Study of Structural and Optical Properties of Titanate Nanotubes with Erbium under Heat Treatment in Different Atmospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation, Ion Exchange Reaction and Thermal Treatment of Titanate Nanotubes
2.2. Characterizations
3. Results
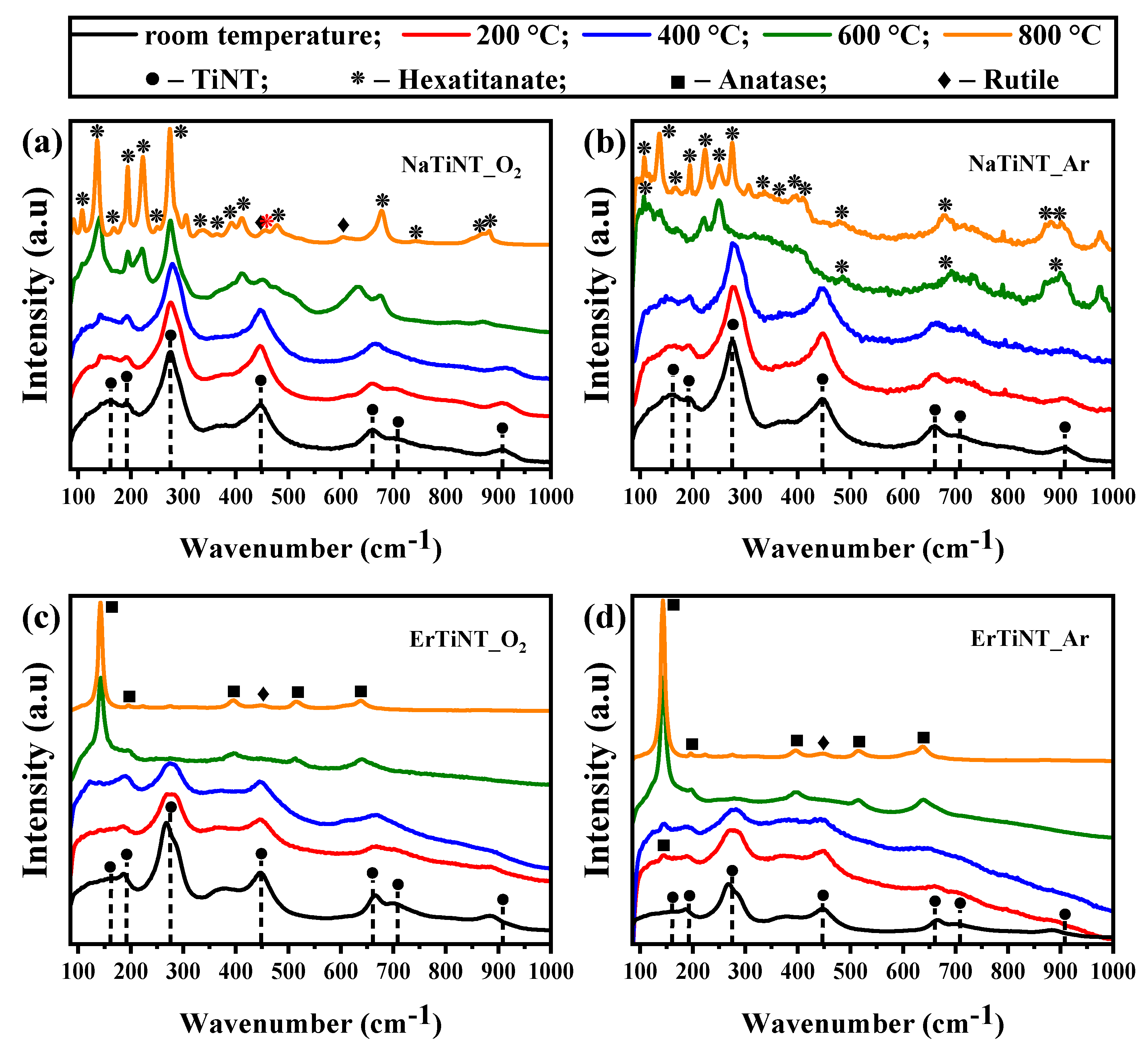
3.1. Raman Spectroscopy
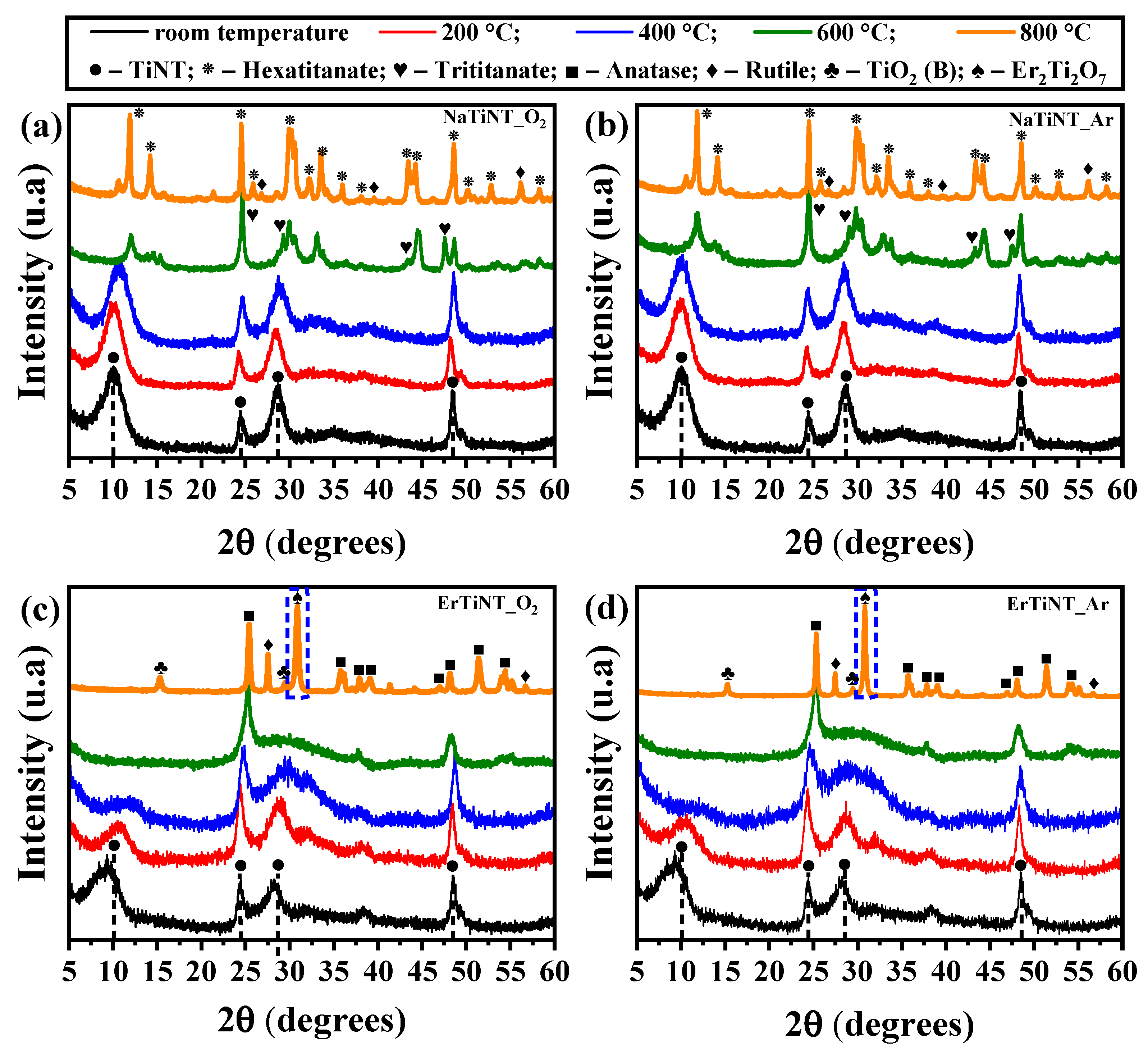
3.2. X-ray Diffraction
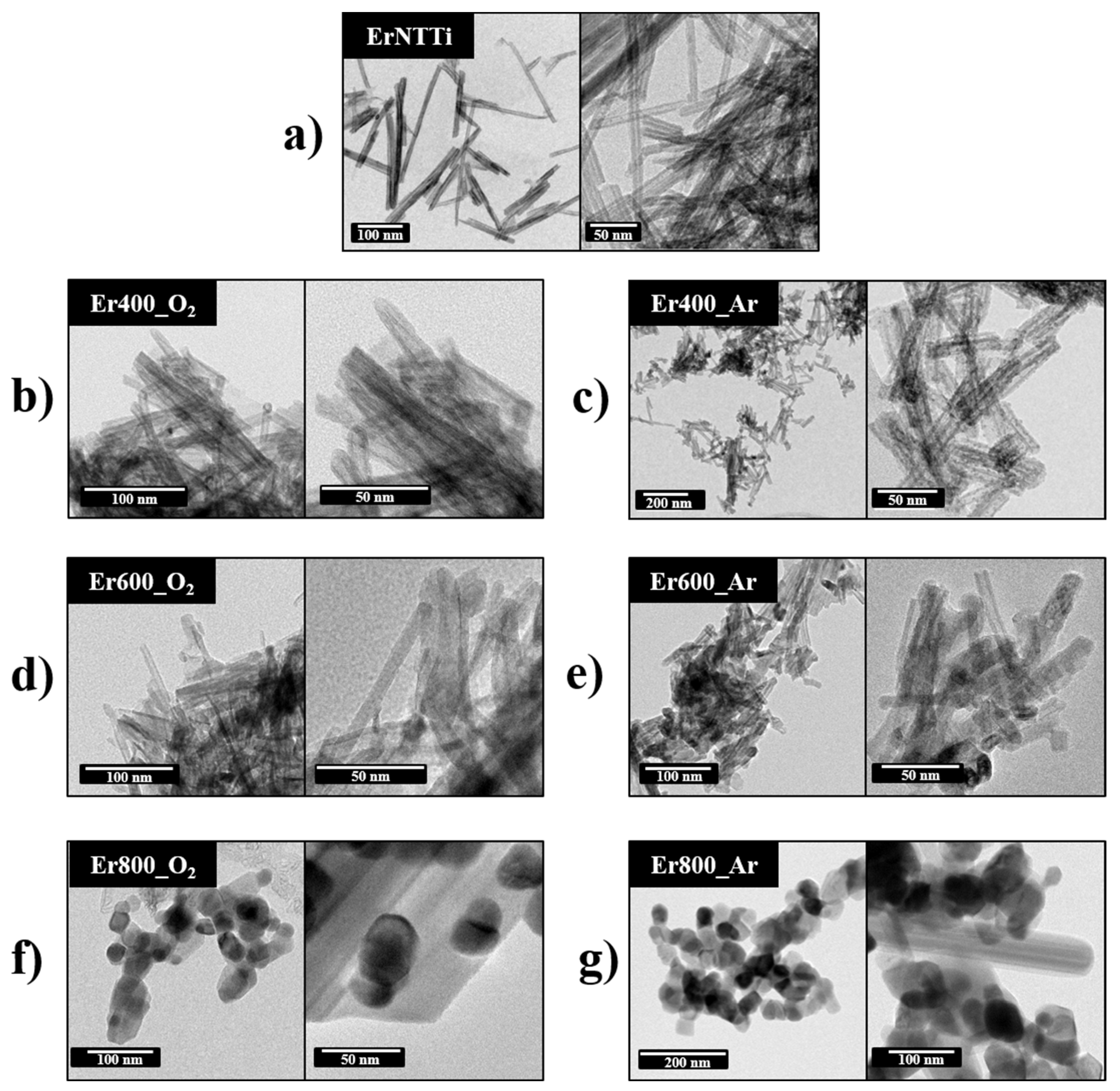
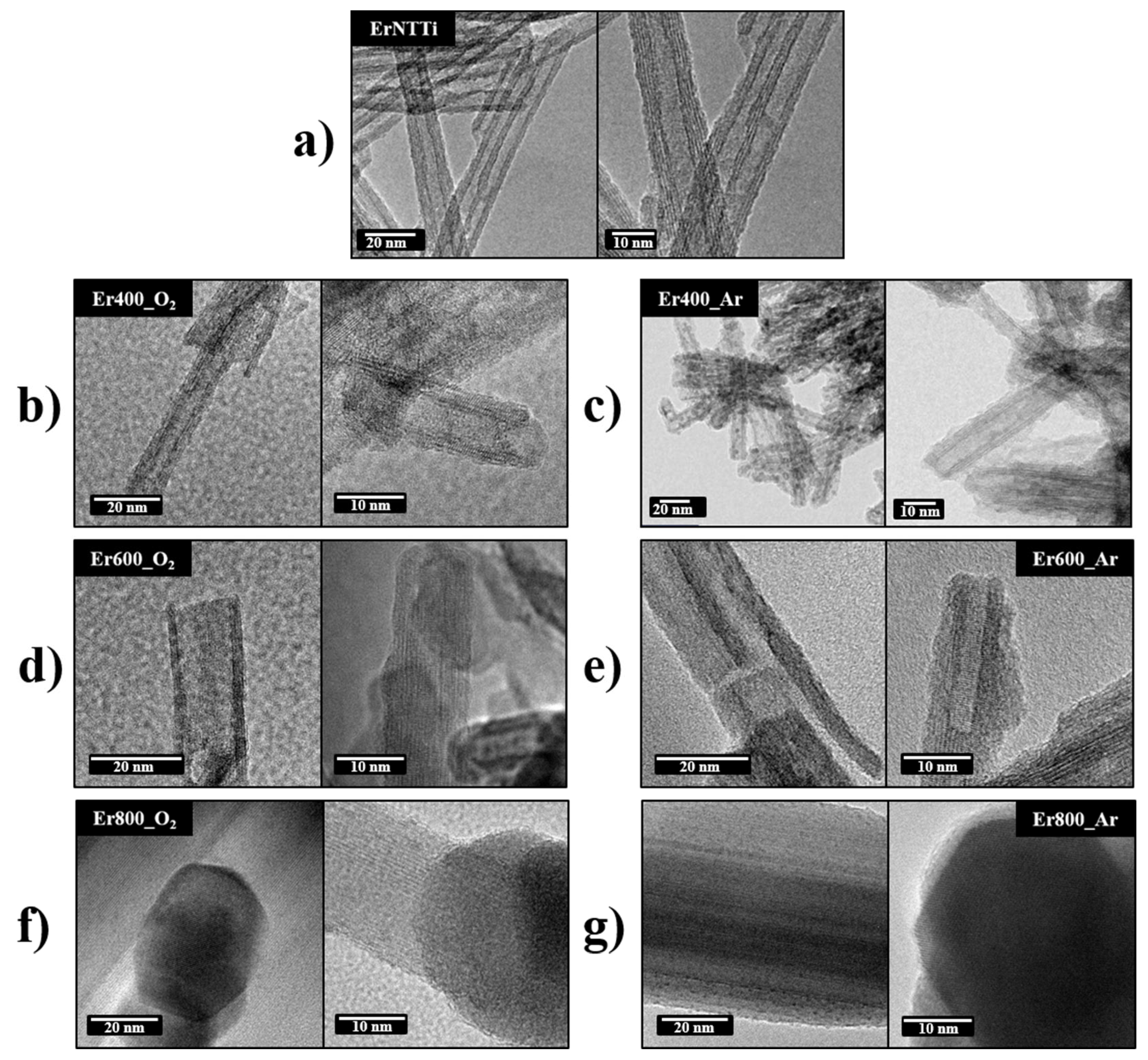
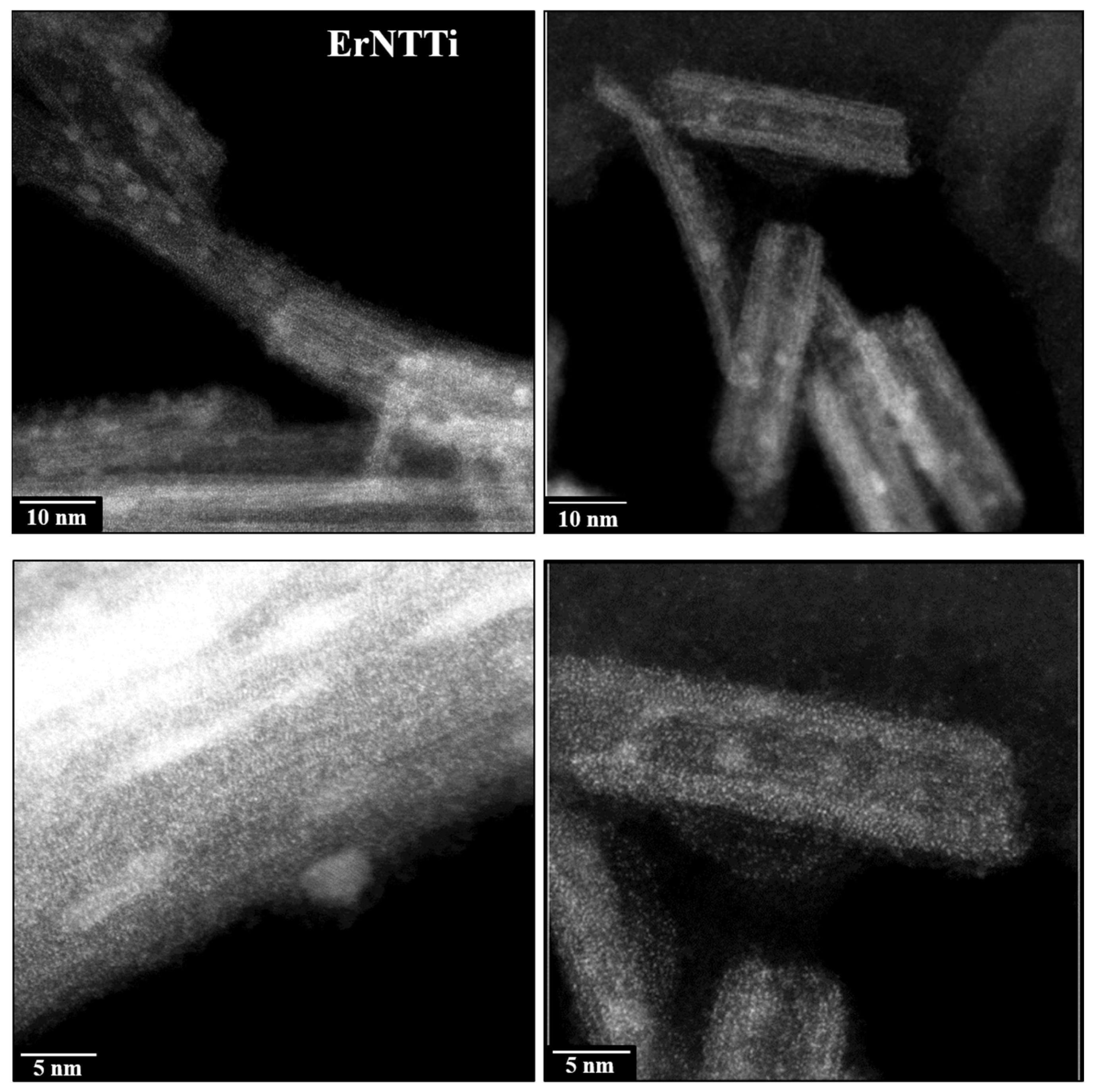
3.3. Morphology and Composition Analysis
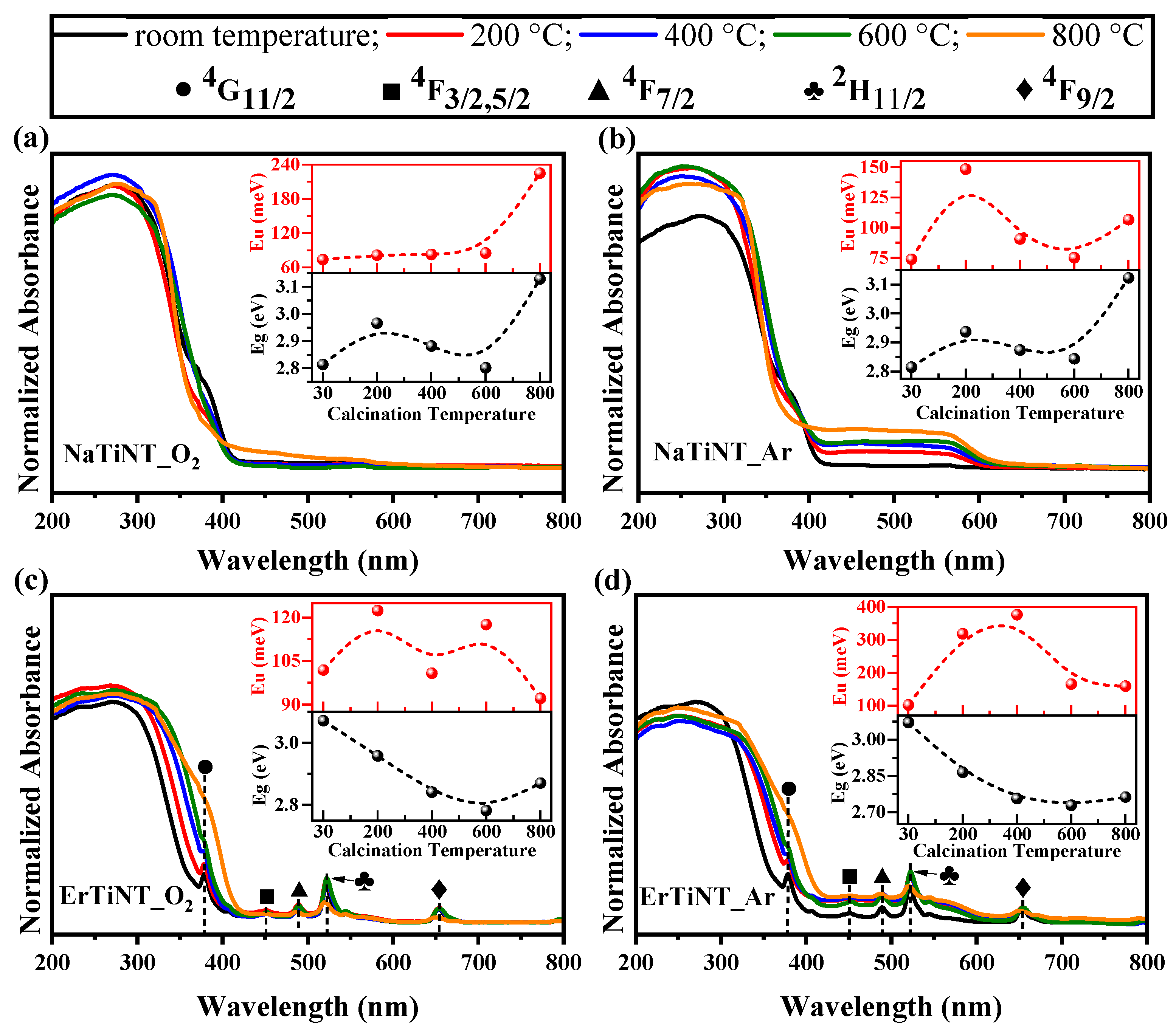
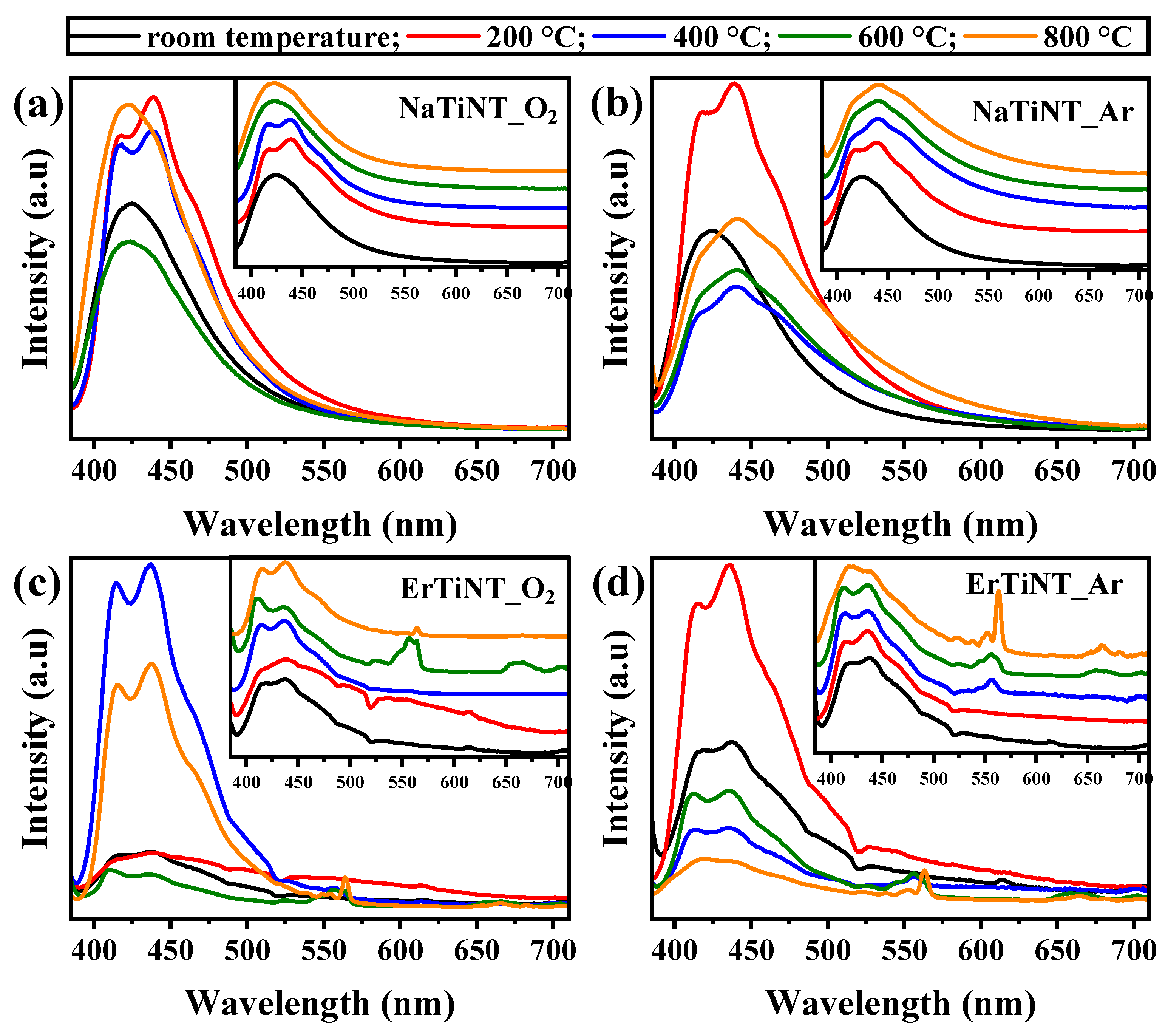
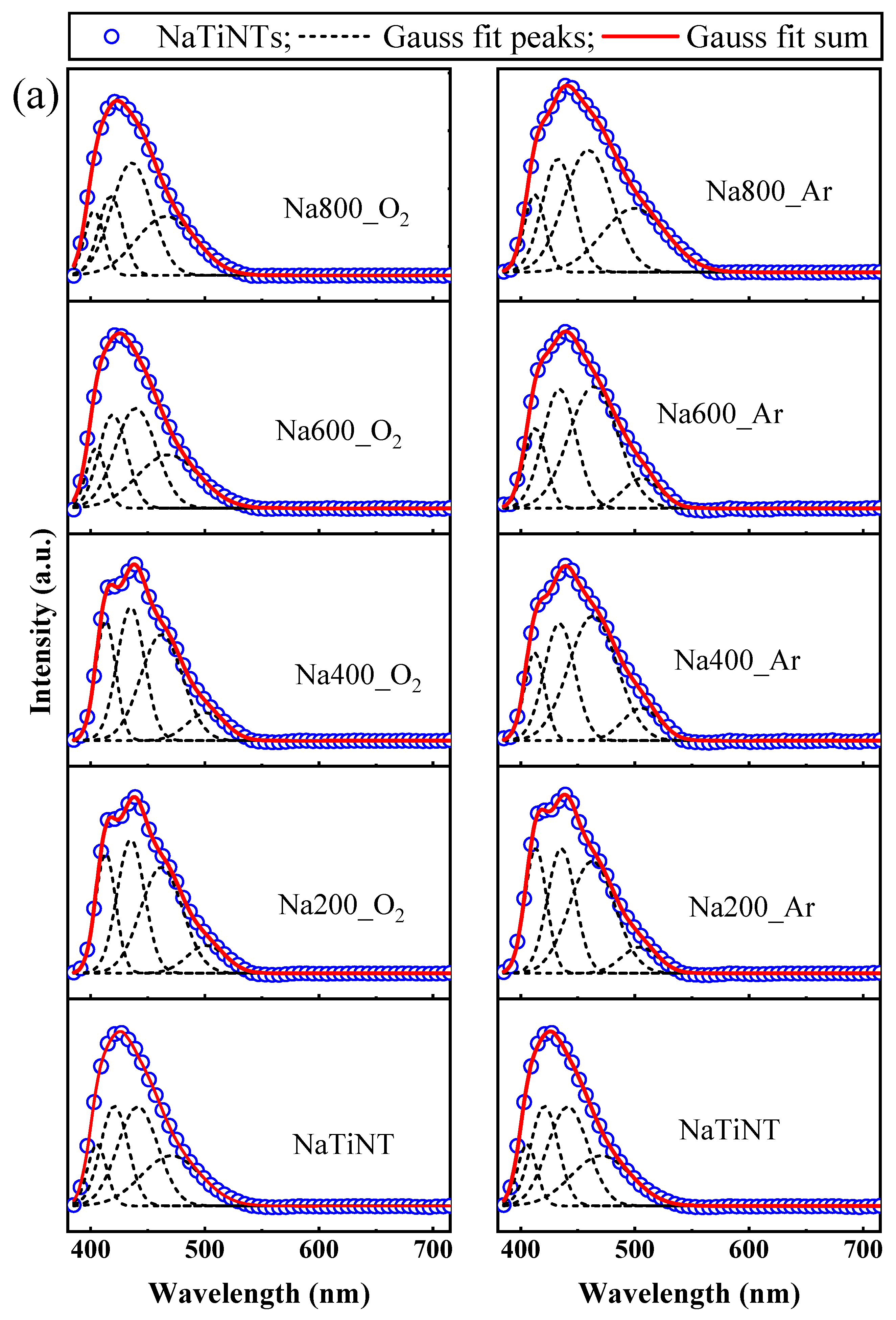
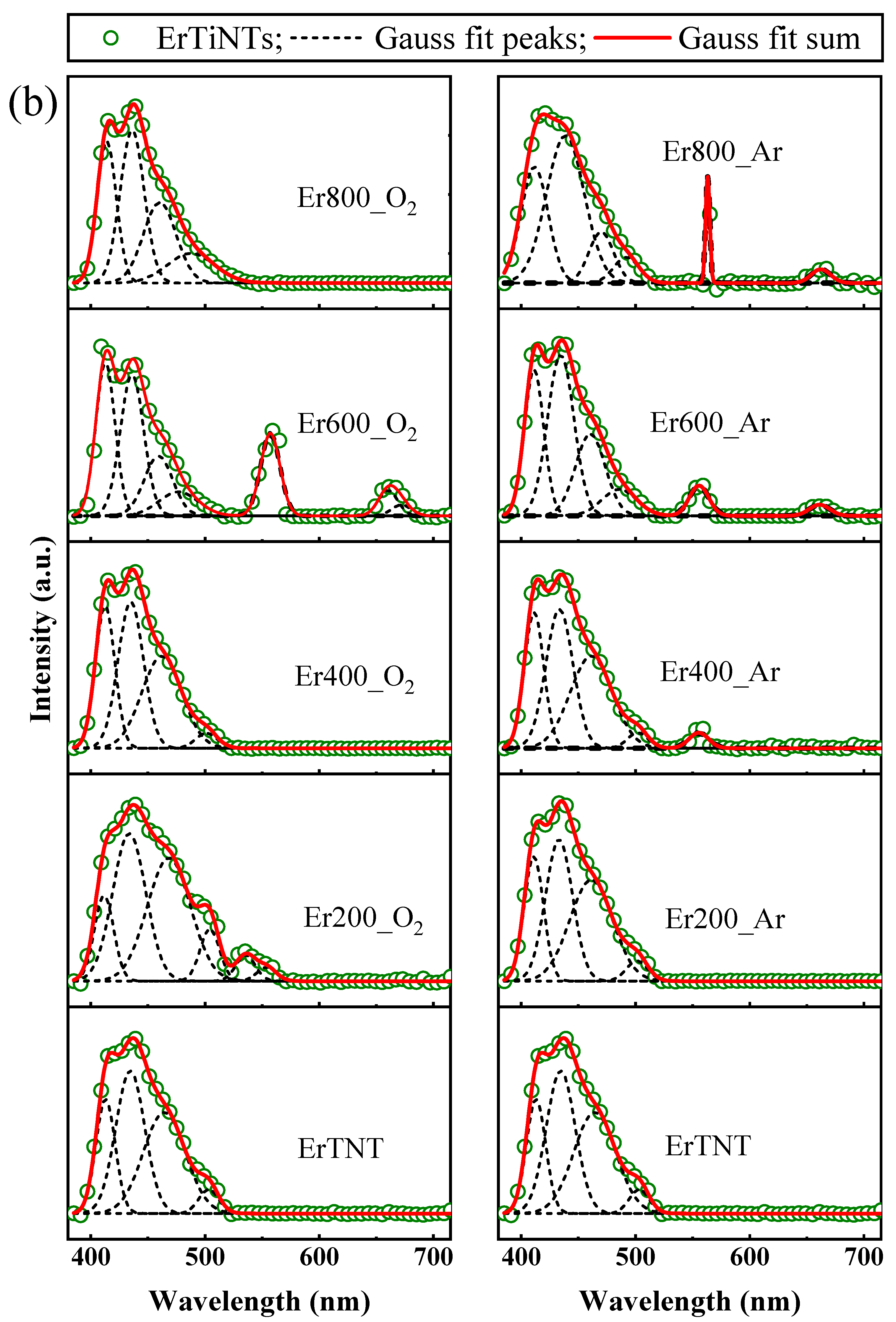
3.4. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Shao, J.; Yan, C.; Qin, R.; Lu, Z.; Geng, H.; Xu, T.; Ju, L. A Review on Optoelectronic Device Applications of 2D Transition Metal Carbides and Nitrides. Mater. Des. 2021, 200, 109452. [Google Scholar] [CrossRef]
- Bablich, A.; Kataria, S.; Lemme, M.C. Graphene and Two-Dimensional Materials for Optoelectronic Applications. Electronics 2016, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Qin, A.; Tang, B.Z. Siloles in Optoelectronic Devices. J. Mater. Chem. C 2017, 5, 7375–7389. [Google Scholar] [CrossRef]
- Prakash, J.; Samriti; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel Rare Earth Metal–Doped One-Dimensional TiO2 Nanostructures: Fundamentals and Multifunctional Applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Khanna, S.; Marathey, P.; Mukhopadhyay, I. Proceedings Titania Coated Zinc Oxide Nanorods: A Study of Structural and Optical Properties for Photocatalytic Applications. Mater. Today Proc. 2021, 47, 682–685. [Google Scholar] [CrossRef]
- Muniyappan, S.; Solaiyammal, T.; Sudhakar, K.; Karthigeyan, A.; Murugakoothan, P. Conventional Hydrothermal Synthesis of Titanate Nanotubes: Systematic Discussions on Structural, Optical, Thermal and Morphological Properties. Mod. Electron. Mater. 2017, 3, 174–178. [Google Scholar] [CrossRef]
- Badawi, A.; Althobaiti, M.G.; Ali, E.E.; Alharthi, S.S.; Alharbi, A.N. A Comparative Study of the Structural and Optical Properties of Transition Metals (M = Fe, Co, Mn, Ni) Doped ZnO Films Deposited by Spray-Pyrolysis Technique for Optoelectronic Applications. Opt. Mater. 2022, 124, 112055. [Google Scholar] [CrossRef]
- Souza, H.T.S.; Oliveira, S.A.A.; Souza, J.S. Modulating the Photocatalytic Activity of Ag Nanoparticles-Titanate Nanotubes Heterojunctions through Control of Microwave-Assisted Synthesis Conditions. J. Photochem. Photobiol. A Chem. 2020, 390, 112264. [Google Scholar] [CrossRef]
- Plodinec, M.; Gajović, A.; Jakša, G.; Žagar, K.; Čeh, M. High-Temperature Hydrogenation of Pure and Silver-Decorated Titanate Nanotubes to Increase Their Solar Absorbance for Photocatalytic Applications. J. Alloy. Compd. 2014, 591, 147–155. [Google Scholar] [CrossRef]
- Buchholcz, B.; Varga, E.; Varga, T.; Plank, K.; Kiss, J.; Kónya, Z. Structure and Stability of Boron Doped Titanate Nanotubes and Nanowires. Vacuum 2017, 138, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Erjavec, B.; Kaplan, R.; Pintar, A. Effects of Heat and Peroxide Treatment on Photocatalytic Activity of Titanate Nanotubes. Catal. Today 2015, 241, 15–24. [Google Scholar] [CrossRef]
- Vranješ, M.; Jakovljević, J.K.; Milošević, M.; Ćirić-Marjanović, G.; Stoiljković, M.; Konstantinović, Z.; Pavlović, V.; Milivojević, D.; Šaponjić, Z. Hydrothermal Synthesis of Mn2+ Doped Titanate Nanotubes: Investigation of Their Structure and Room Temperature Ferromagnetic Behavior. Solid State Sci. 2019, 94, 155–161. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Gusmão, S.B.S.; Ghosh, A.; Marques, T.M.F.; Vieira, L.H.S.; Ferreira, O.P.; Silva-Filho, E.C.; Lobo, A.O.; Osajima, J.A.; Viana, B.C. Titanate-Based One-Dimensional Nano-Heterostructure: Study of Hydrothermal Reaction Parameters for Improved Photocatalytic Application. Solid State Sci. 2019, 98, 106043. [Google Scholar] [CrossRef]
