Manufacture of SiC: Effect of Carbon Precursor
Abstract
1. Introduction
2. Theoretical Background
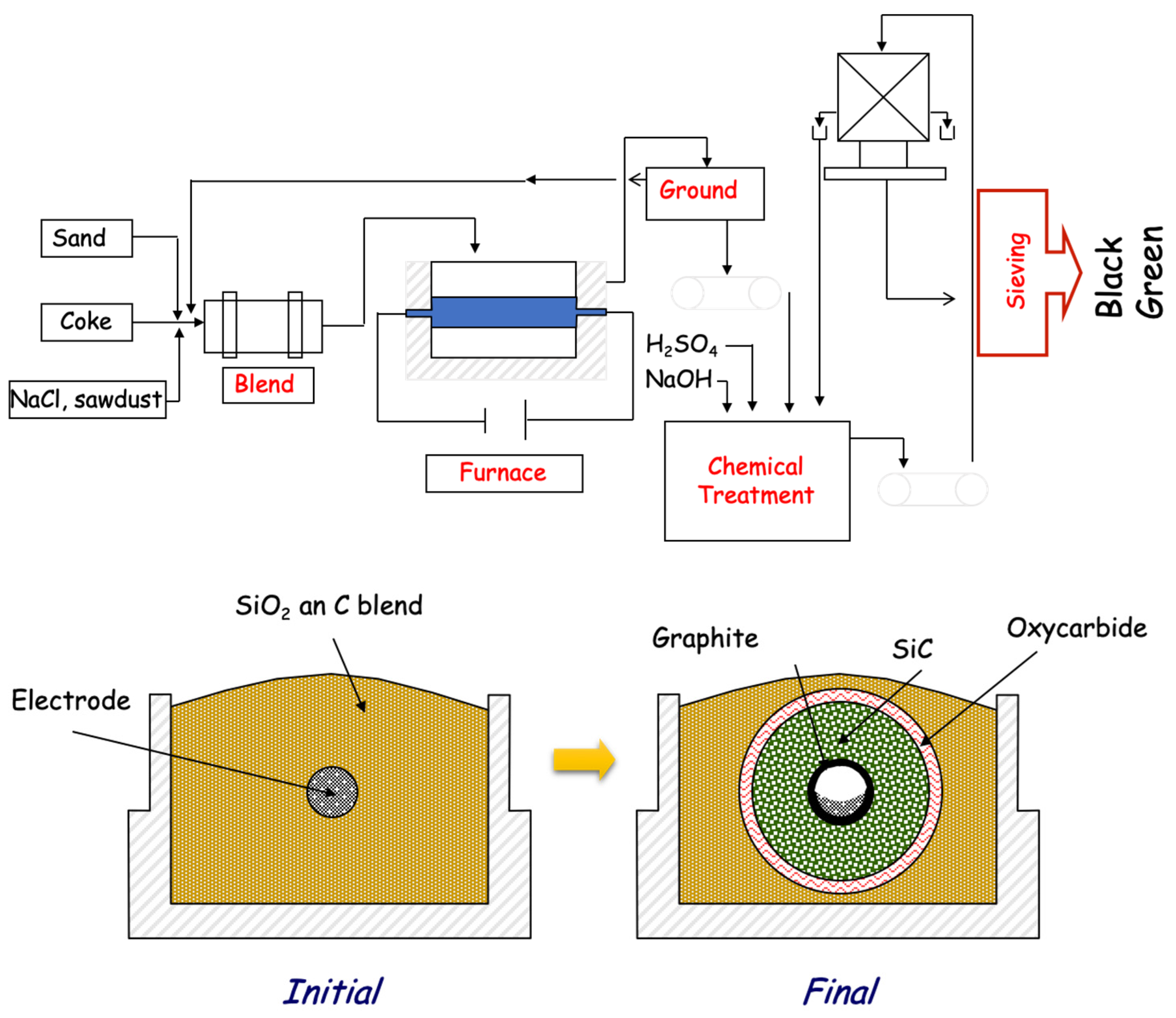
2.1. Industrial Production of SiC
2.2. Laboratory Production of SiC
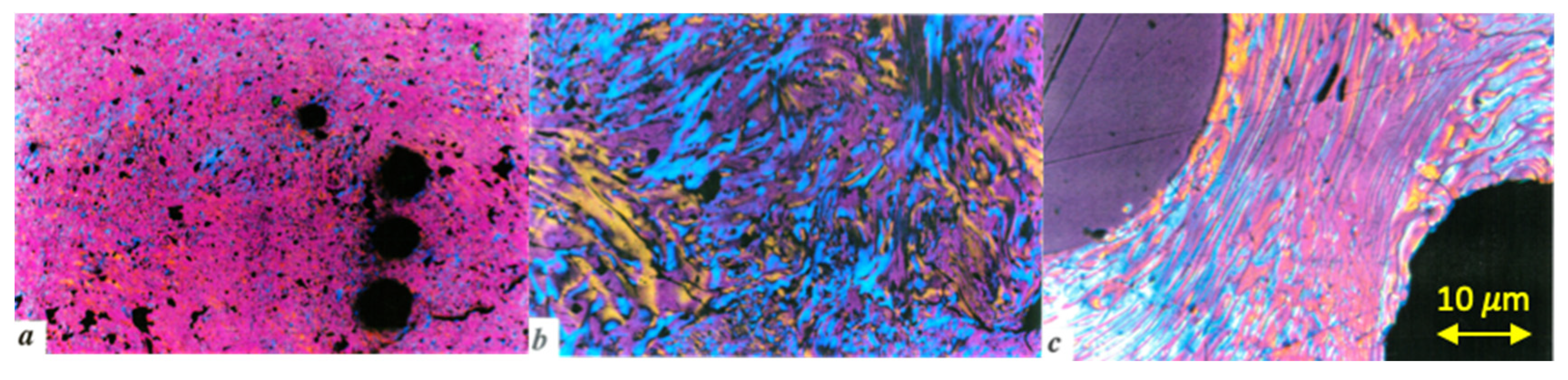
2.3. About the Coke
3. Materials and Methods
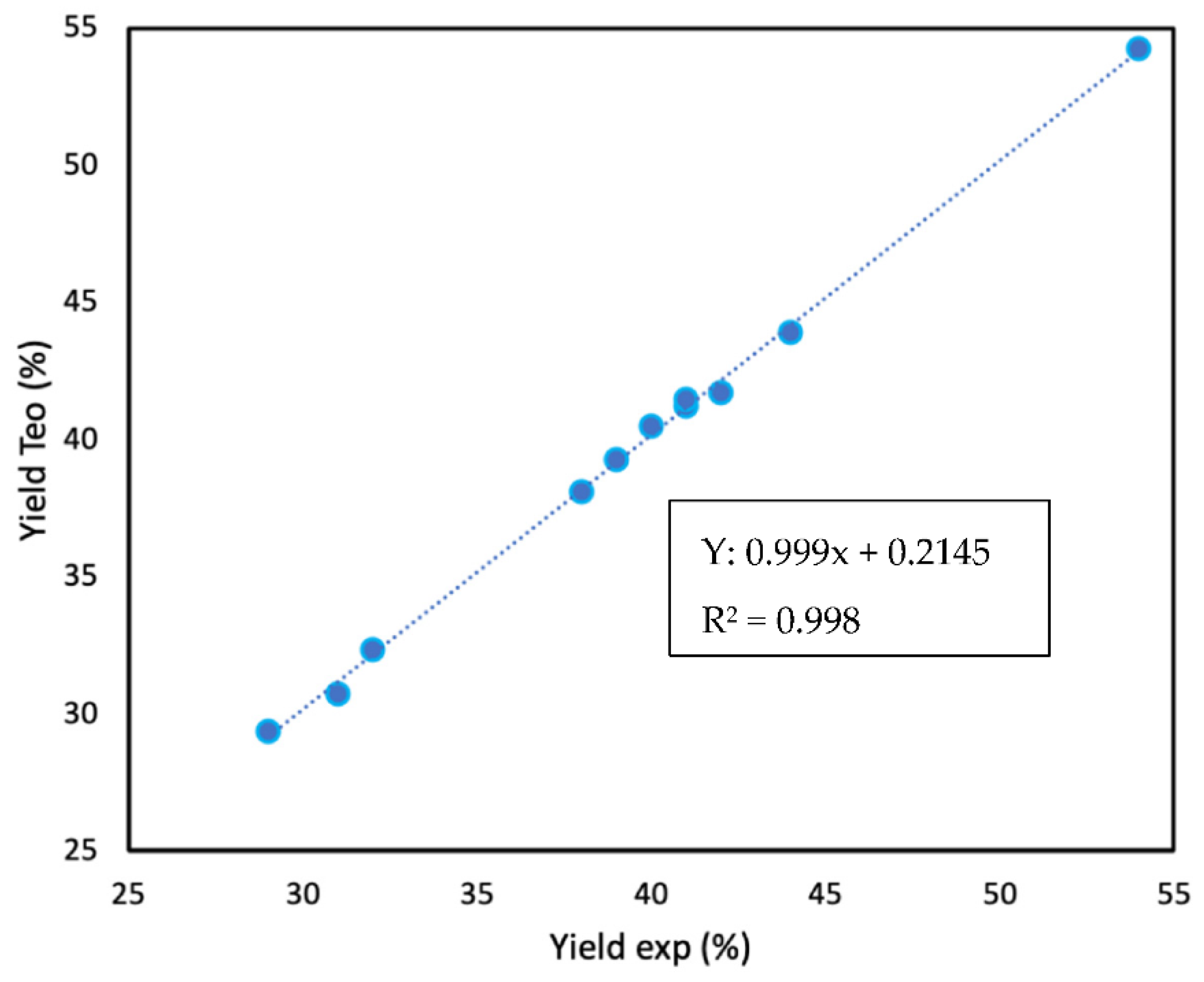
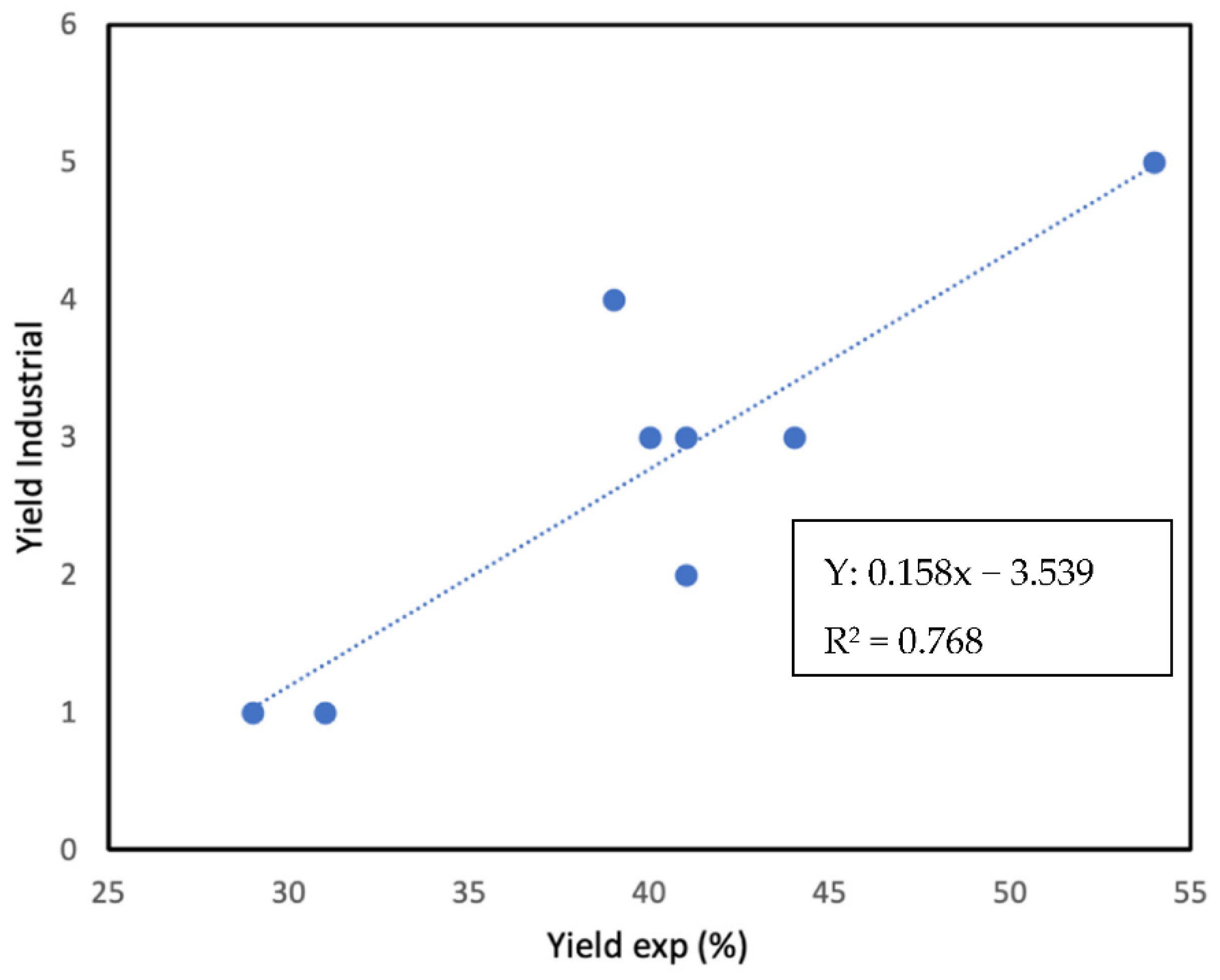
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Textural Component | Symbol | Assignment | OTI |
|---|---|---|---|
| Isotropic | I | No optical activity | 0 |
| Fine mosaics | fM | <1.5 microns | 1 |
| Medium mosaics | nM | 1.5–5 microns | 3 |
| Coarse mosaics | cM | 5–10 microns | 5 |
| Small domains | d | 10–60 microns | 20 |
| Domains | D | >60 microns | 30 |
| Granular flow | Gf | Composed of elongated isochromatic units of coalesced mosaics | 20 |
| Flow anisotropy | E | Elongated isochromatic units of variable size | 20 |
| Flow domains | FD | >10 microns width; >30 microns length | 30 |
References
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Acheson, E.G. Manufacture of Graphite. U.S. Patent 568323, 29 October 1896. [Google Scholar]
- Moissan, H. Nouvelles recherches sur la météorité de Cañon Diablo. Comptes Rendus 1904, 139, 773–786. [Google Scholar]
- Available online: https://www.carbo.com/en-us/our-history (accessed on 1 January 2023).
- Knippenberg, W.F. Growth phenomena in silicon carbide. Philips Res. Repts. 1963, 18, 161–274. [Google Scholar]
- Ramsdell, L.S. Studies on SiC. Am. Mineral. 1947, 32, 64–82. [Google Scholar]
- Caccia, M.; Narciso, J. Production of SiC Materials by Reactive Infiltration. MSF 2014, 783–786, 1863–1866. [Google Scholar] [CrossRef]
