Influence of Hafnium Addition on the Microstructure, Microhardness and Corrosion Resistance of Ti20Ta20Nb20(ZrMo)20−xHfx (where x = 0, 5, 10, 15 and 20 at.%) High Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
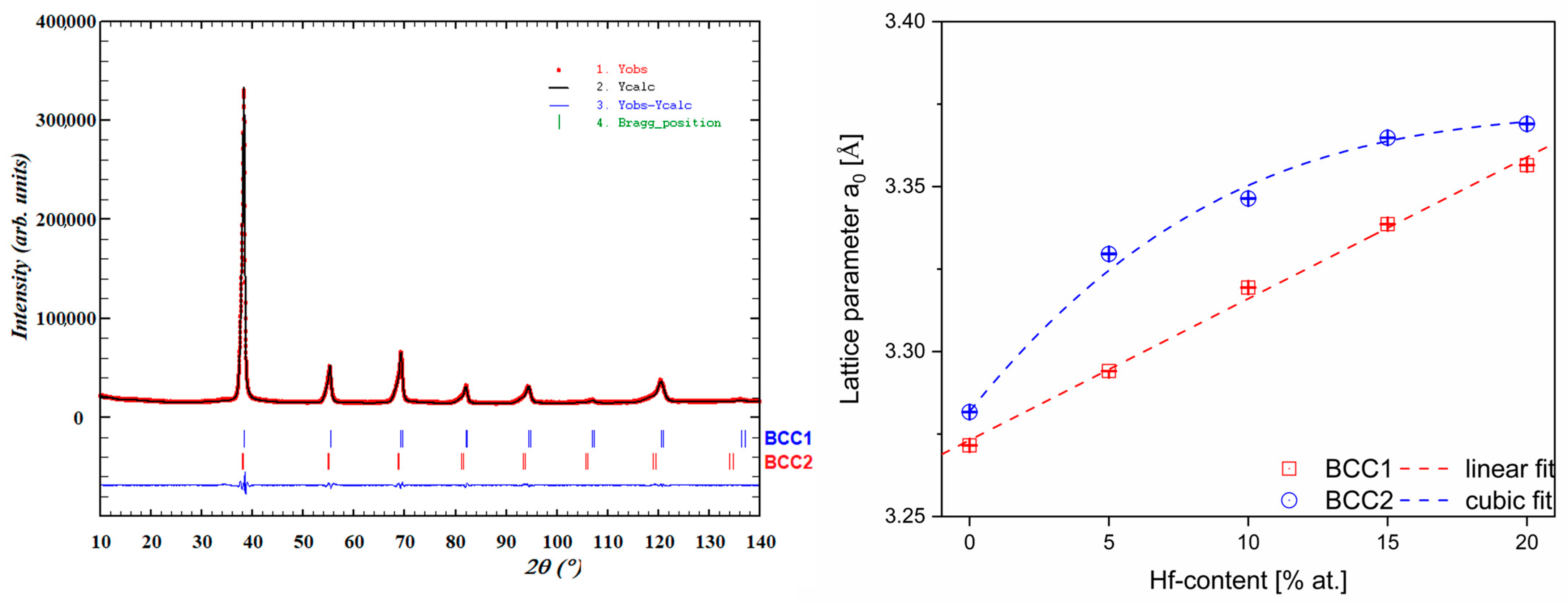
3.1. XRD Phase Analysis of Studied High Entropy Alloys
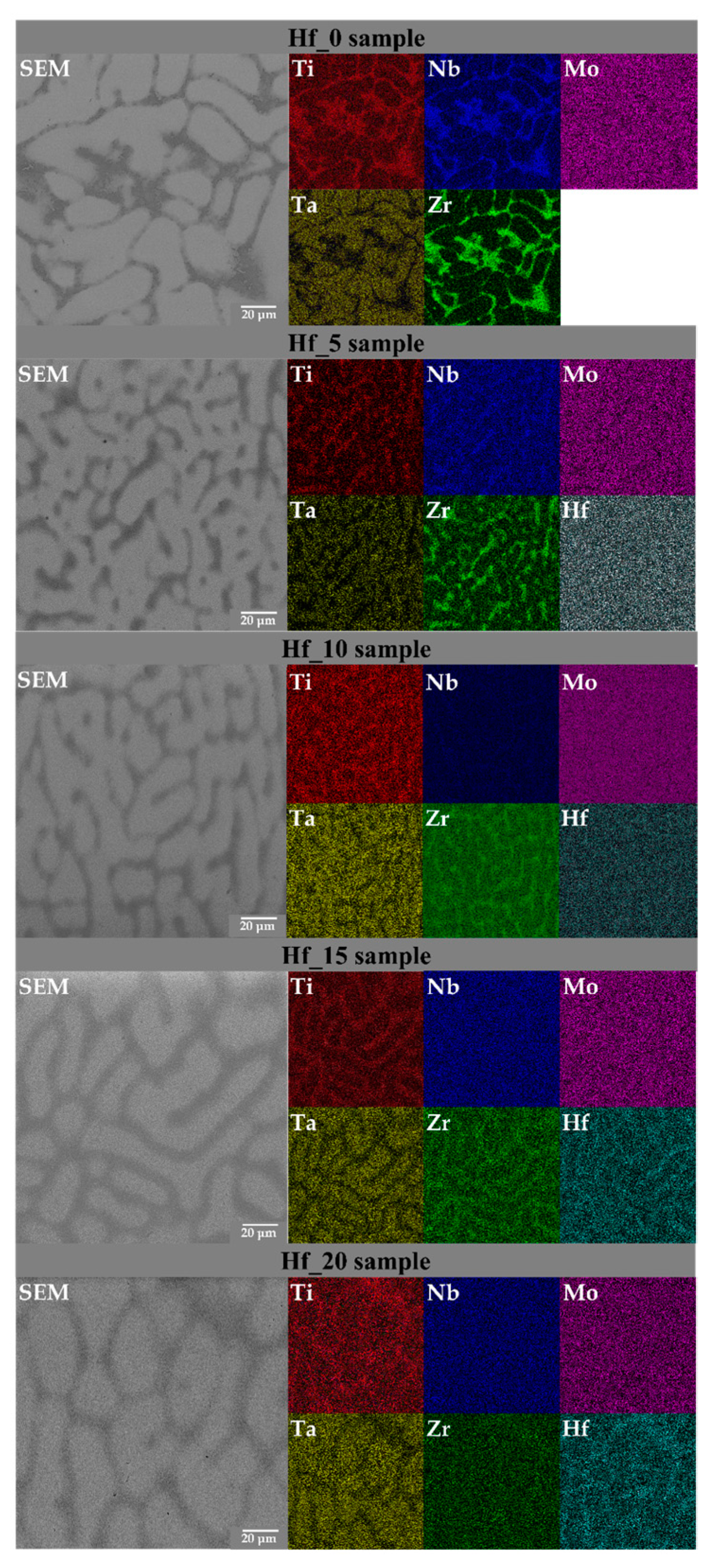
3.2. SEM Microstructure and EDS Chemical Composition Analysis of Studied High Entropy Alloys
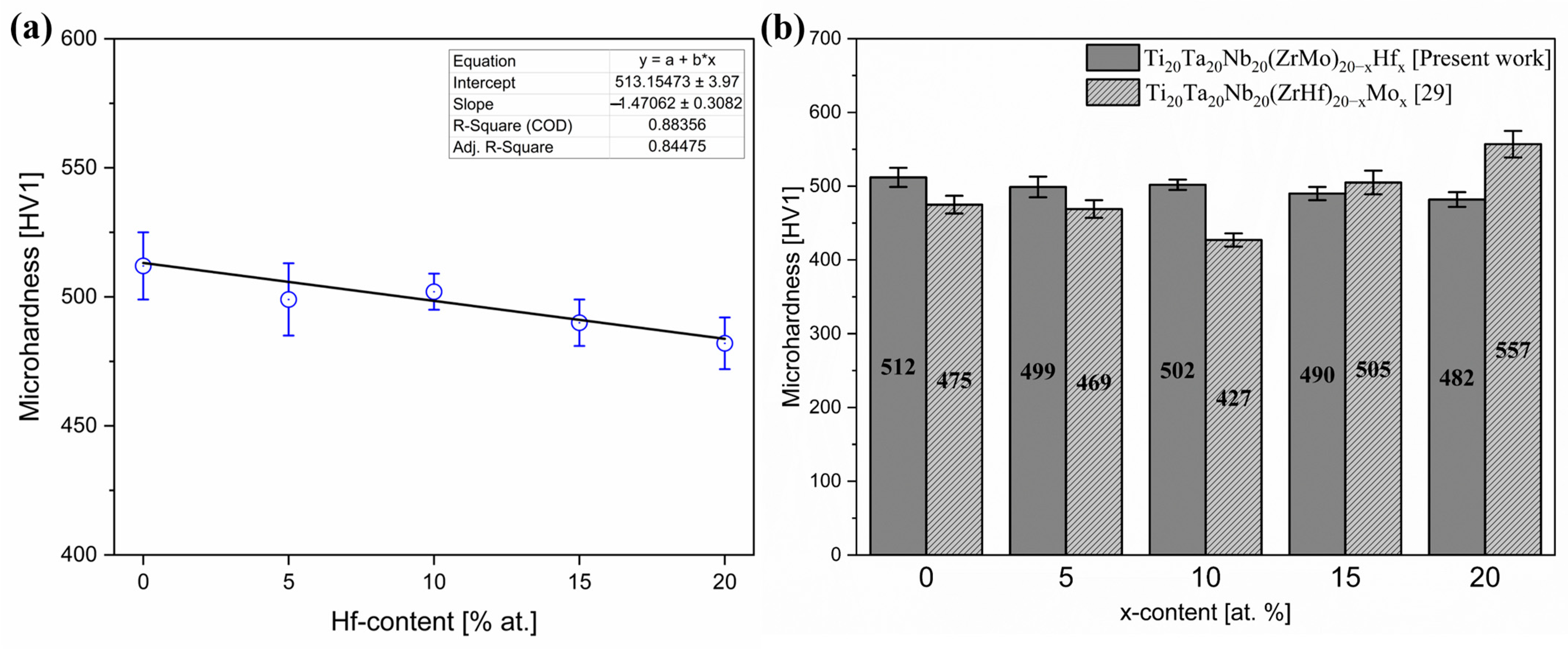
3.3. Microhardness of Investigated HEAs
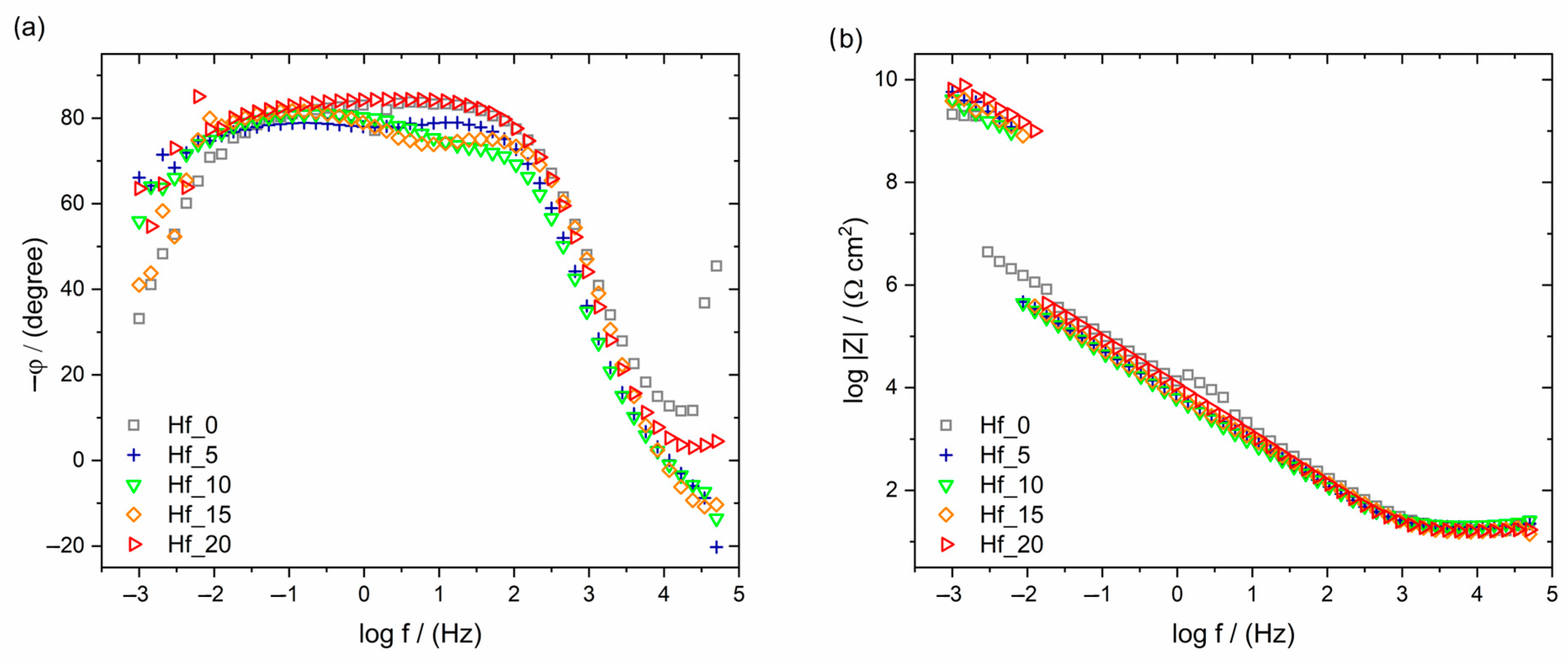
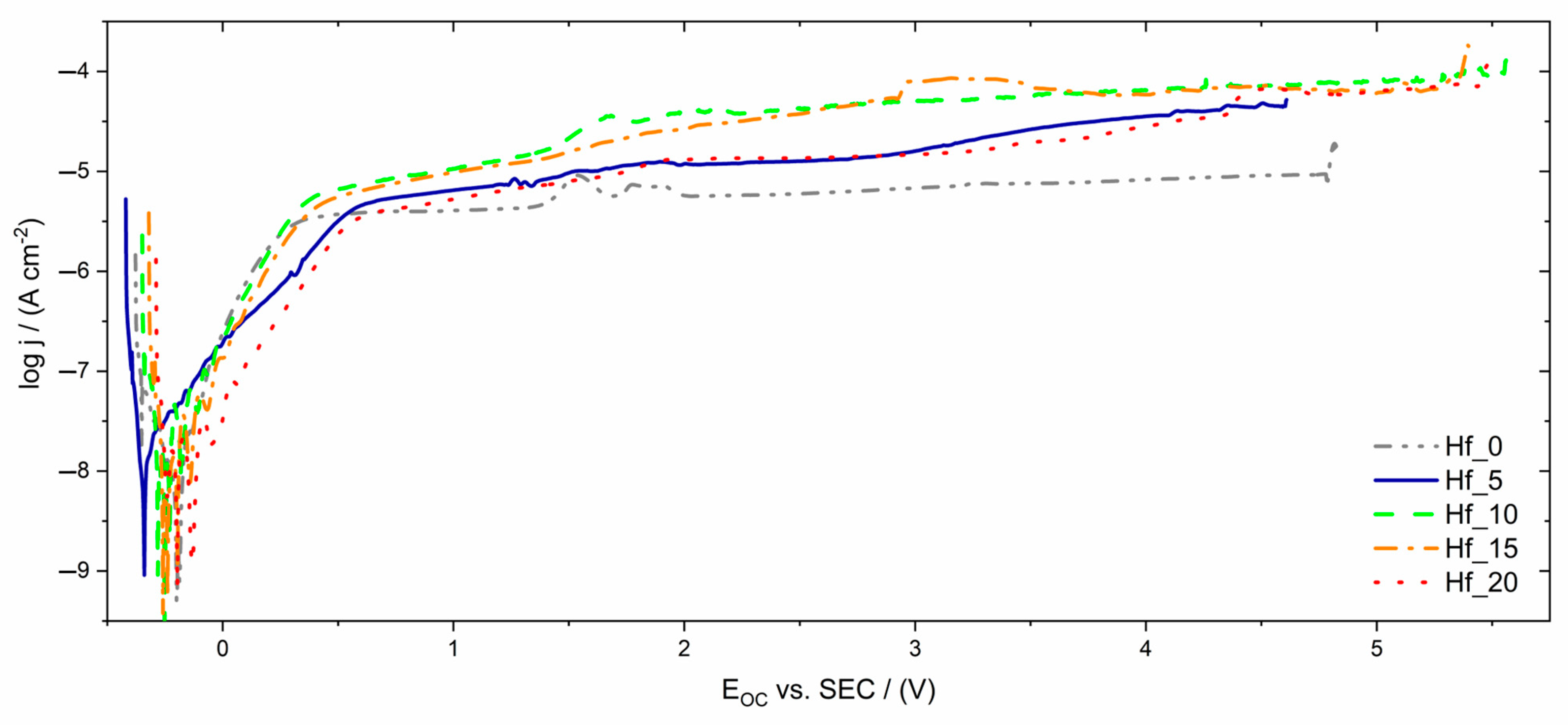
3.4. Corrosion Resistance Properties of Investigated HEAs
| Sample | EOC vs. SCE (mV) | log|Z|f→0.01Hz (Ω∙cm2) | EBD vs. SCE (V) | Reference |
|---|---|---|---|---|
| Hf_0 | −228 | 6.05 | ~5.00 | Present work |
| Hf_5 | −270 | 5.54 | ~4.60 | Present work |
| Hf_10 | −199 | 5.50 | ~5.50 | Present work |
| Hf_15 | −170 | 5.59 | ~5.35 | Present work |
| Hf_20 | −139 | 5.64 | ~5.45 | Present work |
| Ti20Ta20Nb20(ZrHf)10Mo20 | ― | ― | ~6.18 | [29] |
| Ti20Ta20Nb20(ZrHf)12.5Mo15 | ― | ― | ~6.11 | |
| Ti20Ta20Nb20(ZrHf)15Mo10 | ― | ― | 5.57 | |
| Ti15Mo | ― | ― | 5.50 | [39] |
| Ti20Ta20Nb20(ZrHf)17.5Mo5 | ― | ― | 5.15 | [29] |
| TiNbZrTa | ― | ― | 5.00 | [40] |
| Ti15Mo | ― | ― | 4.51 | [41] |
| Ti20Ta20Nb20(ZrHf)20 | ― | ― | 4.33 | [29] |
| Titanium Grade 7 | ― | ― | 2.40 | [42] |
| Ti6Al4V | ― | ― | 1.53 | [43] |
| cp-Ti Grade 2 | ― | ― | 1.48 | |
| Ti6Al7Nb | ― | ― | 1.38 | |
| Ti13Nb13Zr | ― | ― | 1.25 | |
| 316L stainless steel | ― | ― | 0.96 | [44] |
| Pure Ti | ― | ― | 0.50 | [42] |
| Ti15Nb | ― | ― | 0.45 | [45] |
| NiTi SMA | ― | ― | 0.45 | [46] |