- Manique, M.C.; Silva, A.P.; Alves, A.K.; Bergmann, C.P. Titanate nanotubes produced from microwave-assisted hydrothermal synthesis: Characterization, adsorption and photocatalytic activity. Braz. J. Chem. Eng. 2017, 34, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Hafez, H.S.; Saif, M.; McLeskey, J.T., Jr.; Abdel-Mottaleb, M.S.A.; Yahia, I.S.; Story, T.; Knoff, W. Hydrothermal Preparation of Gd3+- Doped Titanate Nanotubes: Magnetic Properties and Photovoltaic Performance. Int. J. Photoenergy 2009, 2009, 240402. [Google Scholar] [CrossRef] [Green Version]
- Phan, T.D.; Vo, C.M.; Tran, T.M.T.; Luu, T.L.A.; Nguyen, X.S. Structural and Bandgap Properties of Titanium Dioxide Nanotube/Graphene Oxide Composites Prepared by a Facile Hydrothermal Method. Mater. Res. Express 2019, 6, 105054. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Luz-Lima, C.; Sacilloti, M.; Fujisawa, K.; Perea-Lopez, N.; Terrones, M.; Silva, E.N.; Ferreira, O.P.; Viana, B.C. Photoluminescence Enhancement of Titanate Nanotubes by Insertion of Rare Earth Ions in Their Interlayer Spaces. J. Nanomater. 2017, 2017, 3809807. [Google Scholar] [CrossRef] [Green Version]
- Sales, D.A.; Marques, T.M.F.; Ghosh, A.; Gusmão, S.B.S.; Vasconcelos, T.L.; Luz-Lima, C.; Ferreira, O.P.; Hollanda, L.M.; Lima, I.S.; Silva-Filho, E.C.; et al. Synthesis of Silver-Cerium Titanate Nanotubes and Their Surface Properties and Antibacterial Applications. Mater. Sci. Eng. C 2020, 115, 111051. [Google Scholar] [CrossRef]
- Gusmão, S.B.S.; Ghosh, A.; Marques, T.M.F.; Gusmão, G.O.M.; Oliveira, T.G.; Cavalcante, L.C.D.; Vasconcelos, T.L.; Abreu, G.J.P.; Guerra, Y.; Peña-Garcia, R.; et al. Structural and Magnetic Properties of Titanate Nano-Heterostructures Decorated with Iron Based Nanoparticles. J. Phys. Chem. Solids 2020, 145, 109561. [Google Scholar] [CrossRef]
- Das, P.; Das, S.; Ratha, S.; Chakraborty, B.; Chatterjee, S. Ion Beam Engineered Hydrogen Titanate Nanotubes for Superior Energy Storage Application. Electrochim. Acta 2021, 371, 137774. [Google Scholar] [CrossRef]
- Chen, L.; Ji, H.; Qi, J.; Huang, T.; Wang, C.; Liu, W. Degradation of Acetaminophen by Activated Peroxymonosulfate Using Co(OH)2 Hollow Microsphere Supported Titanate Nanotubes: Insights into Sulfate Radical Production Pathway through CoOH+ Activation. Chem. Eng. J. 2021, 406, 126877. [Google Scholar] [CrossRef]
- Thi, D.; Thi, H.; Thi, D.; Thu, A.; Quang, P.; Hong, G. Schottky Contacts of (Au, Pt)/ Nanotube-Titanates for Fast Response to NO2 Gas at Room Temperature. Sens. Actuators B Chem. 2017, 244, 941–948. [Google Scholar] [CrossRef]
- Esteves, M.; Fernández-Werner, L.; Pignanelli, F.; Montenegro, B.; Belluzzi, M.; Pistón, M.; Chialanza, M.R.; Faccio, R.; Mombrú, Á.W. Synthesis, Characterization and Simulation of Lithium Titanate Nanotubes for Dye Sensitized Solar Cells. Ceram. Int. 2019, 45, 708–717. [Google Scholar] [CrossRef]
- Yarali, M.; Biçer, E.; Gürsel, S.A.; Yürüm, A. Expansion of Titanate Nanotubes by the Use of a Surfactant and Its Improved Performance as an Anode in Li-Ion Batteries. Electrochim. Acta 2016, 220, 453–464. [Google Scholar] [CrossRef]
- Freitas, T.S.; Marques, T.M.F.; Barros, L.N.L.C.; da Silva, J.H.; Cruz, R.P.; Pereira, R.L.S.; Silva, A.R.P.; Santos, A.T.L.; Ghosh, A.; Agressott, E.V.H.; et al. Synthesis of Cu-TiNT, Characterization, and Antibacterial Properties Evaluation. Mater. Today Chem. 2021, 21, 100539. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Ferreira, O.P.; Da Costa, J.A.P.; Fujisawa, K.; Terrones, M.; Viana, B.C. Study of the Growth of CeO2 Nanoparticles onto Titanate Nanotubes. J. Phys. Chem. Solids 2015, 87, 213–220. [Google Scholar] [CrossRef]
- Rónavári, A.; Kovács, D.; Vágvölgyi, C.; Kónya, Z.; Kiricsi, M.; Pfeiffer, I. Ion Exchange Defines the Biological Activity of Titanate Nanotubes. J. Basic Microbiol. 2016, 56, 557–565. [Google Scholar] [CrossRef]