- Ortega-Trigueros, A.; Narciso, J.; Caccia, M. Synthesis of high-surface area mesoporous SiC with hierarchical porosity for use as catalyst support. J. Amer. Ceram. Soc. 2020, 103, 5966–5977. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, K.; Yoon Yue, C.; Wei, J.; Pan, Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Prog. Mater. Sci. 2015, 72, 1–60. [Google Scholar] [CrossRef]
- Ramesh, P.D.; Vaidhyanathan, B.; Ganguli, M.; Rao, K.J. Synthesis of β-SiC powder by use of microwave radiation. J. Mater. Res. 1994, 9, 3025–3027. [Google Scholar] [CrossRef]
- Jepps, N.W.; Page, T. Polytypic transformations in silicon carbide. Prog. Cryst. Growth Charact. 1983, 7, 259–307. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Tronstad, R.; Ostrvski, O. Synthesis of SiC whiskers by VLS and VS process. Ceram. Int. 2016, 42, 5668–5676. [Google Scholar] [CrossRef]
- Chiew, Y.L.; Cheong, K.Y. A review on the synthesis of SiC from plant-based biomasses. Mater. Sci. Eng. B 2011, 176, 951–964. [Google Scholar] [CrossRef]
- Naslain, R.R. SiC-Matrix Composites: Nonbrittle ceramics for thermostructural apllplication. Appl. Ceram. Tech. 2005, 2, 75–84. [Google Scholar] [CrossRef]
- Yamada, K.; Mohri, M. Properties and applications of silicon carbides ceramics. In Silicon Carbide Ceramics—1; Somiya, S., Inomata, Y., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 13–44. [Google Scholar] [CrossRef]
- Xu, M.; Girish, Y.R.; Rakesh, K.P.; Wu, P.; Manukumar, H.M.; Byrappa, S.M.; Byrappa, U.K. Recent advances and challenges in silicon carbide (SiC) ceramic nanoarchitectures and their applications. Mater. Today Comm. 2021, 28, 102533–102566. [Google Scholar] [CrossRef]
- Tian, J.; Piñero, E.; Narciso, J.; Louis, E. Effects of temperature on pressure infiltration of liquid Al and Al–12wt.%Si alloy into packed SiC particles. Sripta Mater. 2005, 52, 1483–1488. [Google Scholar] [CrossRef]
