Recent Advances in Electro-Optic Response of Polymer-Stabilized Cholesteric Liquid Crystals
Abstract
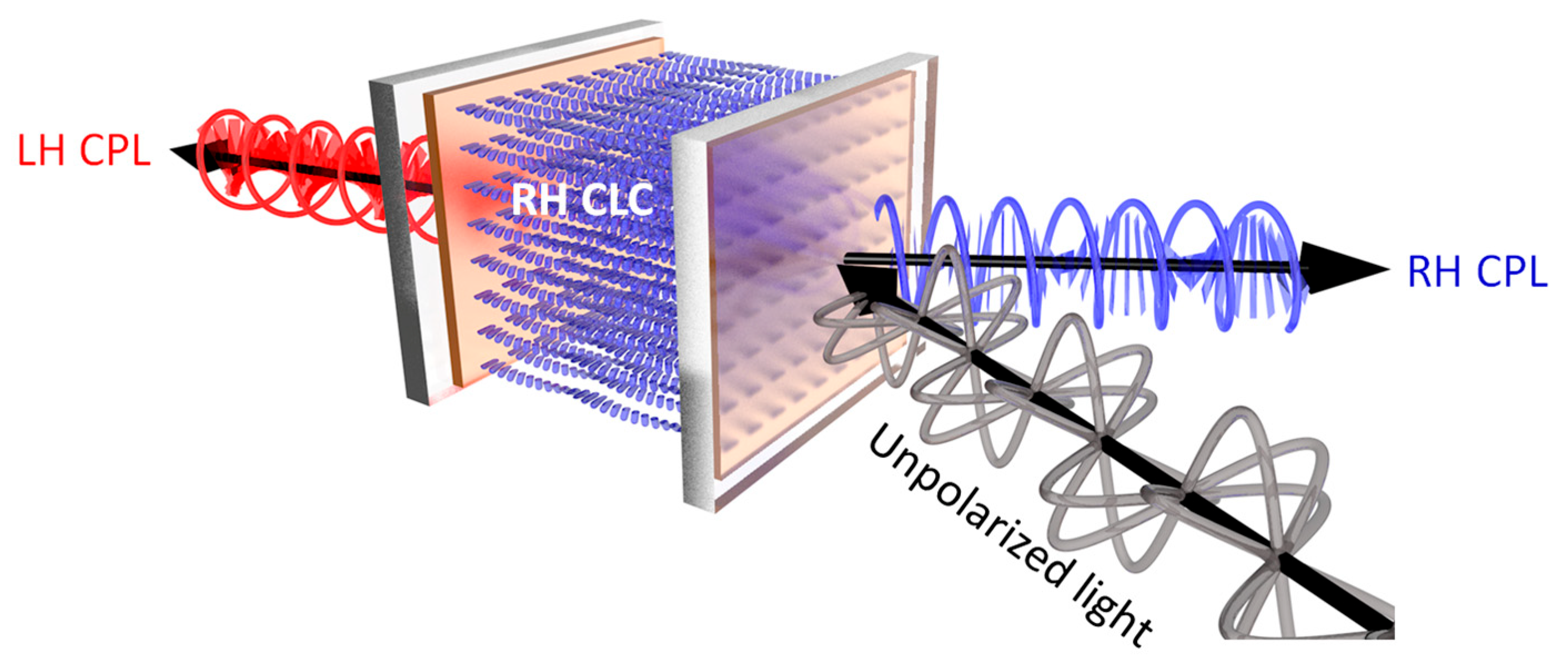
:1. Introduction
2. Electro-Optic Response in PSCLCs
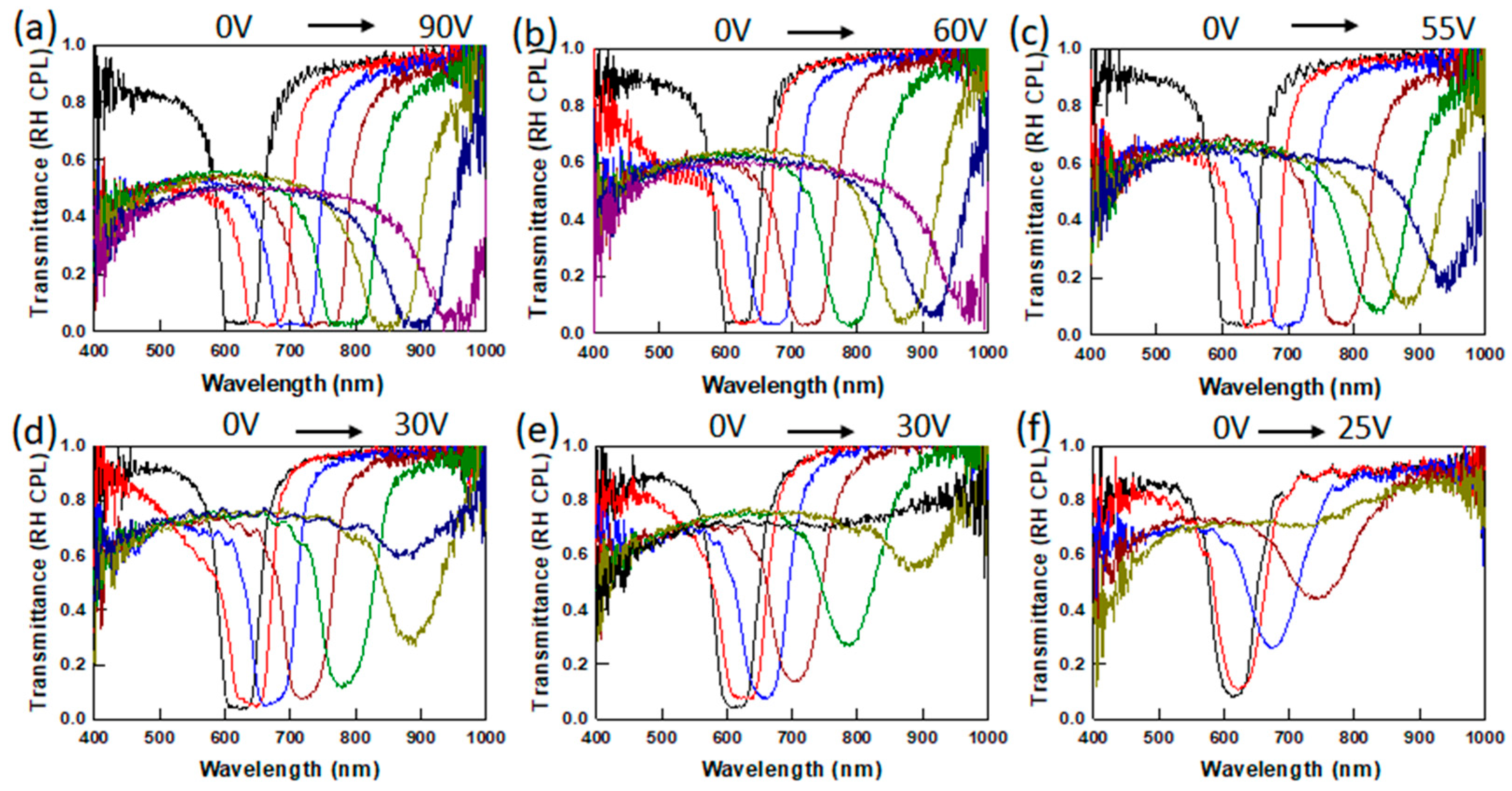
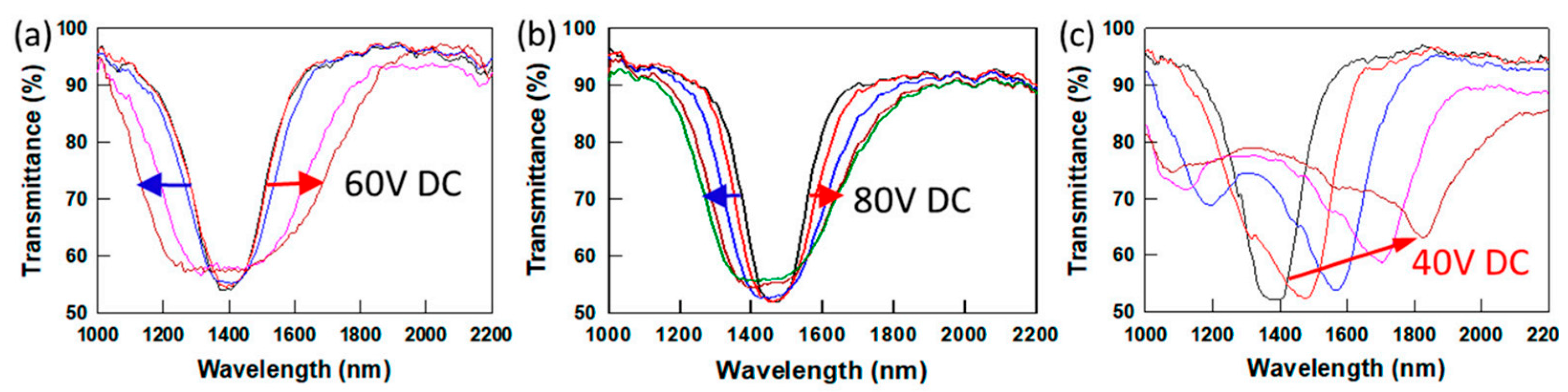
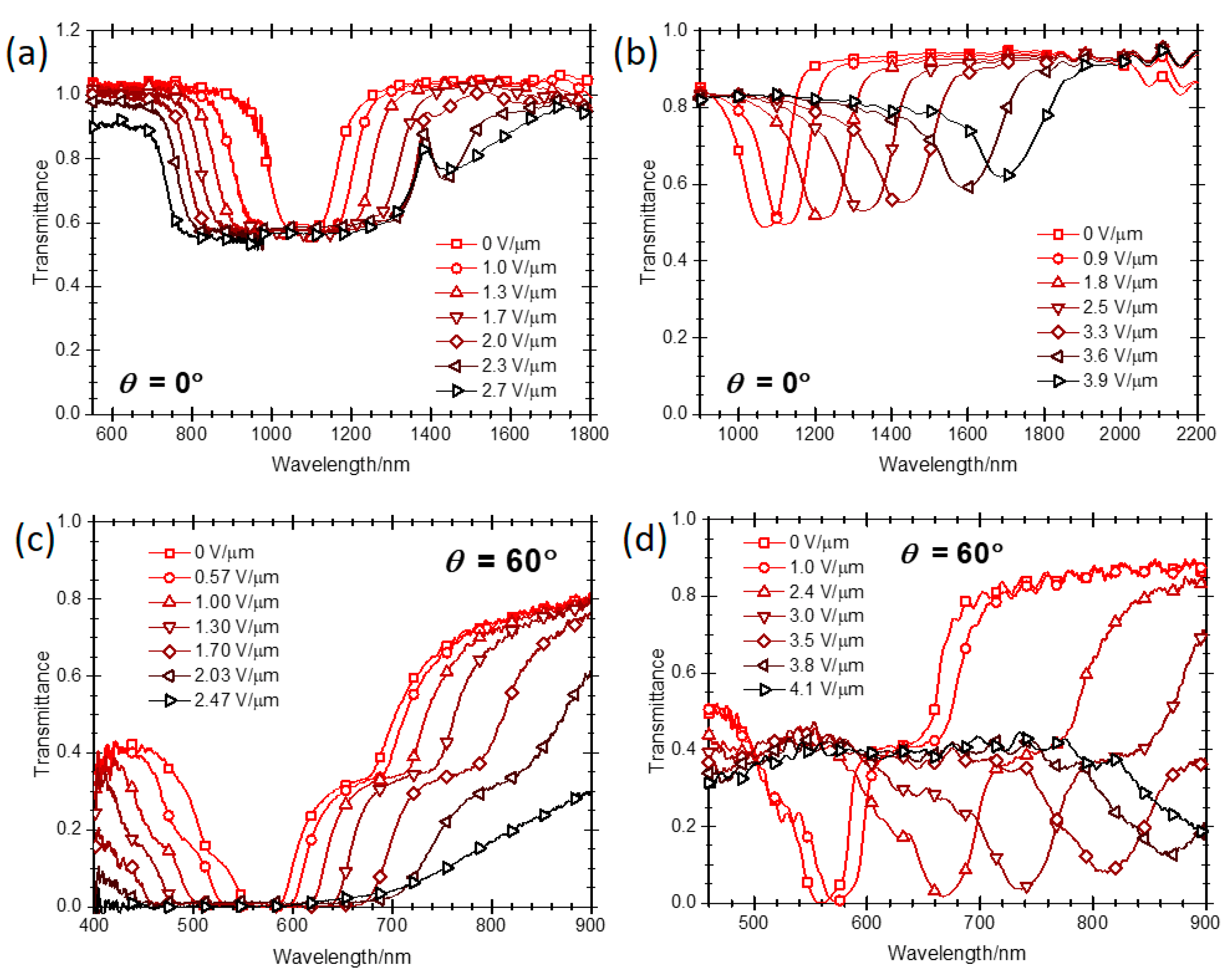
2.1. Bandwidth Broadening
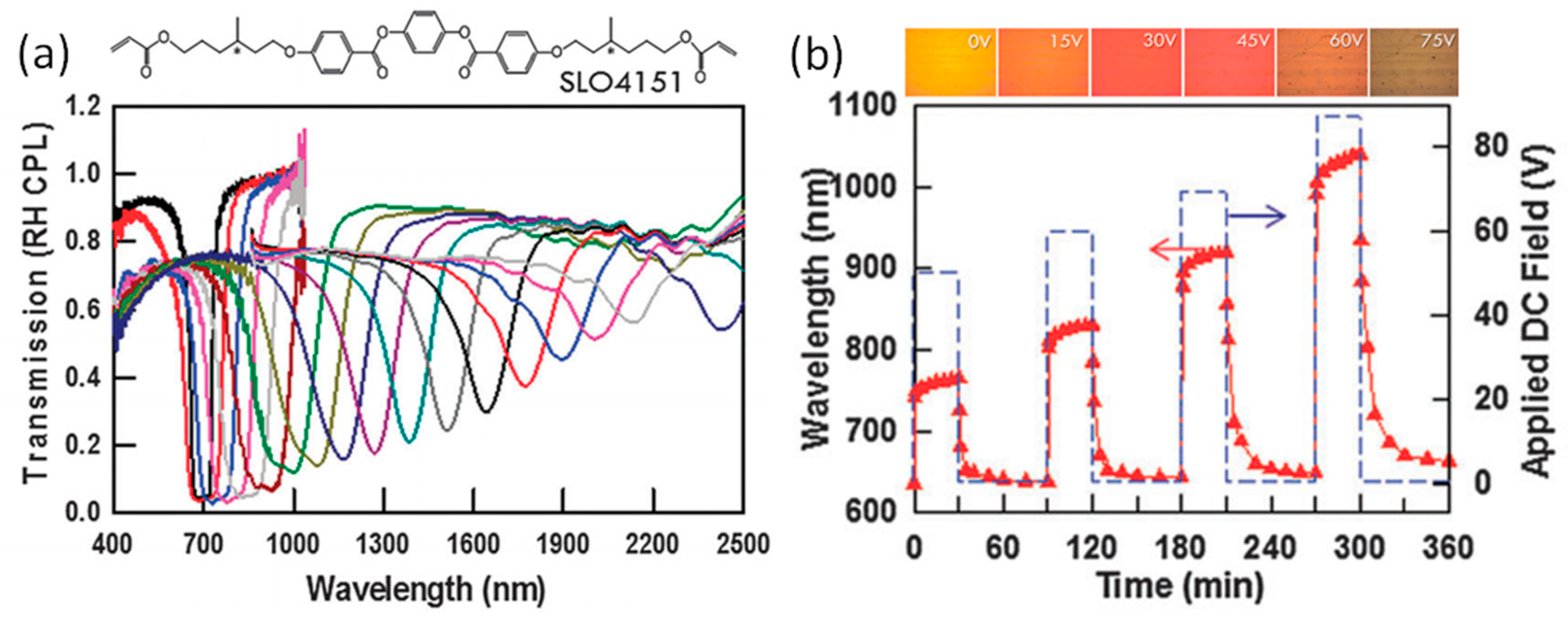
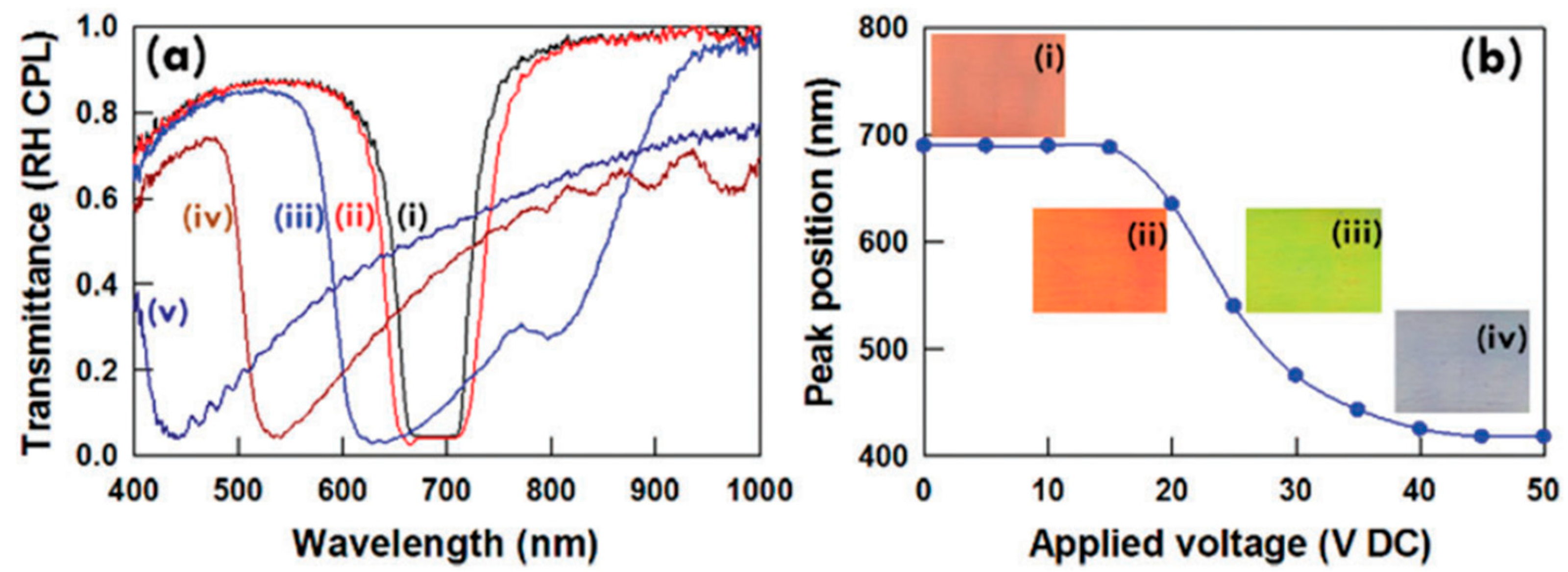
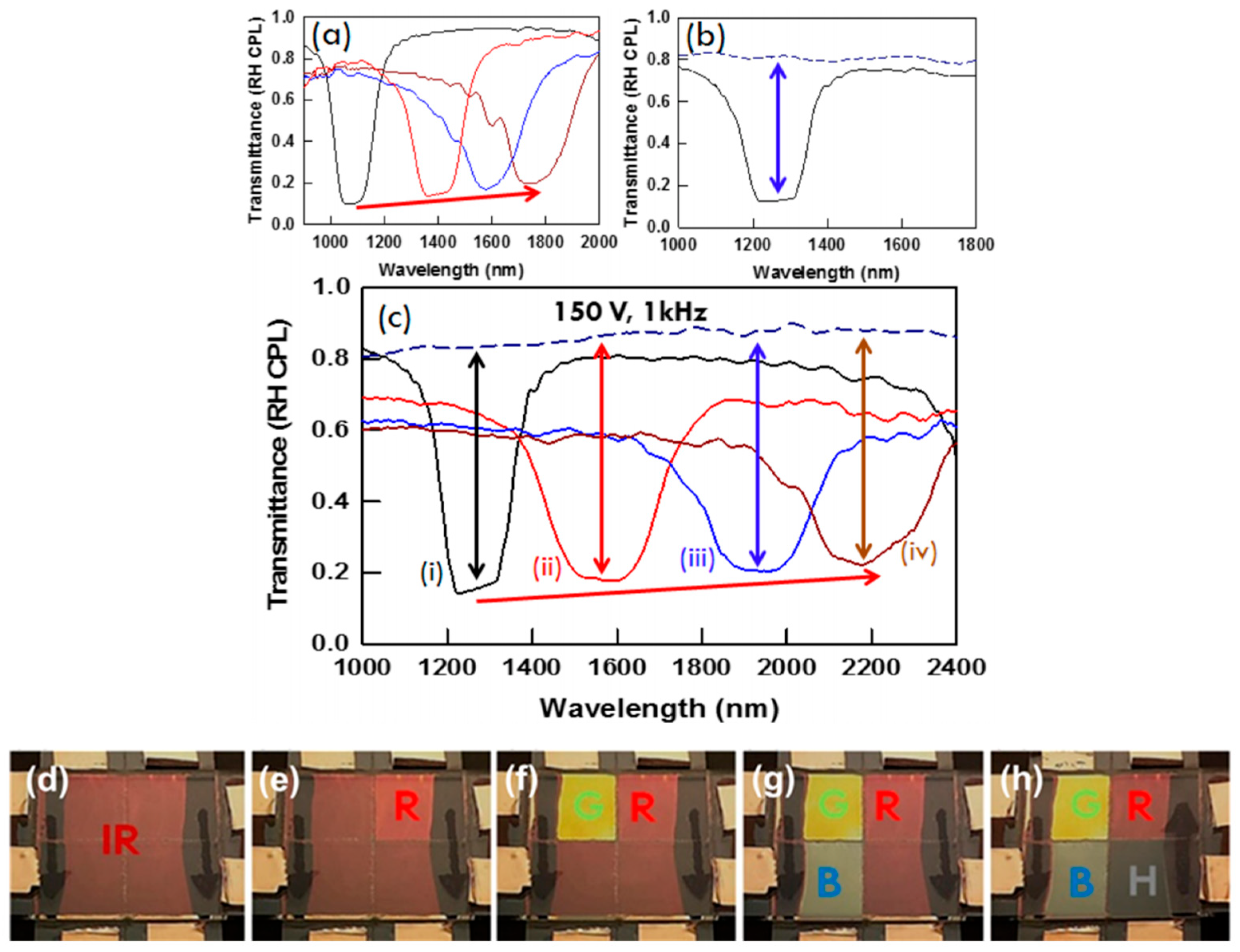
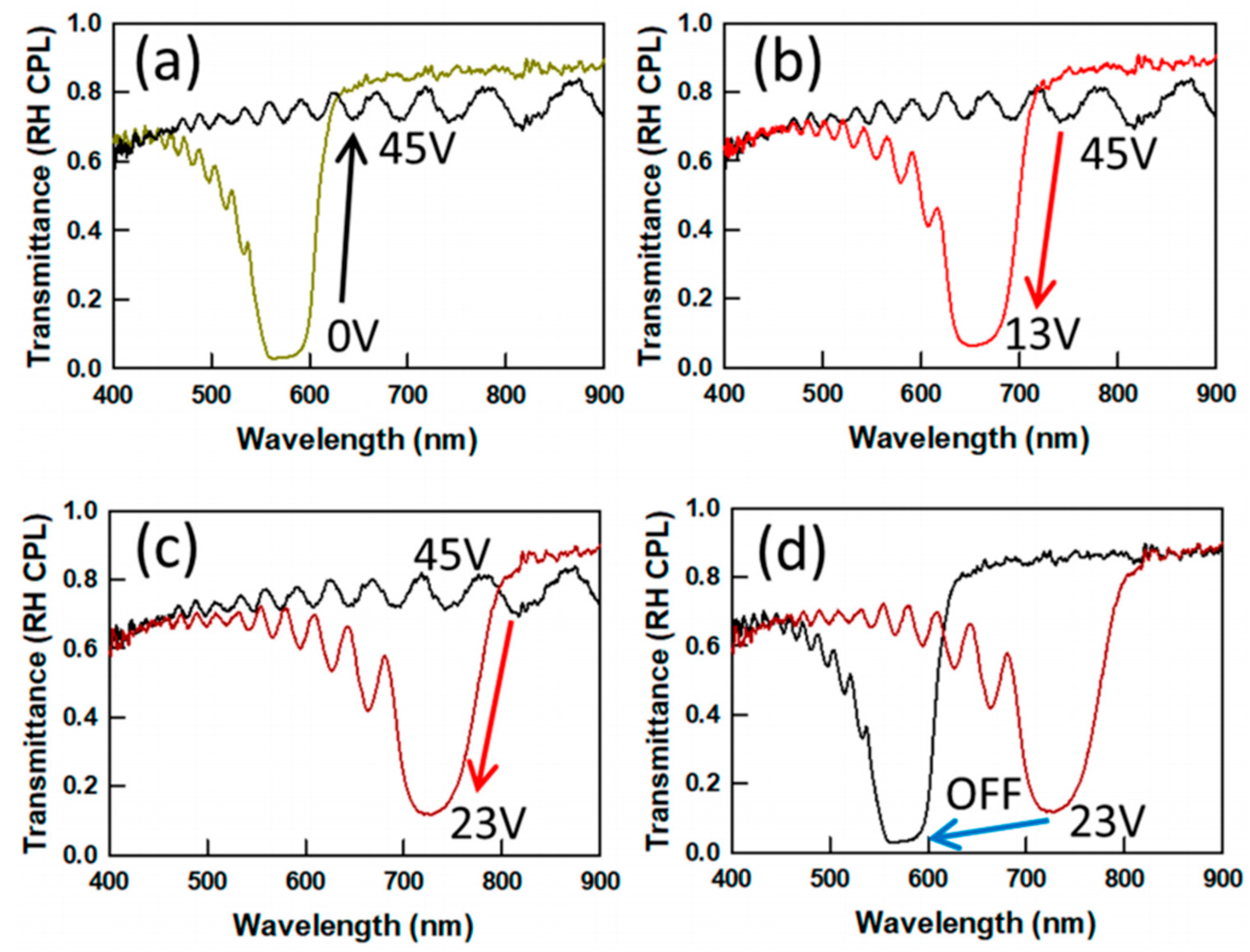
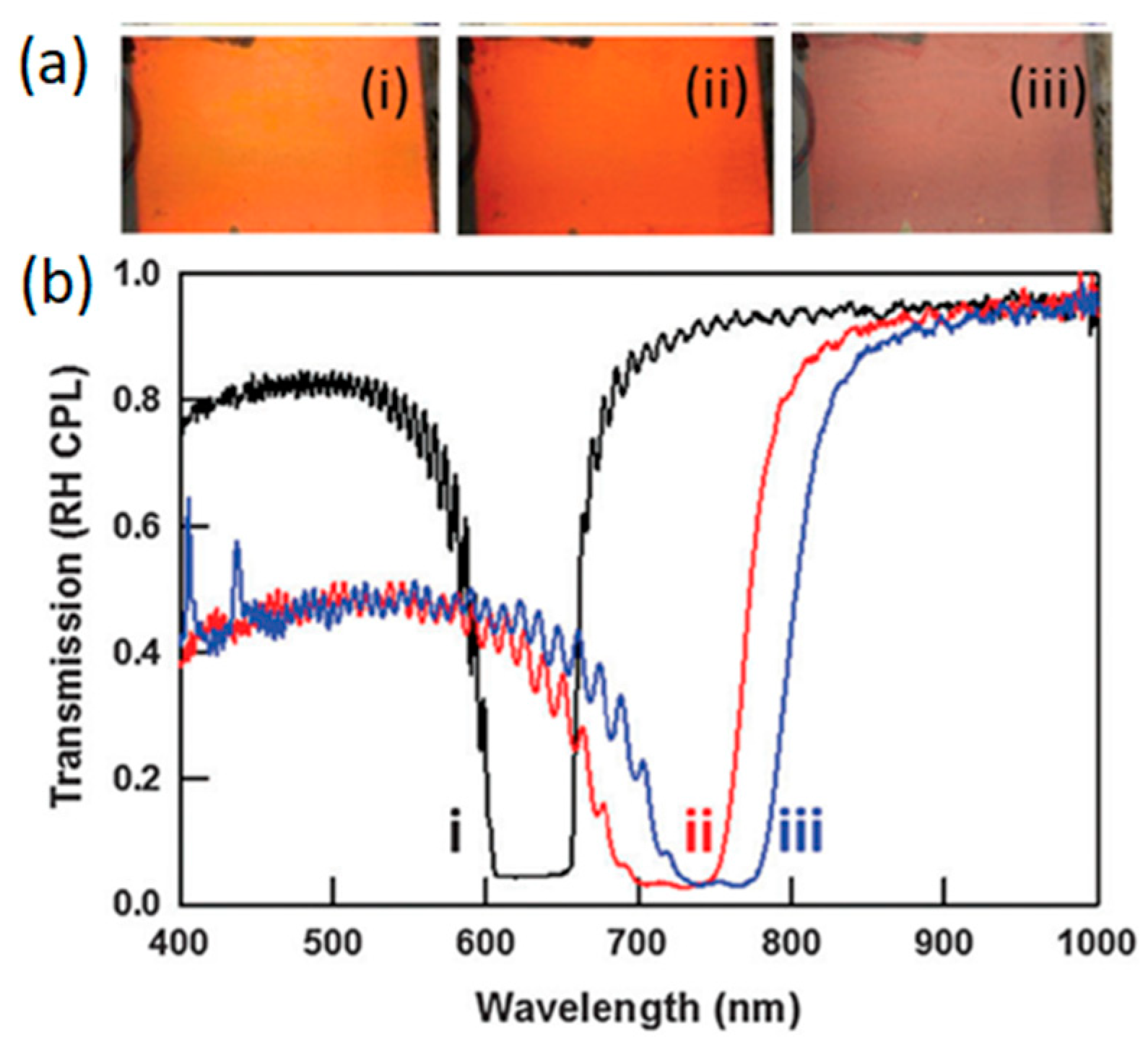
2.2. Reflection Notch Tuning
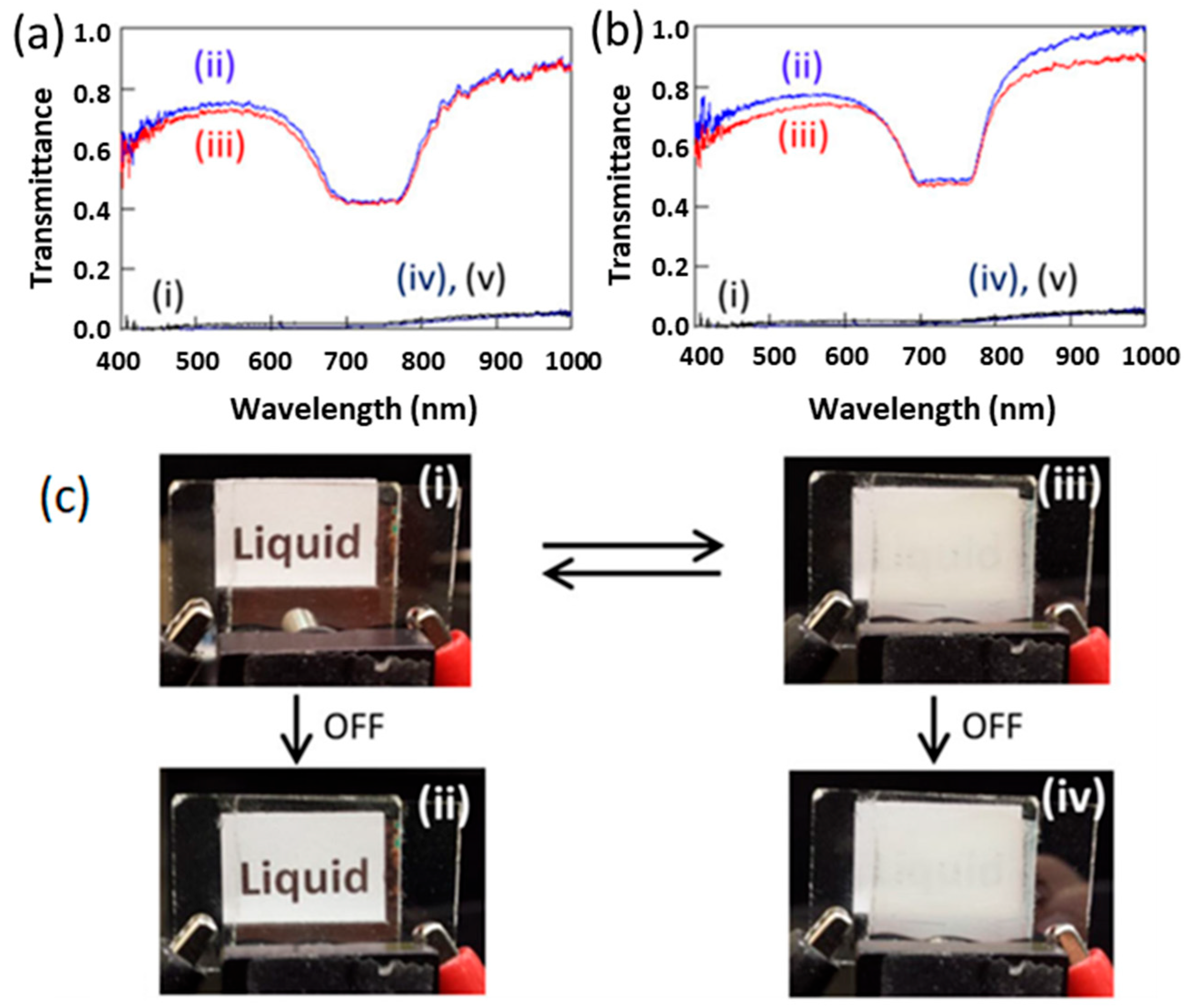
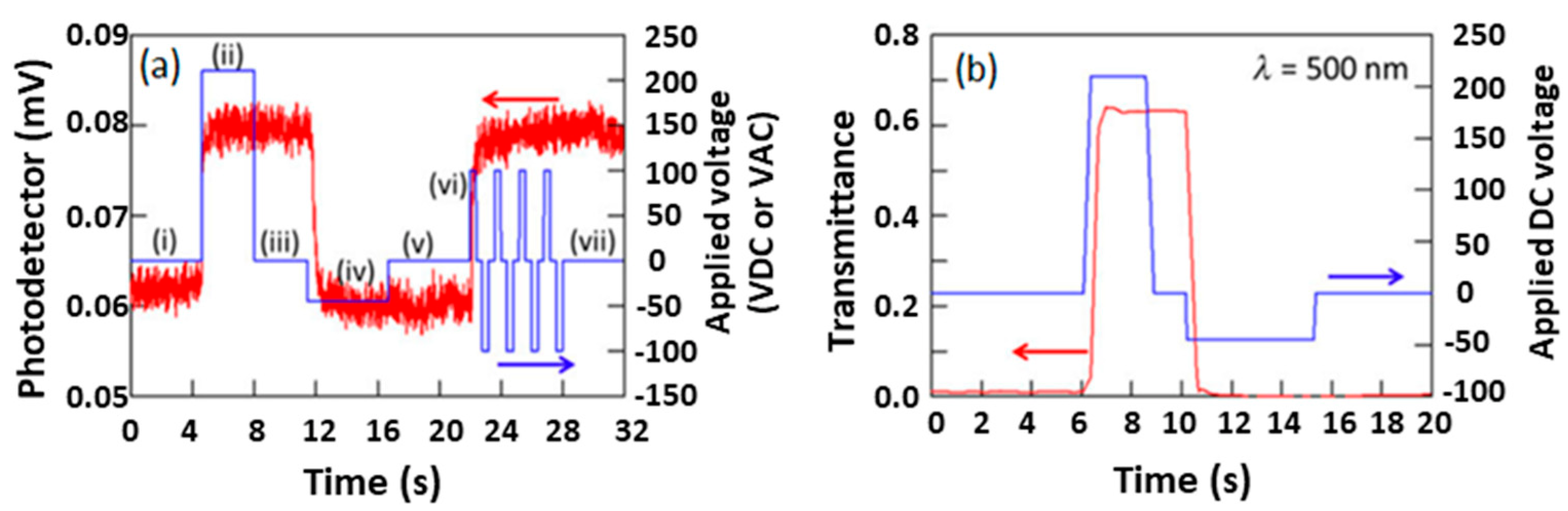
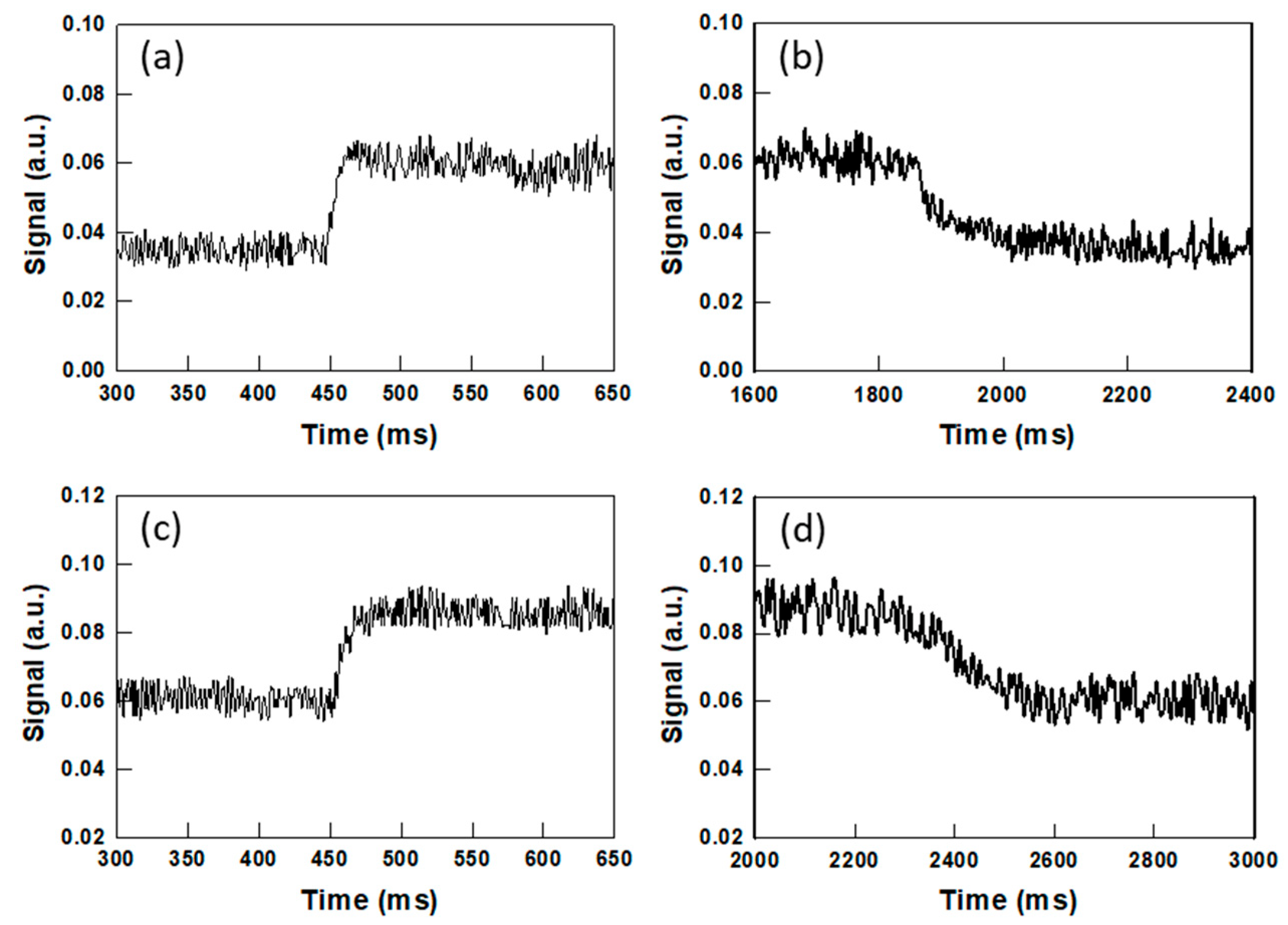
2.3. Switching Response in PSCLCs
2.4. Other Effects on Dynamic Behavior of PSCLCs
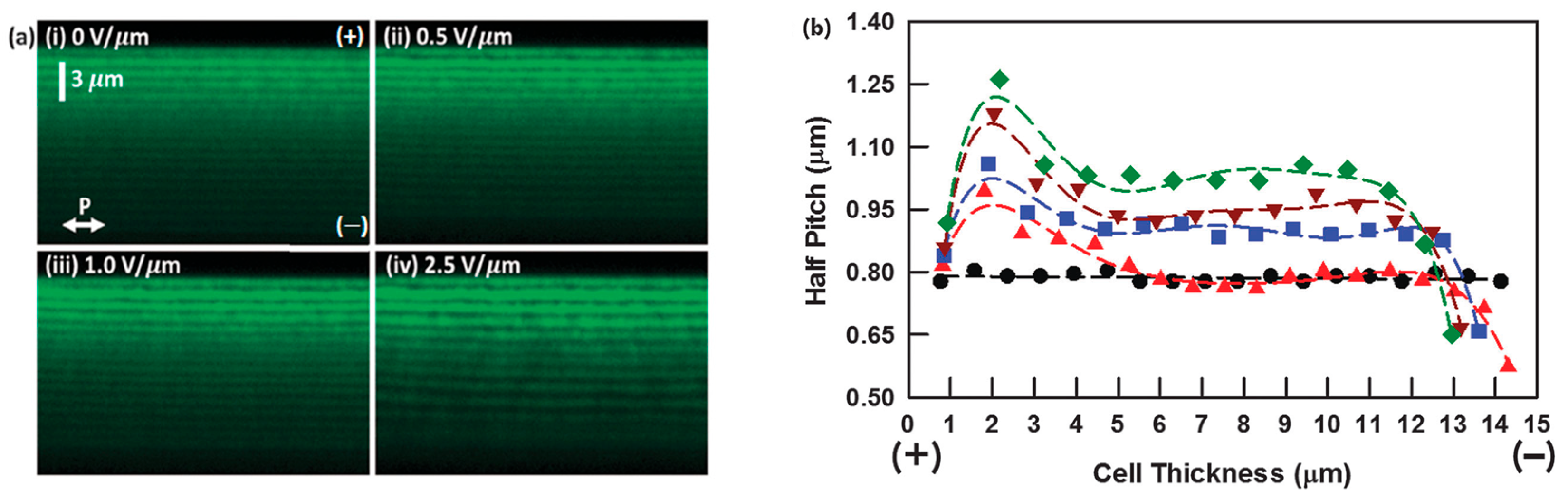
2.4.1. Cell Thickness
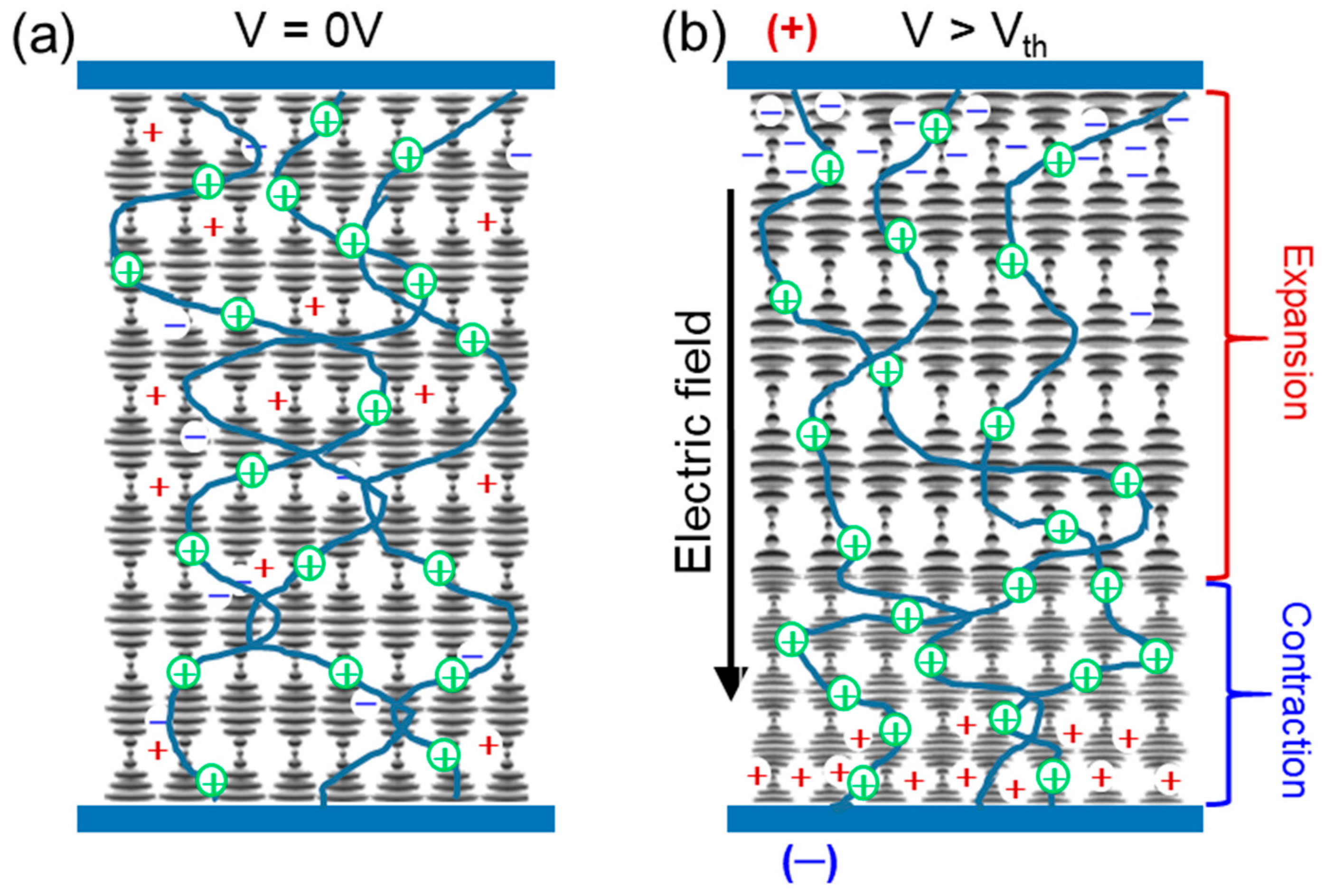
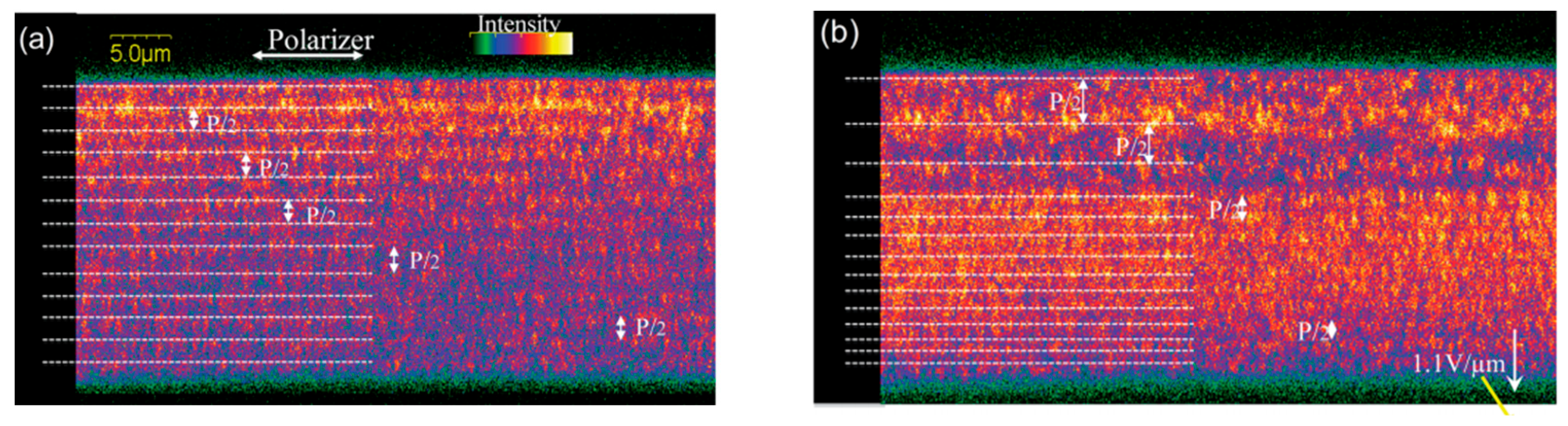
2.4.2. Ion Effect
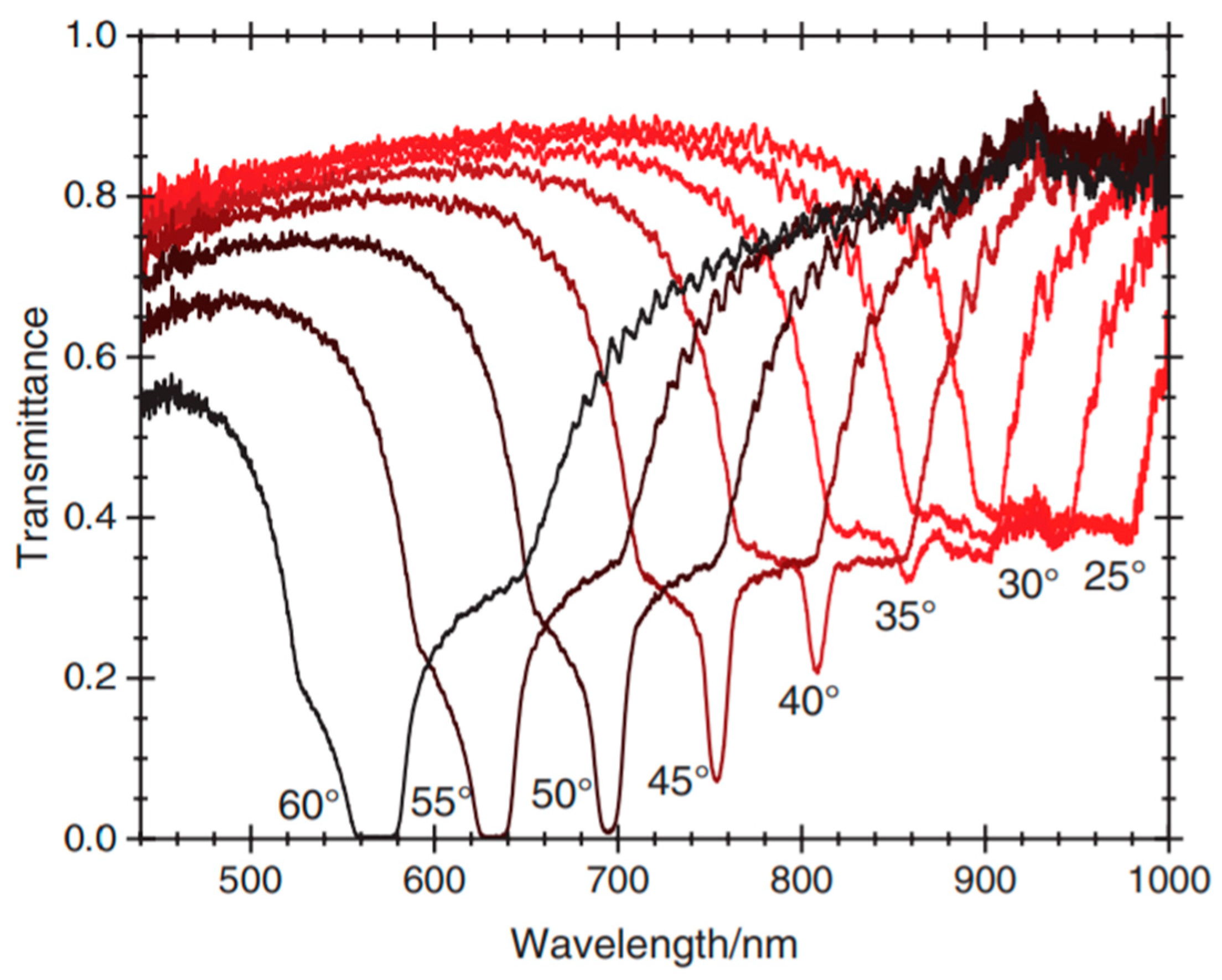
2.4.3. High Reflectivity of a PSCLC at Oblique Incidence
2.5. Potential Mechanism
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geelhaar, T.; Griesar, K.; Reckmann, B. 125 Years of Liquid Crystals—A Scientific Revolution in the Home. Angew. Chem. Int. Ed. 2013, 52, 8798–8809. [Google Scholar] [CrossRef] [PubMed]
- Kitzerow, H.-S.; Bahr, C. Chirality in Liquid Crystals; Springer: New York, NY, USA, 2001. [Google Scholar]