| Ti45Nb | ― | ― | 0.28 | [45] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matuła, I.; Dercz, G.; Sowa, M.; Barylski, A.; Duda, P. Fabrication and Characterization of New Functional Graded Material Based on Ti, Ta, and Zr by Powder Metallurgy Method. Materials 2021, 14, 6609. [Google Scholar] [CrossRef] [PubMed]
- Matyja, E.; Prusik, K.; Zubko, M.; Dercz, G. Microstructure refinement and mechanical properties of the NiCoMnIn alloy obtained by arc melting technique from mechanically alloyed powder. J. Alloys Compd. 2021, 859, 157841. [Google Scholar] [CrossRef]
- Matyja, E.; Prusik, K.; Zubko, M.; Dercz, G.; Glowka, K. Structure of the Ni-Co-Mn-In alloy obtained by mechanical alloying and sintering. J. Alloys Compd. 2019, 801, 529–535. [Google Scholar] [CrossRef]
- Zhang, Y. History of High-Entropy Materials. In High-Entropy Materials: A Brief Introduction; Springer Singapore: Singapore, 2019; pp. 1–33. ISBN 978-981-13-8526-1. [Google Scholar]
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated exploration of multi-principal element alloys with solid solution phases. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.-W. Physical Metallurgy BT-High-Entropy Alloys: Fundamentals and Applications; Gao, M.C., Yeh, J.-W., Liaw, P.K., Zhang, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 51–113. ISBN 978-3-319-27013-5. [Google Scholar]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention - A Review. Recent Patents Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Rituerto Sin, J.; Suñer, S.; Neville, A.; Emami, N. Fretting corrosion of hafnium in simulated body fluids. Tribol. Int. 2014, 75, 10–15. [Google Scholar] [CrossRef]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Rituerto Sin, J.; Neville, A.; Emami, N. Corrosion and tribocorrosion of hafnium in simulated body fluids. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Esposito, M.; Cucu, M.; Ericson, L.E.; Thomsen, P. Tissue response to hafnium. J. Mater. Sci. Mater. Med. 2001, 12, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-P.; Jia, X.; Zheng, D.-L.; Wang, G.; Sun, J.-F.; Yan, M. A novel approach to achieving a low Young’s modulus in titanium-based metallic glasses. Emerg. Mater. Res. 2019, 8, 22–28. [Google Scholar] [CrossRef]
- Rajaraman, V.; Nallaswamy, D.; Ganapathy, D.; Rajeshkumar, S.; Ariga, P.; Ganesh, K. Effect of Hafnium Coating on Osseointegration of Titanium Implants: A Split Mouth Animal Study. J. Nanomater. 2021, 2021. [Google Scholar] [CrossRef]
- Rajaraman, V.; Dhanraj, M.; Jain, A.R. Dental implant biomaterials - Newer metals and their alloys. Drug Invent. Today 2018, 10, 986–989. [Google Scholar]
- Wang, S.P.; Xu, J. TiZrNbTaMo high-entropy alloy designed for orthopedic implants: As-cast microstructure and mechanical properties. Mater. Sci. Eng. C 2017, 73, 80–89. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef]
- Shittu, J.; Pole, M.; Cockerill, I.; Sadeghilaridjani, M.; Reddy, L.V.K.; Manivasagam, G.; Singh, H.; Grewal, H.S.; Arora, H.S.; Mukherjee, S. Biocompatible High Entropy Alloys with Excellent Degradation Resistance in a Simulated Physiological Environment. ACS Appl. Bio Mater. 2020, 3, 8890–8900. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Gurel, S.; Yagci, M.B.; Bal, B.; Canadinc, D. Corrosion behavior of novel Titanium-based high entropy alloys designed for medical implants. Mater. Chem. Phys. 2020, 254, 123377. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of TiZrTaHfNb and Ti1.5ZrTa0.5Hf0.5Nb0.5 refractory high-entropy alloys for biomedical applications. Intermetallics 2019, 113, 106572. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Pang, S.; Liaw, P.K.; Zhang, T. Bio-corrosion behavior and in vitro biocompatibility of equimolar TiZrHfNbTa high-entropy alloy. Intermetallics 2020, 124, 106845. