Ionic Liquids as Working Fluids for Heat Storage Applications: Decomposition Behavior of N-Butyl-N-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HRMAS NMR
2.3. ICP-OES Measurements
2.4. EDX Measurements
3. Results and Discussion
3.1. Sample Appearance after Thermal Treatment
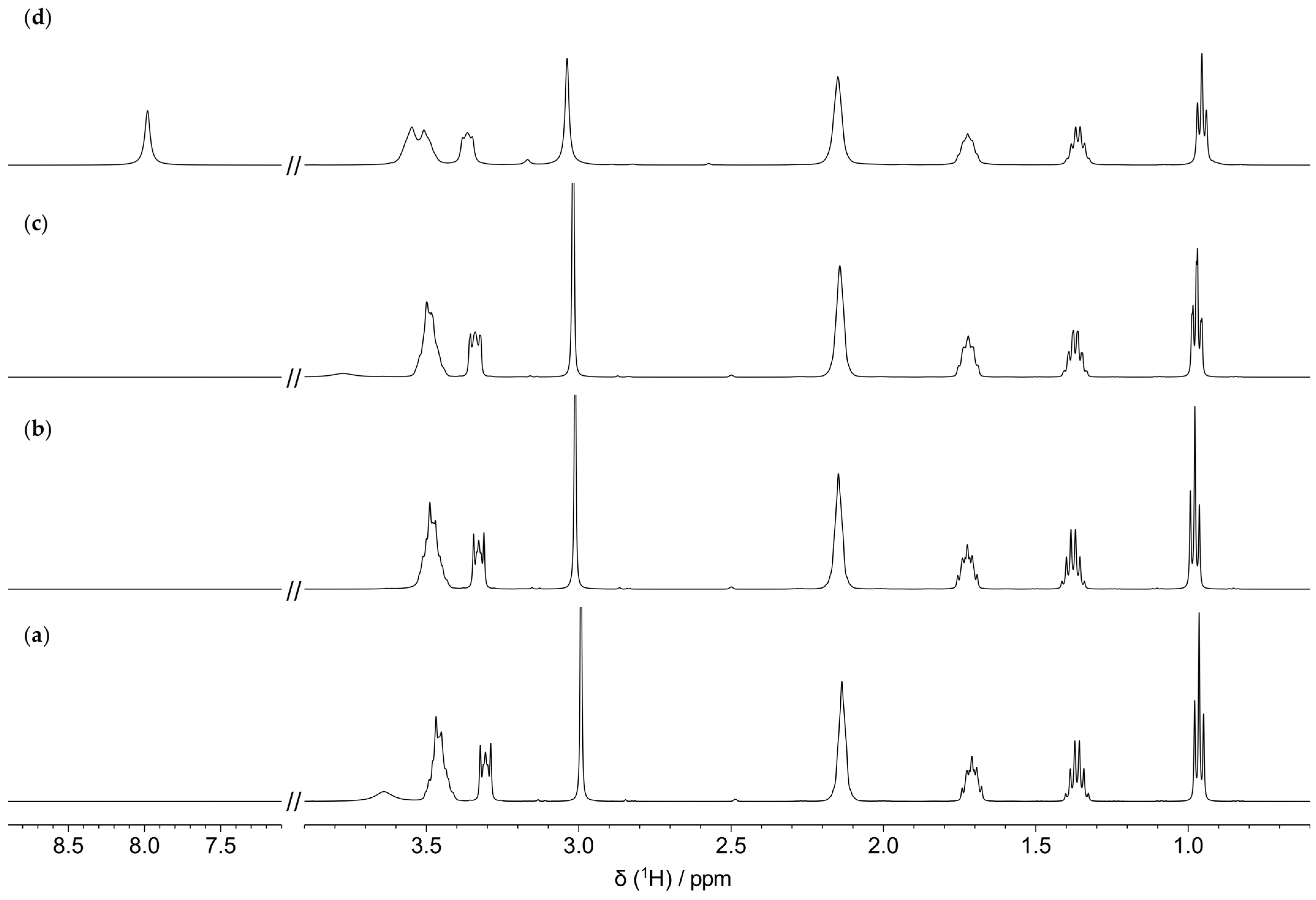
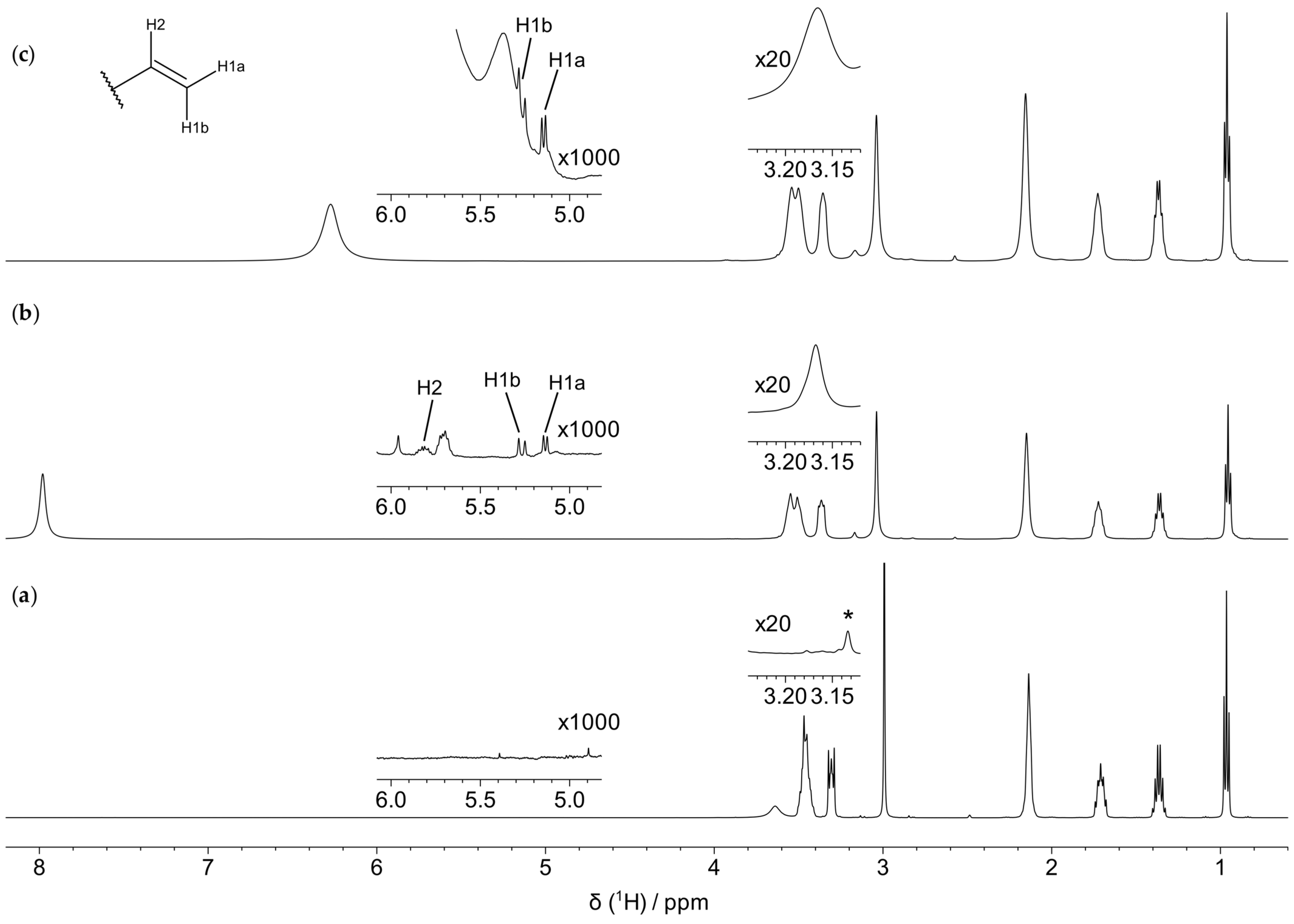
3.2. Characterization of the Thermal Degradation of the Cation
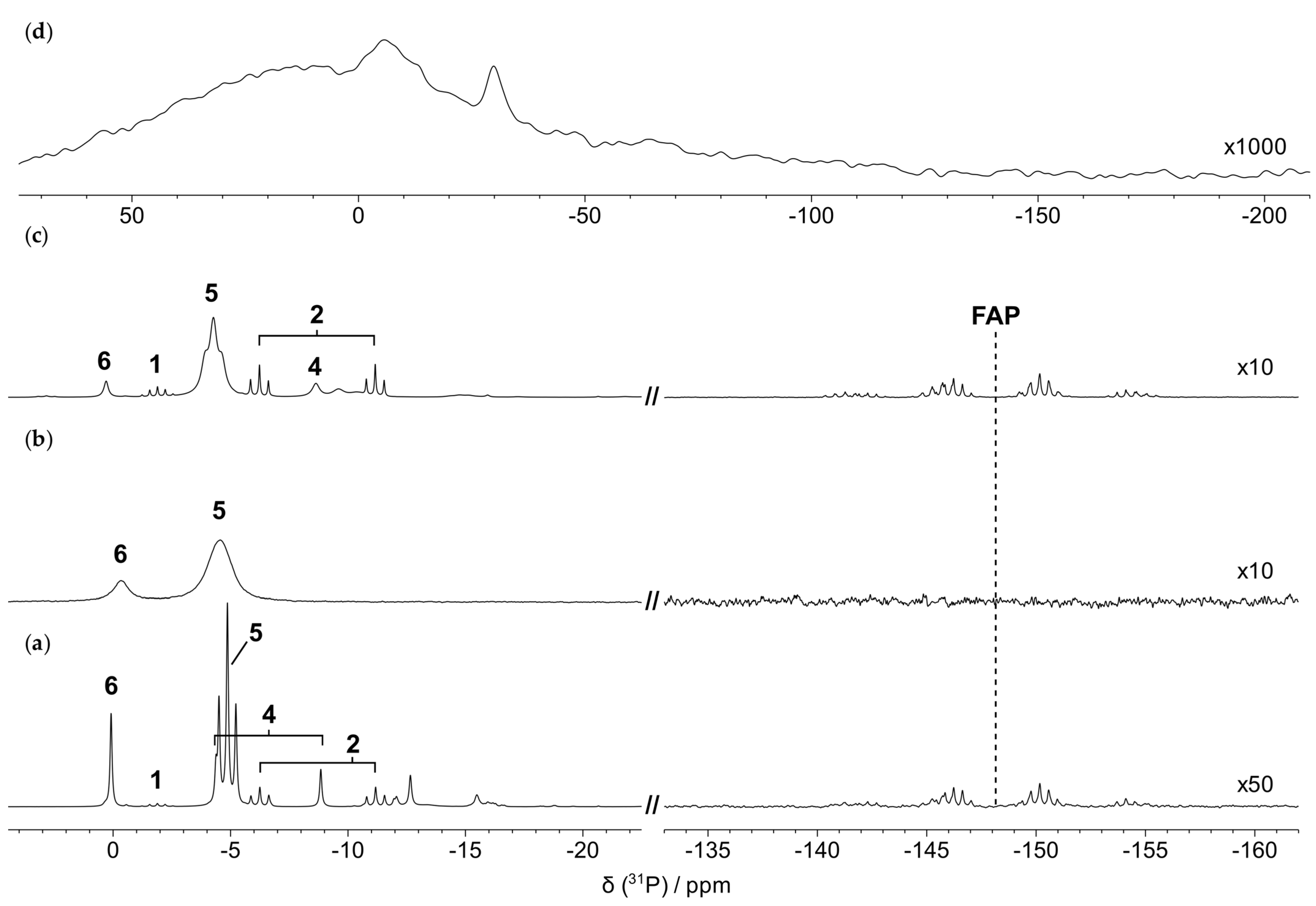
3.2.1. Thermal Degradation of the Cation in the Absence of the Metal/Alloy
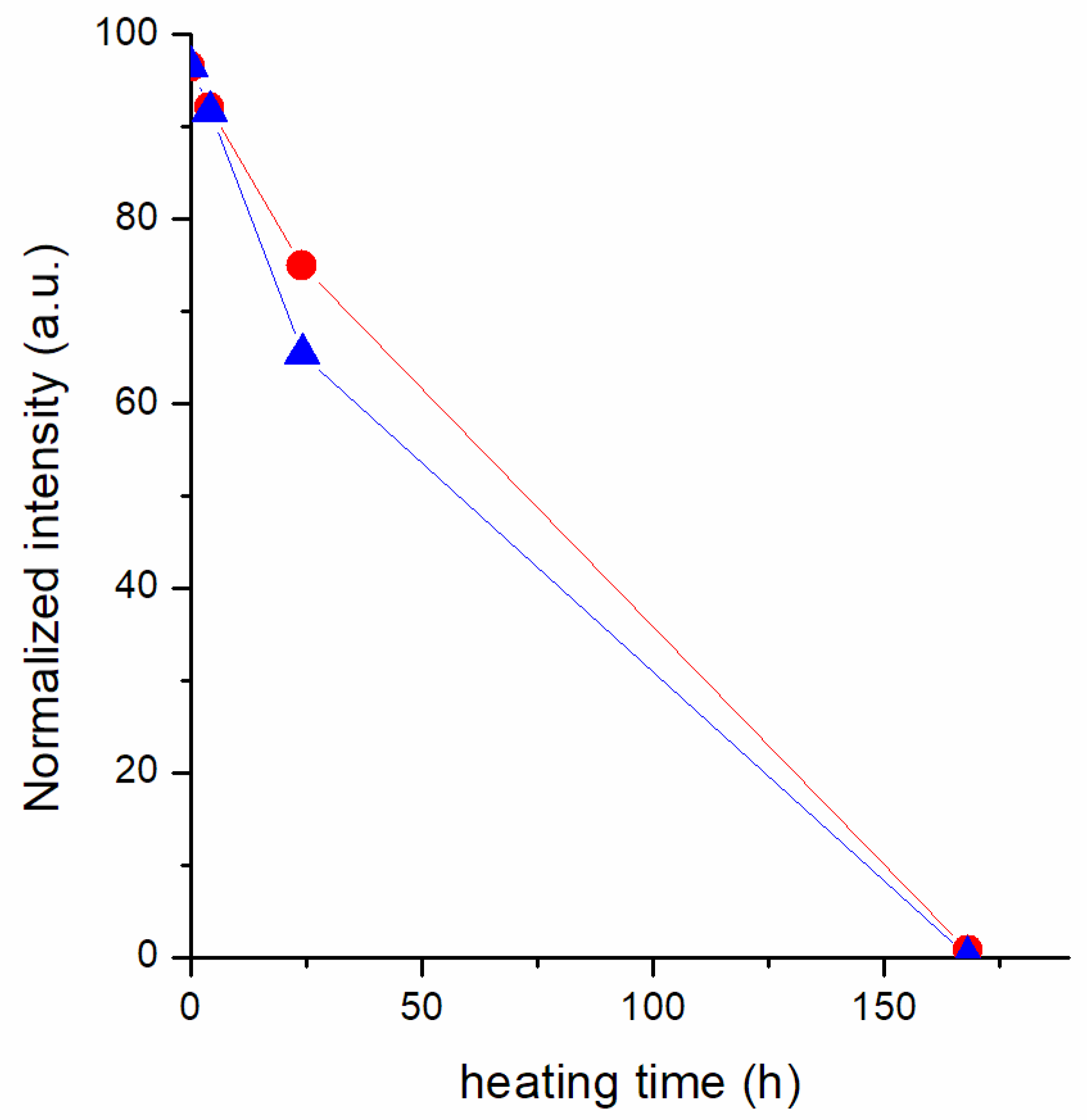
3.2.2. Thermal Degradation of the Cation in the Presence of the Metal/Alloy
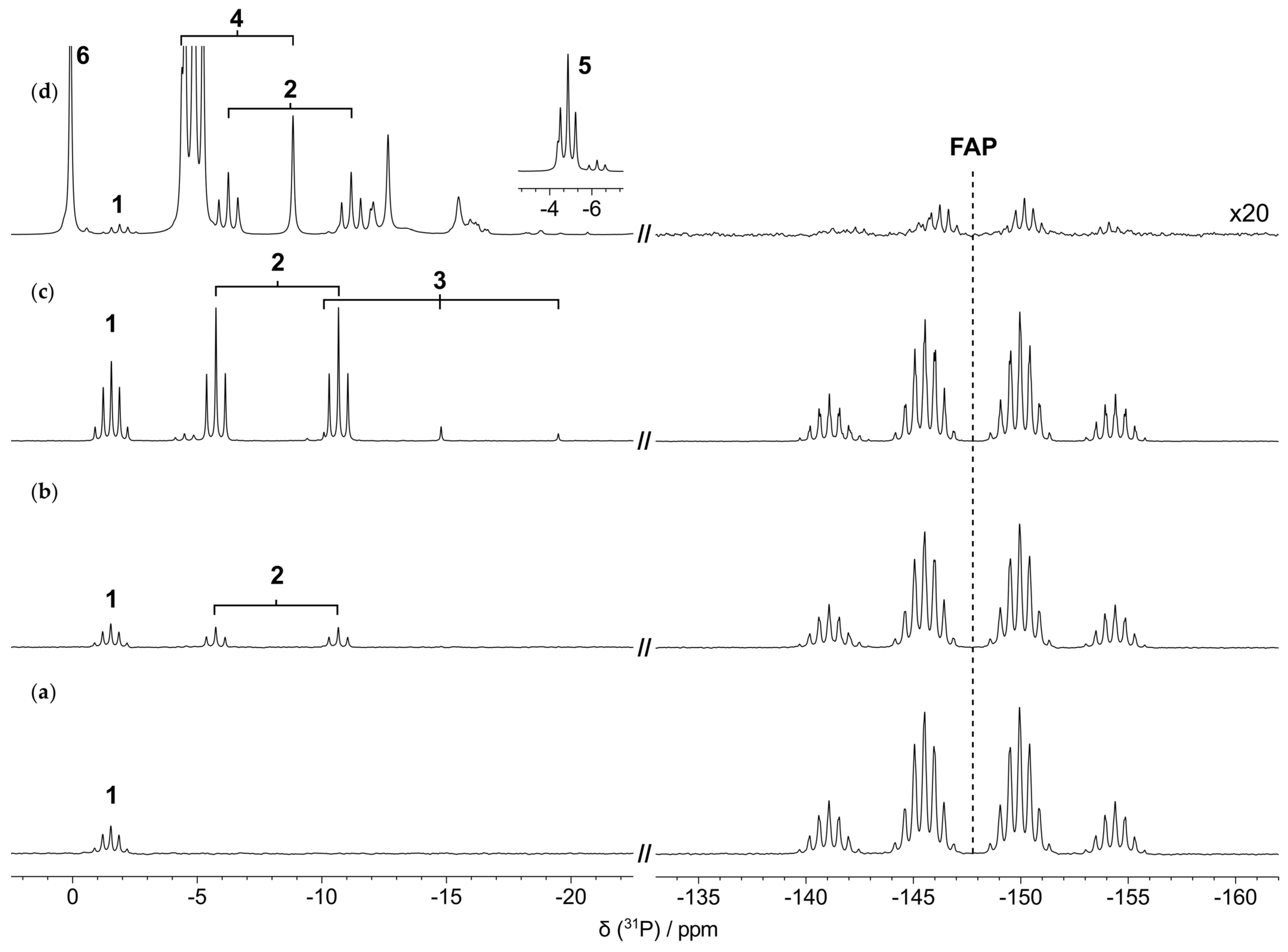
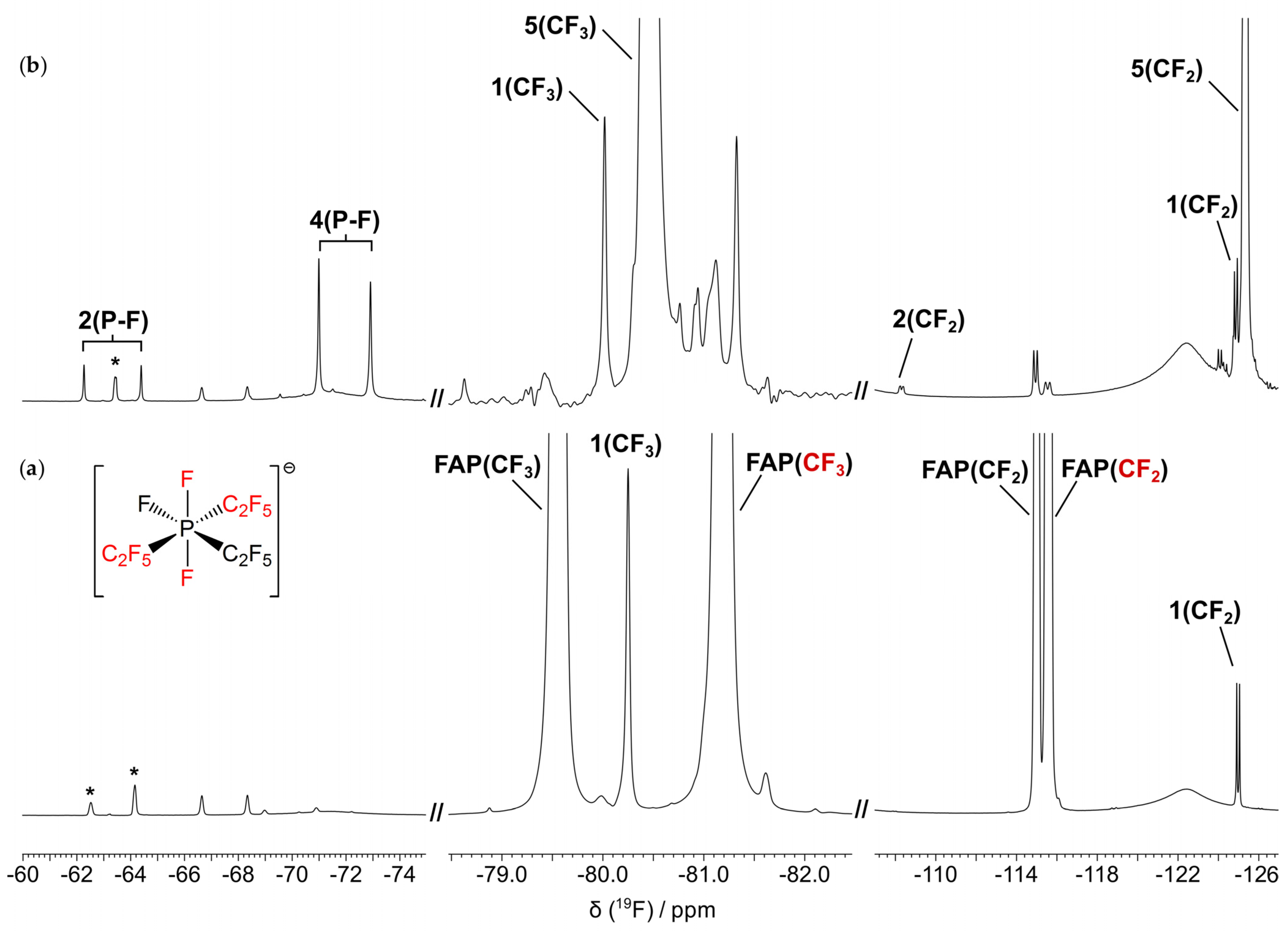
3.3. Characterization of the Thermal Degradation of the Anion
3.3.1. Thermal Degradation of the Anion in the Absence of the Metal/Alloy
3.3.2. Thermal Degradation of the Anion in the Presence of the Metal/Alloy
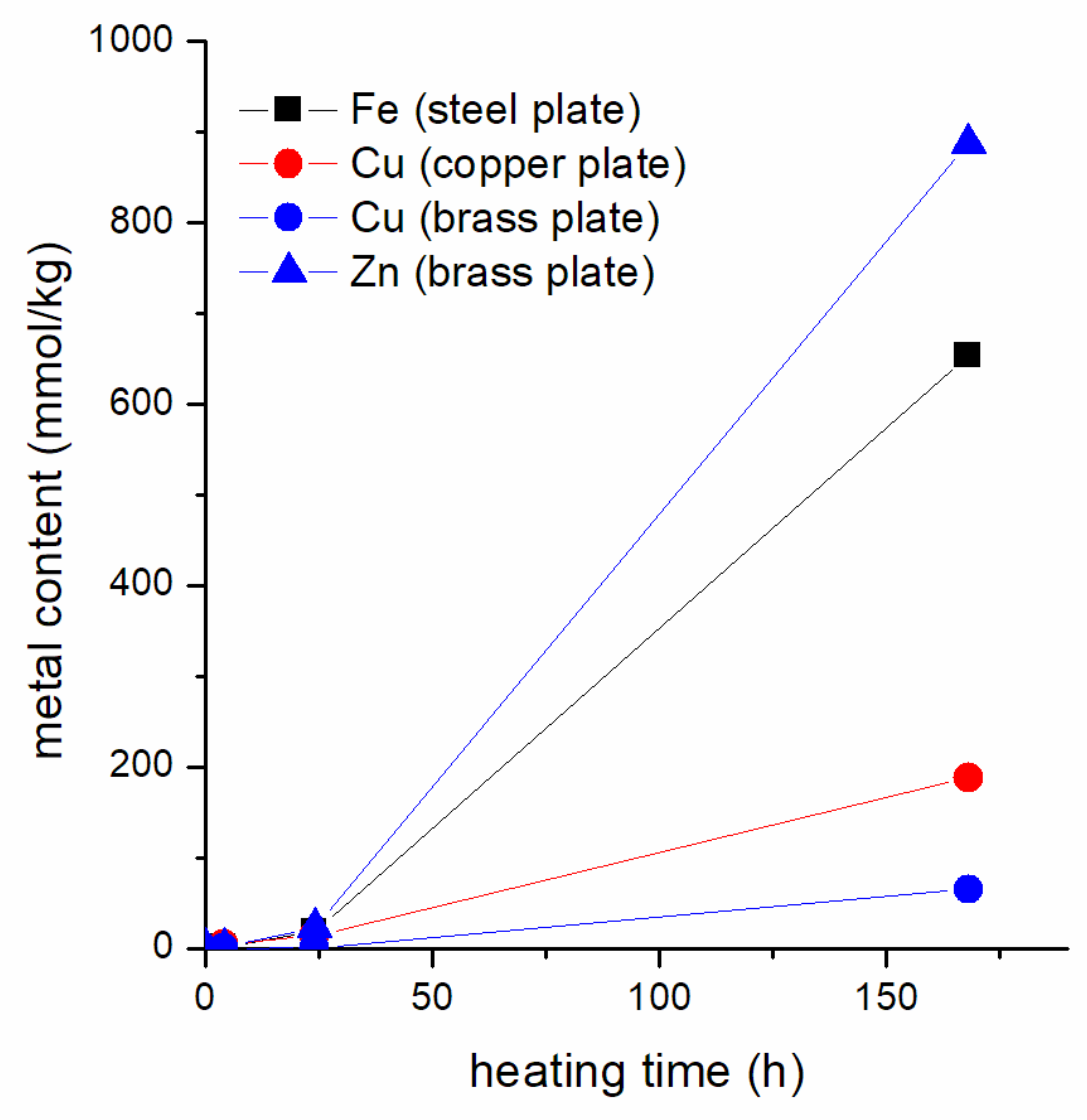
3.4. Detection of Elements in the Heated IL Using ICP-OES and EDX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauer, A. Advances in Energy Storage: Latest Developments from R&D to the Market; WILEY: New York, NY, USA, 2022; ISBN 978-1-119-23935-2. [Google Scholar]
- Cabeza, L.F. Advances in Thermal Energy Storage Systems: Methods and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 978-1-78242-096-5. [Google Scholar]