- Wu, S.T.; Yang, D.-K. Reflective Liquid Crystal Displays; Wiley: Chichester, UK, 2001. [Google Scholar]
- Blinov, L.M. Electro-Optical and Magneto-Optical Properties of Liquid Crystals; Wiley: New York, BY, USA, 1983. [Google Scholar]
- Yang, D.-K.; Chien, C.-C.; Donna, J.W. Cholesteric liquid crystal/ polymer dispersion for hazy-free light shutters. Appl. Phys. Lett. 1992, 60, 3102–3104. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.-K.; Doane, J.W.; Yaniv, Z.; Glasser, J. Cholesteric reflective display: Drive scheme and contrast. Appl. Phys. Lett. 1994, 64, 1905. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.-K.; West, J.L.; Chien, L.C.; Doane, J.W. Control of reflectivity and bistability in displays using cholesteric liquid crystals. J. Appl. Phys. 1994, 76, 1331–1333. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.-H. Bistable reflective cholesteric liquid crystal display. J. Appl. Phys. 1997, 81, 1063–1066. [Google Scholar] [CrossRef]
- Taheri, B.; Doane, J.W.; Davis, D.; St. John, D. Optical properties of bistable cholesteric reflective displays. SID Int. Symp. Dig. Tec. 1996, 27, 39–42. [Google Scholar]
- Anderson, J.; Watson, P.; Ruth, J.; Sergan, V.; Bos, P. Fast frame rate bistable cholesteric texture reflective displays. SID Int. Symp. Dig. Tec. 1998, 29, 806–809. [Google Scholar] [CrossRef]