- Uematsu, E.; Itadani, A.; Uematsu, K.; Toda, K.; Sato, M. IR Study on Adsorption of Carbon Dioxide on Alkaline Earth Metal Ion-Exchanged Titanate Nanotubes at Room Temperature. Vib. Spectrosc. 2020, 109, 103088. [Google Scholar] [CrossRef]
- Méndez-Galván, M.; Celaya, C.A.; Jaramillo-Quintero, O.A.; Muñiz, J.; Díaz, G.; Lara-García, H.A. Tuning the Band Gap of M-Doped Titanate Nanotubes (M = Fe, Co, Ni, and Cu): An Experimental and Theoretical Study. Nanoscale Adv. 2021, 3, 1382–1391. [Google Scholar] [CrossRef]
- Dos Santos, N.M.; Rocha, J.M.; Matos, J.M.E.; Ferreira, O.P.; Filho, J.M.; Viana, B.C.; Oliveira, A.C. Metal Cations Intercalated Titanate Nanotubes as Catalysts for α,β Unsaturated Esters Production. Appl. Catal. A Gen. 2013, 454, 74–80. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Li, Y.; Li, M.; Wu, Y.; Wang, C.; Jiao, H.; Na, P. Enhanced Adsorption of Cesium Ions by Electrochemically Switched Ion Exchange Method: Based on Surface-Synthetic Na2Ti3O7 Nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123712. [Google Scholar] [CrossRef]
- Lopes, J.N.L.; Filho, J.C.S.; Messias, D.N.; Pilla, V.; Dantas, N.O.; Silva, A.C.A.; Andrade, A.A. Nd3+-Doped TiO2 Nanocrystals: Structural Changes, Excited-State Dynamics, and Luminescence Defects. J. Lumin. 2021, 240, 118461. [Google Scholar] [CrossRef]
- Talane, T.E.; Mbule, P.S.; Noto, L.L.; Shingange, K.; Mhlongo, G.H.; Mothudi, B.M. Sol-Gel Preparation and Characterization of Er3+ Doped TiO2 Luminescent Nanoparticles. Mater. Res. Bull. 2018, 108, 234–241. [Google Scholar] [CrossRef]
- Conde-Gallardo, A.; Garcıía-Rocha, M.; Hernández-Calderón, I.; Palomino-Merino, R. Photoluminescence Properties of the Eu3+ Activator Ion in the TiO2 Host Matrix. Appl. Phys. Lett. 2001, 78, 3436–3438. [Google Scholar] [CrossRef]
- Jeon, S.; Braun, P. V Hydrothermal Synthesis of Er-Doped Luminescent TiO2 Nanoparticles. Chem. Mater. 2003, 15, 1256–1263. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Chi, B.; Victorio, E.S.; Jin, T. Synthesis of Eu-Doped Photoluminescent Titania Nanotubes via a Two-Step Hydrothermal Treatment. Nanotechnology 2006, 17, 2234–2241. [Google Scholar] [CrossRef]
- Radha, E.; Komaraiah, D.; Sayanna, R.; Sivakumar, J. Photoluminescence and Photocatalytic Activity of Rare Earth Ions Doped Anatase TiO2 Thin Films. J. Lumin. 2022, 244, 118727. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; James, J.; Kalarikkal, N.; Sivakumar, J.; Ramana Reddy, M.V.; Sayanna, R. Effect of Particle Size and Dopant Concentration on the Raman and the Photoluminescence Spectra of TiO2:Eu3+ Nanophosphor Thin Films. J. Lumin. 2019, 211, 320–333. [Google Scholar] [CrossRef]
- Chang, M.; Song, Y.; Sheng, Y.; Chen, J.; Guan, H.; Shi, Z.; Zhou, X.; Zheng, K.; Zou, H. Photoluminescence and Photocatalysis Properties of Dual-Functional Eu3+ -Doped Anatase Nanocrystals. J. Phys. Chem. C 2017, 121, 2369–2379. [Google Scholar] [CrossRef]
- Meksi, M.; Turki, A.; Kochkar, H.; Bousselmi, L.; Guillard, C.; Berhault, G. The Role of Lanthanum in the Enhancement of Photocatalytic Properties of TiO2 Nanomaterials Obtained by Calcination of Hydrogenotitanate Nanotubes. Appl. Catal. B Environ. 2016, 181, 651–660. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. Evidence of Upconversion Luminescence Contribution to the Improved Photoactivity of Erbium Doped TiO2 Systems. Chem. Commun. 2012, 48, 7865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castañeda-Contreras, J.; Marañón-Ruiz, V.F.; Chiu-Zárate, R.; Pérez-Ladrón de Guevara, H.; Rodriguez, R.; Michel-Uribe, C. Photocatalytic Activity of Erbium-Doped TiO2 Nanoparticles Immobilized in Macro-Porous Silica Films. Mater. Res. Bull. 2012, 47, 290–295. [Google Scholar] [CrossRef]
- Pérez, J.A.B.; Courel, M.; Valderrama, R.C.; Hernández, I.; Pal, M.; Delgado, F.P.; Mathews, N.R. Structural, Optical, and Photoluminescence Properties of Erbium Doped TiO2 Films. Vacuum 2019, 169, 108873. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.-E.; Kang, K.-S.; Yoon, J. Enhanced Photocatalytic Activity of TiO2 Nanotubes Decorated with Erbium and Reduced Graphene Oxide. Appl. Surf. Sci. 2021, 565, 150459. [Google Scholar] [CrossRef]
