Formation of MXene-Derived/NiCoFe-LDH Heterostructures for Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
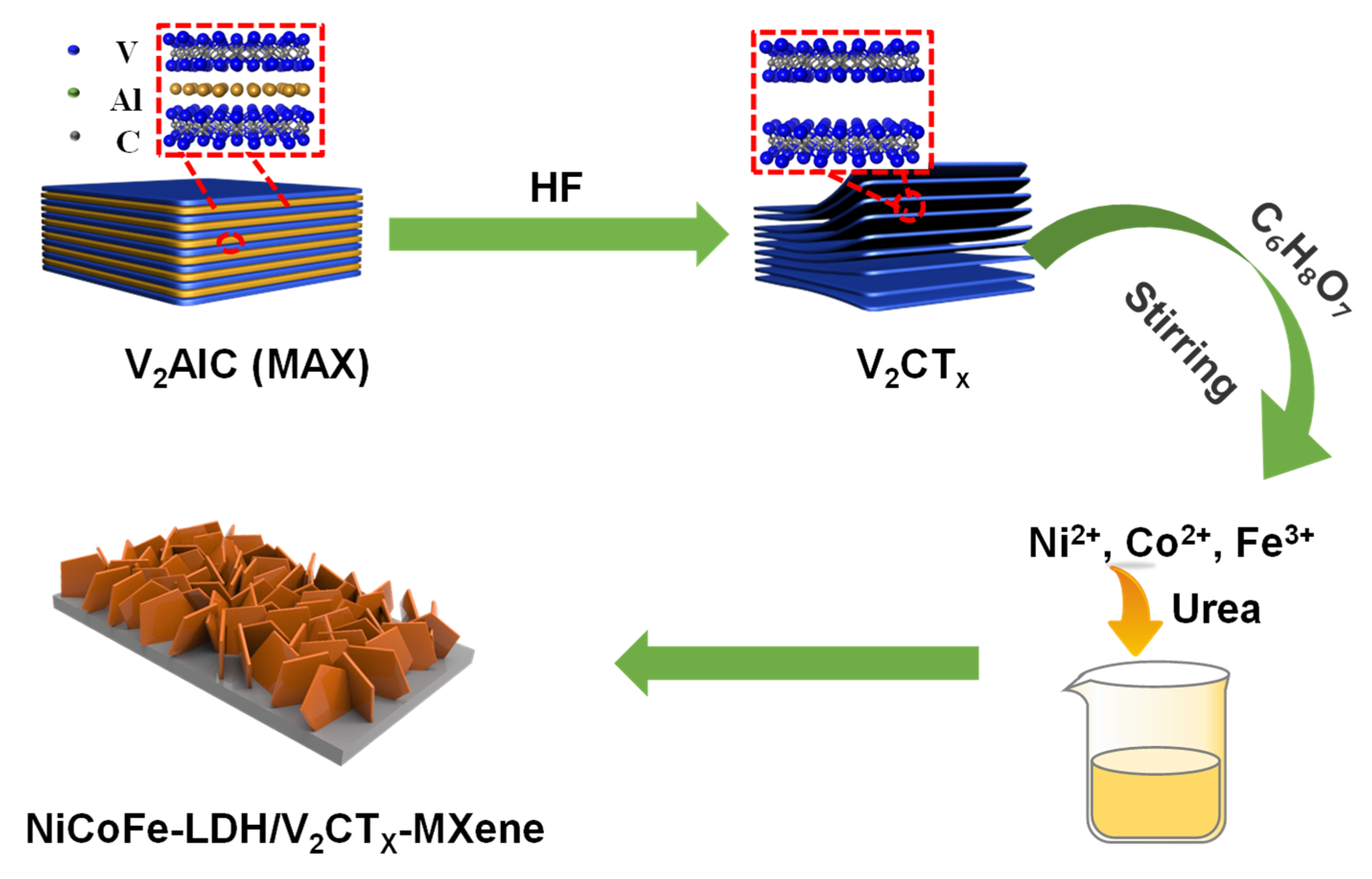
2.1. Material Synthesis
2.1.1. Synthesis of V2CTx–MXene
2.1.2. Synthesis of NiCoFe–LDH/V2CTx–MXene
2.2. Material Characterization
2.3. Electrochemical Performance Tests
3. Results and Discussion
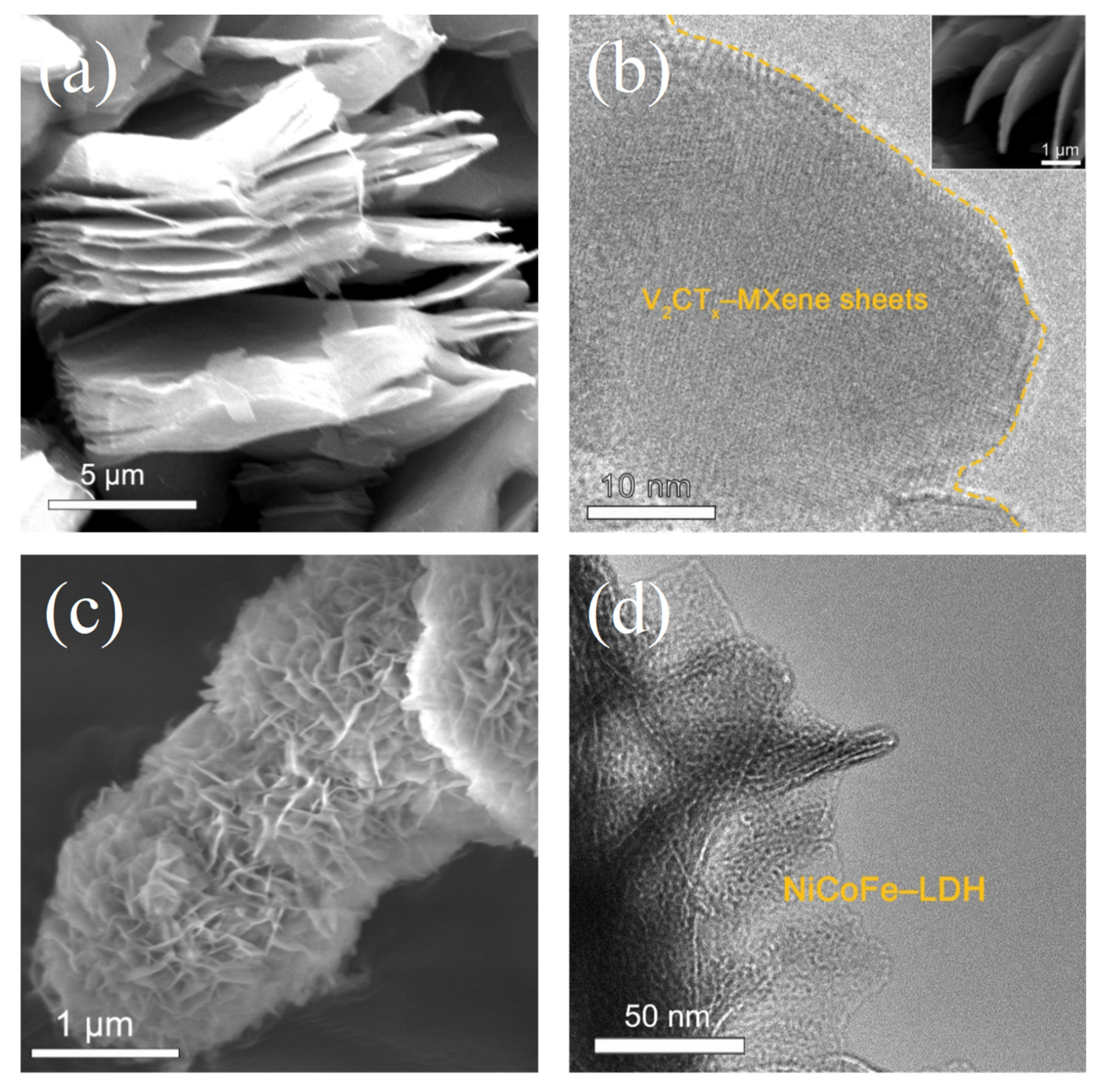
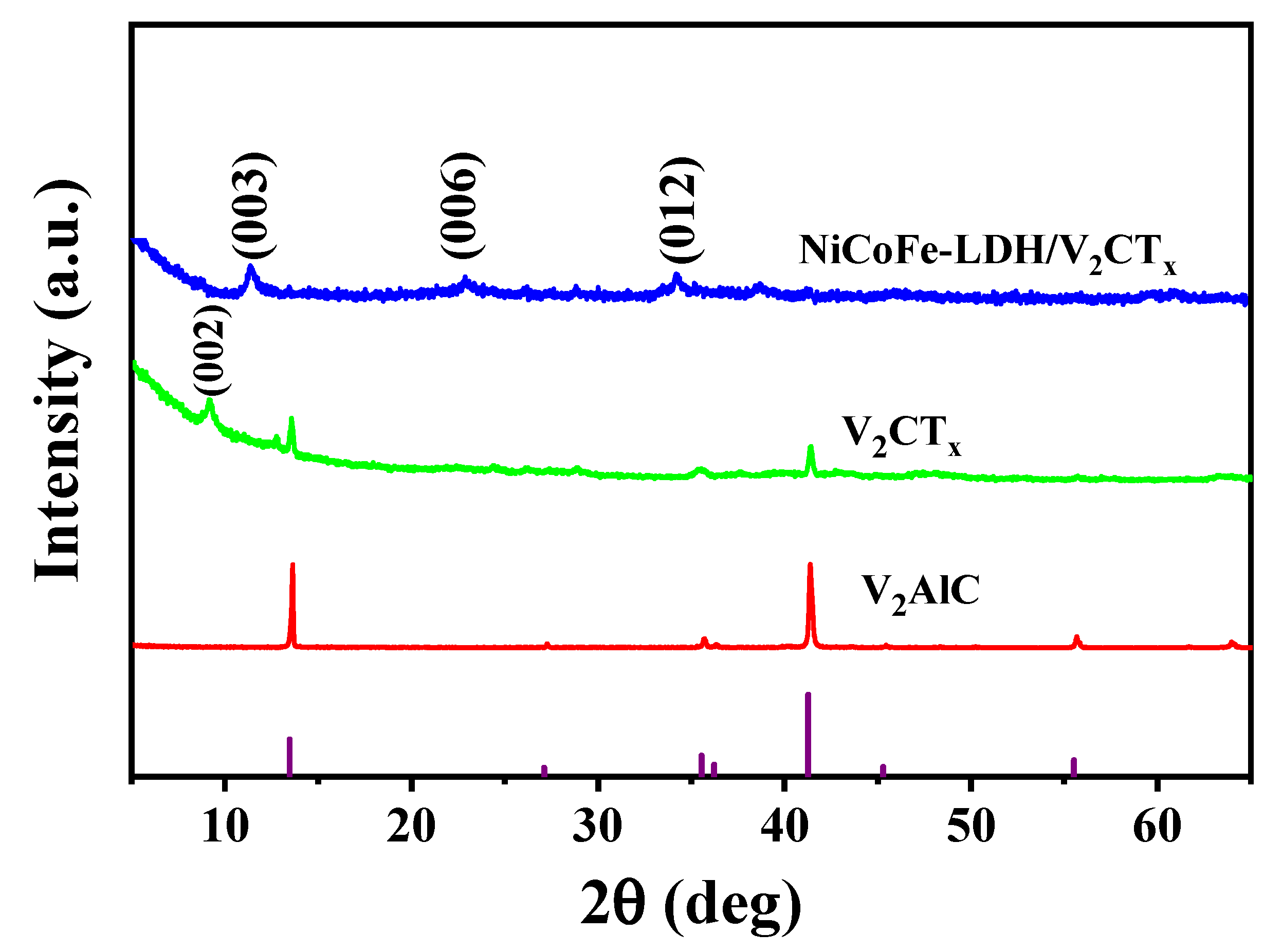
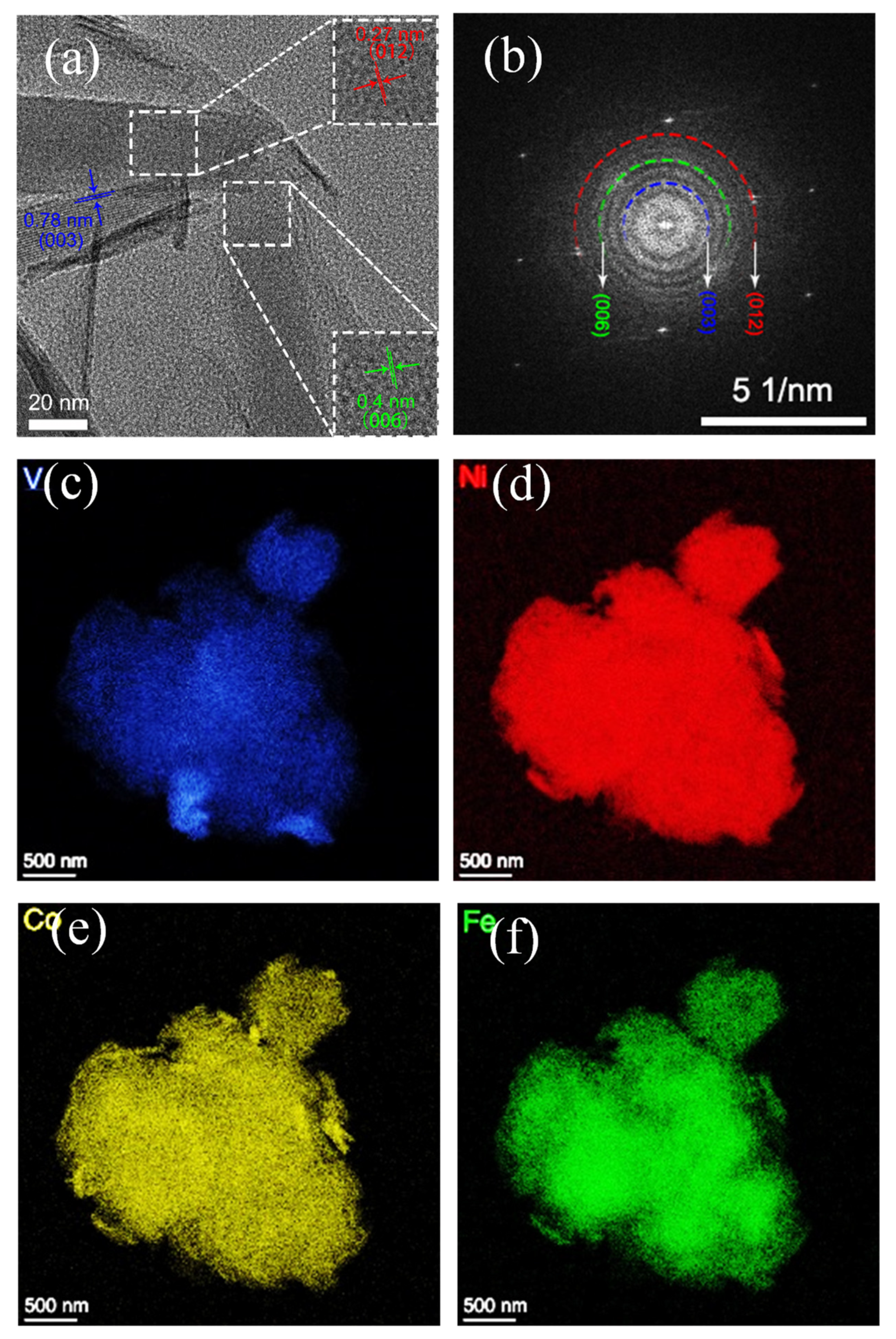
3.1. Material Characterization
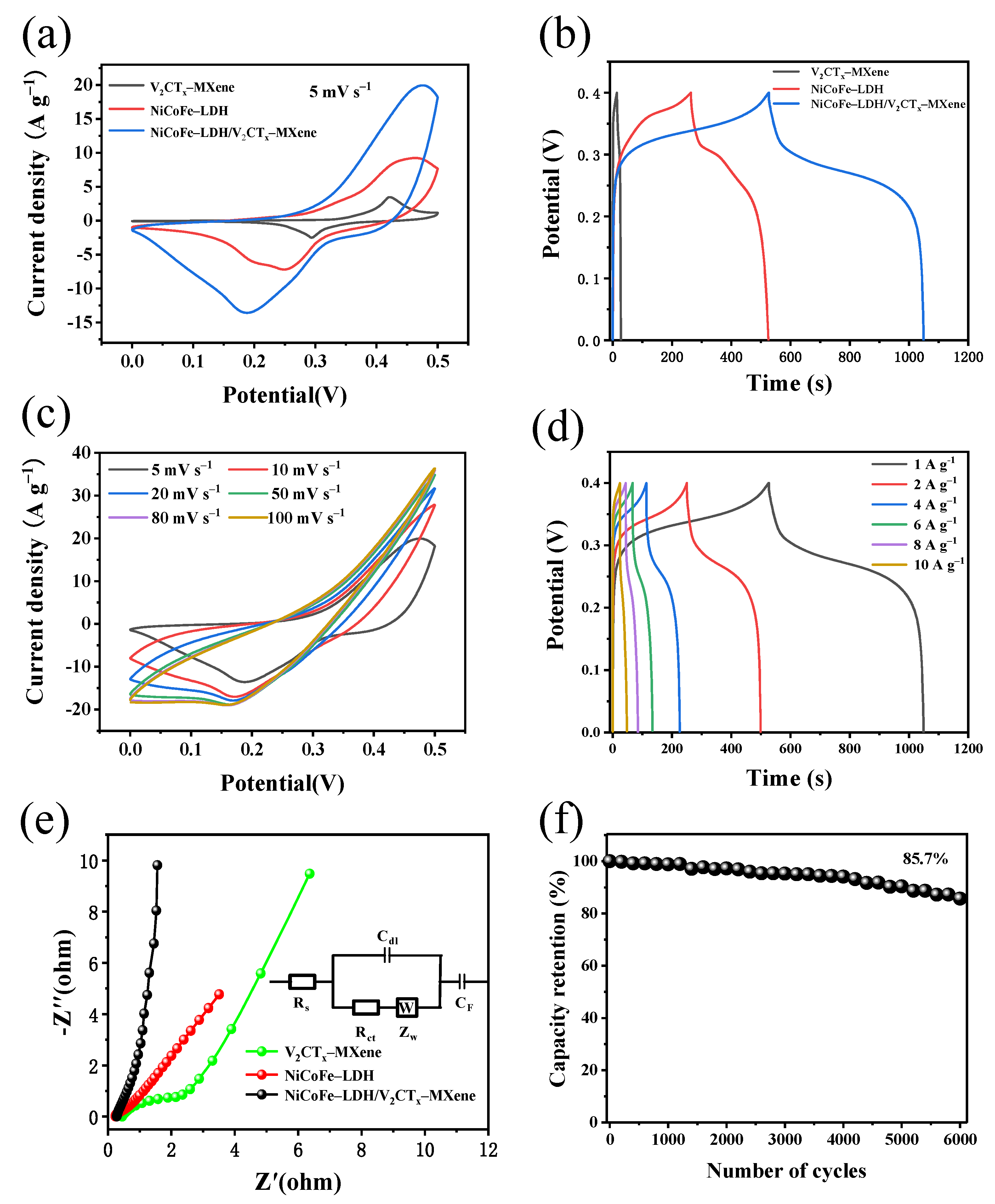
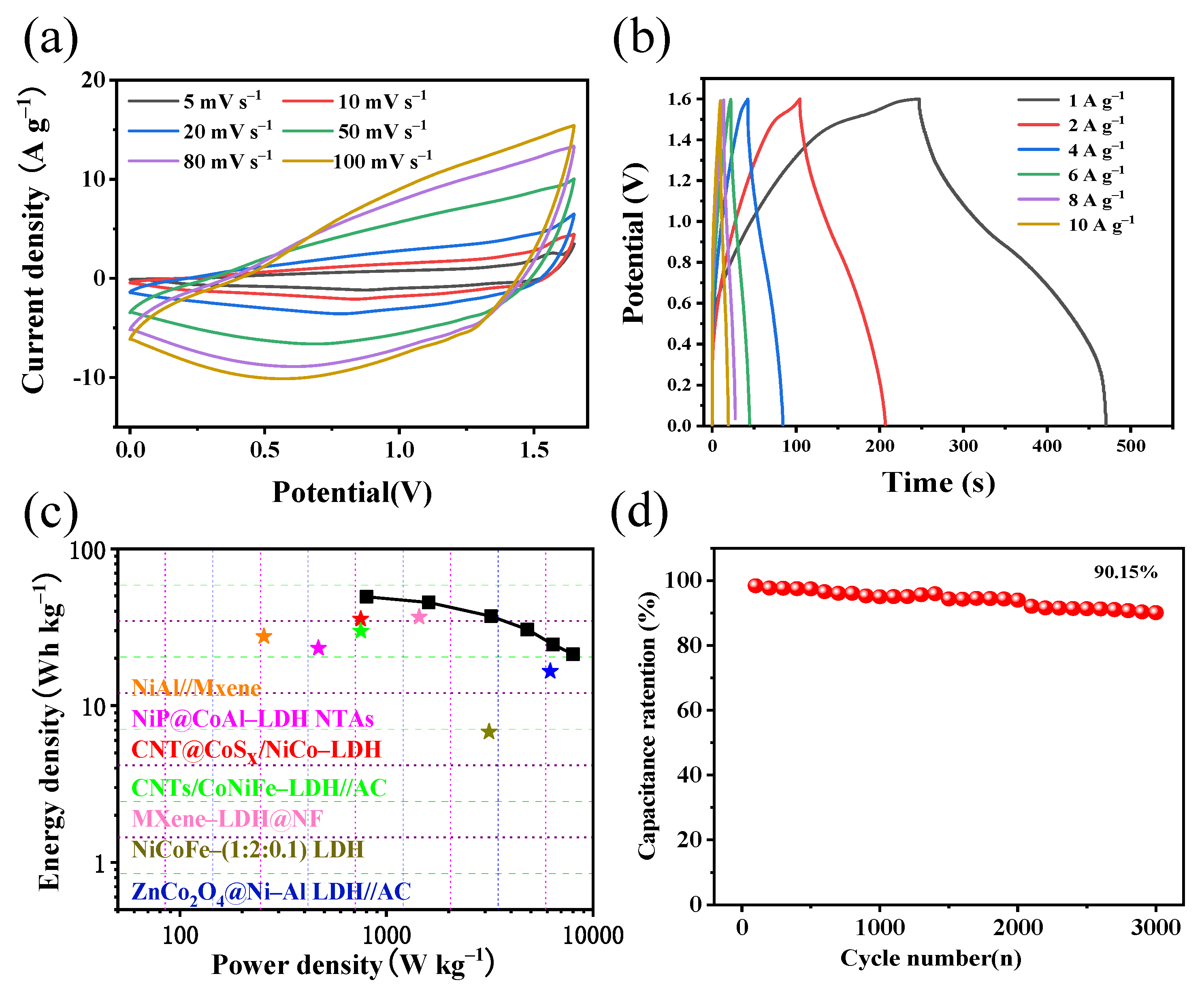
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Miao, M.; Zhang, L. Carbon peaking and carbon neutrality goals and reflections on China’s energy transition Part II—Fossil energy in energy transition. Sino Glob. Energy 2022, 27, 1–7. [Google Scholar]
- Xu, J.; Yang, X.; Zou, Y.; Zhu, L.; Xu, F.; Sun, L.; Xiang, C.; Zhang, J. High density anchoring of NiMoS4 on ultrathin Ti3C2 MXene assisted by dopamine for supercapacitor electrode materials. J. Alloys Compd. 2021, 891, 161945. [Google Scholar] [CrossRef]
- Sui, Q.; Yu, Y.; Xiang, C.; Wang, Q.; Sun, L.; Xu, F.; Zhang, J.; Zou, Y. Static adsorption of MOFs nanosheets on 3D nanocubes for supercapacitor electrode materials. J. Alloys Compd. 2022, 921, 165982. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Xu, J.; Xiang, C.; Yu, S.; Zou, Y.; Fang, S.; Hu, Z.; Xu, F.; Sun, L. Synthesis of Porous Yolk-Shelled NiSe2–MnSe Heterojunctions for High-Cycling-Stability Asymmetric Supercapacitor Electrode Materials. ACS Appl. Energy Mater. 2022, 5, 6194–6205. [Google Scholar] [CrossRef]