- Yang, D.-K. Flexible bistable cholesteric reflective displays. J. Disp. Technol. 2006, 2, 32. [Google Scholar] [CrossRef]
- Li, C.-C.; Tseng, H.-Y.; Pai, T.-W.; Wu, Y.-C.; Hsu, W.-H.; Jau, H.-C.; Chen, C.-W.; Lin, T.H. Bistable cholesteric liquid crystal light shutter with multielectrode driving. Appl. Opt. 2014, 53, E33–E37. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Fu, K.-Y.; Lo, K.-Y.; Tsai, M.-S. Bistable transflective cho lesteric light shutters. Opt. Express 2003, 11, 560–565. [Google Scholar] [CrossRef]
- Lin, F.-C.; Wei, L. Color-reflective dual frequency cholesteric liquid crystal displays and their drive schemes. Appl. Phys. Express 2011, 4, 112201. [Google Scholar] [CrossRef]
- Kumar, P.; Kang, S.-W.; Lee, S.H. Advanced bistable cholesteric light shutter with dual frequency nematic liquid crystal. Opt. Mater. Express 2012, 2, 1121–1134. [Google Scholar] [CrossRef]
- Xu, M.; Yang, D.-K. Dual frequency cholesteric liquid crystals. Appl. Phys. Lett. 1997, 70, 720. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.-H.; Wu, S.T. Dielectric heating effects of dual-frequency liquid crystals. Appl. Phys. Lett. 2005, 86, 231104. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, D.-K. Polymer stabilized cholesteric dichroic dye displays. SID Int. Symp. Digest Tec. 2012, 33, 469–471. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Ren, H.; Fan, Y.-H.; Wu, Y.-H.; Wu, S.T. Polarization-independent and fast-response phase modulation using a normal-mode polymer-stabilized cholesteric texture. J. Appl. Phys. 2005, 98, 043112. [Google Scholar] [CrossRef]