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Yang, Z.; Liang, X.; Lei, Z.; Huang, H.; Wang, H.; Liu, X.; An, K.; Wu, W.; et al. Formation, structure and properties of biocompatible TiZrHfNbTa high-entropy alloys. Mater. Res. Lett. 2019, 7, 225–231. [Google Scholar] [CrossRef]
- Yang, W.; Pang, S.; Liu, Y.; Wang, Q.; Liaw, P.K.; Zhang, T. Design and properties of novel Ti–Zr–Hf–Nb–Ta high-entropy alloys for biomedical applications. Intermetallics 2022, 141, 107421. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Glowka, K.; Zubko, M.; Świec, P.; Prusik, K.; Albrecht, R.; Dercz, G.; Loskot, J.; Witala, B.; Stróż, D. Microstructure and mechanical properties of Co-Cr-Mo-Si-Y-Zr high entropy alloy. Metals 2020, 10, 1456. [Google Scholar] [CrossRef]
- Glowka, K.; Zubko, M.; Świec, P.; Prusik, K.; Szklarska, M.; Chrobak, D.; Lábár, J.L.; Stróż, D. Influence of Molybdenum on the Microstructure, Mechanical Properties and Corrosion Resistance of Ti20Ta20Nb20(ZrHf)20−xMox (Where: x = 0, 5, 10, 15, 20) High Entropy Alloys. Materials 2022, 15, 393. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Wang, L.; Yang, X.; Zeng, T.; Sun, L.; Wang, R. Microstructure evolution and wear resistance of laser cladded 316L stainless steel reinforced with in-situ VC-Cr7C3. Surf. Coatings Technol. 2022, 435, 128264. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wu, S.; Lin, H.; Yang, Y.; Fan, S.; Gu, C.; Wang, J.; Song, C. Customized a Ti6Al4V Bone Plate for Complex Pelvic Fracture by Selective Laser Melting. Materials 2017, 10, 35. [Google Scholar] [CrossRef]
- Prusa, F.; Bernatikova, A.; Palan, J. Ultra-High Strength Ti Grade 4 Prepared by Intensive Plastic Deformation. Manuf. Technol. J. 2017, 17, 819–822. [Google Scholar] [CrossRef]
