Study of Geopolymers Obtained from Wheat Husk Native to Northern Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wheat Husk Calcination
2.3. Preparation of Alkali Solution
2.4. Geopolymerization
2.5. Characterization
3. Results and Discussion
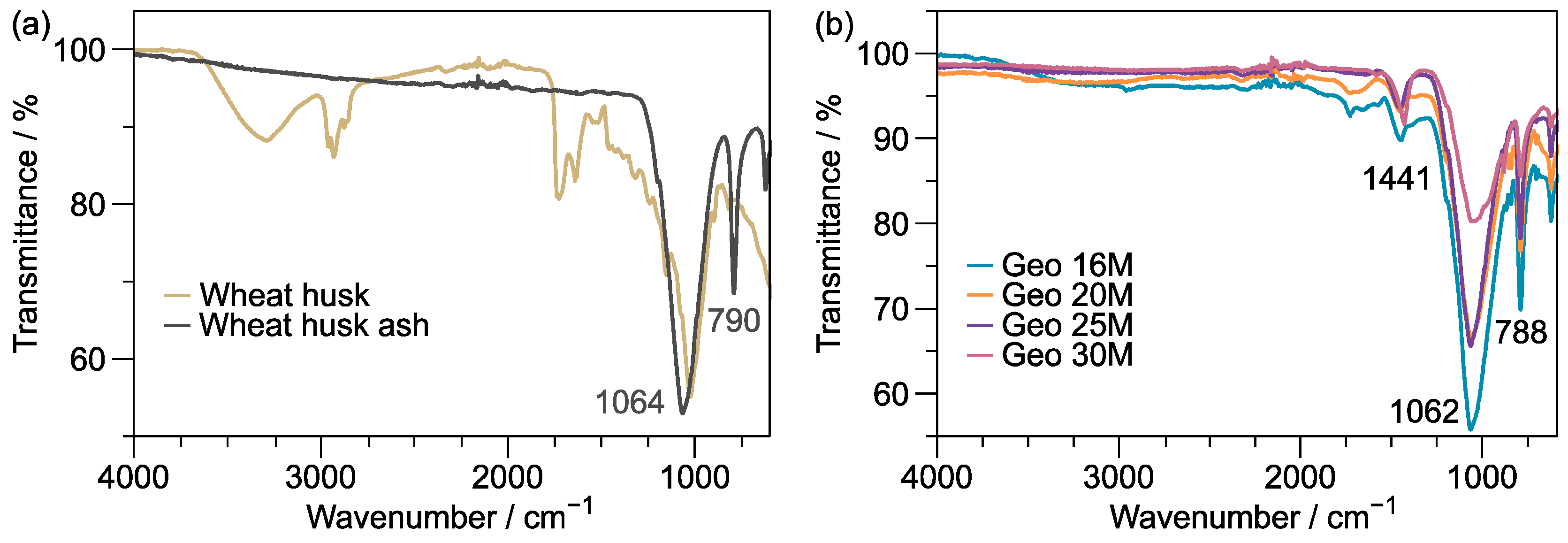
3.1. Functional Group Analysis
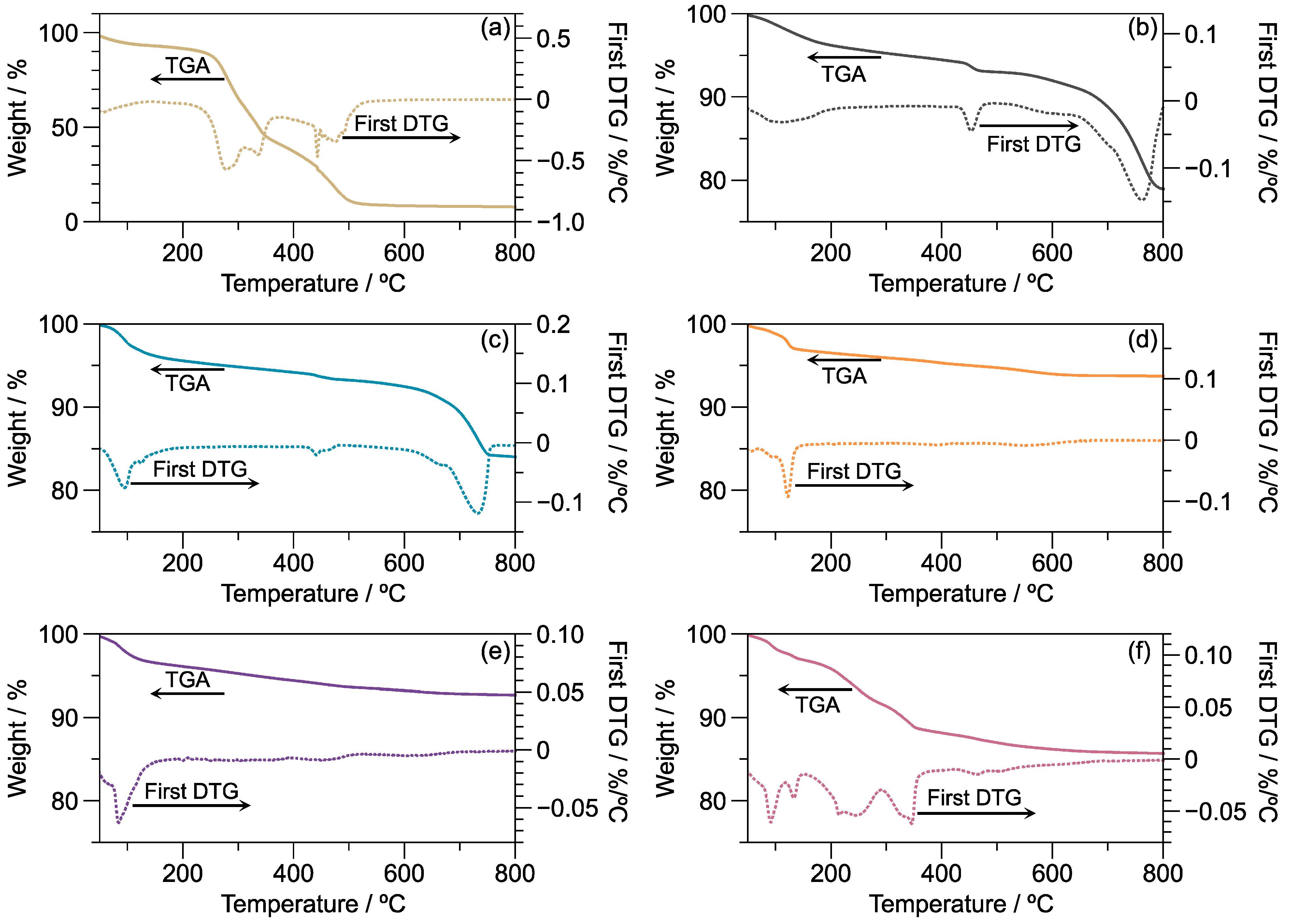
3.2. Thermal Stability
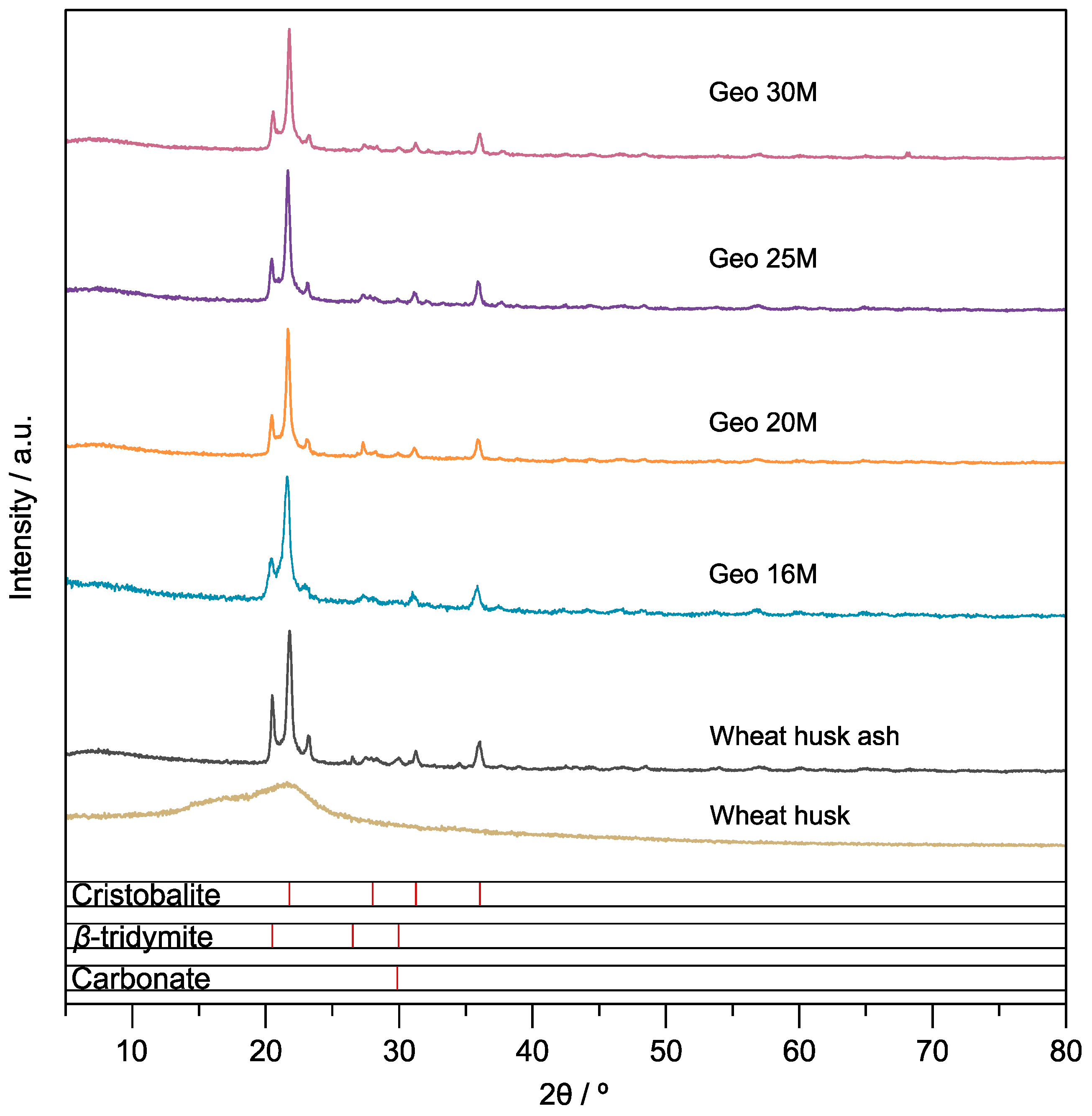
3.3. Crystal Structure
3.4. BET Specific Surface Area and Pore Size Distribution Analysis
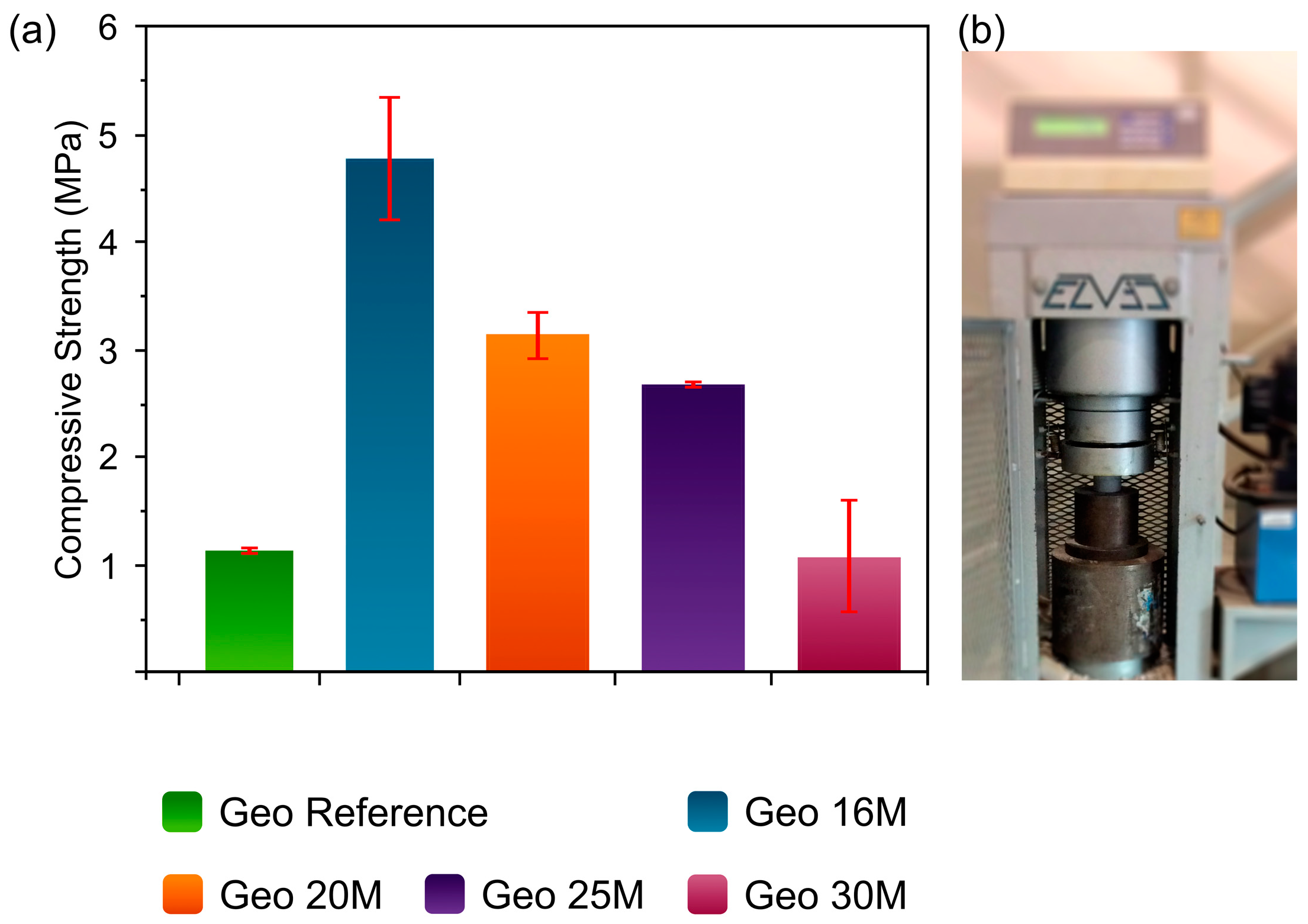
3.5. Mechanical Properties
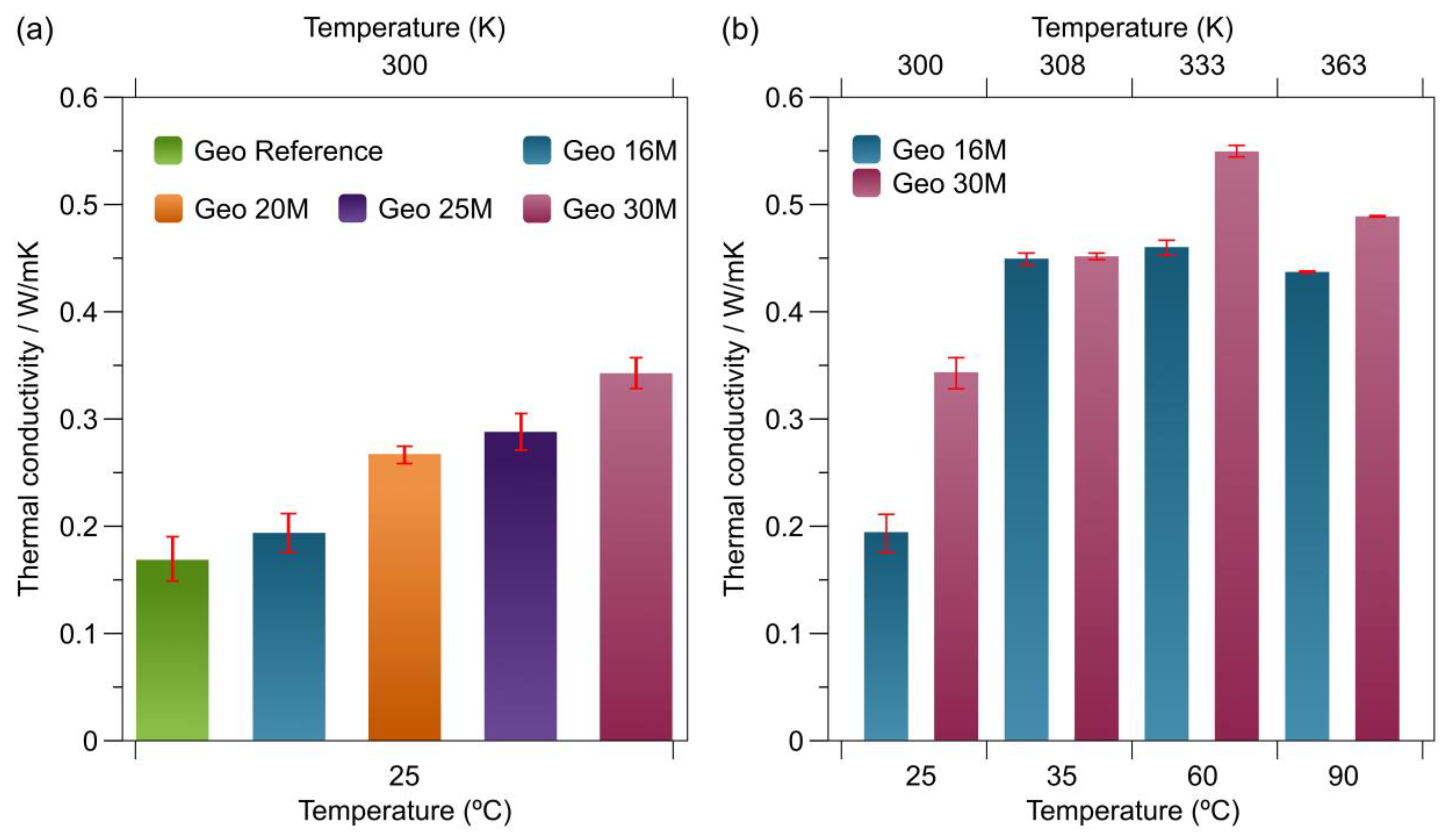
3.6. Thermal Conductivity
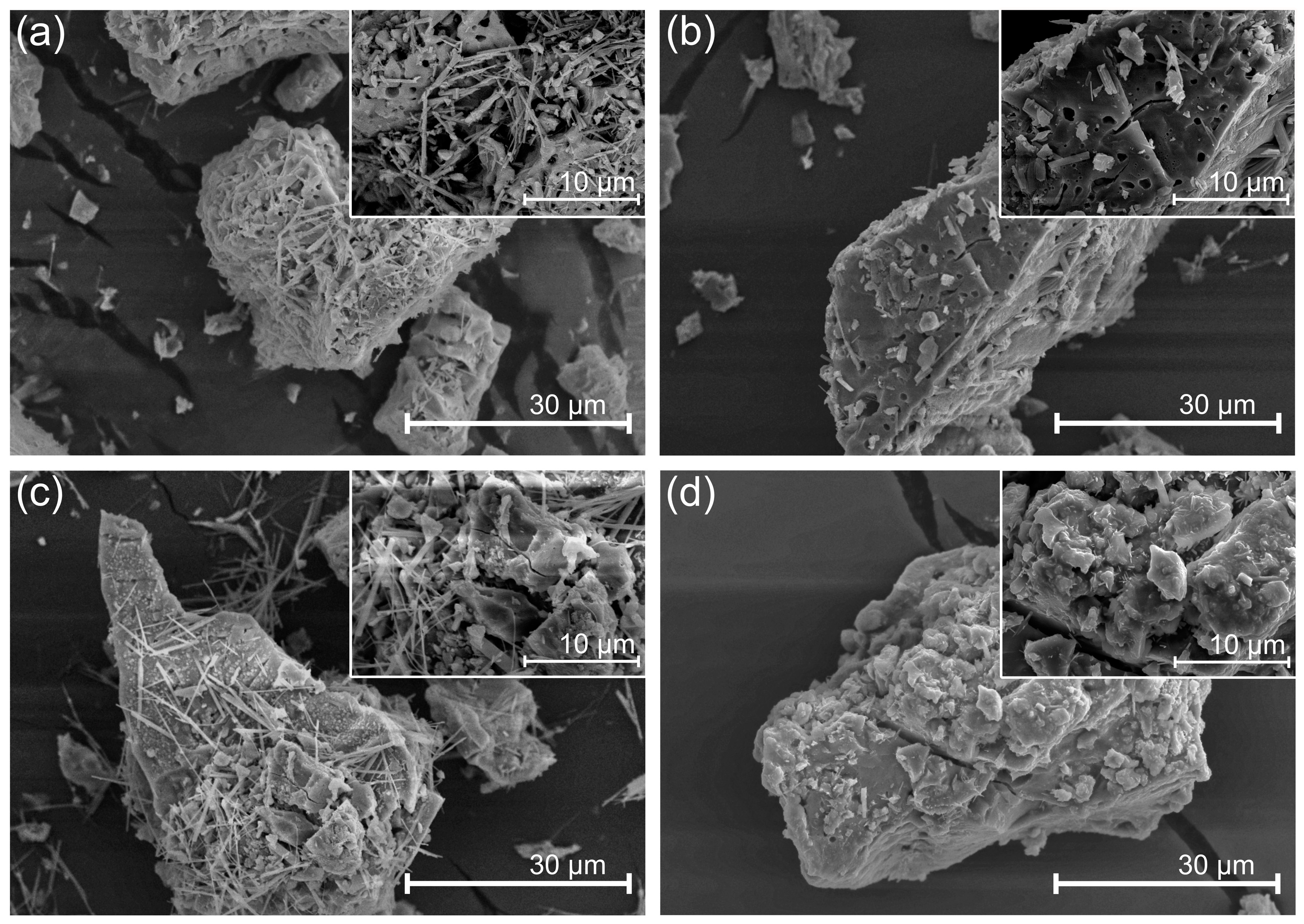
3.7. Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terzioğlu, P.; Yücel, S.; Kuş, Ç. Review on a Novel Biosilica Source for Production of Advanced Silica-Based Materials: Wheat Husk. Asia-Pac. J. Chem. Eng. 2019, 14, e2262. [Google Scholar] [CrossRef] [Green Version]
- Santiago-De la Rosa, N.; Mugica-Álvarez, V.; Cereceda-Balic, F.; Guerrero, F.; Yáñez, K.; Lapuerta, M. Emission Factors from Different Burning Stages of Agriculture Wastes in Mexico. Environ. Sci. Pollut. Res. 2017, 24, 24297–24310. [Google Scholar] [CrossRef] [PubMed]
- Montero, G.; Coronado, M.A.; Torres, R.; Jaramillo, B.E.; García, C.; Stoytcheva, M.; Vázquez, A.M.; León, J.A.; Lambert, A.A.; Valenzuela, E. Higher Heating Value Determination of Wheat Straw from Baja California, Mexico. Energy 2016, 109, 612–619. [Google Scholar] [CrossRef]
- Muthuraj, R.; Lacoste, C.; Lacroix, P.; Bergeret, A. Sustainable Thermal Insulation Biocomposites from Rice Husk, Wheat Husk, Wood Fibers and Textile Waste Fibers: Elaboration and Performances Evaluation. Ind. Crops. Prod. 2019, 135, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Shafiei, N.; Nasrollahzadeh, M.; Iravani, S. Green Synthesis of Silica and Silicon Nanoparticles and Their Biomedical and Catalytic Applications. Comments Inorg. Chem. 2021, 41, 317–372. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J. Effect of Activators on Hybrid Alkaline Binder Based on Tungsten Mining Waste and Ground Granulated Blast Furnace Slag. Constr. Build. Mater. 2020, 232, 117176. [Google Scholar] [CrossRef]
- Maraveas, C. Production of Sustainable Construction Materials Using Agro-Wastes. Materials 2020, 13, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. A Review on Sustainable Use of Agricultural Straw and Husk Biomass Ashes: Transitioning towards Low Carbon Economy. Sci. Total Environ. 2022, 838, 156407. [Google Scholar] [CrossRef]
- Lan, T.; Meng, Y.; Ju, T.; Chen, Z.; Du, Y.; Deng, Y.; Song, M.; Han, S.; Jiang, J. Synthesis and Application of Geopolymers from Municipal Waste Incineration Fly Ash (MSWI FA) as Raw Ingredient—A Review. Resour. Conserv. Recycl. 2022, 182, 106308. [Google Scholar] [CrossRef]
- Joshi, A.; Montes, C.; Salehi, S.; Allouche, E.; Lvov, Y. Optimization of Geopolymer Properties by Coating of Fly-Ash Microparticles with Nanoclays. J. Inorg. Organomet. Polym. Mater. 2015, 25, 282–292. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A. Studying Different Silica Sources for Preparation of Alternative Waterglass Used in Preparation of Binary Geopolymer Binders from Metakaolin/Boiler Slag. Constr. Build. Mater. 2019, 227, 116–621. [Google Scholar] [CrossRef]
- Zulkifly, K.; Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Shee-Ween, O.; Khalid, M.S. bin Effect of Phosphate Addition on Room-Temperature-Cured Fly Ash-Metakaolin Blend Geopolymers. Constr. Build. Mater. 2021, 270, 121486. [Google Scholar] [CrossRef]