- Ren, H.; Wu, S.-T. Reflective reversed-mode polymer stabilized cholesteric texture light switches. J. Appl. Phys. 2002, 92, 797–800. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Cao, H.; Guo, J.; Li, B.; He, S.; Ouyang, C.; Cao, M.; Huang, H.; Yang, H. Effects of monomer structure on the morphology of polymer network and the electro-optical property of reverse-mode polymer stabilized cholesteric texture. J. Appl. Polym. Sci. 2009, 111, 1353–1357. [Google Scholar] [CrossRef]
- Dierking, I. Polymer-Modified Liquid Crystals; Royal Society of Chemistry: Cambridge, UK, 2019. [Google Scholar]
- Fung, Y.K.; Yang, D.-K.; Ying, S.; Chien, L.C.; Zumer, S.; Doane, J.W. Polymer networks formed in liquid crystals. Liq. Cryst. 1995, 19, 797–801. [Google Scholar] [CrossRef]
- Hikmet, R.A.M.; Lub, J. Anisotropic networks and gels obtained by photopolymerization in the liquid crystalline state: Synthesis and applications. Prog. Polym. Sci. 1996, 21, 1165–1209. [Google Scholar] [CrossRef]
- Hikmet, R.A.M. Electrically induced light scattering from anisotropic gels. J. Appl. Phys. 1990, 68, 4406–4412. [Google Scholar] [CrossRef]