- Wang, S.; Fang, S.; Zhang, K.; Zou, Y.; Xiao, Z.; Xu, F.; Sun, L.; Xiang, C. Growth of yolk-shell CuCo2S4 on NiO nanosheets for high-performance flexible supercapacitors. Ceram. Int. 2021, 48, 3636–3646. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene–2D layered electrode materials for energy storage. Prog. Nat. Sci. 2018, 28, 133–147. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yan, X.; Lyu, M.; Wang, L.; Bell, J.; Wang, H. 2-Methylimidazole-Derived Ni–Co Layered Double Hydroxide Nanosheets as High Rate Capability and High Energy Density Storage Material in Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 15510–15524. [Google Scholar] [CrossRef]
- Mao, X.; Zou, Y.; Xu, F.; Sun, L.; Chu, H.; Zhang, H.; Zhang, J.; Xiang, C. Three-Dimensional Self-Supporting Ti3C2 with MoS2 and Cu2O Nanocrystals for High-Performance Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 22664–22675. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Hu, L.; Chen, M.; Yan, Y.; Wu, L. Nickel-Cobalt Layered Double Hydroxide Nanosheets for High-performance Supercapacitor Electrode Materials. Adv. Funct. Mater. 2013, 24, 934–942. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, J.; Li, Z.; Yang, W.; Wang, B.; Hou, M.; He, Y.; Liu, Q.; Mann, T.; Yang, P.; et al. Graphene nanosheet/Ni2+/Al3+ layered double-hydroxide composite as a novel electrode for a supercapacitor. Chem. Mater. 2011, 23, 3509–3516. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, X.; Zhan, J.; Sui, Y.; Qi, J.; Wei, F.; Meng, Q.; He, Y.; Ren, Y.; Zhan, Z.; et al. Hierarchical NiS@CoS with Controllable Core-Shell Structure by Two-Step Strategy for Supercapacitor Electrodes. Adv. Mater. Interfaces 2019, 7, 1901618. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Xu, F.; Xiang, C.; Sui, Q.; Zhang, J.; Sun, L.; Chen, Z. Ultrathin graphene@NiCo2S4@Ni-Mo layered double hydroxide with a 3D hierarchical flowers structure as a high performance positive electrode for hybrid supercapacitor. J. Energy Storage 2022, 52, 105049. [Google Scholar] [CrossRef]
- Liang, J.; Xiang, C.; Zou, Y.; Hu, X.; Chu, H.; Qiu, S.; Xu, F.; Sun, L. Spacing graphene and Ni-Co layered double hydroxides with polypyrrole for high-performance supercapacitors. J. Mater. Sci. Technol. 2020, 55, 190–197. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Wei, X.; Yuan, C.; Liu, T.; Cao, D.; Yin, J.; Wang, G. Preparation and electrochemical performances of nanostructured CoxNi1−x(OH)2 composites for supercapacitors. J. Power Sources 2013, 240, 338–343. [Google Scholar] [CrossRef]
- Cai, X.; Shen, X.; Ma, L.; Ji, Z.; Xu, C.; Yuan, A. Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor. Chem. Eng. J. 2015, 268, 251–259. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, J.; Zhang, W.; Ma, L.; Jiang, Z.; Wang, J.; Huang, Y. Synergistically coupling of 3D FeNi-LDH arrays with Ti3C2Tx-MXene nanosheets toward superior symmetric supercapacitor. Nano Energy 2022, 91, 106633. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Fouad, O.A.; Makhlouf, S.A.; Yusoff, M.M.; Chong, K.F. Co3O4/SiO2 nanocomposites for supercapacitor application. J. Solid State Electrochem. 2014, 18, 2505–2512. [Google Scholar] [CrossRef] [Green Version]
