Phase Relations in the Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) Ternary Oxide Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase Formation in the Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) Systems
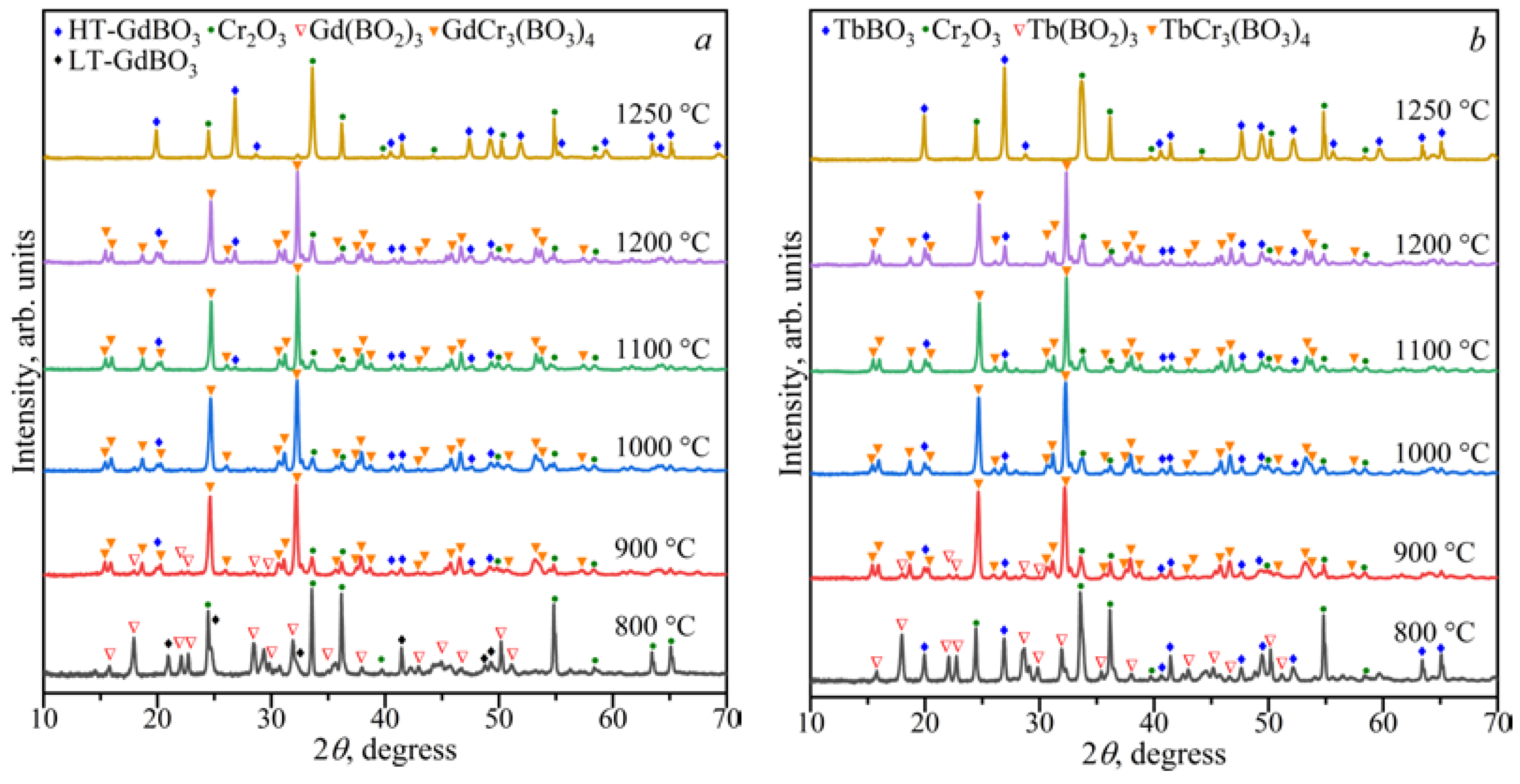
3.1.1. Ln2O3–Cr2O3–B2O3 (Ln = Gd, Tb) Ternary Systems
800–900 °C for Ln = Tb)
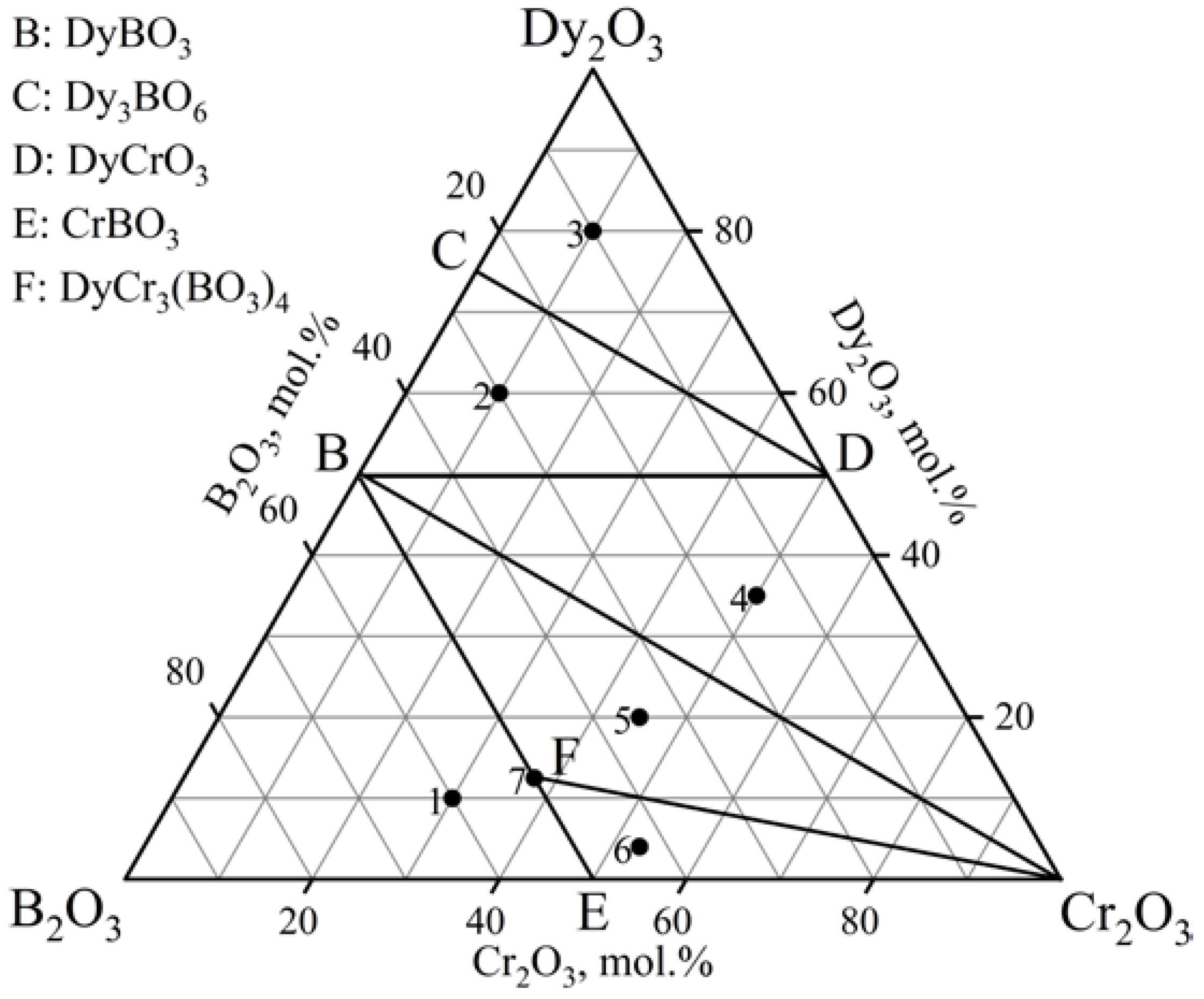
3.1.2. The Dy2O3–Cr2O3–B2O3 Ternary System
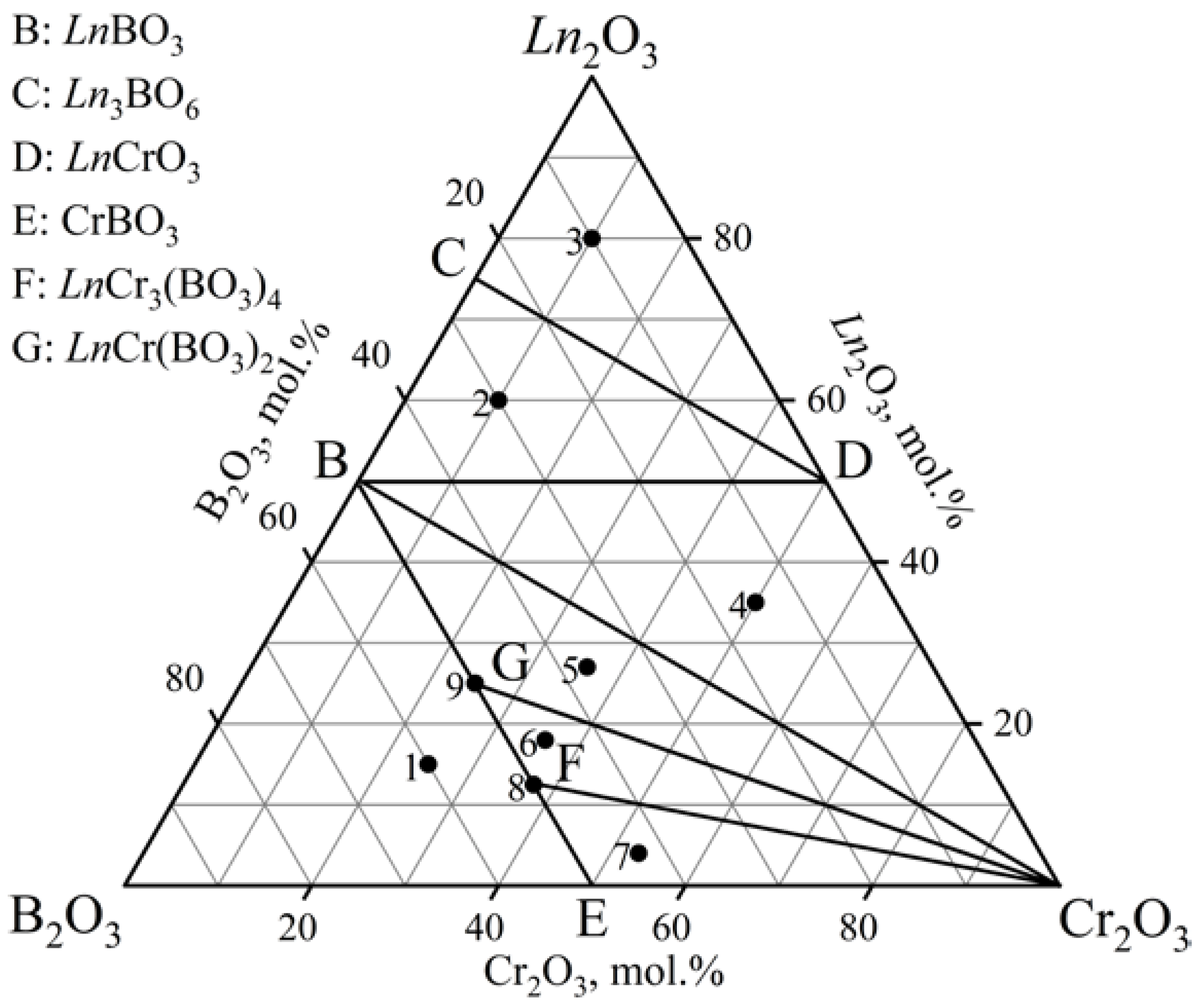
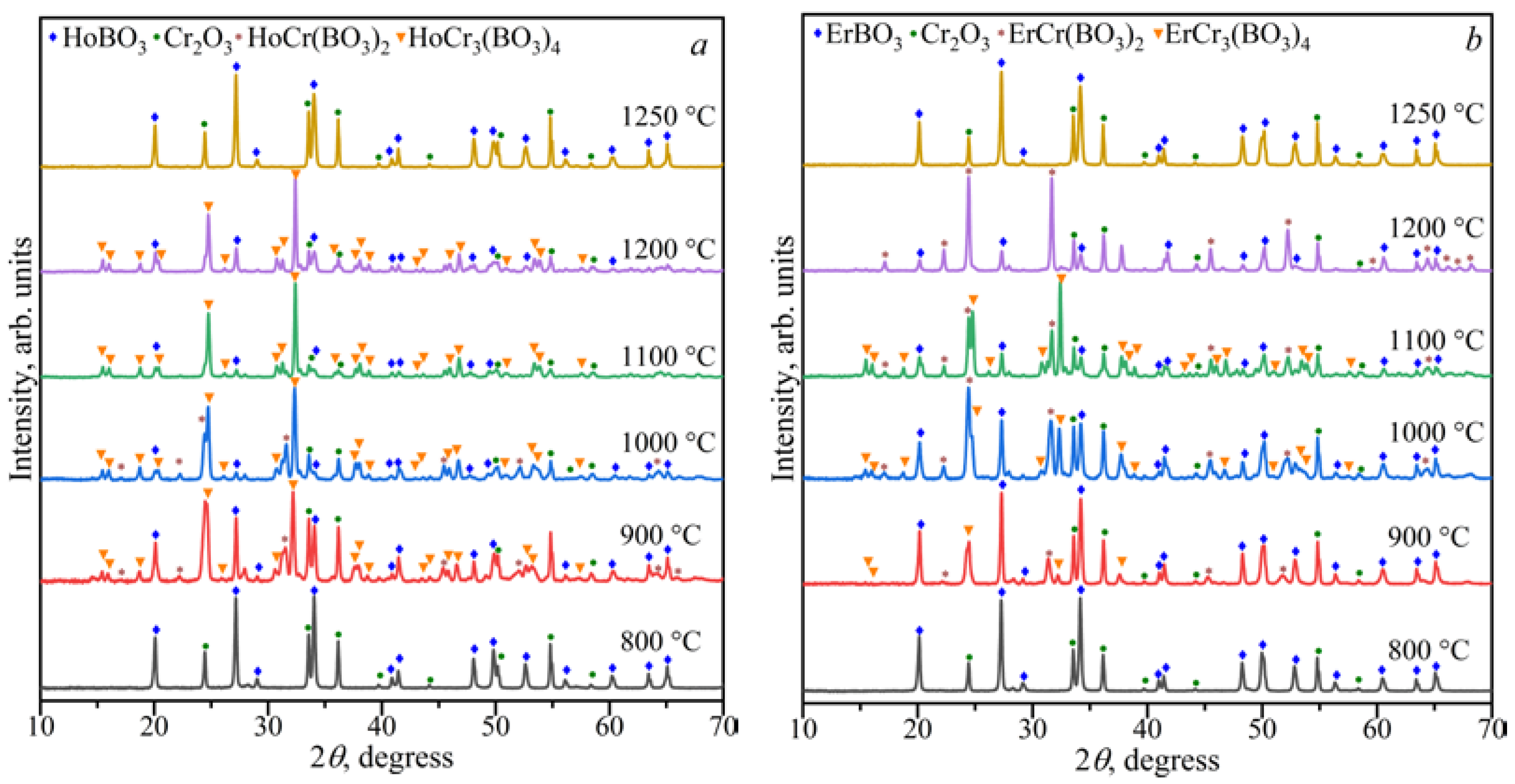
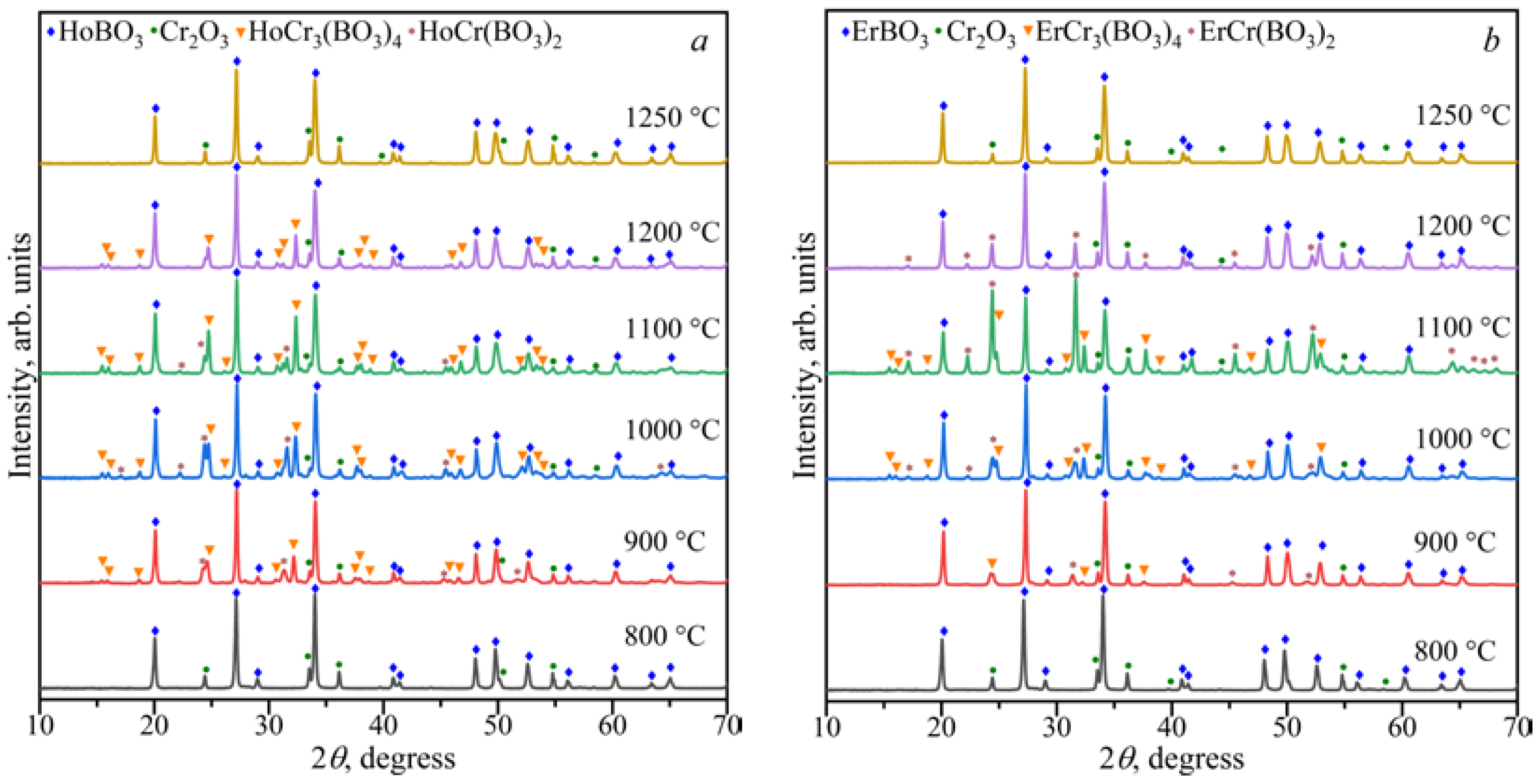
3.1.3. The Ln2O3–Cr2O3–B2O3 (Ln = Ho, Er) Ternary Systems
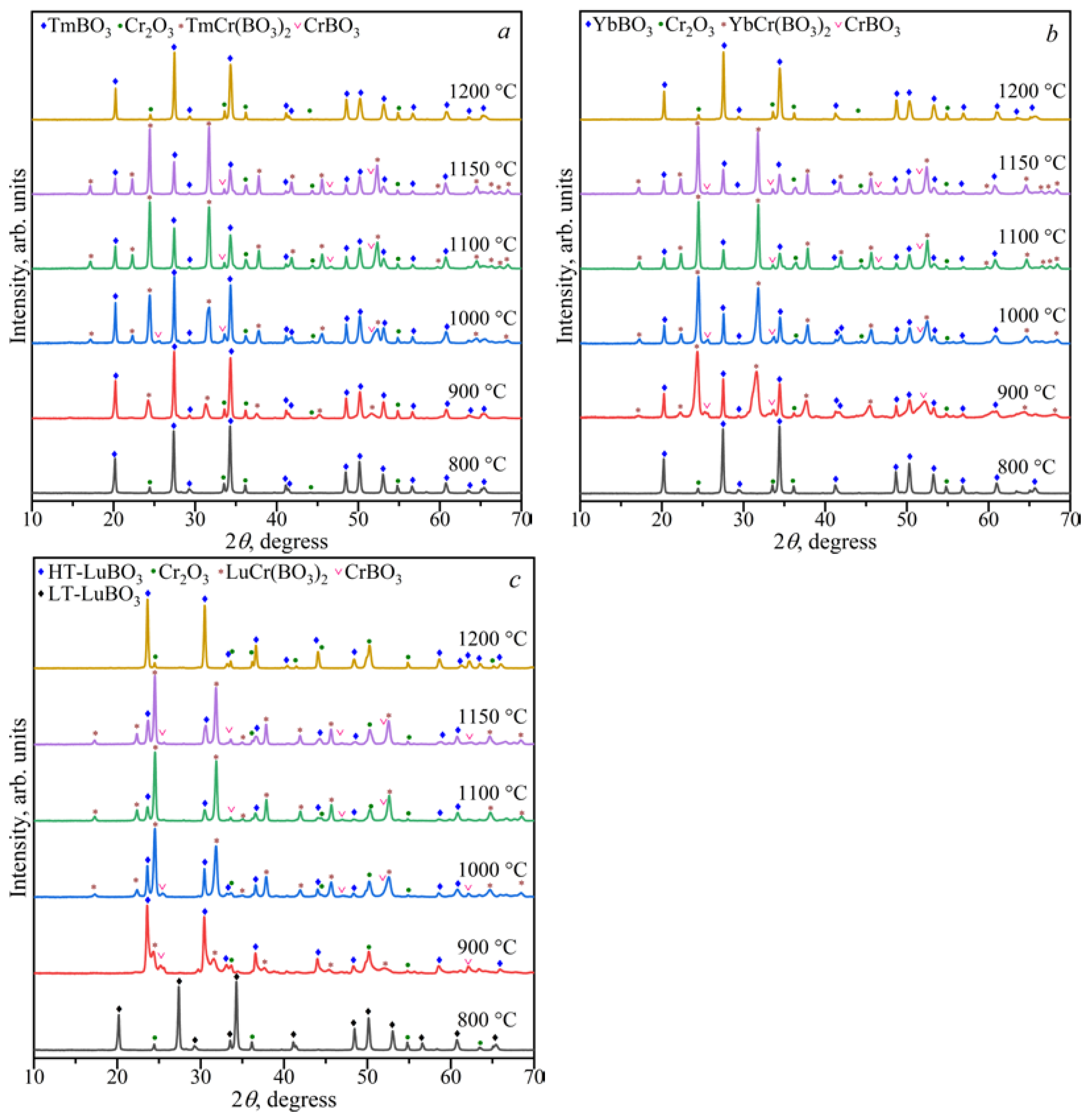
3.1.4. The Ln2O3–Cr2O3–B2O3 (Ln = Tm–Lu) Ternary Systems
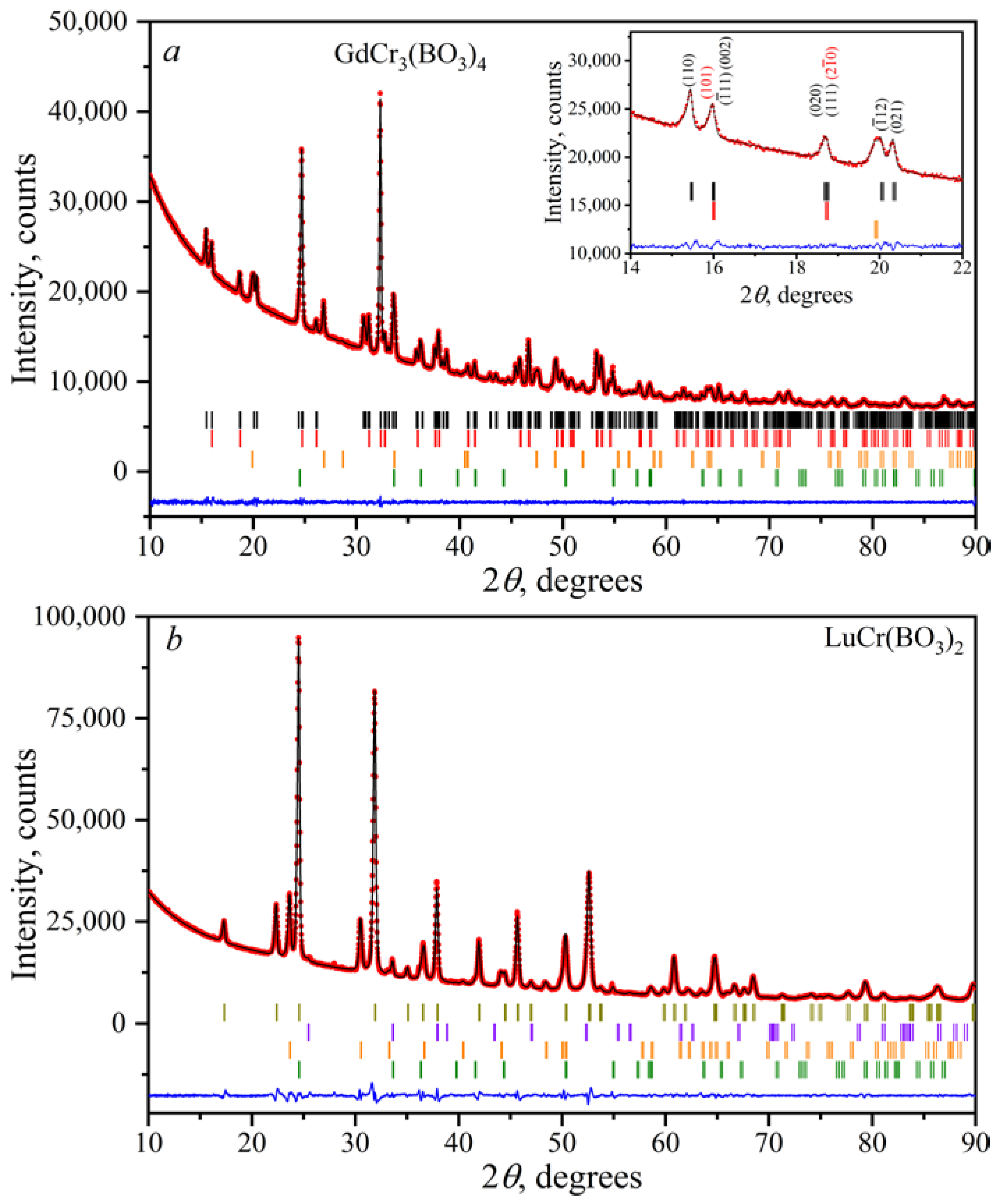
3.2. Powder X-ray Diffraction
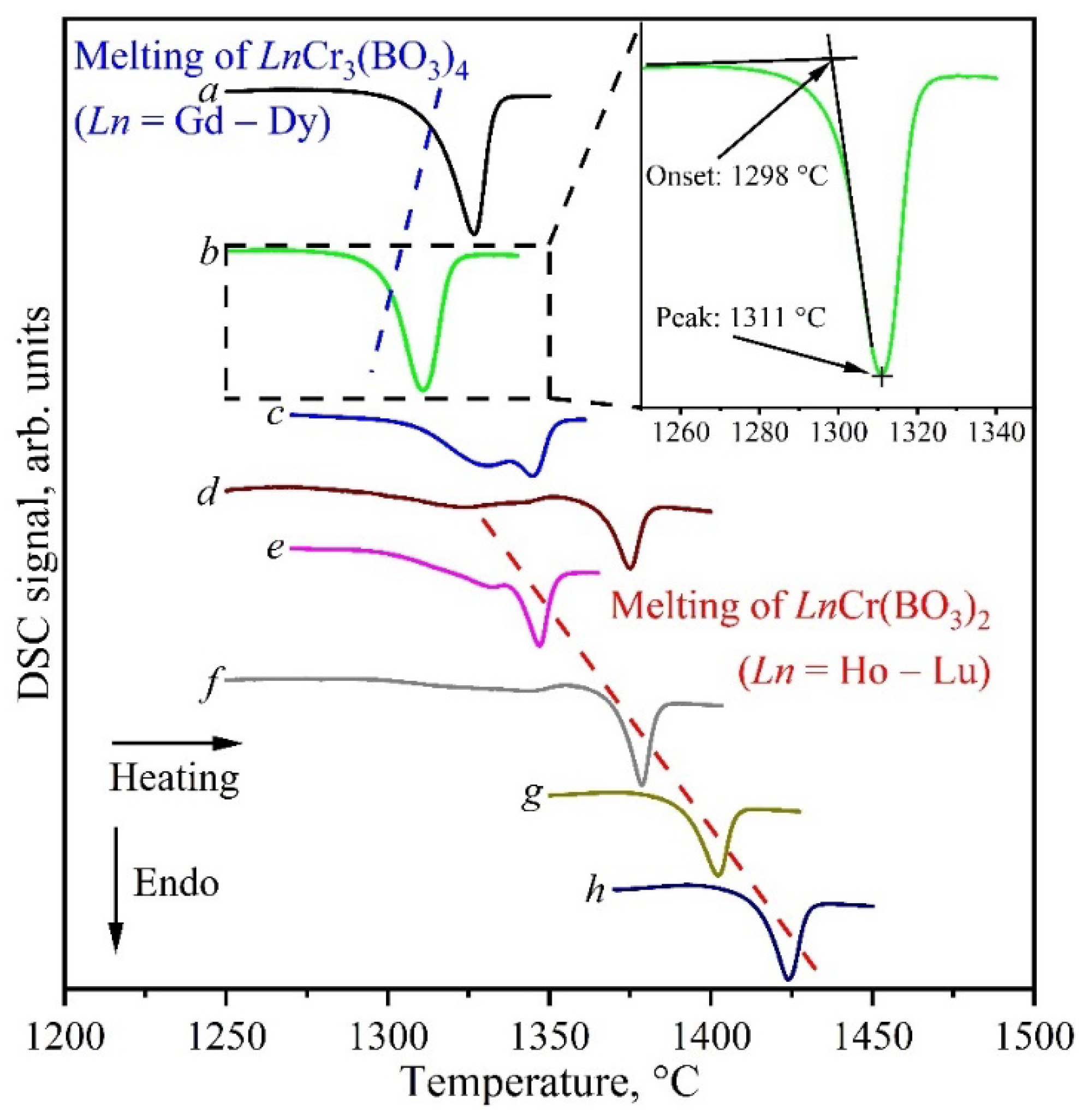
3.3. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levin, E.M. Liquid Immiscibility in Oxide Systems. In Phase Diagrams: Materials Science and Technology; Alper, A.M., Ed.; Academic Press: New York, NY, USA, 1970; Volume 3, pp. 143–236. ISBN 978-0-12-053203-2. [Google Scholar]
- Levin, E.M.; Robbins, C.R.; Waring, J.L. Immiscibility and the System Lanthanum Oxide-Boric Oxide. J. Am. Ceram. Soc. 1961, 44, 87–91. [Google Scholar] [CrossRef]
- Li, L.; Lu, P.; Wang, Y.; Jin, X.; Li, G.; Wang, Y.; You, L.; Lin, J. Synthesis of Rare Earth Polyborates Using Molten Boric Acid as a Flux. Chem. Mater. 2002, 14, 4963–4968. [Google Scholar] [CrossRef]
- Li, L.; Jin, X.; Li, G.; Wang, Y.; Liao, F.; Yao, G.; Lin, J. Novel Rare Earth Polyborates. 2. Syntheses and Structures. Chem. Mater. 2003, 15, 2253–2260. [Google Scholar] [CrossRef]
- Ysker, J.S.; Hoffmann, W. Die Kristallstruktur des La[B3O6]: Ein neuer Ketten-Borat-Typ. Naturwissenschaften 1970, 57, 129. [Google Scholar] [CrossRef]
- Goubin, F.; Montardi, Y.; Deniard, P.; Rocquefelte, X.; Brec, R.; Jobic, S. Optical Properties of CeBO3 and CeB3O6 Compounds: First-Principles Calculations and Experimental Results. J. Solid State Chem. 2004, 177, 89–100. [Google Scholar] [CrossRef]