- Meng, T.; Ahmed, S.; Dai, D.; Yu, Y. Effects of Load Types and Critical Molar Ratios on Strength Properties and Geopolymerization Mechanism. Rev. Adv. Mater. Sci. 2021, 60, 216–222. [Google Scholar] [CrossRef]
- Karuppannan, M.S.; Palanisamy, C.; Farook, M.S.M.; Natarajan, M. Study on Fly Ash and GGBS Based Oven Cured Geopolymer Concrete. In Proceedings of the AIP Conference Proceedings, Coimbatore, India, 17–18 July 2020; American Institute of Physics Inc.: College Park, MD, USA, 2020; p. 2240. [Google Scholar] [CrossRef]
- Suwan, T.; Paphawasit, B.; Fan, M.; Jitsangiam, P.; Chindaprasirt, P. Effect of Microwave-Assisted Curing Process on Strength Development and Curing Duration of Cellular Lightweight Geopolymer Mortar. Mater. Manuf. Process. 2021, 36, 1040–1048. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Taebuanhuad, S. Role of Microwave Radiation in Curing the Fly Ash Geopolymer. Adv. Powder Technol. 2013, 24, 703–707. [Google Scholar] [CrossRef]
- Louie, S.M.; Spielman-Sun, E.R.; Small, M.J.; Tilton, R.D.; Lowry, G.V. Correlation of the Physicochemical Properties of Natural Organic Matter Samples from Different Sources to Their Effects on Gold Nanoparticle Aggregation in Monovalent Electrolyte. Environ. Sci. Technol. 2015, 49, 2188–2198. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, Chemical and Surface Properties of Wheat Husk, Rye Husk and Soft Wood and Their Polypropylene Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Cruz-Medina, R.; Ayala-Hernández, D.A.; Vega-Rios, A.; López-Martínez, E.I.; Mendoza-Duarte, M.E.; Estrada-Monje, A.; Zaragoza-Contreras, E.A. Curing of Cellulose Hydrogels by Uv Radiation for Mechanical Reinforcement. Polymers 2021, 13, 2342. [Google Scholar] [CrossRef]