- Hikmet, R.A.M.; Howard, R. Structure and properties of anisotropic gels and plasticized networks containing molecules with a smectic-A phase. Phys. Rev. E 1993, 48, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Dierking, I. Polymer Network-Stabilized Liquid Crystals. Adv. Mater. 2000, 12, 167–181. [Google Scholar] [CrossRef]
- Dierking, I. Recent development in polymer stabilized liquid crystals. Polym. Chem. 2010, 1, 1153–1159. [Google Scholar] [CrossRef]
- Hikmet, R.A.M.; Boots, H.M.J. Domain structure and switching behavior of anisotropic gels. Phys. Rev. E 1995, 51, 5824–5831. [Google Scholar] [CrossRef]
- Hikmet, R.A.M.; Kemperman, H. Electrically switchable mirrors and optical components made from liquid-crystal gels. Nature 1998, 392, 476–479. [Google Scholar] [CrossRef]
- Guo, J.; Cao, J.; Wei, J.; Zhang, D.; Liu, F.; Pan, G.; Zhao, D.; He, W.; Yang, H. Polymer stabilized liquid crystal films reflecting both right- and left-circularly polarized light. Appl. Phys. Lett. 2008, 93, 201901. [Google Scholar] [CrossRef]
- Robbie, K.; Broer, D.J.; Brett, M.J. Chiral nematic order in liquid crystals imposed by an engineered inorganic nanostructure. Nature 1999, 399, 764–766. [Google Scholar] [CrossRef]
- Guo, J.; Liu, F.; Chen, F.; Wei, J.; Yang, H. Realisation of cholesteric liquid-crystalline materials reflecting both right- and left-circularly polarised light using the wash-out/refill technique. Liq. Cryst. 2010, 37, 171–178. [Google Scholar] [CrossRef]