- Wang, C.T.; Gao, N.; Gee, M.G.; Wood, R.J.K.; Langdon, T.G. Effect of grain size on the micro-tribological behavior of pure titanium processed by high-pressure torsion. Wear 2012, 280–281, 28–35. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R.; Benč, M.; Dvořák, J. Affecting Microstructure and Properties of Additively Manufactured AISI 316L Steel by Rotary Swaging. Materials 2022, 15, 6291. [Google Scholar] [CrossRef]
- Stea, S.; Visentin, M.; Savarino, L.; Ciapetti, G.; Donati, M.E.; Moroni, A.; Caja, V.; Pizzoferrato, A. Microhardness of bone at the interface with ceramic-coated metal implants. J. Biomed. Mater. Res. 1995, 29, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Azambuja, D.S. Corrosion behavior of ti and Ti6Al4V in citrate buffers containing fluoride ions. Mater. Res. 2010, 13, 45–50. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical stability and corrosion resistance of Ti–Mo alloys for biomedical applications. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef]
- Szklarska, M.; Łosiewicz, B.; Dercz, G.; Zubko, M.; Albrecht, R.; Stróz, D. Charac terization of long-term corros ion performance of ti15mo alloy in saline solution. Arch. Metall. Mater. 2019, 64, 773–778. [Google Scholar] [CrossRef]
- Samuel, S.; Nag, S.; Nasrazadani, S.; Ukirde, V.; El Bouanani, M.; Mohandas, A.; Nguyen, K.; Banerjee, R. Corrosion resistance and in vitro response of laser-deposited Ti-Nb-Zr-Ta alloys for orthopedic implant applications. J. Biomed. Mater. Res.—Part A 2010, 94, 1251–1256. [Google Scholar] [CrossRef]
- Szklarska, M.; Dercz, G.; Rak, J.; Simka, W.; Losiewicz, B. The influence of passivation type on corrosion resistance of Ti15Mo alloy in simulated body fluids. Arch. Metall. Mater. 2015, 60, 2687–2693. [Google Scholar] [CrossRef]