- Hernández-Martínez, D.; Leyva-Verduzco, A.A.; Rodríguez-Félix, F.; Acosta-Elías, M.; Wong-Corral, F.J. Obtaining and Characterization of Silicon(Si) from Wheat Husk Ash for Its Possible Application in Solar Cells. J. Clean. Prod. 2020, 271, 122698. [Google Scholar] [CrossRef]
- Alves Fungaro, D.; Valério da Silva, M. Utilization of Water Treatment Plant Sludge and Coal Fly Ash in Brick Manufacturing. Am. J. Environ. Prot. 2014, 2, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Azimi, E.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength Development and Elemental Distribution of Dolomite/Fly Ash Geopolymer Composite under Elevated Temperature. Materials 2020, 13, 1015. [Google Scholar] [CrossRef] [Green Version]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Terzioglu, P.; Yucel, S.; Rababah, T.M.; Özçimen, D. Characterization of Wheat Hull and Wheat Hull Ash as a Potential Source of Sio2. Bioresources 2013, 8, 4406–4420. [Google Scholar] [CrossRef] [Green Version]
- Šupić, S.; Malešev, M.; Radonjanin, V.; Bulatović, V.; Milović, T. Reactivity and Pozzolanic Properties of Biomass Ashes Generated by Wheat and Soybean Straw Combustion. Materials 2021, 14, 1004. [Google Scholar] [CrossRef]
- Wang, J.; Minami, E.; Asmadi, M.; Kawamoto, H. Effect of Delignification on Thermal Degradation Reactivities of Hemicellulose and Cellulose in Wood Cell Walls. J. Wood Sci. 2021, 67, 19. [Google Scholar] [CrossRef]
- Kim, H.-J.; Eom, Y.-G. Thermogravimetric Analysis of Rice Husk Flour for a New Raw Material of Lignocellulosic Fiber-Thermoplastic Polymer Composites. J. Korean Wood Sci. Technol. 2001, 29, 59–67. [Google Scholar]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Extraction, Chemical Composition, and Characterization of Potential Lignocellulosic Biomasses and Polymers from Corn Plant Parts. Bioresources 2019, 14, 6485–6500. [Google Scholar] [CrossRef]