- Sieke, C.; Nikelski, T.; Schleid, T. Pr(BO2)3 Und PrCl(BO2)2: Zwei Meta-Borate Des Praseodyms Im Vergleich. Z. Anorg. Und Allg. Chem. 2002, 628, 819. [Google Scholar] [CrossRef]
- Müller-Bunz, H.; Nikelski, T.; Schleid, T. Einkristalle Des Neodym(III)-Meta-Borats Nd(BO2)3 Und -Ortho-Borats Nd[BO3]/Single Crystals of the Neodymium(III) Meta-Borate Nd(BO2)3 and Ortho-Borate Nd[BO3]. Z. Nat. B 2003, 58, 375–380. [Google Scholar] [CrossRef]
- Goriounova, A.; Held, P.; Becker, P.; Bohatý, L. Monoclinic Modification of Polymorphic TbB3O6. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, i83–i85. [Google Scholar] [CrossRef] [Green Version]
- Bambauer, H.U.; Weidelt, J.; Ysker, J.-S. Röntgenographische Und Optische Untersuchungen an Boraten Seltener Erden SE(BO2)3. Z. Krist. Cryst. Mater. 1969, 130, 207–213. [Google Scholar] [CrossRef]
- Mukherjee, P.; Suard, E.; Dutton, S.E. Magnetic Properties of Monoclinic Lanthanide Metaborates, Ln(BO2)3, Ln = Pr, Nd, Gd, Tb. J. Phys. Condens. Matter 2017, 29, 405807. [Google Scholar] [CrossRef]
- Nikelski, T.; Schleid, T. Synthese Und Kristallstruktur von Terbium(III)-Meta-Oxoborat Tb(BO2)3 (≡ TbB3O6). Z. Anorg. Und Allg. Chem. 2003, 629, 1017–1022. [Google Scholar] [CrossRef]
- Nikelski, T.; Schäfer, M.C.; Huppertz, H.; Schleid, T. Crystal Structure of Dysprosium Meta-Oxoborate, β-Dy(BO2)3, via Normal-Pressure Synthesis. Z. Krist. New Cryst. Struct. 2008, 223, 177–178. [Google Scholar] [CrossRef] [Green Version]
- Levin, E.M.; Roth, R.S.; Martin, J.B. Polymorphism of ABO3 Type Rare Earth Borates. Am. Mineral. 1961, 46, 1030–1055. [Google Scholar]
- Wu, Y.; Ding, D.; Pan, S.; Yang, F.; Ren, G. Research on Phase Transition Behavior of Lutetium Orthoborate LuBO3. Phase Transit. 2011, 84, 315–324. [Google Scholar] [CrossRef]
- Fedorov, P.P. Morphotropism of Rare-Earth Orthoborates RBO3. J. Struct. Chem. 2019, 60, 679–691. [Google Scholar] [CrossRef]
- Ren, M.; Lin, J.H.; Dong, Y.; Yang, L.Q.; Su, M.Z.; You, L.P. Structure and Phase Transition of GdBO3. Chem. Mater. 1999, 11, 1576–1580. [Google Scholar] [CrossRef]