- García-Cordovilla, C.; Louis, E.; Narciso, J. Pressure infiltration of packed ceramic particulates by liquid metals. Acta Mater. 1999, 47, 4461–4479. [Google Scholar] [CrossRef]
- Kumar, M.S.; Begum, S.R.; Pruncu, C.I.; Asl, M.S. Role of homogeneous distribution of SiC reinforcement on the characteristics of stir casted Al–SiC composites. J. Alloys Compd. 2021, 869, 159250–159260. [Google Scholar] [CrossRef]
- Meselhy, A.F.; Reda, M.M. Investigation of mechanical properties of nanostructured Al-SiC composite manufactured by accumulative roll bonding. J. Comp. Mater. 2019, 53, 3951–3961. [Google Scholar] [CrossRef]
- Ozpineci, B.; Tolbert, L.M. Characterization of SiC Schottky Diodes at Different Temperatures. IEEE Power Elec. Lett. 2003, 1, 54–57. [Google Scholar] [CrossRef]
- Rupp, R.; Treu, M.; Voss, S.; Bjork, F.; Reimann, T. “2nd Generation” SiC Schottky diodes: A new benchmark in SiC device ruggedness. In Proceedings of the 18th International Symposium on Power Semiconductor Devices & IC’s, Naples, Italy, 4–8 June 2006. [Google Scholar] [CrossRef]
- Neudeck, P.G. Progress in silicon carbide semiconductor electronics technology. J. Electro. Mater. 1995, 24, 283–288. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, L.; Cheng, X.; Yan, L.; Huang, J.; Liu, Z. Interfacial modification mechanism of ALD-SiO2/4H-SiC heterojunction by synergistic nitrogen–oxygen-atmosphere RTA. Appl. Phys. A 2022, 128, 1132. [Google Scholar] [CrossRef]
- Yazdi, G.; Iakimov, T.; Yakimova, R. Epitaxial Graphene on SiC: A Review of Growth and Characterization. Crystals 2016, 6, 53. [Google Scholar] [CrossRef]
- Caccia, M.; Giuranno, D.; Molina-Jorda, J.M.; Moral, M.; Nowak, R.; Ricci, E.; Sobczak, N.; Narciso, J.; Fernández Sanz, J. Graphene Translucency and Interfacial Interactions in the Gold/Graphene/SiC System. J. Phys. Chem. Lett. 2018, 9, 3850–3855. [Google Scholar] [CrossRef]