- Bernal, S.A.; Juenger, M.C.G.; Ke, X.; Matthes, W.; Lothenbach, B.; de Belie, N.; Provis, J.L. Characterization of Supplementary Cementitious Materials by Thermal Analysis. Mater. Struct./Mater. Et Constr. 2017, 50, 26. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of Elevated Temperatures on Geopolymer Paste, Mortar and Concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Gharzouni, A.; Hyvernaud, E.; Texier-Mandoki, N.; Bourbon, X.; Rossignol, S. Influence of the Nature and Amount of Carbonate Additions on the Thermal Behaviour of Geopolymers: A Model for Prediction of Shrinkage. Constr. Build. Mater. 2021, 296, 123–752. [Google Scholar] [CrossRef]
- Milonjić, S.K.; Čerović, L.S.; Čokeša, D.M.; Zec, S. The influence of cationic impurities in silica on its crystallization and point of zero charge. J. Colloid Interface Sci. 2007, 309, 155–159. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Sahmaran, M. Evaluation of the Tridymite Formation as a Technique for Enhancing Geopolymer Binders Based on Glass Waste. J. Clean. Prod. 2021, 278, 123–983. [Google Scholar] [CrossRef]
- Singh, N.S.; Thokchom, S.; Debbarma, R. Characteristics of Rice Husk Ash-Sodium Aluminate Geopolymer at Elevated Temperature. Emerg. Mater. Res. 2020, 9, 155–162. [Google Scholar] [CrossRef]
- Aboulayt, A.; Riahi, M.; Ouazzani Touhami, M.; Hannache, H.; Gomina, M.; Moussa, R. Properties of Metakaolin Based Geopolymer Incorporating Calcium Carbonate. Adv. Powder Technol. 2017, 28, 2393–2401. [Google Scholar] [CrossRef]