- DeLovato, N.; Sundarnath, K.; Cvijovic, L.; Kota, K.; Kuravi, S. A Review of Heat Recovery Applications for Solar and Geothermal Power Plants. Renew. Sustain. Energy Rev. 2019, 114, 109329. [Google Scholar] [CrossRef]
- Mourad, A.; Aissa, A.; Said, Z.; Younis, O.; Iqbal, M.; Alazzam, A. Recent Advances on the Applications of Phase Change Materials for Solar Collectors, Practical Limitations, and Challenges: A Critical Review. J. Energy Storage 2022, 49, 104186. [Google Scholar] [CrossRef]
- Khan, M.I.; Asfand, F.; Al-Ghamdi, S.G. Progress in Research and Technological Advancements of Thermal Energy Storage Systems for Concentrated Solar Power. J. Energy Storage 2022, 55, 105860. [Google Scholar] [CrossRef]
- Piper, S.L.; Kar, M.; MacFarlane, D.R.; Matuszek, K.; Pringle, J.M. Ionic Liquids for Renewable Thermal Energy Storage—A Perspective. Green Chem. 2022, 24, 102–117. [Google Scholar] [CrossRef]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, L.; Chen, B.; Fei, W. Thermodynamical Properties of Phase Change Materials Based on Ionic Liquids. Chem. Eng. J. 2009, 147, 58–62. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Visser, A.E.; Bridges, N.J.; Khan, J.A. Thermal Performance of Ionic Liquids for Solar Thermal Applications. Exp. Therm. Fluid Sci. 2014, 59, 88–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Salih, A.A.M.; Li, M.; Yang, B. Synthesis and Characterization of Functionalized Ionic Liquids for Thermal Storage. Energy Fuels 2014, 28, 2802–2810. [Google Scholar] [CrossRef]
- Das, L.; Rubbi, F.; Habib, K.; Aslfattahi, N.; Saidur, R.; Baran Saha, B.; Algarni, S.; Irshad, K.; Alqahtani, T. State-of-the-Art Ionic Liquid & Ionanofluids Incorporated with Advanced Nanomaterials for Solar Energy Applications. J. Mol. Liq. 2021, 336, 116563. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic Liquid Thermal Stabilities: Decomposition Mechanisms and Analysis Tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Z. Thermal Stability of Ionic Liquids: Current Status and Prospects for Future Development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Liu, Z.; Li, Y.; Sun, H.; Wang, H.; Wang, J. Thermal Decomposition and Volatility of Ionic Liquids: Factors, Evaluation and Strategies. J. Mol. Liq. 2022, 366, 120336. [Google Scholar] [CrossRef]
- Preibisch, Y.; Horsthemke, F.; Winter, M.; Nowak, S.; Best, A.S. Is the Cation Innocent? An Analytical Approach on the Cationic Decomposition Behavior of N-Butyl-N-Methylpyrrolidinium Bis(Trifluoromethanesulfonyl)Imide in Contact with Lithium Metal. Chem. Mater. 2020, 32, 2389–2398. [Google Scholar] [CrossRef]