- Lara-Avila, S.; Danilov, A.; Golubev, D.; He, H.; Kim, K.H.; Yakimova, R.; Lombardi, F.; Bauch, T.; Cherednichenko, S.; Kubatkin, S. Towards quantum-limited coherent detection of terahertz waves in charge-neutral graphene. Nat. Astron. 2019, 3, 983–988. [Google Scholar] [CrossRef]
- Katoh, Y.; Snead, L.L.; Szlufarska, I.; Weber, W.J. Radiation effects in SiC for nuclear structural applications. Curr. Opin. Solid State Mater. Sci. 2012, 16, 143–152. [Google Scholar] [CrossRef]
- Koyanagi, T.; Katoh, Y.; Nozawa, T.; Snead, L.L.; Kondo, S.; Henager, C.H.; Ferraris, M.; Hinoki, T.; Huang, Q. Recent progress in the development of SiC composites for nuclear fusion applications. J. Nucl. Mater. 2018, 511, 544–555. [Google Scholar] [CrossRef]
- Katoh, Y.; Ozawa, K.; Shih, C.; Nozawa, T.; Shinavski, R.J.; Hasegawa, A.; Snead, L.L. Continuous SiC fiber, CVI SiC matrix composites for nuclear applications: Properties and irradiation effects. J. Nucl. Mater. 2014, 511, 448–476. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Narciso, J. Synthesis of SiC and Si3N4: An overview. Adv. Mater. 1995, 7, 209–211. [Google Scholar] [CrossRef]
- Narciso-Romero, F.J.; Rodríguez-Reinoso, F. Synthesis of SiC from rice husks catalysed by iron, cobalt or nickel. J. Mater. Sci. 1996, 31, 779–784. [Google Scholar] [CrossRef]
- Wang, L.; Wada, H.; Allard, L.F. Synthesis and Characterization of SiC whiskers. J. Mater. Res. 1992, 7, 148–163. [Google Scholar] [CrossRef]
- Milewski, J.V.; Gac, F.D.; Petrovic, J.J.; Skaggs, S.R. Growth of beta-silicon carbide whiskers by the VLS process. J. Mater. Sci. 1985, 20, 1160–1166. [Google Scholar] [CrossRef]
- Yajima, S.; Hasegawa, Y.; Okamura, K.; Matsuszawa, T. Development of high tensile strength silicon carbide fibre using an organosilicon polymer precursor. Nature 1978, 273, 525–527. [Google Scholar] [CrossRef]