- Freire, A.L.; Moura-Nickel, C.D.; Scaratti, G.; de Rossi, A.; Araújo, M.H.; de Noni Júnior, A.; Rodrigues, A.E.; Castellón, E.R.; de Fátima Peralta Muniz Moreira, R. Geopolymers Produced with Fly Ash and Rice Husk Ash Applied to CO2 Capture. J. Clean. Prod. 2020, 273, 122917. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Hu, J.; Liu, B.; Yang, J.; Liang, S.; Xiao, K.; Hou, H. A Review on Microwave Irradiation to the Properties of Geopolymers: Mechanisms and Challenges. Constr. Build. Mater. 2021, 294, 123491. [Google Scholar] [CrossRef]
- Terzioglu, P.; Yucel, S. Synthesis of Magnesium Silicate from Wheat Husk Ash: Effects of Parameters on Structural and Surface Properties. Bioresources 2012, 7, 5435–5447. [Google Scholar] [CrossRef] [Green Version]
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.H.; Macquarrie, D.J. Tuneable Porous Carbonaceous Materials from Renewable Resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous Materials as Elements of Modern Drug Delivery Systems for Anti-Inflammatory Agents: A Review of Recent Achievements. Pharmaceutics 2022, 14, 1452. [Google Scholar] [CrossRef]
- Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Yang, Z. An Excellent Universal Catalyst Support-Mesoporous Silica: Preparation, Modification and Applications in Energy-Related Reactions. Int. J. Hydrogen Energy 2022, 47, 9537–9565. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Liang, Q.; Zhao, J.; Pan, D.; Ma, J. Resource Utilization of Solid Waste in the Field of Phase Change Thermal Energy Storage. J. Energy Storage 2023, 58, 106362. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Tobaldi, D.M.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Synthesis of Porous Biomass Fly Ash-Based Geopolymer Spheres for Efficient Removal of Methylene Blue from Wastewaters. J. Clean. Prod. 2019, 207, 350–362. [Google Scholar] [CrossRef]