- Handzlik, P.; Fitzner, K. Corrosion resistance of Ti and Ti-Pd alloy in phosphate buffered saline solutions with and without H2O2 addition. Trans. Nonferrous Met. Soc. China (English Ed.) 2013, 23, 866–875. [Google Scholar] [CrossRef]
- Cai, Z.; Shafer, T.; Watanabe, I.; Nunn, M.E.; Okabe, T. Electrochemical characterization of cast titanium alloys. Biomaterials 2003, 24, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, T.; Rokosz, K. Corrosion resistance of magnetoelectropolished AISI 316L SS biomaterial. Anti-Corrosion Methods Mater. 2014, 61, 57–64. [Google Scholar] [CrossRef]
- Godley, R.; Starosvetsky, D.; Gotman, I. Corrosion behavior of a low modulus β -Ti-45 % Nb. J. Mater. Scince Med. 2006, 17, 63–67. [Google Scholar]
- Rondelli, G. Corrosion resistance tests on NiTi shape memory alloy. Biomaterials 1996, 17, 2003–2008. [Google Scholar] [CrossRef]
- Chembath, M.; Balaraju, J.N.; Sujata, M. Surface characteristics, corrosion and bioactivity of chemically treated biomedical grade NiTi alloy. Mater. Sci. Eng. C 2015, 56, 417–425. [Google Scholar] [CrossRef]



| Chemical Composition | Abbreviation | δ (%) | ΔHmix (kJ∙mol−1) | ΔSmix (J·(mol·K)−1) | Δχ (eV) | VEC | Ω |
|---|---|---|---|---|---|---|---|
| Ti20Ta20Nb20(ZrMo)20 | Hf_0 | 5.46 | −1.76 | 13.38 | 0.282 | 4.80 (BCC) | 19.77 (multi-phase) |
| Ti20Ta20Nb20(ZrMo)17.5Hf5 | Hf_5 | 5.42 | −1.24 | 14.35 | 0.274 | 4.75 (BCC) | 30.21 (multi-phase) |
| Ti20Ta20Nb20(ZrMo)15Hf10 | Hf_10 | 5.37 | −0.70 | 14.68 | 0.263 | 4.70 (BCC) | 54.51 (multi-phase) |
| Ti20Ta20Nb20(ZrMo)12.5Hf15 | Hf_15 | 5.29 | −0.16 | 14.72 | 0.251 | 4.65 (BCC) | 246.85 (multi-phase) |
| Ti20Ta20Nb20(ZrMo)10Hf20 | Hf_20 | 5.19 | 0.40 | 14.53 | 0.235 | 4.60 (BCC) | 94.45 (multi-phase) |