- McConney, M.E.; Tondiglia, V.P.; Hurtubise, J.M.; White, T.J.; Bunning, T.J. Photoinduced hyper-reflective cholesteric liquid crystals enabled via surface initiated photopolymerization. Chem. Commun. 2011, 47, 505–507. [Google Scholar] [CrossRef]
- McConney, M.E.; Tondiglia, V.P.; Hurtubise, J.M.; Natarajan, L.V.; White, T.J.; Bunning, T.J. Thermally Induced, Multicolored Hyper-Reflective Cholesteric Liquid Crystals. Adv. Mater. 2011, 23, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zeng, J.; Zhu, S.; Zhang, D.; Ma, C.; Zhang, C.; Yu, P.; Miao, Z. A bistable light shutter based on polymer stabilized cholesteric liquid crystals. Opt. Mater. 2023, 136, 113426. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Chen, M.; Li, T.; Cao, H.; Yang, D.-K.; Yang, Z.; Yang, H.; Zhu, S. Bistable polymer-dispersed cholesteric liquid crystal thin film enabled by a stepwise polymerization. RSC Adv. 2015, 5, 58959–58965. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Zhang, M.; Zhang, C.; Niu, R.; Ma, H.; Sun, Y. Colorable Light-Scattering Device Based on Polymer-Stabilized Ion-Doped Cholesteric Liquid Crystal and an Electrochromatic Layer. ACS Appl. Mater. Interfaces 2023, 15, 7184–7195. [Google Scholar] [CrossRef] [PubMed]
- Tondiglia, V.P.; Natarajan, L.V.; Bailey, C.A.; McConney, M.E.; Lee, K.M.; Bunning, T.J.; Zola, R.; Nemati, H.; Yang, D.-K.; White, T.J. Bandwidth broadening induced by ionic interactions in polymer stabilized cholesteric liquid crystals. Opt. Mater. Express 2014, 4, 1465. [Google Scholar] [CrossRef]