- Pyschik, M.; Winter, M.; Nowak, S. Determination and Quantification of Cations in Ionic Liquids by Capillary Electrophoresis-Mass Spectrometry. J. Chromatogr. A 2017, 1485, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pyschik, M.; Schultz, C.; Passerini, S.; Winter, M.; Nowak, S. Aging of Cations of Ionic Liquids Monitored by Ion Chromatography Hyphenated to an Electrospray Ionization Mass Spectrometer. Electrochim. Acta 2015, 176, 1143–1152. [Google Scholar] [CrossRef]
- Ananikov, V.P. Characterization of Molecular Systems and Monitoring of Chemical Reactions in Ionic Liquids by Nuclear Magnetic Resonance Spectroscopy. Chem. Rev. 2011, 111, 418–454. [Google Scholar] [CrossRef]
- Damodaran, K. Recent Advances in NMR Spectroscopy of Ionic Liquids. Prog. Nucl. Magn. Reson. Spectrosc. 2022, 129, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rencurosi, A.; Lay, L.; Russo, G.; Prosperi, D.; Poletti, L.; Caneva, E. HRMAS NMR Analysis in Neat Ionic Liquids: A Powerful Tool to Investigate Complex Organic Molecules and Monitor Chemical Reactions. Green Chem. 2007, 9, 216–218. [Google Scholar] [CrossRef]
- Alam, T.M.; Jenkins, J.E. HR-MAS NMR Spectroscopy in Material Science. In Advanced Aspects of Spectroscopy; Farrukh, M.A., Ed.; IntechOpen: London, UK, 2012; pp. 279–306. [Google Scholar]