- Singh, K.; Harish, S.; Kristy, A.P.; Shivani, V.; Archana, J.; Navaneethan, M.; Shimomura, M.; Hayakawa, Y. Erbium Doped TiO2 Interconnected Mesoporous Spheres as an Efficient Visible Light Catalyst for Photocatalytic Applications. Appl. Surf. Sci. 2018, 449, 755–763. [Google Scholar] [CrossRef]
- Dhanalakshmi, J.; Selvakumari, C. Improved Photo-Induced Charge Carriers Separation through the Addition of Erbium on TiO2 Nanoparticles and Its Effect on Photocatalytic Degradation of Rhodamine B. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 524–533. [Google Scholar] [CrossRef]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.-E.; Kang, K.-S.; Yoon, J. Improved Photoelectrochemical Properties of TiO2 Nanotubes Doped with Er and Effects on Hydrogen Production from Water Splitting. Chemosphere 2021, 267, 129289. [Google Scholar] [CrossRef]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The Role of Lanthanides in TiO2-Based Photocatalysis: A Review. Appl. Catal. B Environ. 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Kiatkittipong, K.; Scott, J.; Amal, R. Hydrothermally Synthesized Titanate Nanostructures: Impact of Heat Treatment on Particle Characteristics and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2011, 3, 3988–3996. [Google Scholar] [CrossRef]
- Akbari, M.; Mirzaei, A.A.; Arsalanfar, M. Microemulsion Based Synthesis of Promoted Fe–Co/MgO Nanocatalyst: Influence of Calcination Atmosphere on the Physicochemical Properties, Activity and Light Olefins Selectivity for Hydrogenation of Carbon Monoxide. Mater. Chem. Phys. 2020, 249, 123003. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Lv, T.; Guo, M.; Li, J.; Okonkwo, C.A.; Liu, Q.; Jia, L. The Effect of Calcination Atmosphere upon the Photocatalytic Performance of Au-La2O3/TiO2 for Hydrogen Production from Formic Acid. Appl. Catal. A Gen. 2017, 547, 96–104. [Google Scholar] [CrossRef]
- Slimen, H.; Lachheb, H.; Qourzal, S.; Assabbane, A.; Houas, A. The Effect of Calcination Atmosphere on the Structure and Photoactivity of TiO2 Synthesized through an Unconventional Doping Using Activated Carbon. J. Environ. Chem. Eng. 2015, 3, 922–929. [Google Scholar] [CrossRef]
- Galetti, A.E.; Gomez, M.F.; Arrua, L.A.; Abello, M.C. Ethanol Steam Reforming over Ni/ZnAl2O4-CeO2. Influence of Calcination Atmosphere and Nature of Catalytic Precursor. Appl. Catal. A Gen. 2011, 408, 78–86. [Google Scholar] [CrossRef]
- Blasco-Tamarit, E.; Solsona, B.; Sánchez-Tovar, R.; García-García, D.; Fernández-Domene, R.M.; García-Antón, J. Influence of Annealing Atmosphere on Photoelectrochemical Response of TiO2 Nanotubes Anodized under Controlled Hydrodynamic Conditions. J. Electroanal. Chem. 2021, 897, 115579. [Google Scholar] [CrossRef]
- Rodrigues, G.L.C.; de Oliveira, T.G.; Gusmão, S.B.S.; Marques, T.M.F.; Ferreira, O.P.; Ghosh, A.; dos Santos, C.C.; Milani, R.; Garcia, R.R.P.; Viana, B.C. Titanate Nanotubes: Effect of Rare Earth Insertion, Thermal Treatment and Their Optical Properties. Opt. Mater. 2022, 127, 112302. [Google Scholar] [CrossRef]
- Gusmão, S.B.S.; Ghosh, A.; Marques, T.M.F.; Ferreira, O.P.; Lobo, A.O.; Osajima, J.A.O.; Luz-Lima, C.; Sousa, R.R.M.; Matos, J.M.E.; Viana, B.C. One-Pot Synthesis of Titanate Nanotubes Decorated with Anatase Nanoparticles Using a Microwave-Assisted Hydrothermal Reaction. J. Nanomater. 2019, 2019, 4825432. [Google Scholar] [CrossRef]
- Jose, M.; Szymańska, K.; Szymański, K.; Moszyński, D.; Mozia, S. Effect of Copper Salts on the Characteristics and Antibacterial Activity of Cu-Modified Titanate Nanotubes. J. Environ. Chem. Eng. 2020, 8, 104550. [Google Scholar] [CrossRef]
- Cech, O.; Castkova, K.; Chladil, L.; Dohnal, P.; Cudek, P.; Libich, J.; Vanysek, P. Synthesis and Characterization of Na2Ti6O13 and Na2Ti6O13/Na2Ti3O7 Sodium Titanates with Nanorod-like Structure as Negative Electrode Materials for Sodium-Ion Batteries. J. Energy Storage 2017, 14, 391–398. [Google Scholar] [CrossRef]