- Barbosa, T.R.; Foletto, E.L.; Dotto, G.L.; Jahn, S.L. Preparation of Mesoporous Geopolymer Using Metakaolin and Rice Husk Ash as Synthesis Precursors and Its Use as Potential Adsorbent to Remove Organic Dye from Aqueous Solutions. Ceram. Int. 2018, 44, 416–423. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.C.; Lai, S.H. Current Development of Geopolymer as Alternative Adsorbent for Heavy Metal Removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Bingxue, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for Use in Heavy Metals Adsorption, and Advanced Oxidative Processes: A Critical Review. J. Clean Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmed, F.; ul Haq, E.; Zain-ul-Abdein, M.; Raza, A.; Afzal, U.; Bibi, S.; Deen, Z.S. Improved Compressive Strength and Fracture Toughness Analysis of Natural Soil Based Geopolymer. J. Build. Eng. 2021, 44, 103241. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.A.; García, S.R.V.; Israde-Alcántara, I. Fabrication and Characterization of Thermally-Insulating Coconut Ash-Based Geopolymer Foam. Waste Manag. 2018, 80, 235–240. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, X. Thermal Conductivity Analysis of High Porosity Structures with Open and Closed Pores. Int. J. Heat Mass Transf. 2022, 183, 122089. [Google Scholar] [CrossRef]
- Walbrück, K.; Drewler, L.; Witzleben, S.; Stephan, D. Factors Influencing Thermal Conductivity and Compressive Strength of Natural Fiber-Reinforced Geopolymer Foams. Open Ceram. 2021, 5, 100065. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical Activation of Fly Ash and Its Influence on Micro and Nano-Structural Behaviour of Resulting Geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Porous Biomass Fly Ash-Based Geopolymers with Tailored Thermal Conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The Evolution of Strength and Crystalline Phases for Alkali-Activated Ground Blast Furnace Slag and Fly Ash-Based Geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]