- Lin, J.H.; Su, M.Z.; Wurst, K.; Schweda, E. The Structure of La26(BO3)8O27: A Structure with a Distorted Fluorite Type Arrangement of Atoms. J. Solid State Chem. 1996, 126, 287–291. [Google Scholar] [CrossRef]
- Noirault, S.; Célérier, S.; Joubert, O.; Caldes, M.T.; Piffard, Y. Effects of Water Uptake on the Inherently Oxygen-Deficient Compounds Ln26O27□(BO3)8 (Ln = La, Nd). Inorg. Chem. 2007, 46, 9961–9967. [Google Scholar] [CrossRef]
- Lin, J.H.; You, L.P.; Lu, G.X.; Yang, L.Q.; Su, M.Z. Structural and Luminescent Properties of Eu3+ Doped Gd17.33(BO3)4(B2O5)2O16. J. Mater. Chem. 1998, 8, 1051–1054. [Google Scholar] [CrossRef]
- Lin, J.H.; Zhou, S.; Yang, L.Q.; Yao, G.Q.; Su, M.Z.; You, L.P. Structure and Luminescent Properties of Y17.33(BO3)4(B2O5)2O16. J. Solid State Chem. 1997, 134, 158–163. [Google Scholar] [CrossRef]
- Yang, M.; Li, K.; Su, J.; Huang, Q.; Bao, W.; You, L.; Li, Z.; Wang, Y.; Jiang, Y.; Liao, F.; et al. Study on the Crystal Structure of the Rare Earth Oxyborate Yb26B12O57 from Powder X-ray and Neutron Diffraction. J. Alloys Compd. 2011, 509, 4707–4713. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Zhang, X.; Marathe, A.; Cordes, D.B.; Weeks, B.; Chaudhuri, J. Tunable Photoluminescence and Energy Transfer of YBO3:Tb3+, Eu3+ for White Light Emitting Diodes. J. Mater. Chem. C 2013, 1, 7202. [Google Scholar] [CrossRef]
- Yang, R.; Sun, X.; Jiang, P.; Gao, W.; Cong, R.; Yang, T. Sol-Gel Syntheses of Pentaborate β-LaB5O9 and the Photoluminescence by Doping with Eu3+, Tb3+, Ce3+, Sm3+, and Dy3+. J. Solid State Chem. 2018, 258, 212–219. [Google Scholar] [CrossRef]
- Zhu, Q.; Fan, Z.; Wang, S.; Xiahou, J.; Li, J. Uniform Colloidal Spheres for RE3BO6 (RE = Eu-Yb, Y) and Excitation-dependent Luminescence of Y3BO6:Eu3+ Red Phosphor. J. Am. Ceram. Soc. 2019, 102, 7448–7461. [Google Scholar] [CrossRef]
- Tombs, N.C.; Croft, W.J.; Mattraw, H.C. Preparation and Properties of Chromium Borate. Inorg. Chem. 1963, 2, 872–873. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Nazar, L.F. Synthesis, Structure, and Solid-State Electrochemical Properties of Cr3BO6: A New Chromium(III) Borate with the Norbergite Structure. J. Mater. Chem. 2001, 11, 3228–3233. [Google Scholar] [CrossRef]