- Nardelli, F.; Bramanti, E.; Lavacchi, A.; Pizzanelli, S.; Campanella, B.; Forte, C.; Berretti, E.; Freni, A. Thermal Stability of Ionic Liquids: Effect of Metals. Appl. Sci. 2022, 12, 1652. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Welz-Biermann, U.; Kucheryna, A.; Bissky, G.; Willner, H. New Ionic Liquids with Tris(Perfluoroalkyl)Trifluorophosphate (FAP) Anions. J. Fluor. Chem. 2005, 126, 1150–1159. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Bader, J.; Koppe, K.; Hoge, B.; Willner, H. Recent Progress in Perfluoroalkyl-Phosphorus Chemistry. J. Fluor. Chem. 2015, 171, 36–45. [Google Scholar] [CrossRef]
- Noori, S.; Diamanti, M.V.; Pedeferri, M.; Brenna, A.; Ormellese, M. Effect of Water Content on the Corrosiveness of Imidazolium-Based Ionic Liquids. Mater. Corros. 2018, 69, 1658–1668. [Google Scholar] [CrossRef]
- Perissi, I.; Bardi, U.; Caporali, S.; Fossati, A.; Lavacchi, A. Ionic Liquids as Diathermic Fluids for Solar Trough Collectors’ Technology: A Corrosion Study. Sol. Energy Mater. Sol. Cells 2008, 92, 510–517. [Google Scholar] [CrossRef]
- Salgado, J.; Parajó, J.J.; Fernández, J.; Villanueva, M. Long-Term Thermal Stability of Some 1-Butyl-1-Methylpyrrolidinium Ionic Liquids. J. Chem. Thermodyn. 2014, 74, 51–57. [Google Scholar] [CrossRef]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.C.; Chu, Y.H. On the Chemical Stabilities of Ionic Liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef]
