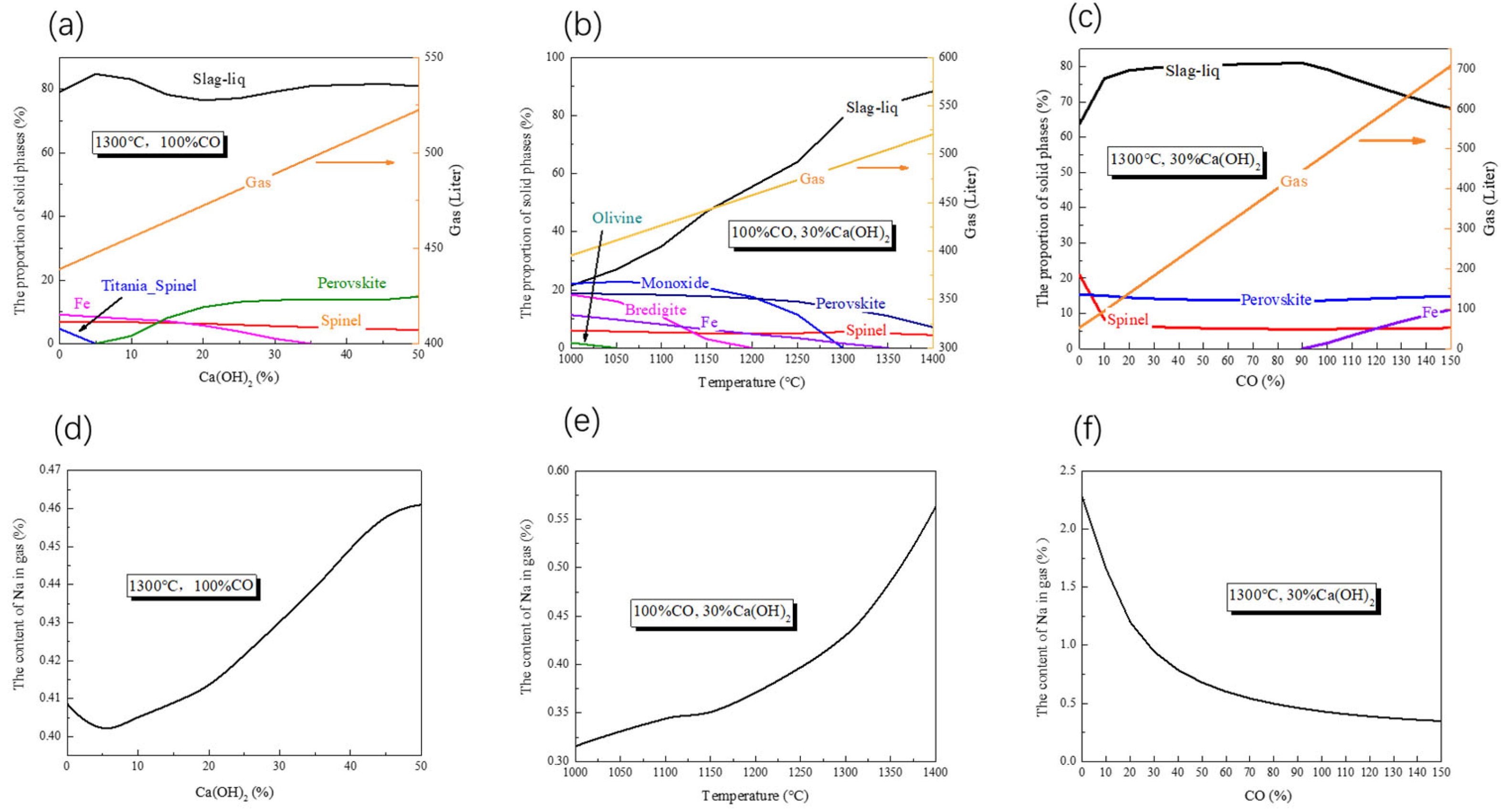
3.1. Thermodynamic Analysis
3.2. Removal Behavior of Sodium
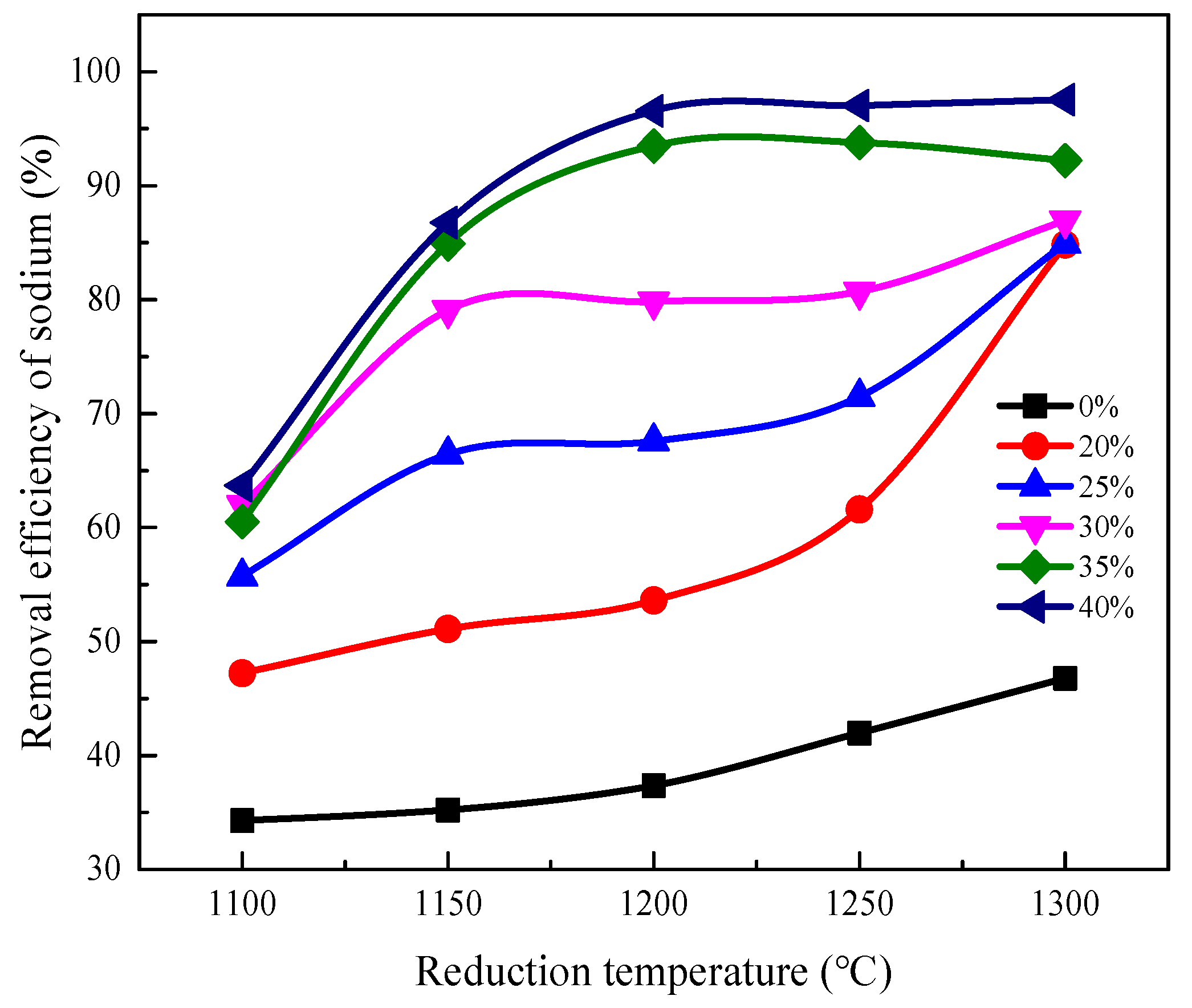
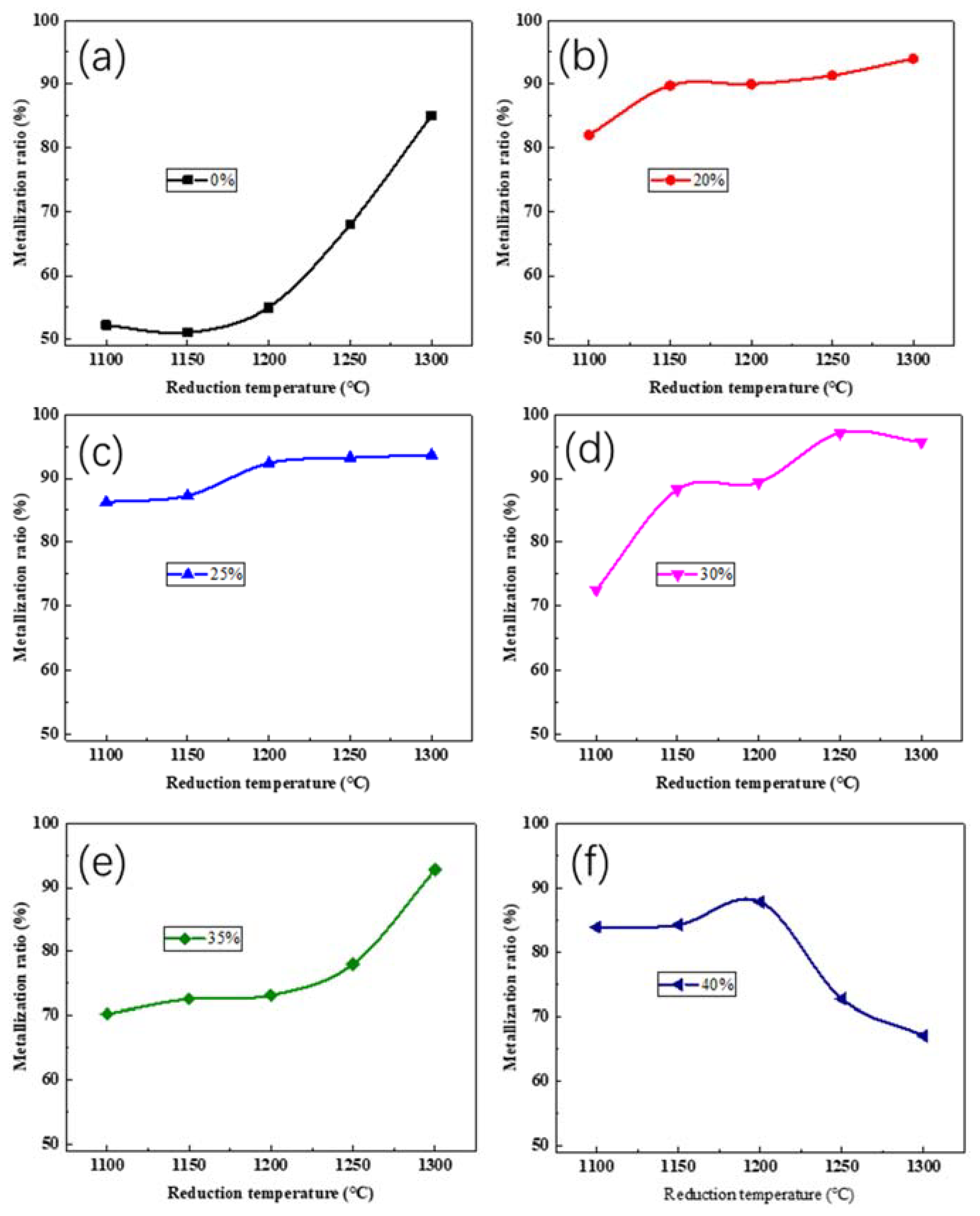
3.2.1. Effect of Reduction Temperature
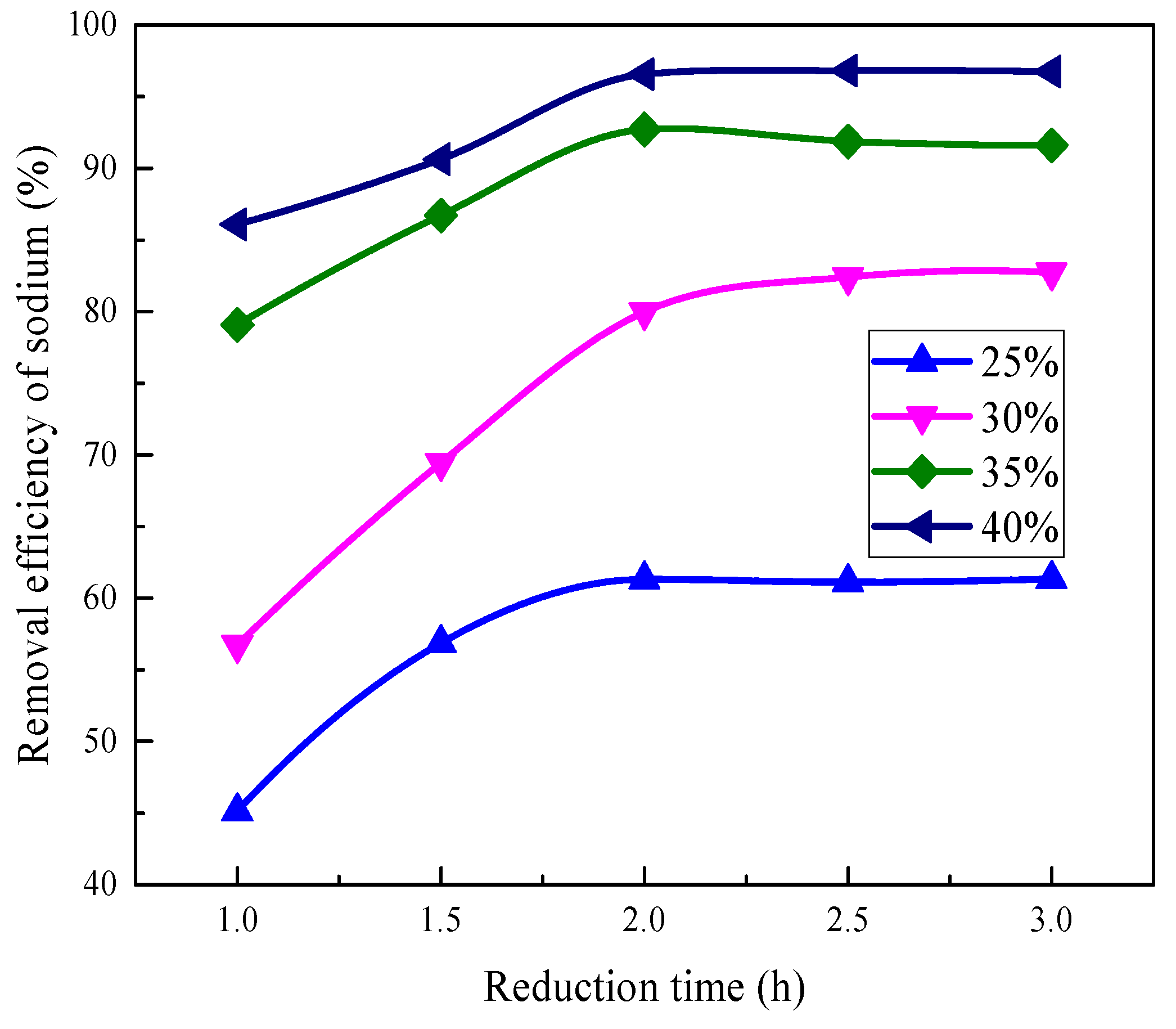
3.2.2. Effect of Reduction Time
3.3. Reduction Behavior of Iron Oxides
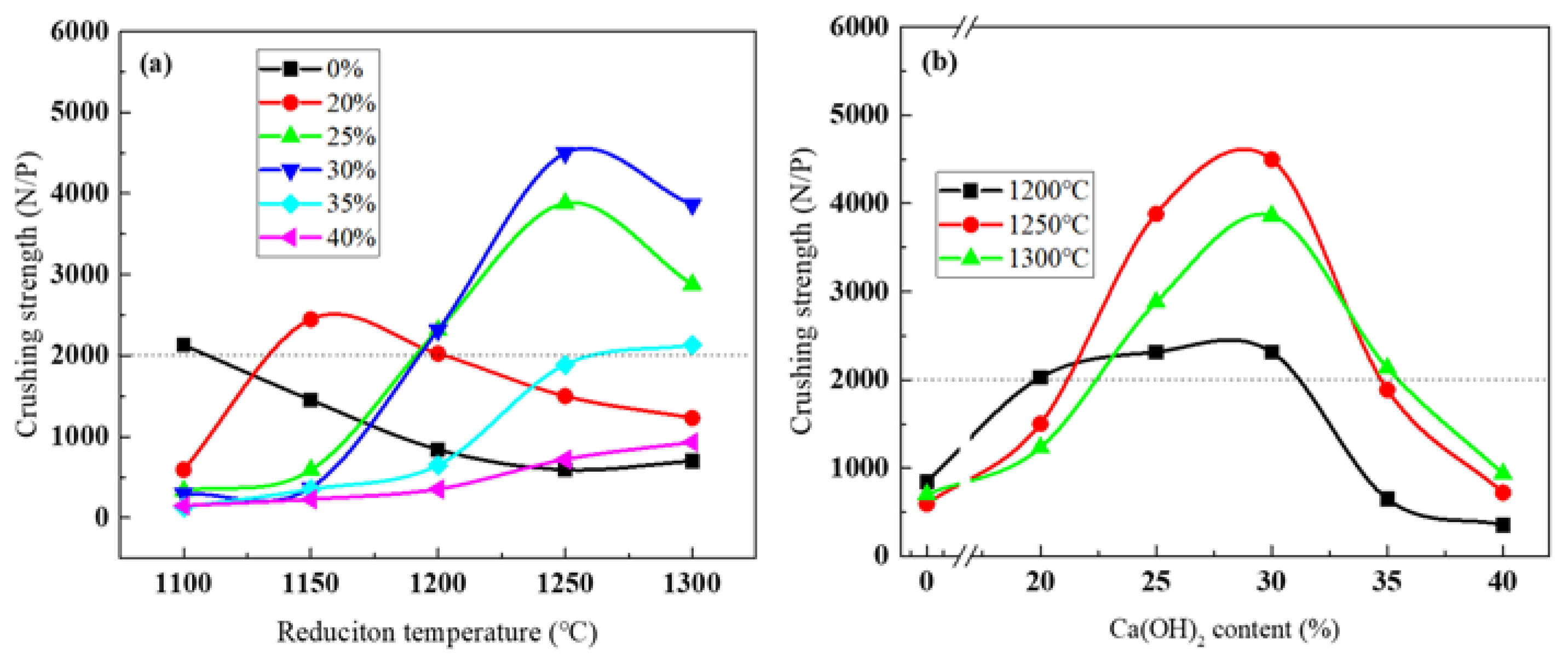
3.4. Compression Strength of Reduced Pellets
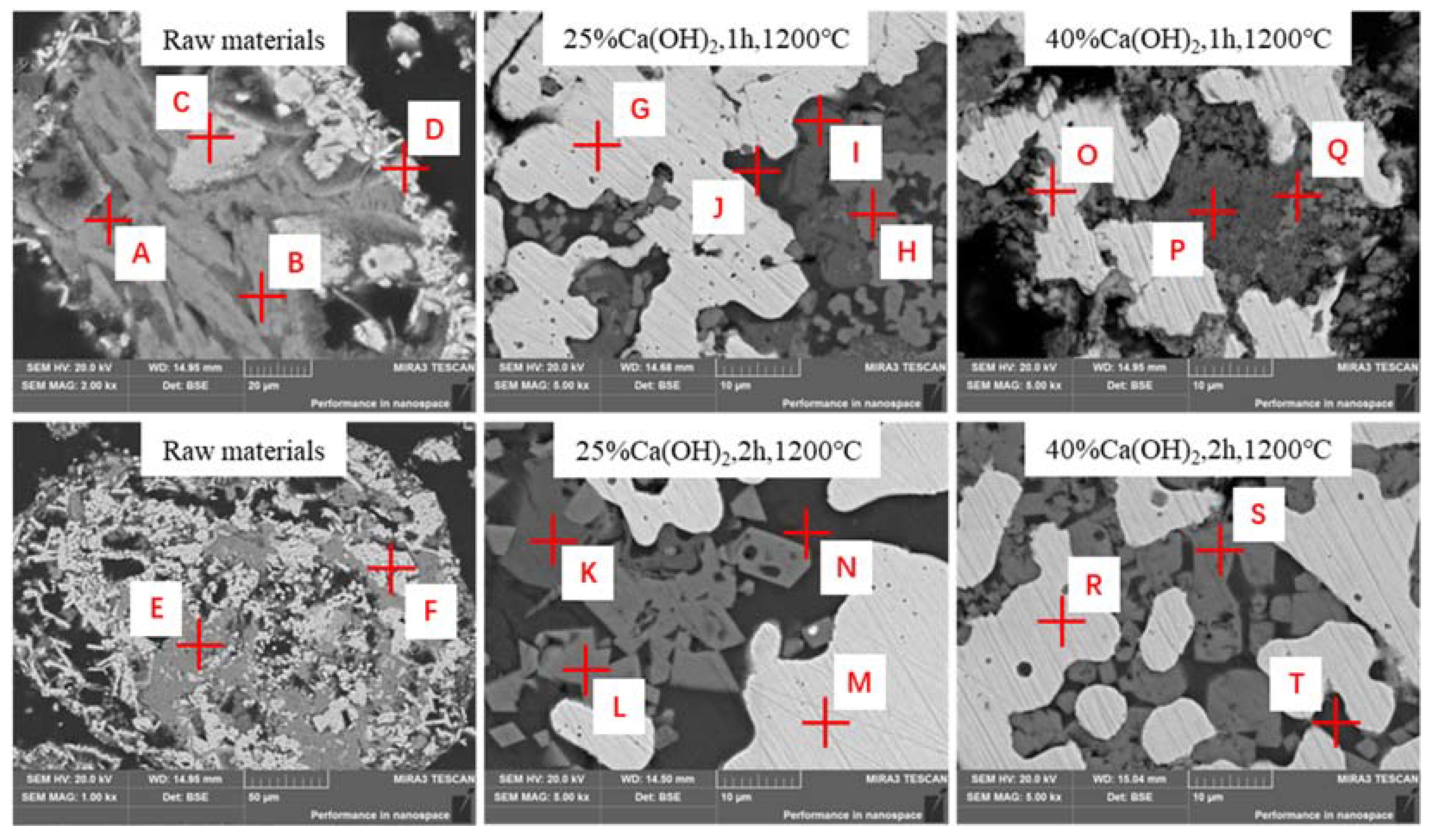
3.5. Microstructure of Roasted Products
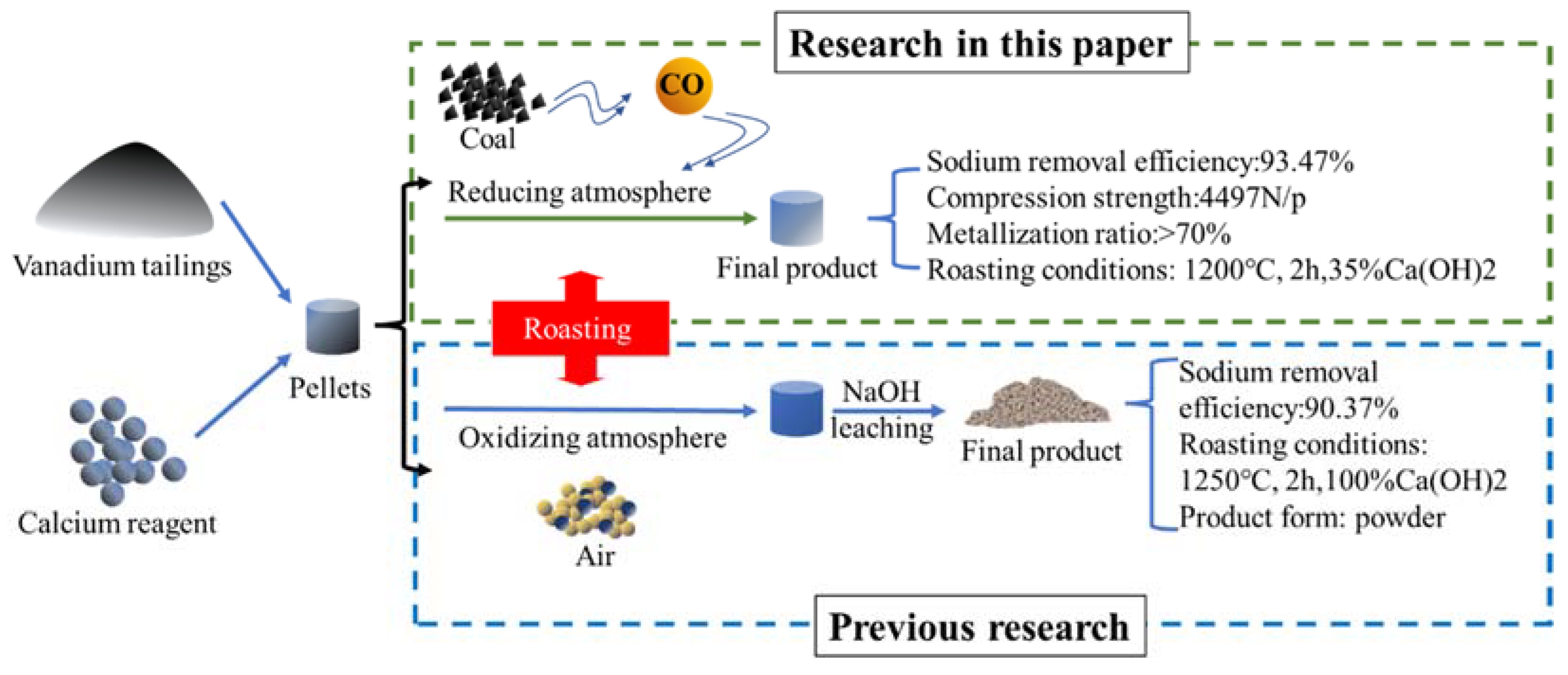
3.6. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.F.; Fang, D.; Song, S.Z.; Cheng, G.J.; Xue, X.X. Selective leaching of vanadium over iron from vanadium slag. J. Hazard. Mater. 2019, 368, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiang, J.Y.; Ling, J.W.; Huang, Q.Y.; Lv, X.W. Comprehensive utilization of vanadium extraction tailings: A brief review. In Energy Technology 2020: Recycling, Carbon Dioxide Management, and Other Technologies; Springer: Cham, Switzerland, 2020; pp. 327–334. [Google Scholar]
