Isolating the Role of Bone Lacunar Morphology on Static and Fatigue Fracture Progression through Numerical Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Design
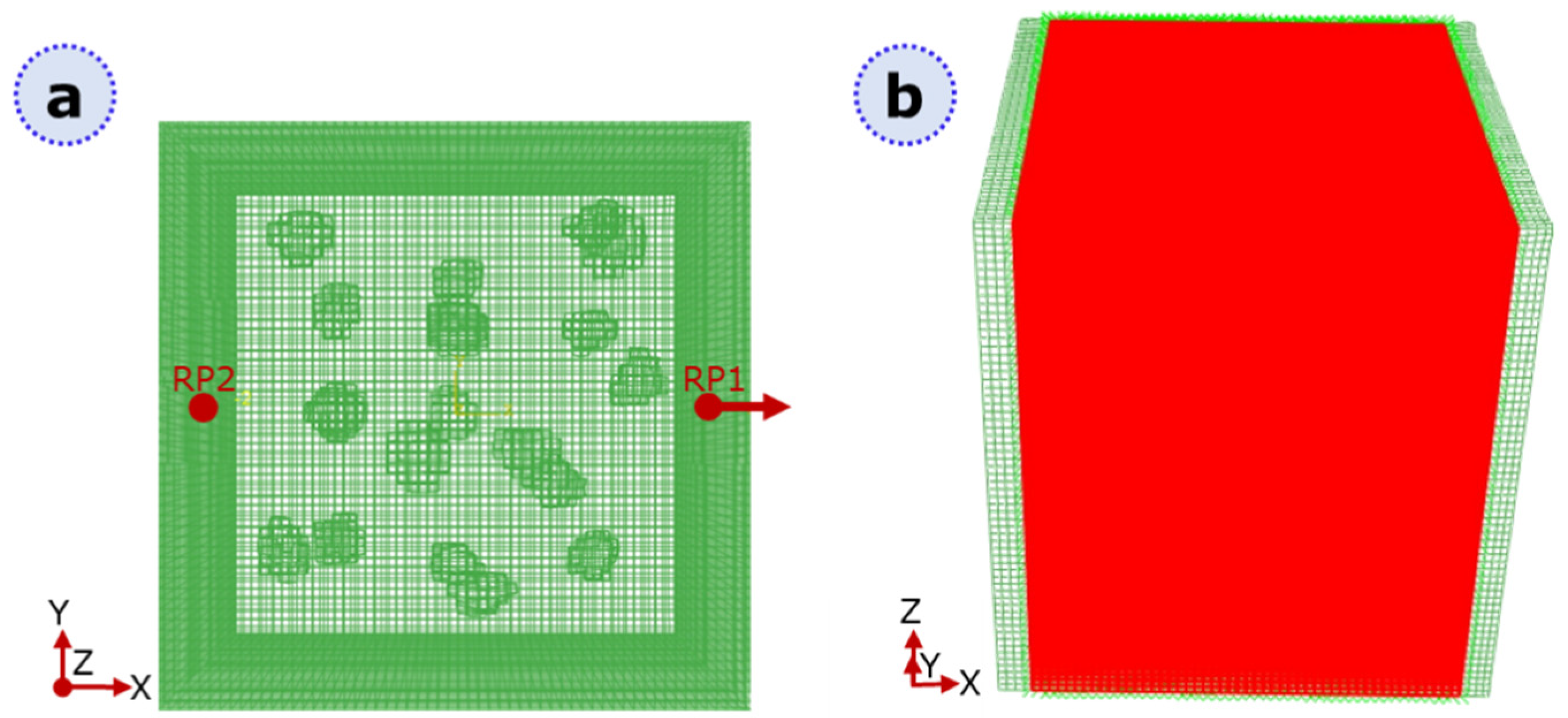
2.2. Static XFEM Analysis
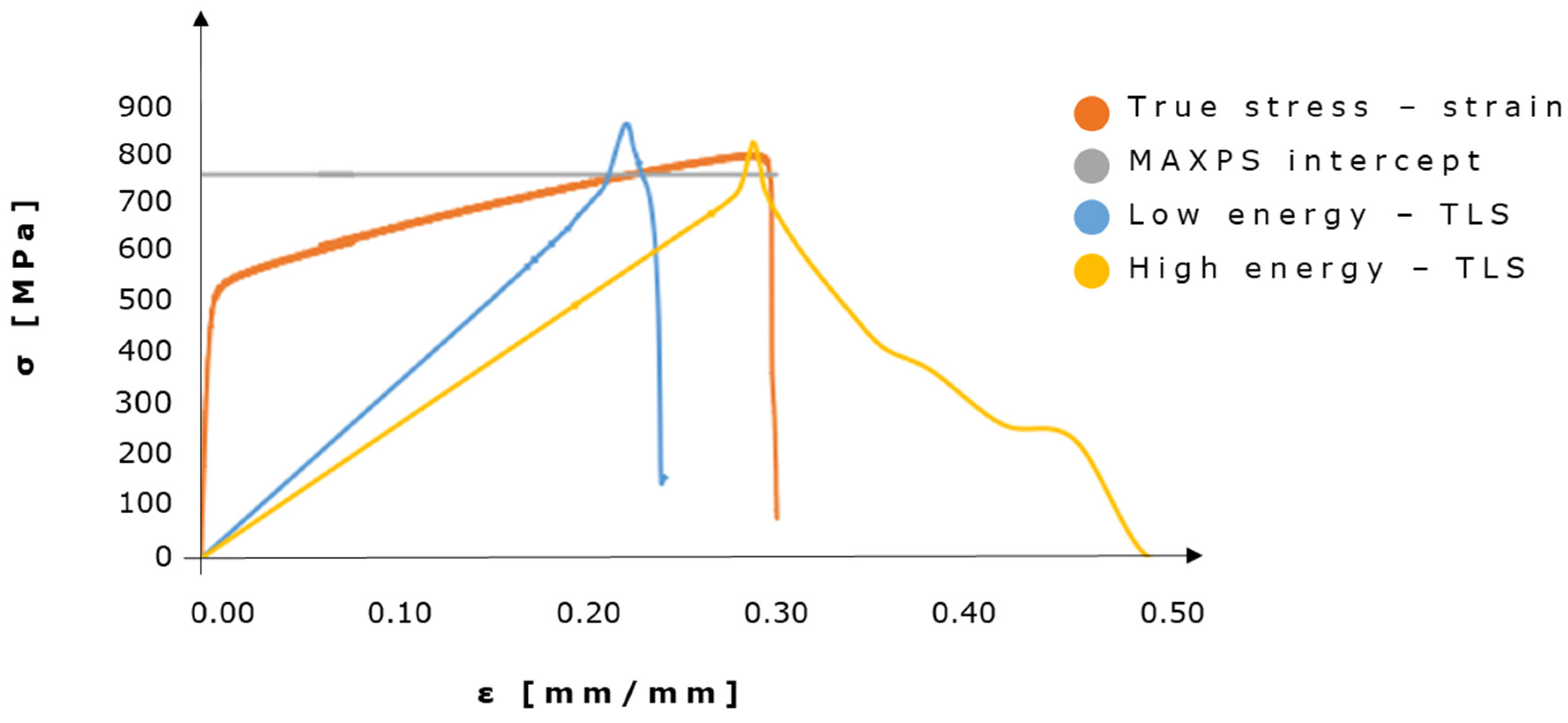
2.3. Fatigue Analysis
3. Results
3.1. Effects of Damage Evolution Parameters on Crack Initiation
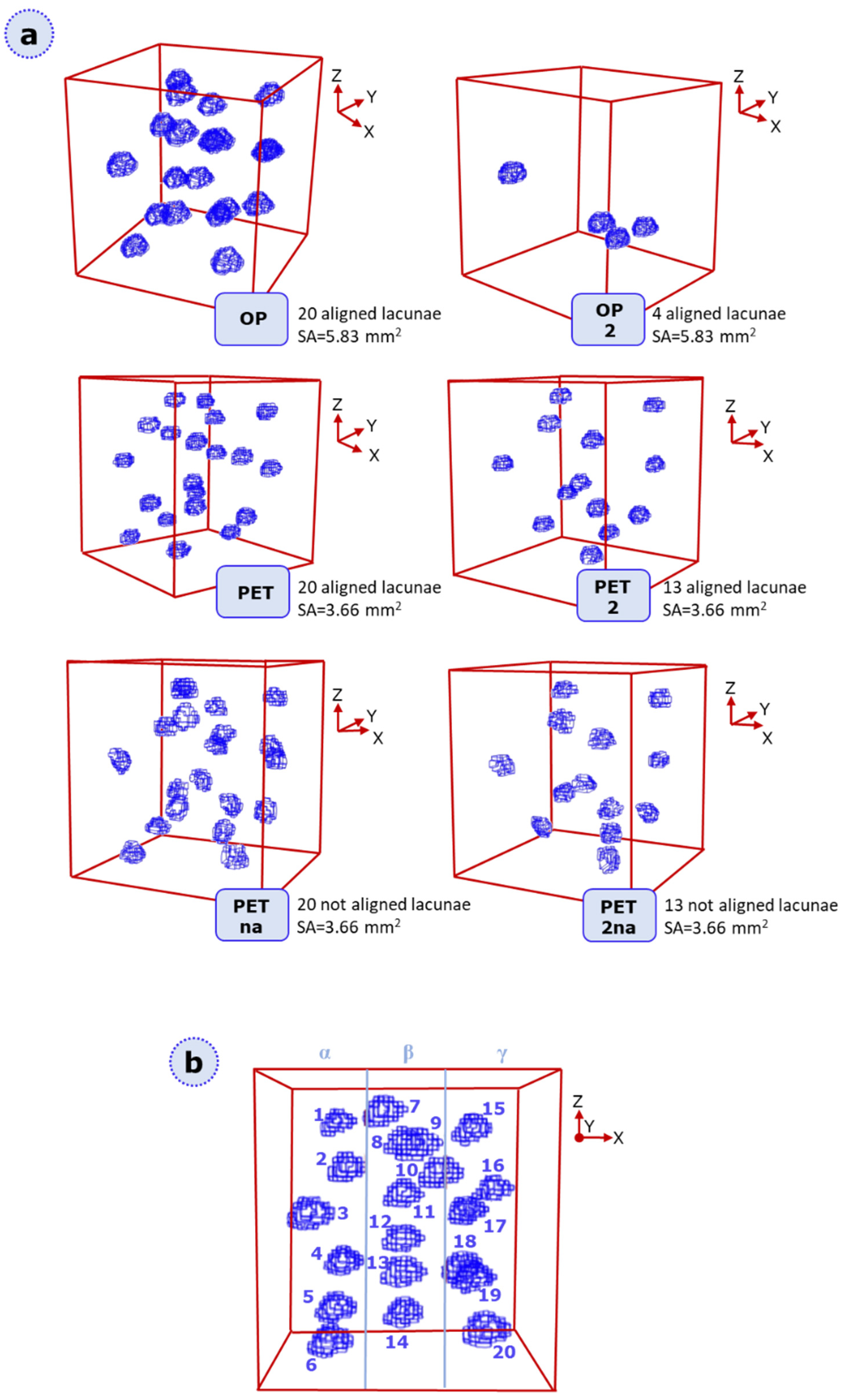
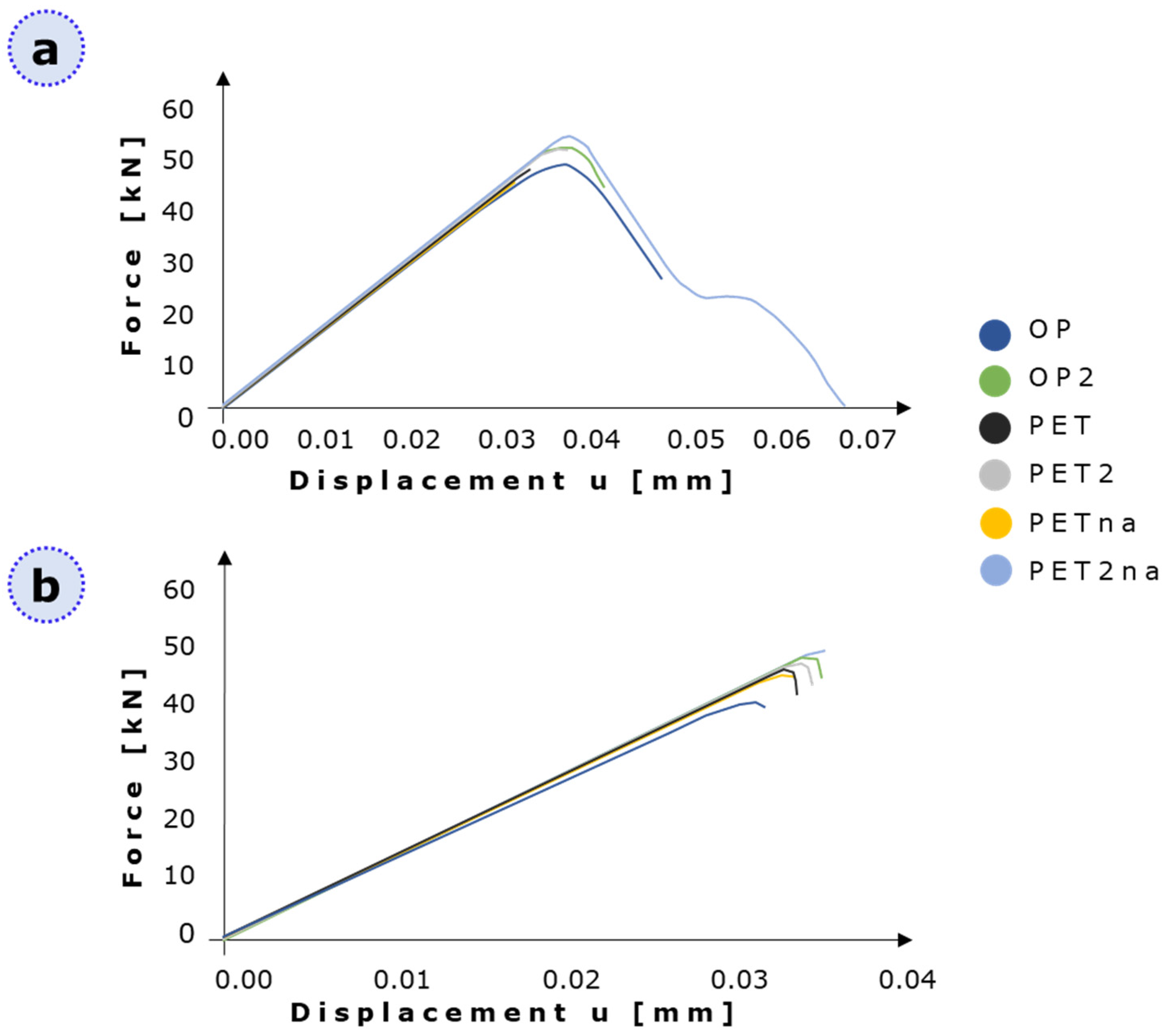
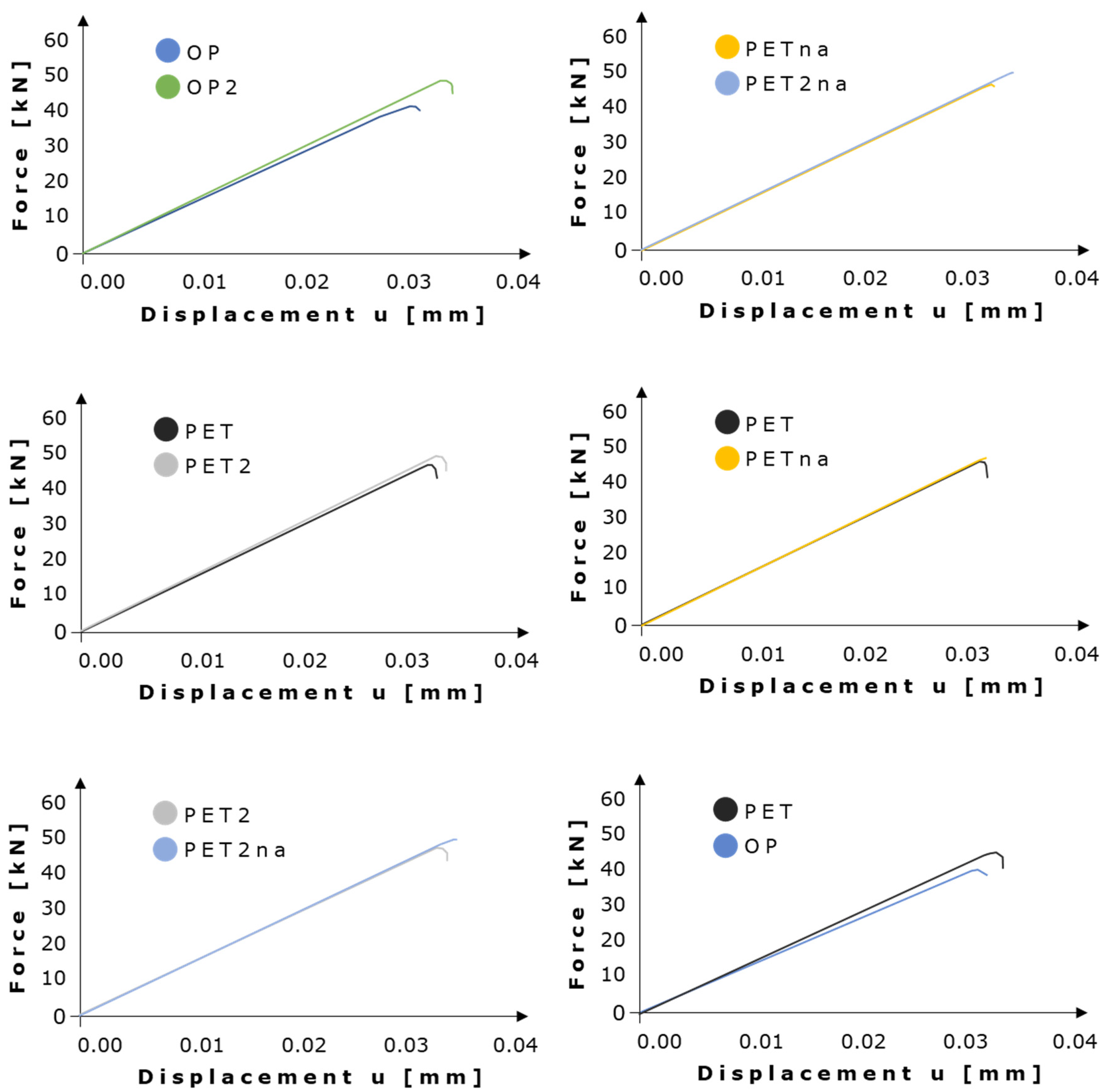
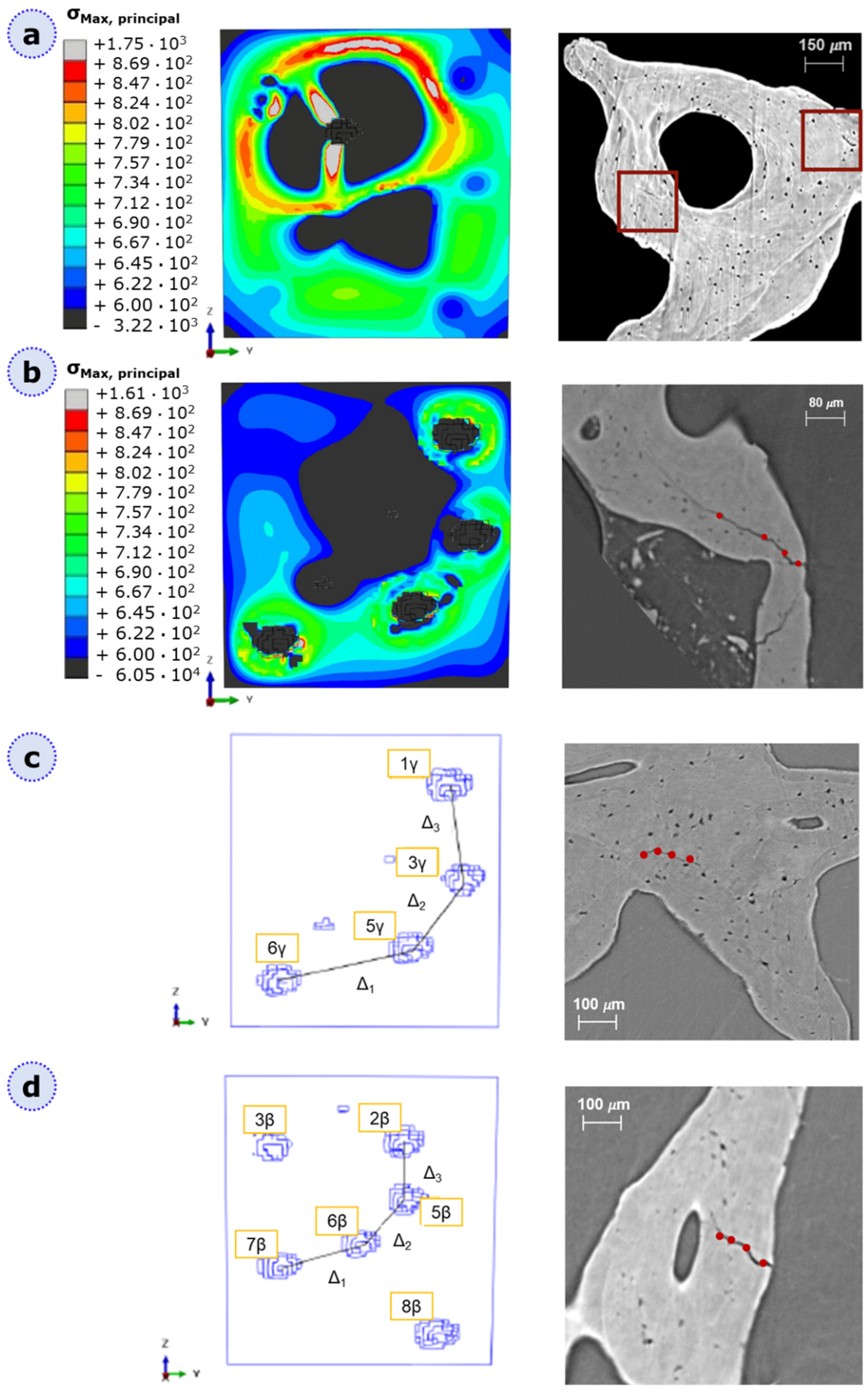
3.2. XFEM Static Analysis of Lacuna-Embedded Geometries
3.3. Fatigue Analysis of Lacuna-Embedded Geometries
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Odén, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef]
- Buccino, F.; Colombo, C.; Vergani, L.M. A review on multiscale bone damage: From the clinical to the research perspective. Materials 2021, 14, 1240. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef]
- Buccino, F.; Zagra, L.; Savadori, P.; Colombo, C.; Grossi, G.; Banfi, G.; Vergani, L. Mapping Local Mechanical Properties of Human Healthy and Osteoporotic Femoral Heads. SSRN Electron. J. 2021, 20, 101229. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Libonati, F.; Ferrario, D.; Rinaudo, L.; Messina, C.; Ulivieri, F.M.; Cesana, B.M.; Strano, M.; Vergani, L. Determinants of bone damage: An ex-vivo study on porcine vertebrae. PLoS ONE 2018, 13, e0202210. [Google Scholar] [CrossRef] [PubMed]
- Mirzaali, M.J.; Mussi, V.; Vena, P.; Libonati, F.; Vergani, L.; Strano, M. Mimicking the loading adaptation of bone microstructure with aluminum foams. Mater. Des. 2017, 126, 207–218. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Busse, B.; Ritchie, R.O. The fracture mechanics of human bone: Influence of disease and treatment. Bonekey Rep. 2015, 4, 743. [Google Scholar] [CrossRef] [PubMed]
- Libonati, F.; Vergani, L. Bone Toughness and Crack Propagation: An Experimental Study. Procedia Eng. 2014, 74, 464–467. [Google Scholar] [CrossRef]
- Colombo, C.; Libonati, F.; Rinaudo, L.; Bellazzi, M.; Ulivieri, F.M.; Vergani, L. A new finite element based parameter to predict bone fracture. PLoS ONE 2019, 14, e0225905. [Google Scholar] [CrossRef]
- Schneider, P.; Meier, M.; Wepf, R.; Müller, R. Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone 2010, 47, 848–858. [Google Scholar] [CrossRef]