- Bither, T.A.; Frederick, C.G.; Gier, T.E.; Weiher, J.F.; Young, H.S. Ferromagnetic VBO3 and Antiferromagnetic CrBO3. Solid State Commun. 1970, 8, 109–112. [Google Scholar] [CrossRef]
- Schneider, S.J.; Roth, R.S.; Waring, J.L. Solid State Reactions Involving Oxides of Trivalent Cations. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1961, 65A, 345–374. [Google Scholar] [CrossRef] [PubMed]
- Quezel-Ambrunaz, S.; Mareschal, M. Paramètres cristallins des chromites de terres rares. Bull. Soc. Fr. Mineral. Cristallogr. 1963, 86, 204–205. [Google Scholar] [CrossRef]
- Geller, S. Crystallographic Studies of Perovskite-like Compounds. IV. Rare Earth Scandates, Vanadites, Galliates, Orthochromites. Acta Crystallogr. 1957, 10, 243–248. [Google Scholar] [CrossRef]
- Berjoan, R. Contribution to the Study of the Interactions between Oxygen and Lanthanum Oxide, Chromic Oxide, or Lanthanum Chromite. Rev. Int. Des Hautes Temp. Des Refract. 1976, 13, 119–135. [Google Scholar]
- Du, Y.; Cheng, Z.X.; Wang, X.-L.; Dou, S.X. Structure, Magnetic, and Thermal Properties of Nd1-xLaxCrO3 (0≤x≤1.0). J. Appl. Phys. 2010, 108, 093914. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Coondoo, I.; Rao, A.; Lu, B.-H.; Kuo, Y.-K.; Kholkin, A.L.; Panwar, N. Impact of Low Level Praseodymium Substitution on the Magnetic Properties of YCrO3 Orthochromites. Phys. B Condens. Matter 2017, 510, 104–108. [Google Scholar] [CrossRef]
- Serrao, C.R.; Kundu, A.K.; Krupanidhi, S.B.; Waghmare, U.V.; Rao, C.N.R. Biferroic YCrO3. Phys. Rev. B 2005, 72, 220101. [Google Scholar] [CrossRef] [Green Version]
- Sahu, J.R.; Serrao, C.R.; Ray, N.; Waghmare, U.V.; Rao, C.N.R. Rare Earth Chromites: A New Family of Multiferroics. J. Mater. Chem. 2007, 17, 42–44. [Google Scholar] [CrossRef]
- Siemons, M.; Simon, U. High Throughput Screening of the Propylene and Ethanol Sensing Properties of Rare-Earth Orthoferrites and Orthochromites. Sens. Actuators B Chem. 2007, 126, 181–186. [Google Scholar] [CrossRef]