- Volkov, A.; Kologrieva, U.; Kovalev, A.; Wainstein, D.; Vakhrushev, V. Vanadium chemical compounds forms in wastes of vanadium pentoxide production. Materials 2020, 13, 4889. [Google Scholar] [CrossRef] [PubMed]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Li, L.J.; Zhao, B.B.; Wang, H.X.; Bai, R.G.; Chen, D.H. The process of high efficiency dealkalization and ore blending in ironmaking of the extracted vanadium residue. Chin. J. Process Eng. 2017, 17, 138–143. [Google Scholar]
- Monakhov, I.N.; Khromov, S.V.; Chernousov, P.I.; Yusfinm, Y.S. The flow of vanadium-bearing materials in industry. Metallurgist 2004, 48, 381–385. [Google Scholar] [CrossRef]
- Deng, R.R.; Xiao, H.; Xie, Z.M.; Liu, Z.H.; Yu, Q.; Chen, G.; Tao, C.Y. A novel method for extracting vanadium by low temperature sodium roasting from converter vanadium slag. Chin. J. Chem. Eng. 2020, 28, 2208–2213. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Huang, Q.Y.; Lv, W.; Pei, G.S.; Lv, X.W.; Bai, C.G. Recovery of tailings from the vanadium extraction process by carbothermic reduction method: Thermodynamic, experimental and hazardous potential assessment. J. Hazard. Mater. 