- Gajović, A.; Friščić, I.; Plodinec, M.; Iveković, D. High Temperature Raman Spectroscopy of Titanate Nanotubes. J. Mol. Struct. 2009, 924–926, 183–191. [Google Scholar] [CrossRef]
- Zárate, R.A.; Fuentes, S.; Cabrera, A.L.; Fuenzalida, V.M. Structural Characterization of Single Crystals of Sodium Titanate Nanowires Prepared by Hydrothermal Process. J. Cryst. Growth 2008, 310, 3630–3637. [Google Scholar] [CrossRef]
- Zhu, K.R.; Yuan, Y.; Zhang, M.S.; Hong, J.M.; Deng, Y.; Yin, Z. Structural Transformation from NaHTi3O7 Nanotube to Na2Ti6O13 Nanorod. Solid State Commun. 2007, 144, 450–453. [Google Scholar] [CrossRef]
- Rónavári, A.; Buchholcz, B.; Kukovecz, Á.; Kónya, Z. Structure and Stability of Pristine and Bi and/or Sb Decorated Titanate Nanotubes. J. Mol. Struct. 2013, 1044, 104–108. [Google Scholar] [CrossRef]
- Ferreira, O.P.; Souza Filho, A.G.; Mendes Filho, J.; Alves, O.L. Unveiling the Structure and Composition of Titanium Oxide Nanotubes through Ion Exchange Chemical Reactions and Thermal Decomposition Processes. J. Braz. Chem. Soc. 2006, 17, 393–402. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Morais, R.N.; Nobre, F.X.; Rocha, J.M.; Ghosh, A.; Soares, T.A.S.; Viana, B.C.; Machado, G.; Costa, J.C.S.; De Matos, J.M.E. Hydrogen Production from Aqueous Glycerol Using Titanate Nanotubes Decorated with Au Nanoparticles as Photocatalysts. An. Acad. Bras. Ciências 2019, 91, 1–15. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Strayer, M.E.; Ghosh, A.; Silva, A.; Ferreira, O.P.; Fujisawa, K.; Alves da Cunha, J.R.; Abreu, G.J.P.; Terrones, M.; Mallouk, T.E.; et al. Homogeneously Dispersed CeO2 Nanoparticles on Exfoliated Hexaniobate Nanosheets. J. Phys. Chem. Solids 2017, 111, 335–342. [Google Scholar] [CrossRef]
- Xiao, N.; Li, Z.; Liu, J.; Gao, Y. Effects of Calcination Temperature on the Morphology, Structure and Photocatalytic Activity of Titanate Nanotube Thin Films. Thin Solid Film. 2010, 519, 541–548. [Google Scholar] [CrossRef]
- Wang, S.; Shi, H.; Xia, Y.; Liang, S.; Pei, M.; Xu, Z.; Pei, X.; Hu, Y.; Wu, X. Electrospun-Based Nanofibers for Sodium and Potassium Ion Storage: Structure Design for Alkali Metal Ions with Large Radius. J. Alloy. Compd. 2022, 918, 165680. [Google Scholar] [CrossRef]
- Junaid, M.; Kousar, I.; Gulbadan, S.; Khan, M.A.; Yousuf, M.A.; Baig, M.M.; Ashraf, G.A.; Somaily, H.H.; Morsi, M. Structural, Microstructural, Spectral, and Dielectric Properties of Erbium Substituted Spinel Ferrites. Phys. B Condens. Matter 2022, 641, 414120. [Google Scholar] [CrossRef]
- Ali, I.O.; Salama, T.M.; El-Gawad, A.A.; El-Henawy, A.A.; Ghazy, M.B.; Bakr, M.F. Silver Nanoparticles @ Titanate Nanotubes Composite: Synthesis, Characterization, Applications and Docking. Inorg. Chem. Commun. 2022, 137, 109187. [Google Scholar] [CrossRef]
- Viana, B.C.; Ferreira, O.P.; Souza Filho, A.G.; Mendes Filho, J.; Alves, O.L. Structural, Morphological and Vibrational Properties of Titanate Nanotubes and Nanoribbons. J. Braz. Chem. Soc. 2009, 20, 167–175. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Sales, D.A.; Silva, L.S.; Bezerra, R.D.S.; Silva, M.S.; Osajima, J.A.; Ferreira, O.P.; Ghosh, A.; Silva Filho, E.C.; Viana, B.C.; et al. Amino-Functionalized Titanate Nanotubes for Highly Efficient Removal of Anionic Dye from Aqueous Solution. Appl. Surf. Sci. 2020, 512, 145659. [Google Scholar] [CrossRef]
- Viana, B.C.; Ferreira, O.P.; Filho, A.G.S.; Hidalgo, A.A.; Filho, J.M.; Alves, O.L. Alkali Metal Intercalated Titanate Nanotubes: A Vibrational Spectroscopy Study. Vib. Spectrosc. 2011, 55, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Manfroi, D.C.; dos Anjos, A.; Cavalheiro, A.A.; Perazolli, L.A.; Varela, J.A.; Zaghete, M.A. Titanate Nanotubes Produced from Microwave-Assisted Hydrothermal Synthesis: Photocatalytic and Structural Properties. Ceram. Int. 2014, 40, 14483–14491. [Google Scholar] [CrossRef]