- Ballman, A.A. A New Series of Synthetic Borates Isostructural with the Carbonate Mineral Huntite. Am. Mineral. 1962, 47, 1380–1383. [Google Scholar]
- Leonyuk, N.I.; Leonyuk, L.I. Growth and Characterization of RM3(BO3)4 Crystals. Prog. Cryst. Growth Charact. Mater. 1995, 31, 179–278. [Google Scholar] [CrossRef]
- Vicat, J.; Aléonard, S. Etude de borates MeMe′(BO3)2 de structure dolomite. Mater. Res. Bull. 1968, 3, 611–620. [Google Scholar] [CrossRef]
- Vicat, J.; Aléonard, S. Borates de terres rares TCr(BO3)2 de structure dolomite. Bull. Soc. Fr. Mineral. Crystallogr. 1968, 91, 293–295. [Google Scholar] [CrossRef]
- Kuzmin, N.N.; Maltsev, V.V.; Volkova, E.A.; Leonyuk, N.I.; Boldyrev, K.N.; Bludov, A.N. Growth and Spectroscopic and Magnetic Properties of TbCr3(BO3)4 Crystals. Inorg. Mater. 2020, 56, 828–835. [Google Scholar] [CrossRef]
- Gondek, Ł.; Szytuła, A.; Przewoźnik, J.; Żukrowski, J.; Prokhorov, A.; Chernush, L.; Zubov, E.; Dyakonov, V.; Duraj, R.; Tyvanchuk, Y. On the Peculiar Properties of Triangular-Chain EuCr3(BO3)4 Antiferromagnet. J. Solid State Chem. 2014, 210, 30–35. [Google Scholar] [CrossRef]
- Kurazhkovskaya, V.S.; Borovikova, E.Y.; Leonyuk, N.I.; Koporulina, E.V.; Belokoneva, E.L. Infrared Spectroscopy and the Structure of Polytypic Modifications of RM3(BO3)4 Borates (R—Nd, Gd, Y; M—Al, Ga, Cr, Fe). J. Struct. Chem. 2008, 49, 1035–1041. [Google Scholar] [CrossRef]
- Kurazhkovskaya, V.S.; Dobretsova, E.A.; Borovikova, E.Y.; Mal’tsev, V.V.; Leonyuk, N.I. Infrared Spectroscopy and the Structure of Rare-Earth Chromium Borates RCr3(BO3)4 (R = La—Er). J. Struct. Chem. 2011, 52, 699–707. [Google Scholar] [CrossRef]
- Borovikova, E.Y.; Dobretsova, E.A.; Boldyrev, K.N.; Kurazhkovskaya, V.S.; Maltsev, V.V.; Leonyuk, N.I. Vibrational Spectra and Factor Group Analysis of Rare-Earth Chromium Borates, RCr3(BO3)4, with R = La–Ho. Vib. Spectrosc. 2013, 68, 82–90. [Google Scholar] [CrossRef]