- Yajima, S.; Josaburo, H.; Mamoru, O. Continuous silicon carbide fiber of high tensile strength. Chem. Lett. 1975, 4, 931–934. [Google Scholar] [CrossRef]
- Zhang, R.J.; Yang, Y.Q.; Shen, W.T.; Wang, C.; Luo, X. Microstructure of SiC fiber fabricated by two-stage chemical vapor deposition on tungsten filament. J. Crys. Gro. 2010, 313, 56–61. [Google Scholar] [CrossRef]
- Dicarlo, J.A. Creep of chemically vapour deposited SiC fibres. J. Mater. Sci. 1986, 21, 217–224. [Google Scholar] [CrossRef]
- Ishikawa, T. Recent developments of the SiC fiber Nicalon and its composites, including properties of the SiC fiber Hi-Nicalon for ultra-high temperature. Comp. Sci. Tech. 1994, 51, 135–144. [Google Scholar] [CrossRef]
- Greil, P.; Lifka, T.; Kaindl, A. Biomorphic cellular silicon carbide ceramics from wood: I. processing and microstructure. J. Eur. Ceram Soc. 1998, 18, 1961–1973. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Xu, Q.; Gao, K.; Dang, C.; Li, P.; Luo, Q.; Zheng, H.; Song, C.; Tian, Y.; et al. Bamboo derived SiC ceramics-phase change composites for efficient, rapid, and compact solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 240, 111726. [Google Scholar] [CrossRef]
- Singh, S.K.; Raj, S.; Debasish, D. Preparation of SiC from Rice Husk: Past, Present, and Future. In Sustainable Chemical, Mineral and Material Processing; Chinthapudi, E., Basu, S., Thorat, B.N., Eds.; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Galvagno, S.; Portofino, S.; Casciaro, G.; Casu, S.; Martino, M.; Russo, A. Synthesis of beta silicon carbide powders from biomass gasification residue. J. Mater. Sci. 2007, 42, 6878–6886. [Google Scholar] [CrossRef]
- Calderon, N.R.; Martinez-Escandell, M.; Narciso, J.; Rodríguez-Reinoso, F. The role of carbon biotemplate density in mechanical properties of biomorphic SiC. J. Eur. Ceram. Soc. 2009, 29, 465–472. [Google Scholar] [CrossRef]