2018, 257, 128–137. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhen, Y.L.; He, X.B.; Wang, L.J.; Chou, K.C. Recovery and separation of Fe and Mn from simulated chlorinated vanadium slag by molten salt electrolysis. Int. J. Miner. Metall. Mater. 2020, 27, 1678–1686. [Google Scholar] [CrossRef]
- Hao, J.Z.; Liu, A.Q.; Ma, M.L. Research of Vanadium Tailings Based Far-Infrared Radiation Coatings. Paint. Coat. Ind. 2009, 39, 13. [Google Scholar]
- Xiu, D.P.; Wang, Q.C.; Yang, Y.G.; Gu, S.L.; Sun, Z.Q. Techniques of making vanadium and titanium black porcelain and its application in modern industry. Chin. Ceram. 2008, 44, 44. [Google Scholar]
- Yang, S.L.; Ma, L.; Liu, J.F.; Lei, Z.J. Prospect of Researching and Developing Vanadium Functional Materials Using Vanadium Resources in Panxi Area. Iron Steel Vanadium Titan. 2016, 37, 84. [Google Scholar]
- Wei, B.; Zhang, Y.M.; Bao, S.X. Preparation of geopolymers from vanadium tailings by mechanical activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- Li, L.J.; Chen, D.H.; Bai, R.G.; Zheng, S.L.; Du, H.; Wang, S.N.; Zhang, Y. Leaching of vanadium from vanadium-containing residue by NaOH sub-molten salt. Chin. J. Process Eng. 2011, 11, 747–754. [Google Scholar]
- Ge, H.W.; Wei, C.; Fan, G.; Li, M.T.; Deng, Z.G.; Li, C.X. Pathbreaking experimentation study of a new leaching technology of extracted vanadium residue by acid leaching under Oxygen pressure. Shanxi Metall. 2008, 116, 17. [Google Scholar]
- Fan, G.; Wei, C.; Ge, H.W.; Li, M.T.; Deng, Z.G.; Li, C.X. Vanadium recovery from extracted vanadium residue by atmospheric pressure acid leaching. Nonferrous Met. 2010, 62, 65. [Google Scholar]
- Zhang, Y.; Zhang, T.A.; Dreisinger, D.; Lv, C.X.; Lv, G.Z.; Zhang, W.G. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J. Hazard. Mater. 2019, 369, 632–641. [Google Scholar] [CrossRef]
- Mazurek, K. Recovery of vanadium, potassium and iron from a spent vanadium catalyst by oxalic acid solution leaching, precipitation and ion exchange processes. Hydrometallurgy 2013, 134, 26–31. [Google Scholar] [CrossRef]