- Tondiglia, V.P.; Natarajan, L.V.; Bailey, C.A.; Duning, M.M.; Sutherland, R.L.; Yang, D.-K.; Voevodin, A.; White, T.J.; Bunning, T.J. Electrically induced bandwidth broadening in polymer stabilized cholesteric liquid crystals. J. Appl. Phys. 2011, 110, 053109. [Google Scholar] [CrossRef]
- Nemati, H.; Liu, S.; Zola, R.S.; Tondiglia, V.P.; Lee, K.M.; White, T.J.; Bunning, T.J.; Yang, D.-K. Mechanism of electrically induced photonic band gap broadening in polymer stabilized cholesteric liquid crystals with negative dielectric anisotropies. Soft Matter 2015, 11, 1208. [Google Scholar] [CrossRef]
- Lee, K.M.; Tondiglia, V.P.; McConney, M.E.; Natarajan, L.V.; Bunning, T.J.; White, T.J. Color-tunable mirrors based on electrically regulated bandwidth broadening in polymer-stabilized cholesteric liquid crystals. ACS Photonics 2014, 1, 1033. [Google Scholar] [CrossRef]
- Radka, B.; Lee, K.M.; Godman, N.P.; White, T.J. Electro-optic characteristics of stabilized cholesteric liquid crystals with non-liquid crystalline polymer networks. Soft Matter 2022, 18, 3013–3018. [Google Scholar] [CrossRef]
- McConney, M.E.; Tondiglia, V.P.; Natarajan, L.V.; Lee, K.M.; White, T.J.; Bunning, T.J. Electrically induced color changes in polymer-stabilized cholesteric liquid crystals. Adv. Opt. Mater. 2013, 1, 417–421. [Google Scholar] [CrossRef]