- Calderon, N.R.; Martinez-Escandell, M.; Narciso, J.; Rodríguez-Reinoso, F. Manufacture of Biomorphic SiC Components with Homogeneous Properties from Sawdust by Reactive Infiltration with Liquid Silicon. J. Amer. Ceram. Soc. 2010, 93, 1003–1009. [Google Scholar] [CrossRef]
- Narciso-Romero, F.J.; Rodríguez-Reinoso, F.; Díez, M.A. Influence of the carbon material on the synthesis of silicon carbide. Carbon 1999, 37, 1771–1778. [Google Scholar] [CrossRef]
- Calderon, N.R.; Voytovych, R.; Narciso, J.; Eustathopoulos, N. Pressureless infiltration versus wetting in AlSi/graphite system. J. Mater. Sci. 2010, 45, 4345–4350. [Google Scholar] [CrossRef]
- Caccia, M.; Narciso, J. Key Parameters in the Manufacture of SiC-Based Composite Materials by Reactive Melt Infiltration. Materials 2019, 12, 2425. [Google Scholar] [CrossRef]
- Dezellus, O.; Jacques, S.; Hodaj, F.; Eustathopoulos, N. Wetting and infiltration of carbon by liquid silicon. J. Marer Sci. 2005, 40, 2307–2311. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. Wettability at High Temperatures; Pergamon Materials Series; Pergamon Press: Oxford, UK, 1999; Volume 3. [Google Scholar]
- Eustathopoulos, N. Progress in understanding and modeling reactive wetting of metals on ceramics. Curr. Opin. Solid State Mater. Sci. 2005, 9, 152–160. [Google Scholar] [CrossRef]
- Forrest, R.A.; Marsh, H. Reflection interference colours in optical microscopy of carbon. Carbon 1977, 15, 348–349. [Google Scholar] [CrossRef]
- Calderon, N.R.; Martínez-Escandell, M.; Narciso, J.; Rodríguez-Reinoso, F. The combined effect of porosity and reactivity of the carbon preforms on the properties of SiC produced by reactive infiltration with liquid Si. Carbon 2009, 47, 2200–2210. [Google Scholar] [CrossRef]