- Buccino, F.; Aiazzi, I.; Casto, A.; Liu, B.; Sbarra, M.C.; Ziarelli, G.; Banfi, G.; Vergani, L.M. The synergy of synchrotron imaging and convolutional neural networks towards the detection of human micro-scale bone architecture and damage. J. Mech. Behav. Biomed. Mater. 2023, 137, 105576. [Google Scholar] [CrossRef] [PubMed]
- Goff, E.; Buccino, F.; Bregoli, C.; McKinley, J.P.; Aeppli, B.; Recker, R.R.; Shane, E.; Cohen, A.; Kuhn, G.; Müller, R. Large-scale quantification of human osteocyte lacunar morphological biomarkers as assessed by ultra-high-resolution desktop micro-computed tomography. Bone 2021, 152, 116094. [Google Scholar] [CrossRef]
- Buccino, F.; Bagherifard, S.; D’Amico, L.; Zagra, L.; Banfi, G.; Tromba, G.; Vergani, L.M. Assessing the intimate mechanobiological link between human bone micro-scale trabecular architecture and micro-damages. Eng. Fract. Mech. 2022, 270, 108582. [Google Scholar] [CrossRef]
- Goff, E.; Cohen, A.; Shane, E.; Recker, R.R.; Kuhn, G.; Müller, R. Large-scale osteocyte lacunar morphological analysis of transiliac bone in normal and osteoporotic premenopausal women. Bone 2022, 160, 116424. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, P.; Busse, B. Inter-site Variability of the Human Osteocyte Lacunar Network: Implications for Bone Quality. Curr. Osteoporos. Rep. 2019, 17, 105–115. [Google Scholar] [CrossRef]
- Bonivtch, A.R.; Bonewald, L.F.; Nicolella, D.P. Tissue strain amplification at the osteocyte lacuna: A microstructural finite element analysis. J. Biomech. 2007, 40, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.M.; Van Der Linden, J.C.; Weinans, H.; Prendergast, P.J. Stress-concentrating effect of resorption lacunae in trabecular bone. J. Biomech. 2006, 39, 734–741. [Google Scholar] [CrossRef]
- Qiu, S.; Rao, D.S.; Fyhrie, D.P.; Palnitkar, S.; Parfitt, A.M. The morphological association between microcracks and osteocyte lacunae in human cortical bone. Bone 2005, 37, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Buccino, F.; Colombo, C.; Duarte, D.H.L.; Rinaudo, L.; Ulivieri, F.M.; Vergani, L.M. 2D and 3D numerical models to evaluate trabecular bone damage. Med. Biol. Eng. Comput. 2021, 59, 2139–2152. [Google Scholar] [CrossRef]
- Buccino, F. Isolating trabecular morphology to study bone damage. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1038, 012039. [Google Scholar] [CrossRef]