- Preibisch, Y.; Peschel, C.; Dohmann, J.F.; Winter, M.; Nowak, S. Application of Gas Chromatography Hyphenated to Atmospheric Pressure Chemical Ionization-Quadrupole-Time-of-Flight-Mass Spectrometry (GC-APCI-Q-TOF-MS) for Structure Elucidation of Degradation Products Based on the Cation in Pyr14TFSI. J. Electrochem. Soc. 2021, 168, 026501. [Google Scholar] [CrossRef]
- Föhrenbacher, S.A.; Krahfuss, M.J.; Zapf, L.; Friedrich, A.; Ignat’ev, N.V.; Finze, M.; Radius, U. Tris(Pentafluoroethyl)Difluorophosphorane: A Versatile Fluoride Acceptor for Transition Metal Chemistry. Chem.–Eur. J. 2021, 27, 3504–3516. [Google Scholar] [CrossRef]
- Mahmood, T.; Shreeve, J.M. New Perfluoroalkylphosphonic and Bis(Perfluoroalkyl)Phosphinic Acids and Their Precursors. Inorg. Chem. 1986, 25, 3128–3131. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Willner, H.; Sartori, P. Electrochemical Fluorination (Simons Process)—A Powerful Tool for the Preparation of New Conducting Salts, Ionic Liquids and Strong Brønsted Acids. J. Fluor. Chem. 2009, 130, 1183–1191. [Google Scholar] [CrossRef]



| Metal/Alloy | 4 h at 200 °C | 24 h at 200 °C | 168 h at 200 °C |
|---|---|---|---|
| steel | steel_4h | steel_24h | steel_168h |
| copper | copper_4h | copper_24h | copper_168h |
| brass | brass_4h | brass_24h | brass_168h |
| - | blank_4h | blank_24h | blank_168h |