- Senthil, R.A.; Pan, J.; Yang, X.; Sun, Y. Nickel foam-supported NiFe layered double hydroxides nanoflakes array as a greatly enhanced electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 21824–21834. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Mojtabavi, M.; Caffrey, N.M.; Wanunu, M.; Beidaghi, M. Assembling 2D MXenes into Highly Stable Pseudocapacitive Electrodes with High Power and Energy Densities. Adv. Mater. 2019, 31, 1806931. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Kim, B.J.; Chen, Z. One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754–4762. [Google Scholar] [CrossRef]
- Xia, D.; Chen, H.; Jiang, J.; Zhang, L.; Zhao, Y.; Guo, D.; Yu, J. Facilely synthesized α phase nickel–cobalt bimetallic hydroxides: Tuning the composition for high pseudocapacitance. Electrochimica Acta 2015, 156, 108–114. [Google Scholar] [CrossRef]
- Ma, K.; Cheng, J.; Zhang, J.; Li, M.; Liu, F.; Zhang, X. Dependence of Co/Fe ratios in Co-Fe layered double hydroxides on the structure and capacitive properties. Electrochimica Acta 2016, 198, 231–240. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Liu, Z.; Wang, L.; Han, P.; Xu, H.; Zhang, K.; Dong, S.; Yao, J.; Cui, G. Nitrogen-doped graphene nanosheets with excellent lithium storage properties. J. Mater. Chem. 2011, 21, 5430–5434. [Google Scholar] [CrossRef]
- Lu, X.; Han, Y.; Lu, T. Structure Characterization and Application of Graphdiyne in Photocatalytic and Electrocatalytic Reactions. Acta Phys. Chim. Sin. 2018, 34, 1014–1028. [Google Scholar] [CrossRef]