- Idkaidek, A.; Jasiuk, I. Cortical bone fracture analysis using XFEM—Case study. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2809. [Google Scholar] [CrossRef]
- Heidari-Rarani, M.; Sayedain, M. Finite element modeling strategies for 2D and 3D delamination propagation in composite DCB specimens using VCCT, CZM and XFEM approaches. Theor. Appl. Fract. Mech. 2019, 103, 102246. [Google Scholar] [CrossRef]
- Yin, D.; Chen, B.; Lin, S. Finite element analysis on multi-toughening mechanism of microstructure of osteon. J. Mech. Behav. Biomed. Mater. 2021, 117, 104408. [Google Scholar] [CrossRef] [PubMed]
- Buccino, F.; Martinoia, G.; Vergani, L.M. Torsion—Resistant structures: A nature addressed solution. Materials 2021, 14, 5368. [Google Scholar] [CrossRef]
- Marco, M.; Giner, E.; Larraínzar-Garijo, R.; Caeiro, J.R.; Miguélez, M.H. Modelling of femur fracture using finite element procedures. Eng. Fract. Mech. 2018, 196, 157–167. [Google Scholar] [CrossRef]
- Gasser, T.C.; Holzapfel, G.A. A numerical framework to model 3-D fracture in bone tissue with application to failure of the proximal femur. In Proceedings of the IUTAM Symposium on Discretization Methods for Evolving Discontinuities, Lyon, France, 4–7 September 2006; Solid Mechanics and Its Applications. Springer: Berlin/Heidelberg, Germany, 2007; Volume 5, pp. 199–211. [Google Scholar]
- Mirzaali, M.J.; Libonati, F.; Böhm, C.; Rinaudo, L.; Cesana, B.M.; Ulivieri, F.M.; Vergani, L. Fatigue-caused damage in trabecular bone from clinical, morphological and mechanical perspectives. Int. J. Fatigue 2020, 133, 105451. [Google Scholar] [CrossRef]
- Hao, L.; Rui-Xin, L.; Biao, H.; Bin, Z.; Bao-Hui, H.; Ying-Jie, L.; Xi-Zheng, Z. Effect of athletic fatigue damage and the associated bone targeted remodeling in the rat ulna. Biomed. Eng. Online 2017, 16, 99. [Google Scholar] [CrossRef]
- van Hove, R.P.; Nolte, P.A.; Vatsa, A.; Semeins, C.M.; Salmon, P.L.; Smit, T.H.; Klein-Nulend, J. Osteocyte morphology in human tibiae of different bone pathologies with different bone mineral density—Is there a role for mechanosensing? Bone 2009, 45, 321–329. [Google Scholar] [CrossRef]
- Belytschko, T.; Black, T. Elastic crack growth in finite elements with minimal remeshing. Int. J. Numer. Methods Eng. 1999, 45, 601–620. [Google Scholar] [CrossRef]