- Tian, L.; Xu, Z.F.; Chen, L.J.; Liu, Y.; Zhang, T.A. Effect of microwave heating on the pressure leaching of vanadium from converter slag. Hydrometallurgy 2019, 184, 45–54. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhang, T.A.; Lv, G.Z.; Zhang, Y.; Liu, Y.; Xie, G. Extraction of vanadium from LD converter slag by pressure leaching process with titanium white waste acid. Rare Met. Mater. Eng. 2015, 44, 1894–1898. [Google Scholar]
- Du, W.; Chen, H. Resource utilization of vanadium tailings back into the steel process. North V-Ti-Bear 2011, 9, 22. [Google Scholar]
- Li, L.J. Fundamental Applied Research on Resource Utilization of Vanadium Tailings; Northeastern University: Boston, MA, USA, 2013. [Google Scholar]
- Guo, Y.F.; Wang, C.; Wang, S.; Chen, F.; Wang, X.Y.; An, Z.W.; Yang, L.Z. Research on the removal of sodium from vanadium tailings by calcification roasting and NaOH leaching. Sustainability 2022, 14, 9051. [Google Scholar] [CrossRef]
- Li, H.K.; Wang, Y.J.; Jiao, K.X.; Zhang, J.L.; Zhu, R. Guo, H.J. Study on alkali circulation process and its influence on coke ratio in blast furnace. In 10th International Symposium on High-Temperature Metallurgical Processing; Springer: Cham, Switzerland, 2019; pp. 15–24. [Google Scholar]
- Leimalm, U.; Forsmo, S.; Dahlstedt, A.; Ökvist, L.S.; Björkman, B. Blast furnace pellet textures during reduction and correlation to strength. ISIJ Int. 2010, 50, 1396. [Google Scholar] [CrossRef]
- Odo, J.U.; Nwoke, V.U. Effect of core diameter on the compressive strength and porosity of itakpe iron ore pellets. In 10th International Symposium on High-Temperature Metallurgical Processing; Springer: Cham, Switzerland, 2019; pp. 511–521. [Google Scholar]

| FCad | Aad | Vad | Mad |
|---|---|---|---|
| 84.79 | 13.23 | 1.98 | 0.76 |
| Na2O | K2O | Total-Fe | SiO2 | TiO2 | MnO | Cr2O3 | Al2O3 | CaO | MgO | V2O5 | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.45 | 0.02 | 32.32 | 13.64 | 11.92 | 6.33 | 4.18 | 2.78 | 1.88 | 1.69 | 1.34 | 0.47 |
| Point | Atoms (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | Ti | Ca | Cr | Mn | Fe | Al | Si | Na | |