| Species | 31P δ (ppm) a | JP–F (Hz) |
|---|---|---|
| 1 | −1.9 (p) | 66 (2 bonds) |
| 2 | −8.7 (dt) | 998 (1 bond), 74 (2 bonds) |
| 3 | −14.8 (t) | 951 (1 bond) |
| 4 | −6.6 (d) | 902 (1 bond) |
| 5 | −4.9 (t) | 73 (2 bonds) |
| 6 | 0.1 (s) | - |
| Species | 19F δ (ppm) a | JP–F (Hz) |
|---|---|---|
| 1 (CF2) | −124.9 (d) | 66 (2 bonds) |
| 1 (CF3) | −80.0 (s) | - |
| 2 (P–F) | −63.3 (d) | 998 (1 bond) |
| 2 (CF2) | −108.3 (d) | 74 (2 bonds) |
| 4 (P–F) | −71.9 (d) | 902 (1 bond) |
| 5 (CF2) | −125.3 (d) | 73 (2 bonds) |
| 5 (CF3) | −80.5 (s) | - |
| F− | −148.2 (s) | - |
| Sample | Fe | Cr | Cu | Zn |
|---|---|---|---|---|
| steel_4h | 97.2 | n.d. | ||
| steel_24h | 1033 | n.d. | ||
| steel_168h | 36,500 | 11.2 | ||
| copper_4h | 304 | |||
| copper_24h | 896 | |||
| copper_168h | 11,954 | |||
| brass_4h | 23.2 | 70.5 | ||
| brass_24h | 57.7 | 1510 | ||
| brass_168h | 4146 | 58,021 |
| Element | Atomic% | |||||
|---|---|---|---|---|---|---|
| Steel_168h | Copper_168h | Brass_168h | [BmPyrr]FAP a | [BmPyrr] [Compound 5] a | [BmPyrr] [Compound 6] a | |
| C | 40.2 | 38.8 | 43.5 | 43 | 52 | 60 |
| N | 6.7 | 8.3 | 4.8 | 3 | 5 | 7 |
| O | 28.4 | 30.8 | 21.3 | 0 | 14 | 27 |
| F | 19.7 | 16.9 | 24.7 | 51 | 24 | / |
| P | 4.2 | 4.7 | 4.8 | 3 | 5 | 7 |
| Fe | 0.8 (32) b | / | / | / | / | / |
| Cu | / | 0.4 (16) b | / | / | / | / |