- Ragan, S.; Marsh, H. The influence of oxidation upon strength and structure of a needle-coke and coal extract coke. Carbon 1983, 21, 157–165. [Google Scholar] [CrossRef]
- Dziembaj, R.; Baranski, A.; Kagan, J.M.; Malczyk, J.; Pawlowski, K. Oxygen corrosion of calcined cokes: I. Isothermal kinetics and texture variation. Carbon 1988, 26, 309–315. [Google Scholar] [CrossRef]
- Rouzand, J.N.; Duval, B.; Leroy, J. Specific Reactivities of Pure Carbon of Diverse Origins. In Fundamental Issues in Control of Carbon Gasification Reactivity; Lahaye, J., Ehrburger, P., Eds.; NATO ASI Serie E; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; Volume 192, p. 257. [Google Scholar]
- Marsh, H.; Diez, M.A.; Kuo, K. Coke Microtexture: One Key for Coke Reactivity. In Fundamental Issues in Control of Carbon Gasification Reactivity; Lahaye, J., Ehrburger, P., Eds.; NATO ASI Serie E; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; Volume 192, p. 205. [Google Scholar]
- Muñoz-Guillena, M.J.; Linares-Solano, A.; Salinas-Martinez de Lecea, C. Determination of calorific values of coals by differential thermal analysis. Fuel 1992, 71, 579–583. [Google Scholar] [CrossRef]
- Clayton-Suiter, R. Silicon Carbide Synthesis Using High-Sulphur Petroleum Fluid Coke and Montana Silica. Ph.D. Thesis, Montana University, Missoula, MT, USA, 1965. [Google Scholar]



| SiO2 | Fe2O3 | TiO2 | Al2O3 |
|---|---|---|---|
| 99.8 | 0.025 | 0.020 | 0.060 |
| Coke | Volatile (wt%) | Ash (wt%) | Fixed C (wt%) | Sulphur (wt%) |
|---|---|---|---|---|
| 1 | 9.76 | 1.14 | 89.10 | 6.21 |
| 2 | 7.22 | 0.78 | 92.10 | 5.31 |
| 3 | 10.11 | 0.44 | 89.45 | 5.70 |
| 4 @ | 6.64 | 0.12 | 93.24 | 1.40 |
| 5 | 8.00 | 0.44 | 91.56 | 6.38 |
| 6 @ | 8.52 | 0.21 | 91.27 | 1.54 |
| 7 | 9.43 | 0.62 | 89.25 | 3.83 |
| 8 | 9.53 | 0.37 | 90.10 | 4.03 |