- Boldyrev, K.N.; Kuz’min, N.N.; Mukhin, A.A.; Ivanov, V.Y.; Dobretsova, E.A.; Popova, E.A.; Gavrilkin, S.Y.; Leonyuk, N.I.; Maltsev, V.V.; Malkin, B.Z.; et al. Thermal and Magnetic Properties and Optical Spectroscopy of SmCr3(BO3)4. Phys. Rev. Mater. 2021, 5, 104413. [Google Scholar] [CrossRef]
- Bludov, A.; Savina, Y.; Kobets, M.; Khrustalyov, V.; Savitsky, V.; Gnatchenko, S.; Zajarniuk, T.; Lynnyk, A.; Gutowska, M.U.; Szewczyk, A.; et al. Features of Magnetic and Magnetoelectric Properties, H-T Phase Diagram of GdCr3(BO3)4. J. Magn. Magn. Mater. 2020, 512, 167010. [Google Scholar] [CrossRef]
- Bludov, A.N.; Savina, Y.O.; Pashchenko, V.A.; Gnatchenko, S.L.; Zajarniuk, T.; Lynnyk, A.; Gutowska, M.U.; Szewczyk, A.; Kolodiy, I.V.; Mal’tsev, V.V.; et al. Magnetic Properties of DyCr3(BO3)4. Low Temp. Phys. 2020, 46, 697–703. [Google Scholar] [CrossRef]
- Doi, Y.; Satou, T.; Hinatsu, Y. Crystal Structures and Magnetic Properties of Lanthanide Containing Borates LnM(BO3)2 (Ln=Y, Ho–Lu; M=Sc, Cr). J. Solid State Chem. 2013, 206, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, R.; Zhou, H.D.; Lee, M.; Choi, E.S.; Li, G.; Hong, T.; Calder, S. Magnetic Ground States and Magnetodielectric Effect in RCr(BO3)2 (R = Y and Ho). Phys. Rev. B 2017, 95, 174410. [Google Scholar] [CrossRef] [Green Version]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General Features. Z. Für Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Cohen-Adad, M.T.; Aloui-Lebbou, O.; Goutaudier, C.; Panczer, G.; Dujardin, C.; Pedrini, C.; Florian, P.; Massiot, D.; Gerard, F.; Kappenstein, C. Gadolinium and Yttrium Borates: Thermal Behavior and Structural Considerations. J. Solid State Chem. 2000, 154, 204–213. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
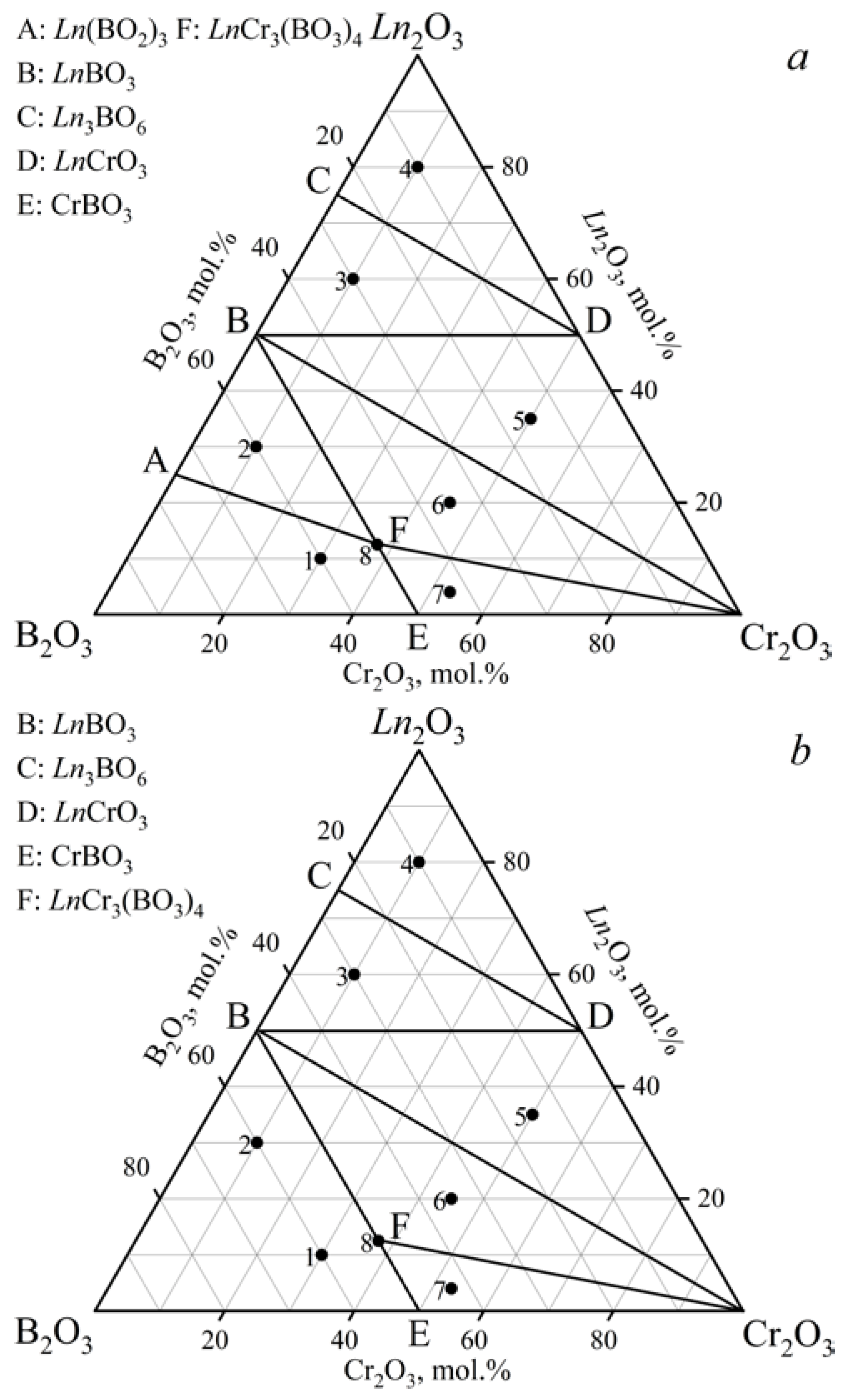

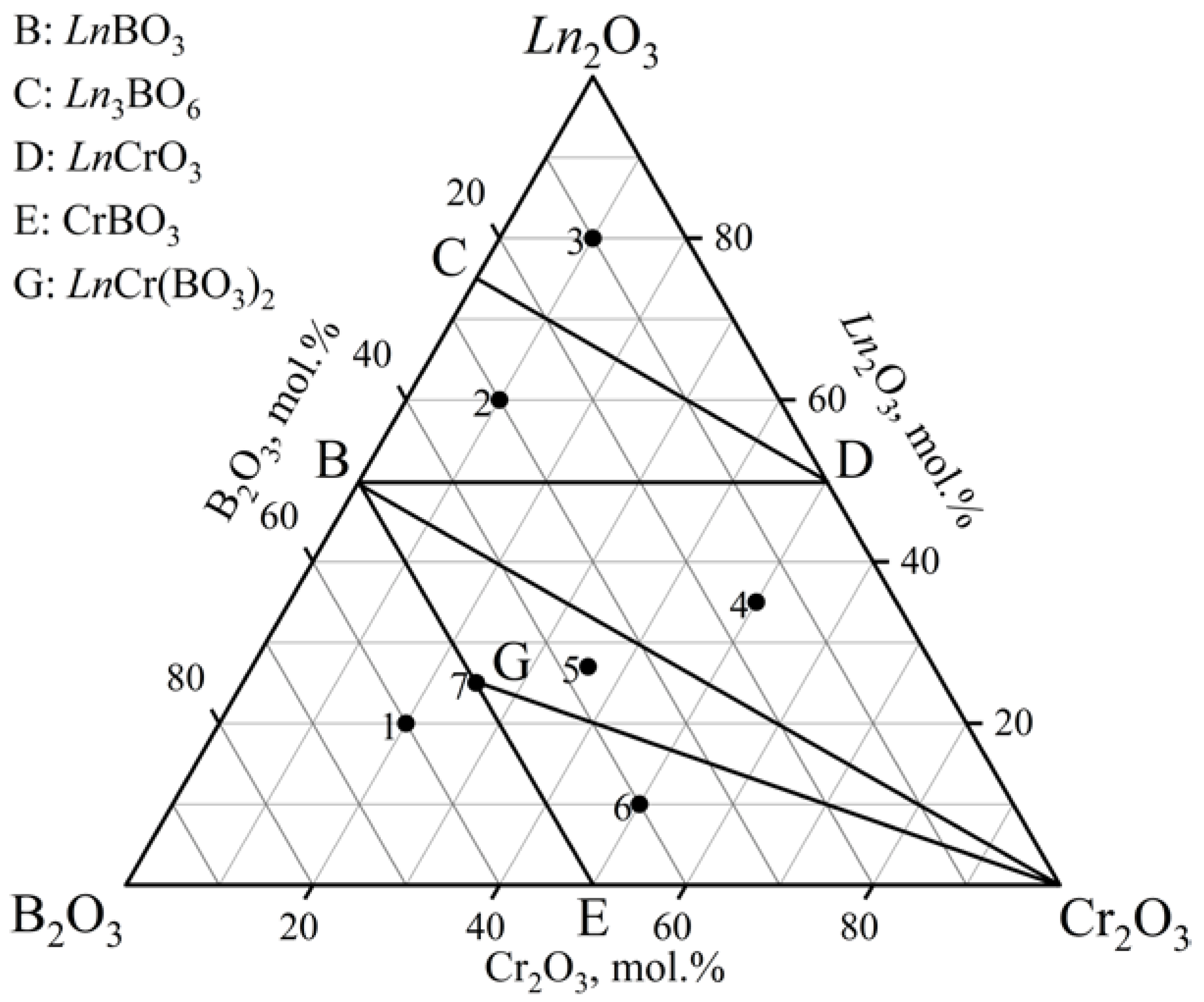

| Samples | Ln2O3, mol.% | Cr2O3, mol.% | B2O3, mol.% | Phase Composition |
|---|---|---|---|---|
| 1 | 10 | 30 | 60 | LnCr3(BO3)4 + CrBO3 + Ln(BO2)3 a + LnBO3 b,c |
| 2 | 30 | 10 | 60 | LnCr3(BO3)4 + Ln(BO2)3 a,d + LnBO3 |
| 3 | 60 | 10 | 30 | LnBO3 + LnCrO3 + Ln3BO6 |