| Zn | / | / | 0.9 (39) b | / | / | / |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardelli, F.; Berretti, E.; Lavacchi, A.; Pitzalis, E.; Freni, A.; Pizzanelli, S. Ionic Liquids as Working Fluids for Heat Storage Applications: Decomposition Behavior of N-Butyl-N-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate. Materials 2023, 16, 1762. https://doi.org/10.3390/ma16051762
Nardelli F, Berretti E, Lavacchi A, Pitzalis E, Freni A, Pizzanelli S. Ionic Liquids as Working Fluids for Heat Storage Applications: Decomposition Behavior of N-Butyl-N-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate. Materials. 2023; 16(5):1762. https://doi.org/10.3390/ma16051762
Chicago/Turabian StyleNardelli, Francesca, Enrico Berretti, Alessandro Lavacchi, Emanuela Pitzalis, Angelo Freni, and Silvia Pizzanelli. 2023. "Ionic Liquids as Working Fluids for Heat Storage Applications: Decomposition Behavior of N-Butyl-N-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate" Materials 16, no. 5: 1762. https://doi.org/10.3390/ma16051762
APA StyleNardelli, F., Berretti, E., Lavacchi, A., Pitzalis, E., Freni, A., & Pizzanelli, S. (2023). Ionic Liquids as Working Fluids for Heat Storage Applications: Decomposition Behavior of N-Butyl-N-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate. Materials, 16(5), 1762. https://doi.org/10.3390/ma16051762