| 9 | 8.90 | 0.45 | 90.65 | 4.85 |
| Regular | 9.95 | 0.15 | 93.76 | 1.09 |
| Combustion | 12.34 | 0.30 | 87.44 | 6.05 |
| Coke | Fe2O3 (wt%) | NiO (wt%) | V2O5 (wt%) | CaO (wt%) |
| 1 | 0.250 | 0.291 | 0.142 | 0.001 |
| 2 | 0.011 | 0.010 | 0.200 | 0.000 |
| 3 | 0.011 | 0.019 | 0.201 | 0.001 |
| 4 @ | 0.021 | 0.029 | 0.000 | 0.000 |
| 5 | 8.00 | 0.44 | 91.56 | 0.002 |
| 6 @ | 0.049 | 0.098 | 0.051 | 0.001 |
| 7 | 0.102 | 0.205 | 0.109 | 0.003 |
| 8 | 0.084 | 0.150 | 0.150 | 0.002 |
| 9 | 0.151 | 0.199 | 0.102 | 0.001 |
| Regular | 0.03 | 0.004 | 0.02 | 0.001 |
| Combustion | 0.06 | 0.06 | 0.145 | 0.000 |
| Coke | OTI | Ratio aro/ali | DTA peak ( °C ) | Yield (%) | Industrial |
|---|---|---|---|---|---|
| 1 | 19 | 1.3 | 650 | 54 | Very Good |
| 2 | 13 | 0.9 | 600 | 31 | Bad |
| 3 | 12 | 0.9 | 584 | 29 | Bad |
| 4@ | 22 | 1.4 | 689 | 39 | Good |
| 5 | 19 | 1.2 | 665 | 40 | Med–Good |
| 6@ | 20 | 1.3 | 698 | 41 | Med–Good |
| 7 | 17 | 1.2 | 655 | 44 | Med–Good |
| 8 | 13 | 1.0 | 603 | 38 | Not used |
| 9 | 13 | 0.9 | 595 | 41 | Medium |
| Regular | 24 | 1.2 | 702 | 42 | Not used |
| Combustion | 12 | 0.9 | 590 | 32 | Not used |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Fernández, E.V.; Narciso, J. Manufacture of SiC: Effect of Carbon Precursor. Materials 2023, 16, 2034. https://doi.org/10.3390/ma16052034
Ramos-Fernández EV, Narciso J. Manufacture of SiC: Effect of Carbon Precursor. Materials. 2023; 16(5):2034. https://doi.org/10.3390/ma16052034
Chicago/Turabian StyleRamos-Fernández, Enrique V., and Javier Narciso. 2023. "Manufacture of SiC: Effect of Carbon Precursor" Materials 16, no. 5: 2034. https://doi.org/10.3390/ma16052034
APA StyleRamos-Fernández, E. V., & Narciso, J. (2023). Manufacture of SiC: Effect of Carbon Precursor. Materials, 16(5), 2034. https://doi.org/10.3390/ma16052034