- Melenk, J.M.; Babuška, I. The partition of unity finite element method: Basic theory and applications. In Computer Methods in Applied Mechanics and Engineering; Elsevier: Amsterdam, The Netherlands, 1996; Volume 139, pp. 289–314. [Google Scholar]
- Barenblatt, G.I. The formation of equilibrium cracks during brittle fracture. General ideas and hypotheses. Axially-symmetric cracks. J. Appl. Math. Mech. 1959, 23, 622–636. [Google Scholar] [CrossRef]
- Barenblatt, G.I. The Mathematical Theory of Equilibrium Cracks in Brittle Fracture. In Advances in Applied Mechanics; Elsevier: Amsterdam, The Netherlands, 1962; Volume 7, pp. 55–129. [Google Scholar]
- Hillerborg, A.; Modeer, M.; Petersson, P.E. Analysis of crack formation and crack growth in concrete by means of fracture mechanics and finite elements. Cem. Concr. Res. 2008, 6, 225–237. [Google Scholar] [CrossRef]
- Systèmes, D. Abaqus/Standard Version 6.12 User’s Manual; Simulia Corp.: Providence, RI, USA, 2019. [Google Scholar]
- AISI Type 316L Stainless Steel Mechanical Characteristics. Available online: https://www.matweb.com/search/DataSheet.aspx?MatGUID=a2d0107bf958442e9f8db6dc9933fe31 (accessed on 30 June 2022).