| Sample | Phase | Lattice Parameters a0, (Å) |
|---|---|---|
| Hf_0 | BCC1 | 3.2716(1) |
| BCC2 | 3.2817(1) | |
| Hf_5 | BCC1 | 3.2941(2) |
| BCC2 | 3.3296(1) | |
| Hf_10 | BCC1 | 3.3194(1) |
| BCC2 | 3.3464(2) | |
| Hf_15 | BCC1 | 3.3386(2) |
| BCC2 | 3.3648(2) | |
| Hf_20 | BCC1 | 3.3565(1) |
| BCC2 | 3.3690(1) |
| Studied Alloy | Elements | Ti | Ta | Nb | Zr | Mo | Hf | Phase Contribution |
|---|---|---|---|---|---|---|---|---|
| Hf_0 | Nominal | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | ― | ― |
| BCC1 | 12.9(2) | 26.2(4) | 24.7(4) | 8.2(2) | 25.5(4) | ― | 81(8)% | |
| BCC2 | 8.4(1) | 15.9(4) | 20.6(1) | 45.6(6) | 9.5(3) | ― | 19(8)% | |
| Hf_5 | Nominal | 20.0 | 20.0 | 20.0 | 17.5 | 17.5 | 5.0 | ― |
| BCC1 | 13.3(2) | 23.7(8) | 26.9(1) | 10.6(6) | 21.5(2) | 3.9(3) | 67(8)% | |
| BCC2 | 20.4(1) | 7.9(2) | 22.2(1) | 31.0(3) | 10.2(2) | 8.4(1) | 33(8)% | |
| Hf_10 | Nominal | 20.0 | 20.0 | 20.0 | 15.0 | 15.0 | 10.0 | ― |
| BCC1 | 12.6(1) | 28.0(3) | 26.0(1) | 8.5(2) | 18.4(1) | 6.5(2) | 67(7)% | |
| BCC2 | 18.4(1) | 11.2(4) | 22.0(1) | 25.7(3) | 10.6(2) | 12.2(2) | 33(7)% | |
| Hf_15 | Nominal | 20.0 | 20.0 | 20.0 | 12.5 | 12.5 | 15.0 | ― |
| BCC1 | 12.2(1) | 26.2(3) | 22.5(1) | 7.2(1) | 15.8(1) | 16.0(2) | 68(10)% | |
| BCC2 | 17.2(1) | 10.8(3) | 19.1(1) | 17.7(2) | 10.3(1) | 24.9(2) | 32(10)% | |
| Hf_20 | Nominal | 20.0 | 20.0 | 20.0 | 10.0 | 10.0 | 20.0 | ― |
| BCC1 | 13.5(1) | 28.2(2) | 23.1(1) | 4.6(1) | 13.6(1) | 17.0(2) | 73(8)% | |
| BCC2 | 20.2(1) | 12.6(3) | 19.0(1) | 9.7(1) | 9.6(1) | 28.9(3) | 27(8)% |
| Sample | Microhardness [HV1] |
|---|---|
| Hf_0 | 512 (13) |
| Hf_5 | 499 (14) |
| Hf_10 | 502 (7) |
| Hf_15 | 490 (9) |
| Hf_20 | 482 (10) |
| Sample | Microhardness [HV1] | Reference |
|---|---|---|
| Ti20Ta20Nb20(ZrMo)20 | 512(13) | Present work |