- Yu, L.; Yi, Q.; Yang, X.; Li, G. A Facile Synthesis of C-N Hollow Nanotubes as High Electroactivity Catalysts of Oxygen Reduction Reaction Derived from Dicyandiamide. Chemistryselect 2018, 3, 12603–12612. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [Green Version]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourfarzad, H.; Shabani-Nooshabadi, M.; Ganjali, M.R.; Kashani, H. Synthesis of Ni-Co-Fe layered double hydroxide and Fe2O3/Graphene nanocomposites as actively materials for high electrochemical performance supercapacitors. Electrochim. Acta 2019, 317, 83–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Li, J.; Yuan, Z.; Li, D.; Wang, L.; Han, W. Self-assembled Cobalt-doped NiMn-layered double hydroxide (LDH)/V2CTX MXene hybrids for advanced aqueous electrochemical energy storage properties. Chem. Eng. J. 2022, 430, 132992. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, H.; Wang, J.; Ding, B.; Xu, Y.; Chang, Z.; Hao, X. Three-dimensional porous MXene/layered double hydroxide composite for high performance supercapacitors. J. Power Sources 2016, 327, 221–228. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.; Ling, Z.; Qiu, J.; Gogotsib, Y. Facile fabrication of MWCNT-doped NiCoAl-layered double hydroxide nanosheets with enhanced electrochemical performances. J. Mater. Chem. A 2013, 1, 1963–1968. [Google Scholar] [CrossRef]
- Zhong, Y.; Liao, Y.; Gao, A.; Hao, J.; Shu, D.; Huang, Y.; Zhong, J.; He, C.; Zeng, R. Supercapacitive behavior of electrostatic self-assembly reduced graphene oxide/CoAl-layered double hydroxides nanocomposites. J. Alloys Compd. 2016, 669, 146–155. [Google Scholar] [CrossRef]
- Guo, J.; Bian, Z.; Ye, L.; Shang, Y.; Guo, F.; Zhang, Y.; Xu, J. Double layers combined with MXene and in situ grown NiAl-LDH arrays on nickel foam for enhanced asymmetric supercapacitors. Ionics 2022, 28, 2967–2977. [Google Scholar] [CrossRef]