| A | 60.52 | 3.75 | / | 3.19 | 7.6 | 24.58 | / | / | / |
| B | 41.11 | 1.75 | 9.39 | / | 2.82 | 13.24 | 2.93 | 24.97 | 3.79 |
| C | 38.91 | 10.28 | / | 6.15 | 3.52 | 37.92 | / | / | 3.22 |
| D | 53.65 | 3.75 | / | 3.19 | 7.6 | 31.22 | / | / | / |
| E | 37.49 | 2.76 | 4.80 | / | 1.76 | 10.99 | 1.58 | 26.38 | 10.26 |
| F | 62.74 | 2.91 | / | 4.33 | 4.26 | 20.98 | / | 2.84 | 1.80 |
| G | / | / | / | 1.14 | 1.62 | 94.87 | / | / | / |
| H | 49.88 | 3.10 | / | 12.36 | 11.21 | / | 2.72 | / | / |
| I | 66.88 | 13.86 | 14.35 | / | / | / | / | / | 0.87 |
| J | 63.28 | / | 11.55 | / | 2.02 | 1.41 | 2.38 | 14.66 | 2.32 |
| K | 62.83 | 17.03 | 18.41 | 0.67 | / | 0.37 | / | / | / |
| L | 54.83 | 2.15 | / | 16.42 | 8.09 | / | 2.28 | / | / |
| M | / | / | / | / | / | 100.00 | / | / | / |
| N | 63.03 | / | 12.18 | / | 1.27 | / | 1.87 | 14.96 | 2.05 |
| O | / | / | / | 1.82 | 1.05 | 96.40 | / | / | / |
| P | 63.89 | 9.57 | 15.38 | / | / | / | 1.54 | 5.97 | 1.10 |
| Q | 56.09 | / | 4.31 | / | / | / | 4.77 | 4.32 | 1.34 |
| R | / | / | / | 2.77 | 2.81 | 91.61 | / | / | / |