| Sample | WHA (g) | NaOH [M] | Na2SiO3 (g) |
|---|---|---|---|
| Geo Reference | 35.7 | 0 | 0 |
| Geo 16M | 35.7 | 16 | 10.67 |
| Geo 20M | 35.7 | 20 | 10.67 |
| Geo 25M | 35.7 | 25 | 10.67 |
| Geo 30M | 35.7 | 30 | 10.67 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | MgO | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|
| WHA | 81.22 | 1.06 | 0.356 | 5.86 | 0.247 | 0.730 | 0.461 | 0.954 |
| WH | N.D. | 0.027 | 0.001 | 0.826 | 0.006 | 0.328 | 1.062 | 0.251 |
| Sample | Average Nano Pore D (nm) BJH Method | ABET (m2/g) Multi-Point BET |
|---|---|---|
| Geo 16M | 3.142 | 0.7723 |
| Geo 30M | 3.506 | 0.5999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Escobar, C.A.; Conejo-Dávila, A.S.; Vega-Rios, A.; Zaragoza-Contreras, E.A.; Farias-Mancilla, J.R. Study of Geopolymers Obtained from Wheat Husk Native to Northern Mexico. Materials 2023, 16, 1803. https://doi.org/10.3390/ma16051803
Hernández-Escobar CA, Conejo-Dávila AS, Vega-Rios A, Zaragoza-Contreras EA, Farias-Mancilla JR. Study of Geopolymers Obtained from Wheat Husk Native to Northern Mexico. Materials. 2023; 16(5):1803. https://doi.org/10.3390/ma16051803
Chicago/Turabian StyleHernández-Escobar, Claudia Alejandra, Alain Salvador Conejo-Dávila, Alejandro Vega-Rios, Erasto Armando Zaragoza-Contreras, and José Rurik Farias-Mancilla. 2023. "Study of Geopolymers Obtained from Wheat Husk Native to Northern Mexico" Materials 16, no. 5: 1803. https://doi.org/10.3390/ma16051803
APA StyleHernández-Escobar, C. A., Conejo-Dávila, A. S., Vega-Rios, A., Zaragoza-Contreras, E. A., & Farias-Mancilla, J. R. (2023). Study of Geopolymers Obtained from Wheat Husk Native to Northern Mexico. Materials, 16(5), 1803. https://doi.org/10.3390/ma16051803