- Li, P.; Wang, P.; Qian, S.; Yu, H.; Lin, X.; Shui, M.; Zheng, X.; Long, N.; Shu, J. Synthesis of Na2Ti6O13 Nanorods as Possible Anode Materials for Rechargeable Lithium Ion Batteries. Electrochim. Acta 2016, 187, 46–54. [Google Scholar] [CrossRef]
- Liang, J.; Wang, J.; Song, K.; Wang, X.; Yu, K.; Liang, C. Enhanced Photocatalytic Activities of Nd-Doped TiO2 under Visible Light Using a Facile Sol-Gel Method. J. Rare Earths 2020, 38, 148–156. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Qi, Z.; Chai, S.Y.; Lee, C.; Park, S.-Y.; Jang, D.-J.; Lee, W.I. Formation of Highly Crystallized TiO2(B) and Its Photocatalytic Behavior. Appl. Catal. B Environ. 2010, 93, 368–375. [Google Scholar] [CrossRef]
- Kobwittaya, K.; Oishi, Y.; Torikai, T.; Yada, M.; Watari, T.; Luitel, H.N. Bright Red Upconversion Luminescence from Er3+ and Yb3+ Co-Doped ZnO-TiO2 Composite Phosphor Powder. Ceram. Int. 2017, 43, 13505–13515. [Google Scholar] [CrossRef]
- Lin, C.; Chao, J.; Liu, C.; Chang, J.; Wang, F. Effect of Calcination Temperature on the Structure of a Pt/TiO2 (B) Nanofiber and Its Photocatalytic Activity in Generating H 2. Langmuir 2008, 24, 9907–9915. [Google Scholar] [CrossRef]
- Sreekantan, S.; Wei, L.C. Study on the Formation and Photocatalytic Activity of Titanate Nanotubes Synthesized via Hydrothermal Method. J. Alloy. Compd. 2010, 490, 436–442. [Google Scholar] [CrossRef]
- de Carvalho, D.C.; Oliveira, A.C.; Ferreira, O.P.; Filho, J.M.; Tehuacanero-Cuapa, S.; Oliveira, A.C. Titanate Nanotubes as Acid Catalysts for Acetalization of Glycerol with Acetone: Influence of the Synthesis Time and the Role of Structure on the Catalytic Performance. Chem. Eng. J. 2017, 313, 1454–1467. [Google Scholar] [CrossRef]
- Anbharasi, L.; Bhanu Rekha, E.A.; Rahul, V.R.; Roy, B.; Gunaseelan, M.; Yamini, S.; Adusumalli, V.N.K.B.; Sarkar, D.; Mahalingam, V.; Senthilselvan, J. Tunable Emission and Optical Trapping of Upconverting LiYF4:Yb,Er Nanocrystal. Opt. Laser Technol. 2020, 126, 106109. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible Light Activity of Rare Earth Metal Doped (Er3+, Yb3+ or Er3+/Yb3+) Titania Photocatalysts. Appl. Catal. B Environ. 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Xu, Y.; Wang, H.; Li, X.; Wang, C. Electrospun Er:TiO2 Nanofibrous Films as Efficient Photocatalysts under Solar Simulated Light. Mater. Lett. 2010, 64, 147–150. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.-H.; Lim, S.K.; Kim, S. Effects of Ion Exchange and Calcinations on the Structure and Photocatalytic Activity of Hydrothermally Prepared Titanate Nanotubes. Cryst. Res. Technol. 2012, 47, 1190–1194. [Google Scholar] [CrossRef]
- Turki, A.; Kochkar, H.; Guillard, C.; Berhault, G.; Ghorbel, A. Effect of Na Content and Thermal Treatment of Titanate Nanotubes on the Photocatalytic Degradation of Formic Acid. Appl. Catal. B Environ. 2013, 138–139, 401–415. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Obregón-Alfaro, S.; Lozano-Sánchez, L.M.; Lee, S.-W. Rapid Microwave-Assisted Synthesis of One-Dimensional Silver–H2Ti3O7 Nanotubes. J. Mol. Catal. A Chem. 2012, 353–354, 163–170. [Google Scholar] [CrossRef]
- Loan, T.T.; Huong, V.H.; Huyen, N.T.; Van Quyet, L.; Bang, N.A.; Long, N.N. Anatase to Rutile Phase Transformation of Iron-Doped Titanium Dioxide Nanoparticles: The Role of Iron Content. Opt. Mater. 2021, 111, 110651. [Google Scholar] [CrossRef]
- Park, H.; Goto, T.; Cho, S.; Nishida, H.; Sekino, T. Enhancing Visible Light Absorption of Yellow-Colored Peroxo-Titanate Nanotubes Prepared Using Peroxo Titanium Complex Ions. ACS Omega 2020, 5, 21753–21761. [Google Scholar] [CrossRef]
- Barbieriková, Z.; Lončarević, D.; Papan, J.; Vukoje, I.D.; Stoiljković, M.; Phillip Ahrenkiel, S.; Nedeljković, J.M. Photocatalytic Hydrogen Evolution over Surface-Modified Titanate Nanotubes by 5-Aminosalicylic Acid Decorated with Silver Nanoparticles. Adv. Powder Technol. 2020, 31, 4683–4690. [Google Scholar] [CrossRef]
- Gao, T.; Wu, Q.; Fjellvåg, H.; Norby, P. Topological Properties of Titanate Nanotubes. J. Phys. Chem. C 2008, 112, 8548–8552. [Google Scholar] [CrossRef]