- Wu, W.; Shuai, M.; Kuang, H.; Zhang, H.; Zhang, W.; Chen, H.; Ling, Q. Synthesis of NiCo-layered double hydroxide/multilayer graphene composite via the ozone oxidation combined microwave-assisted synthesis strategy. Diam. Relat. Mater. 2021, 120, 108636. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Zadeh, M.H.R.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloys Compd. 2020, 860, 158497. [Google Scholar] [CrossRef]
- Miao, C.; Yin, X.; Xia, G.; Zhu, K.; Ye, K.; Wang, Q.; Yan, J.; Cao, D.; Wang, G. Facile microwave-assisted synthesis of cobalt diselenide/reduced graphene oxide composite for high-performance supercapacitors. Appl. Surf. Sci. 2020, 543, 148811. [Google Scholar] [CrossRef]
- Mao, X.; Zou, Y.; Liang, J.; Xiang, C.; Chu, H.; Yan, E.; Zhang, H.; Xu, F.; Hu, X.; Sun, L. Facile synthesis of hierarchical Co–Mo–O–S porous microspheres for high-performance supercapacitors. Ceram. Int. 2020, 46, 1448–1456. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, B.; Wang, C.; Xia, X.; Lei, W.; Hao, Q. Bimetallic metal-organic framework derived porous NiCo2S4 nanosheets arrays as binder-free electrode for hybrid supercapacitor. Appl. Surf. Sci. 2020, 542, 148621. [Google Scholar] [CrossRef]
- Sui, Q.; Li, J.; Xiang, C.; Xu, F.; Zhang, J.; Sun, L.; Zou, Y. Nickel metal-organic framework microspheres loaded with nickel-cobalt sulfides for supercapacitor electrode materials. J. Energy Storage 2022, 55, 105525. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2020, 409, 128216. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, R.; Xu, W.; Ding, J.; Wang, Y.; Yan, X.; Shi, W.; Wang, M. Soluble salt assisted synthesis of hierarchical porous carbon-encapsulated Fe3C based on MOFs gel for all-solid-state hybrid supercapacitor. Chem. Eng. J. 2021, 419, 129576. [Google Scholar] [CrossRef]
- Sun, H.; Yang, X.; Zhang, L.; Zhao, L.; Lian, J. Reusable CoxNi1-x dye adsorbents as supercapacitor electrode materials. J. Mater. Chem. A 2017, 5, 8095–8107. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Z.; Li, R.; Zheng, X.; Lu, F.; He, T. Template-assisted synthesis of NiP@CoAl-LDH nanotube arrays with superior electrochemical performance for supercapacitors. Electrochim. Acta 2016, 204, 160–168. [Google Scholar] [CrossRef]
- Cheng, T. All-solid high-performance asymmetric supercapacitor based onyolk−shell NiMoO4/V2CTx@reduced graphene oxide and hierarchical bamboo-shaped MoO2@Fe2O3/N-doped carbon. Energy Fuels 2021, 35, 10250–10261. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Q.; Bai, C.; Wang, F.; Sun, S.; Xu, Y.; Li, H. Synthesis of CNTs/CoNiFe-LDH nanocomposite with high specific surface area for asymmetric supercapacitor. Nanomaterials 2021, 11, 2155. [Google Scholar] [CrossRef]
- Li, H.; Musharavati, F.; Zalenezhad, E.; Chen, X.; Hui, K. Electrodeposited Ni Co layered double hydroxides on titanium carbide as a binder-free electrode for supercapacitors. Electrochimica Acta 2018, 261, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Ni, C.-S.; Liu, S.-F.; Lee, J.-F.; Pao, C.-W.; Chen, J.-L.; Chen, H.-Y.; Huang, J.-H. Binder-free NiCoFe layered double hydroxide nanosheets for flexible energy storage devices with high-rate-retention characteristics. Electrochimica Acta 2021, 384, 138415. [Google Scholar] [CrossRef]