| 4 | 80 | 10 | 10 | Ln2O3 + LnCrO3 + Ln3BO6 |
| 5 | 35 | 50 | 15 | LnBO3 + LnCrO3 + Cr2O3 |
| 6 | 20 | 45 | 35 | LnCr3(BO3)4 + Cr2O3 + LnBO3 |
| 7 | 4 | 53 | 43 | LnCr3(BO3)4 + CrBO3 + Cr2O3 |
| 8 | 12.5 | 37.5 | 50 | LnCr3(BO3)4 + LnBO3 + Cr2O3 + Ln(BO2)3 d,e |
| Samples | Dy2O3, mol.% | Cr2O3, mol.% | B2O3, mol.% | Phase Composition |
|---|---|---|---|---|
| 1 | 10 | 30 | 60 | DyCr3(BO3)4 + CrBO3 + DyBO3 |
| 2 | 60 | 10 | 30 | DyBO3 + DyCrO3 + Dy3BO6 |
| 3 | 80 | 10 | 10 | Dy2O3 + DyCrO3 + Dy3BO6 |
| 4 | 35 | 50 | 15 | DyBO3 + DyCrO3 + Cr2O3 |
| 5 | 20 | 45 | 35 | DyCr3(BO3)4 + Cr2O3 + DyBO3 |
| 6 | 4 | 53 | 43 | DyCr3(BO3)4 + CrBO3 + Cr2O3 |
| 7 | 12.5 | 37.5 | 50 | DyCr3(BO3)4 + DyBO3 + Cr2O3 |
| Samples | Ln2O3, mol.% | Cr2O3, mol.% | B2O3, mol.% | Phase Composition |
|---|---|---|---|---|
| 1 | 15 | 25 | 60 | LnCr3(BO3)4 + LnCr(BO3)2 + CrBO3 + LnBO3 |
| 2 | 60 | 10 | 30 | LnBO3 + LnCrO3 + Ln3BO6 |
| 3 | 80 | 10 | 10 | Ln2O3 + LnCrO3 + Ln3BO6 |
| 4 | 35 | 50 | 15 | LnBO3 + LnCrO3 + Cr2O3 |
| 5 | 27 | 36 | 37 | LnCr(BO3)2 + Cr2O3 + LnBO3 |
| 6 | 18 | 36 | 46 | LnCr3(BO3)4 + LnCr(BO3)2 + Cr2O3 |
| 7 | 4 | 53 | 43 | LnCr3(BO3)4 + CrBO3 + Cr2O3 |
| 8 | 12.5 | 37.5 | 50 | LnCr3(BO3)4 + LnBO3+ Cr2O3 |
| 9 | 25 | 25 | 50 | LnCr3(BO3)4 + LnCr(BO3)2 + Cr2O3 + LnBO3 |
| Samples | Ln2O3, mol.% | Cr2O3, mol.% | B2O3, mol.% | Phase Composition |
|---|---|---|---|---|
| 1 | 20 | 20 | 60 | LnCr(BO2)3 + CrBO3 + LnBO3 a |
| 2 | 60 | 10 | 30 | LnBO3 b + LnCrO3 + Ln3BO6 |
| 3 | 80 | 10 | 10 | Ln2O3 + LnCrO3 + Ln3BO6 |
| 4 | 35 | 50 | 15 | LnBO3 b + LnCrO3 + Cr2O3 |
| 5 | 27 | 36 | 37 | LnCr(BO3)2 + Cr2O3 + LnBO3 a |
| 6 | 10 | 50 | 40 | LnCr(BO3)2 + CrBO3 + Cr2O3 |
| 7 | 25 | 25 | 50 | LnCr(BO3)2 + LnBO3 a + CrBO3 + Cr2O3 |
| Refined Parameters | β-GdCr3(BO3)4 | β-TbCr3(BO3)4 | β-DyCr3(BO3)4 | β-HoCr3(BO3)4 | β-ErCr3(BO3)4 |
|---|---|---|---|---|---|
| a (Å) | 7.3939 | 7.3912 | 7.3828 | 7.3741 | 7.3712 |
| b (Å) | 9.4873 | 9.4846 | 9.4763 | 9.4728 | 9.4647 |
| c (Å) | 11.4031 | 11.3934 | 11.3782 | 11.3773 | 11.3731 |
| β (°) | 103.853 | 103.861 | 103.847 | 103.843 | 103.852 |