- Bem, V.; Neves, M.C.; Nunes, M.R.; Silvestre, A.J.; Monteiro, O.C. Influence of the Sodium/Proton Replacement on the Structural, Morphological and Photocatalytic Properties of Titanate Nanotubes. J. Photochem. Photobiol. A Chem. 2012, 232, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chou, C. The Effects of Sodium Content and Hydrogenation of TiO2 Nanotubes on Photocatalytic Activity. J. Photochem. Photobiol. A Chem. 2018, 358, 226–235. [Google Scholar] [CrossRef]
- Peng, Y.-P.; Lo, S.-L.; Ou, H.-H.; Lai, S.-W. Microwave-Assisted Hydrothermal Synthesis of N-Doped Titanate Nanotubes for Visible-Light-Responsive Photocatalysis. J. Hazard. Mater. 2010, 183, 754–758. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, H.; Wang, N.; Lin, C.; Li, J. The Evolution of Morphology and Crystal Form of Titanate Nanotubes under Calcination and Its Mechanism. J. Alloy. Compd. 2007, 431, 230–235. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Qin, L. Reduction in the Electronic Band Gap of Titanium Oxide Nanotubes. Solid State Commun. 2007, 141, 168–171. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kordás, K.; Kiss, J.; Kónya, Z. Atomic Scale Characterization and Surface Chemistry of Metal Modified Titanate Nanotubes and Nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef] [Green Version]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Kim, D.H.; Jho, J.H.; Lee, K.S.; Lee, W.J.; Lee, H.G.; Kim, S.J. Effect of Post Treatments on the Structure and Thermal Stability of Titanate Nanotubes. Nanotechnology 2006, 17, 5922–5929. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Mandal, G.; Vasundhara, M. Visible Range Optical Absorption, Urbach Energy Estimation and Paramagnetic Response in Cr-Doped TiO2 Nanocrystals Derived by a Sol–Gel Method. Phys. Chem. Chem. Phys. 2019, 21, 12991–13004. [Google Scholar] [CrossRef]
- Bokare, A.; Pai, M.; Athawale, A.A. Surface Modified Nd Doped TiO2 Nanoparticles as Photocatalysts in UV and Solar Light Irradiation. Sol. Energy 2013, 91, 111–119. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of Photoluminescence Performance of Nano-Sized Semiconductor Materials and Its Relationships with Photocatalytic Activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, Z.; Wang, P.; Li, Q.; Wang, H.; Wu, Z. Enhanced CO2 Photocatalytic Reduction Performance on Alkali and Alkaline Earth Metal Ion-Exchanged Hydrogen Titanate Nanotubes. Appl. Surf. Sci. 2019, 463, 456–462. [Google Scholar] [CrossRef]
- Pal, M.; Pal, U.; Jiménez, J.M.G.Y.; Pérez-Rodríguez, F. Effects of Crystallization and Dopant Concentration on the Emission Behavior of TiO2:Eu Nanophosphors. Nanoscale Res. Lett. 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Reszczyńska, J.; Grzyb, T.; Wei, Z.; Klein, M.; Kowalska, E.; Ohtani, B.; Zaleska-Medynska, A. Photocatalytic Activity and Luminescence Properties of RE3+-TiO2 Nanocrystals Prepared by Sol-Gel and Hydrothermal Methods. Appl. Catal. B Environ. 2016, 181, 825–837. [Google Scholar] [CrossRef]
- Barrocas, B.; Conceição Oliveira, M.; Nogueira, H.I.S.; Fateixa, S.; Monteiro, O.C. A Comparative Study on Emergent Pollutants Photo-Assisted Degradation Using Ruthenium Modified Titanate Nanotubes and Nanowires as Catalysts. J. Environ. Sci. 2020, 92, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, L.J.; Zhu, Z.Q.; Liu, Q.J. Synthesis and Properties of (Yb, N)-TiO2 Photocatalyst for Degradation of Methylene Blue (MB) under Visible Light Irradiation. Mater. Res. Bull. 2015, 70, 358–364. [Google Scholar] [CrossRef]
- Bandi, V.R.; Raghavan, C.M.; Grandhe, B.K.; Kim, S.S.; Jang, K.; Shin, D.-S.; Yi, S.-S.; Jeong, J.-H. Synthesis, Structural and Optical Properties of Pure and Rare-Earth Ion Doped TiO2 Nanowire Arrays by a Facile Hydrothermal Technique. Thin Solid Film. 2013, 547, 207–211. [Google Scholar] [CrossRef]