| S | 53.14 | 7.01 | / | 12.66 | 13.54 | 1.03 | / | / | / |
| T | 60.97 | 1.25 | 11.73 | / | 4.40 | 1.22 | 1.13 | 13.66 | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Guo, Y.; Wang, S.; Chen, F.; Yang, L.; Zheng, Y. Removal of Sodium from Vanadium Tailings by Calcification Roasting in Reducing Atmosphere. Materials 2023, 16, 986. https://doi.org/10.3390/ma16030986
Wang C, Guo Y, Wang S, Chen F, Yang L, Zheng Y. Removal of Sodium from Vanadium Tailings by Calcification Roasting in Reducing Atmosphere. Materials. 2023; 16(3):986. https://doi.org/10.3390/ma16030986
Chicago/Turabian StyleWang, Chao, Yufeng Guo, Shuai Wang, Feng Chen, Lingzhi Yang, and Yu Zheng. 2023. "Removal of Sodium from Vanadium Tailings by Calcification Roasting in Reducing Atmosphere" Materials 16, no. 3: 986. https://doi.org/10.3390/ma16030986
APA StyleWang, C., Guo, Y., Wang, S., Chen, F., Yang, L., & Zheng, Y. (2023). Removal of Sodium from Vanadium Tailings by Calcification Roasting in Reducing Atmosphere. Materials, 16(3), 986. https://doi.org/10.3390/ma16030986