| V (Å3) | 776.63 | 775.45 | 772.90 | 771.66 | 770.38 |
| α-GdCr3(BO3)4 | α-TbCr3(BO3)4 | α-DyCr3(BO3)4 | α-HoCr3(BO3)4 | α-ErCr3(BO3)4 | |
| a (Å) | 9.4700 | 9.4693 | 9.4657 | 9.4650 | 9.4637 |
| c (Å) | 7.4976 | 7.4959 | 7.4801 | 7.4747 | 7.4684 |
| V (Å3) | 582.31 | 582.09 | 580.42 | 579.92 | 579.26 |
| Rwp (%) | 1.01 | 1.13 | 1.13 | 1.33 | 1.51 |
| Rp (%) | 0.76 | 0.85 | 0.83 | 0.95 | 1.04 |
| Tsynthesis (°C) | 1200 | 1200 | 1200 | 1200 | 1100 |
| Refined Parameters | HoCr(BO3)2 | ErCr(BO3)2 | TmCr(BO3)2 | YbCr(BO3)2 | LuCr(BO3)2 |
|---|---|---|---|---|---|
| a (Å) | 4.7664 | 4.7591 | 4.7528 | 4.7471 | 4.7442 |
| c (Å) | 15.5415 | 15.490 | 15.4835 | 15.4061 | 15.3471 |
| V (Å3) | 305.77 | 303.84 | 302.90 | 300.66 | 299.14 |
| Rwp (%) | 1.75 | 3.19 | 3.15 | 2.83 | 2.36 |
| Rp (%) | 1.20 | 2.00 | 2.04 | 1.79 | 1.58 |
| Tsynthesis (°C) | 1000 | 1200 | 1100 | 1100 | 1100 |
| Compound | Peak (°C) | Onset (°C) |
|---|---|---|
| GdCr3(BO3)4 | 1327 | 1312 |
| TbCr3(BO3)4 | 1321 | 1303 |
| DyCr3(BO3)4 | 1311 | 1298 |
| HoCr3(BO3)4 | 1331 | 1302 |
| ErCr3(BO3)4 | 1343 | 1263 |
| HoCr(BO3)2 | 1347 | 1333 |
| ErCr(BO3)2 | 1379 | 1366 |
| TmCr(BO3)2 | 1402 | 1391 |
| YbCr(BO3)2 | 1424 | 1413 |
| LuCr(BO3)2 | >1500 | 1448 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzmin, N.; Maltsev, V.; Mikliaeva, E.; Volkova, E.; Boldyrev, K.; Koporulina, E. Phase Relations in the Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) Ternary Oxide Systems. Materials 2023, 16, 1831. https://doi.org/10.3390/ma16051831
Kuzmin N, Maltsev V, Mikliaeva E, Volkova E, Boldyrev K, Koporulina E. Phase Relations in the Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) Ternary Oxide Systems. Materials. 2023; 16(5):1831. https://doi.org/10.3390/ma16051831
Chicago/Turabian StyleKuzmin, Nikolai, Victor Maltsev, Elizaveta Mikliaeva, Elena Volkova, Kirill Boldyrev, and Elizaveta Koporulina. 2023. "Phase Relations in the Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) Ternary Oxide Systems" Materials 16, no. 5: 1831. https://doi.org/10.3390/ma16051831