- Liqiang, J.; Xiaojun, S.; Baifu, X.; Baiqi, W.; Weimin, C.; Honggang, F. The Preparation and Characterization of La Doped TiO2 Nanoparticles and Their Photocatalytic Activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, T.G.; Shul, Y.-G. Photoluminescence of La/Ti Mixed Oxides Prepared Using Sol–Gel Process and Their PCBA Photodecomposition. J. Photochem. Photobiol. A Chem. 2007, 185, 156–160. [Google Scholar] [CrossRef]
- Yildirim, S.; Yurddaskal, M.; Dikici, T.; Aritman, I.; Ertekin, K.; Celik, E. Structural and Luminescence Properties of Undoped, Nd3+ and Er3+ Doped TiO2 Nanoparticles Synthesized by Flame Spray Pyrolysis Method. Ceram. Int. 2016, 42, 10579–10586. [Google Scholar] [CrossRef]
- Zhao, J.-G.; Zhang, W.-Y.; Ma, Z.-W.; Xie, E.-Q.; Zhao, A.-K.; Liu, Z.-J. Structure and Photoluminescence Properties of Er3+ -Doped TiO2 -SiO2 Powders Prepared by Sol—Gel Method. Chin. Phys. B 2011, 20, 87701. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Ramana Reddy, M.V.; Sayanna, R. Structural, Optical and Photoluminescence Studies of Sol-Gel Synthesized Pure and Iron Doped TiO2 Photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Amy, L.; Favre, S.; Gau, D.L.; Faccio, R. The Effect of Morphology on the Optical and Electrical Properties of Sodium Titanate Nanostructures. Appl. Surf. Sci. 2021, 555, 149610. [Google Scholar] [CrossRef]
- Montoncello, F.; Carotta, M.C.; Cavicchi, B.; Ferroni, M.; Giberti, A.; Guidi, V.; Malagù, C.; Martinelli, G.; Meinardi, F. Near-Infrared Photoluminescence in Titania: Evidence for Phonon-Replica Effect. J. Appl. Phys. 2003, 94, 1501–1505. [Google Scholar] [CrossRef]
- Fang, D.; Huang, K.; Liu, S.; Huang, J. Fabrication and Photoluminiscent Properties of Titanium Oxide Nanotube Arrays. J. Braz. Chem. Soc. 2008, 19, 1059–1064. [Google Scholar] [CrossRef] [Green Version]
- Viana, B.C.; Ferreira, O.P.; Souza Filho, A.G.; Rodrigues, C.M.; Moraes, S.G.; Mendes Filho, J.; Alves, O.L. Decorating Titanate Nanotubes with CeO2 Nanoparticles. J. Phys. Chem. C 2009, 113, 20234–20239. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Mayyas, M.; Koshy, P.; Hart, J.N.; Sorrell, C.C. Planar-Dependent Oxygen Vacancy Concentrations in Photocatalytic CeO2 Nanoparticles. CrystEngComm 2018, 20, 204–212. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Kaneti, Y.V.; Li, S. Tuning the Surface Oxygen Concentration of {111} Surrounded Ceria Nanocrystals for Enhanced Photocatalytic Activities. Nanoscale 2016, 8, 378–387. [Google Scholar] [CrossRef]

| Room Temperature | 200 °C | 400 °C | 600 °C | 800 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Atomic Mean of the Samples (%) | Ratio Na or Er/Ti (%) | Na/Ti | Er/Ti | Na/Ti | Er/Ti | Na/Ti | Er/Ti | Na/Ti | Er/Ti |
| NaTiNT_O2 | 8.50 | 0.84 | 0.44 | - | 0.75 | - | 0.45 | - | 0.63 | - |
| NaTiNT_Ar | 8.50 | 0.84 | 1.03 | - | 0.74 | - | 0.64 | - | 0.11 | - |
| ErTiNT_O2 | 6.20 | 0.30 | 0.27 | 0.12 | 0.0297 | 0.12 | 0.00 | 0.10 | 0.11 | 0.11 |
| ErTiNT_Ar | 6.20 | 0.30 | 0.14 | 0.11 | 0.09 | 0.10 | 0.00 | 0.11 | 0.16 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, G.L.C.; Oliveira, T.G.d.; Gusmão, S.B.S.; Ferreira, O.P.; Vasconcelos, T.L.; Guerra, Y.; Milani, R.; Peña-Garcia, R.; Viana, B.C. Study of Structural and Optical Properties of Titanate Nanotubes with Erbium under Heat Treatment in Different Atmospheres. Materials 2023, 16, 1842. https://doi.org/10.3390/ma16051842
Rodrigues GLC, Oliveira TGd, Gusmão SBS, Ferreira OP, Vasconcelos TL, Guerra Y, Milani R, Peña-Garcia R, Viana BC. Study of Structural and Optical Properties of Titanate Nanotubes with Erbium under Heat Treatment in Different Atmospheres. Materials. 2023; 16(5):1842. https://doi.org/10.3390/ma16051842
Chicago/Turabian StyleRodrigues, Gelson L. C., Tainara G. de Oliveira, Suziete B. S. Gusmão, Odair P. Ferreira, Thiago L. Vasconcelos, Yuset Guerra, Raquel Milani, Ramón Peña-Garcia, and Bartolomeu C. Viana. 2023. "Study of Structural and Optical Properties of Titanate Nanotubes with Erbium under Heat Treatment in Different Atmospheres" Materials 16, no. 5: 1842. https://doi.org/10.3390/ma16051842