| Ti20Ta20Nb20(ZrMo)15Hf10 | 502(7) | Present work |
| Ti20Ta20Nb20(ZrMo)17.5Hf5 | 499(14) | Present work |
| Ti20Ta20Nb20(ZrMo)12.5Hf15 | 490(9) | Present work |
| Ti20Ta20Nb20(ZrMo)10Hf20 | 482(10) | Present work |
| Ti20Ta20Nb20 (ZrHf)10Mo20 | 557 | [29] |
| Ti20Ta20Nb20 (ZrHf)12.5Mo15 | 505 | |
| Ti20Ta20Nb20(ZrHf)20 | 475 | |
| Ti20Ta20Nb20(ZrHf)17.5Mo5 | 469 | |
| 316L SS (laser cladded) | 467.8 | [31] |
| Ti20Ta20Nb20 (ZrHf)15Mo10 | 427 | [29] |
| Ti6Al4V (selective laser melted) | 390 | [32] |
| Titanium Grade 4 (after SPD process) | 330 | [33] |
| Titanium Grade 4 (in an initial state) | 170 | |
| cp-Ti (after HPT) | 305 | [34] |
| 316L SS (additive manufactured) | 300 | [35] |
| Lamellar bone | 88.8 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glowka, K.; Zubko, M.; Gębura, S.; Świec, P.; Prusik, K.; Szklarska, M.; Stróż, D. Influence of Hafnium Addition on the Microstructure, Microhardness and Corrosion Resistance of Ti20Ta20Nb20(ZrMo)20−xHfx (where x = 0, 5, 10, 15 and 20 at.%) High Entropy Alloys. Materials 2023, 16, 1456. https://doi.org/10.3390/ma16041456
Glowka K, Zubko M, Gębura S, Świec P, Prusik K, Szklarska M, Stróż D. Influence of Hafnium Addition on the Microstructure, Microhardness and Corrosion Resistance of Ti20Ta20Nb20(ZrMo)20−xHfx (where x = 0, 5, 10, 15 and 20 at.%) High Entropy Alloys. Materials. 2023; 16(4):1456. https://doi.org/10.3390/ma16041456
Chicago/Turabian StyleGlowka, Karsten, Maciej Zubko, Sandra Gębura, Paweł Świec, Krystian Prusik, Magdalena Szklarska, and Danuta Stróż. 2023. "Influence of Hafnium Addition on the Microstructure, Microhardness and Corrosion Resistance of Ti20Ta20Nb20(ZrMo)20−xHfx (where x = 0, 5, 10, 15 and 20 at.%) High Entropy Alloys" Materials 16, no. 4: 1456. https://doi.org/10.3390/ma16041456