- Bai, X.; Cao, D.; Zhang, H. Constructing ZnCo2O4@LDH Core–Shell hierarchical structure for high performance supercapacitor electrodes. Ceram. Int. 2019, 45, 14943–14952. [Google Scholar] [CrossRef]



| Composites | Specific Capacitances at Different Current Densities (F g−1) | Current Density (A g−1), Number of Cycles | Capacitance Retention (%) | Reference | ||
|---|---|---|---|---|---|---|
| 1 A g−1 | 2 A g−1 | 10 A g−1 | ||||
| Ti3C2T10/NiCo–LDH | - | 730 | 580 | 4, 2000 | 81 | [15] |
| NiMn–LDH/V2CTx–Mxene | 1005 | 836 | 570 | - | - | [33] |
| M30/LDH | 1061 | - | - | 4, 4000 | 70 | [34] |
| NiCoAl–LDH–MWCNT | 1035 | 974 | 597 | 6, 1000 | 83 | [35] |
| RGO/CoAl–LDH | 825 | 752 | - | 4, 4000 | 89.3 | [36] |
| NiAl–LDH/Mxene | 1600 | 1453 | - | 10, 3000 | 78 | [37] |
| NiCo–LDH/MLG | 1212.75 | 1163.5 | - | 6, 3000 | 80.5 | [38] |
| Present study | 1305 | 1245 | 605 | 10, 6000 | 85.7 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Chen, T.; Zou, Y. Formation of MXene-Derived/NiCoFe-LDH Heterostructures for Supercapacitor Applications. Materials 2023, 16, 1643. https://doi.org/10.3390/ma16041643
Guo Y, Chen T, Zou Y. Formation of MXene-Derived/NiCoFe-LDH Heterostructures for Supercapacitor Applications. Materials. 2023; 16(4):1643. https://doi.org/10.3390/ma16041643
Chicago/Turabian StyleGuo, Yihan, Tongxiang Chen, and Yongjin Zou. 2023. "Formation of MXene-Derived/NiCoFe-LDH Heterostructures for Supercapacitor Applications" Materials 16, no. 4: 1643. https://doi.org/10.3390/ma16041643