- Cai, W.; Jiang, J.; Li, G.Q. Analysis and simulation on fracture of structural steel at elevated temperatures based on extended finite element method. Fire Saf. J. 2021, 120, 103022. [Google Scholar] [CrossRef]
- Lin, M.; Agbo, S.; Duan, D.-M.; Cheng, J.J.R.; Adeeb, S. Simulation of Crack Propagation in API 5L X52 Pressurized Pipes Using XFEM-Based Cohesive Segment Approach. J. Pipeline Syst. Eng. Pract. 2020, 11, 04020009. [Google Scholar] [CrossRef]
- Rezanezhad, M.; Lajevardi, S.A.; Karimpouli, S. Effects of pore-crack relative location on crack propagation in porous media using XFEM method. Theor. Appl. Fract. Mech. 2019, 103, 102241. [Google Scholar] [CrossRef]
- Shet, C.; Chandra, N. Analysis of energy balance when using Cohesive Zone Models to simulate fracture processes. J. Eng. Mater. Technol. 2002, 124, 440–450. [Google Scholar] [CrossRef]
- Alrayes, O.; Könke, C.; Ooi, E.T.; Hamdia, K.M. Modeling Cyclic Crack Propagation in Concrete Using the Scaled Boundary Finite Element Method Coupled with the Cumulative Damage-Plasticity Constitutive Law. Materials 2023, 16, 863. [Google Scholar] [CrossRef] [PubMed]
- Tvergaard, V.; Hutchinson, J.W. Effect of T-STRESS on mode I crack growth resistance in a ductile solid. Int. J. Solids Struct. 1994, 31, 823–833. [Google Scholar] [CrossRef]
- Tvergaard, V.; Hutchinson, J.W. The relation between crack growth resistance and fracture process parameters in elastic-plastic solids. J. Mech. Phys. Solids 1992, 40, 1377–1397. [Google Scholar] [CrossRef]
- Scheider, I.; Brocks, W. The effect of the traction separation law on the results of cohesive zone crack propagation analyses. Key Eng. Mater. 2003, 251–252, 313–318. [Google Scholar] [CrossRef]
- Gustafsson, A.; Khayyeri, H.; Wallin, M.; Isaksson, H. An interface damage model that captures crack propagation at the microscale in cortical bone using XFEM. J. Mech. Behav. Biomed. Mater. 2019, 90, 556–565. [Google Scholar] [CrossRef]
- Ali, A.A.; Cristofolini, L.; Schileo, E.; Hu, H.; Taddei, F.; Kim, R.H.; Rullkoetter, P.J.; Laz, P.J. Specimen-specific modeling of hip fracture pattern and repair. J. Biomech. 2014, 47, 536–543. [Google Scholar] [CrossRef]
- Duarte, A.P.C.; Díaz Sáez, A.; Silvestre, N. Comparative study between XFEM and Hashin damage criterion applied to failure of composites. Thin-Walled Struct. 2017, 115, 277–288. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhou, H.W.; Ji, H.W.; Zhang, X.C. Application of extended finite element method in damage progress simulation of fiber reinforced composites. Mater. Des. 2014, 55, 191–196. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Y.; Zhao, J. Elastoplastic fracture analysis of the P91 steel welded joint under repair welding thermal shock based on XFEM. Metals 2020, 10, 1285. [Google Scholar] [CrossRef]
- AISI 304 Stainless Steel Properties, SS304 Composition, Density, Yield Strength, Thermal Conductivity, Hardness, Modulus of Elasticity. Available online: https://www.valin-ss.com/products/stainless_steel_plate_sheet_257182/?gclid=Cj0KCQiAgOefBhDgARIsAMhqXA5EjziwKrTAUzneTJjzycVZ-dqLzImj04nPIkaYs5UpP6nS3CjM18kaAtTUEALw_wcB (accessed on 15 January 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buccino, F.; Cervellera, F.; Ghidini, M.; Marini, R.; Bagherifard, S.; Vergani, L.M. Isolating the Role of Bone Lacunar Morphology on Static and Fatigue Fracture Progression through Numerical Simulations. Materials 2023, 16, 1931. https://doi.org/10.3390/ma16051931
Buccino F, Cervellera F, Ghidini M, Marini R, Bagherifard S, Vergani LM. Isolating the Role of Bone Lacunar Morphology on Static and Fatigue Fracture Progression through Numerical Simulations. Materials. 2023; 16(5):1931. https://doi.org/10.3390/ma16051931
Chicago/Turabian StyleBuccino, Federica, Francesco Cervellera, Marta Ghidini, Riccardo Marini, Sara Bagherifard, and Laura Maria Vergani. 2023. "Isolating the Role of Bone Lacunar Morphology on Static and Fatigue Fracture Progression through Numerical Simulations" Materials 16, no. 5: 1931. https://doi.org/10.3390/ma16051931
APA StyleBuccino, F., Cervellera, F., Ghidini, M., Marini, R., Bagherifard, S., & Vergani, L. M. (2023). Isolating the Role of Bone Lacunar Morphology on Static and Fatigue Fracture Progression through Numerical Simulations. Materials, 16(5), 1931. https://doi.org/10.3390/ma16051931