- White, T.J.; Lee, K.M.; McConney, M.E.; Tondiglia, V.P.; Natarajan, L.V.; Bunning, T.J. Stimuli-Responsive Cholesteric Liquid Crystal Composites for Optics and Photonics. SID Int. Symp. Dig. Tec. 2014, 45, 555. [Google Scholar] [CrossRef]
- Lee, K.M.; Tondiglia, V.P.; Lee, T.; Smalyukh, I.I.; White, T.J. Large range electrically-induced reflection notch tuning in polymer stabilized cholesteric liquid crystals. J. Mater. Chem. C 2015, 3, 8788–8793. [Google Scholar] [CrossRef]
- Lee, K.M.; Tondiglia, V.P.; Rumi, M.; White, T.J. Time-dependent deformation of structurally chiral polymer networks in stabilized cholesteric liquid crystals. J. Polym. Sci. Part B 2018, 56, 1087–1093. [Google Scholar] [CrossRef]
- Lee, K.M.; Tondiglia, V.P.; Godman, N.P.; Middleton, C.M.; White, T.J. Blue-shifting Tuning of the Selective Reflection of Polymer Stabilized Cholesteric Liquid Crystals. Soft Matter 2017, 13, 5842–5848. [Google Scholar]
- Lee, K.M.; Tondiglia, V.P.; White, T.J. Bistable switching of polymer stabilized cholesteric liquid crystals between transparent and scattering modes. MRS Commun. 2015, 5, 223–227. [Google Scholar] [CrossRef]
- Lee, K.M.; Crenshaw, E.P.; Rumi, M.; White, T.J.; Bunning, T.J.; McConney, M.E. Effect of Cell Thickness on the Electro-optic Response of Polymer Stabilized Cholesteric Liquid Crystals with Negative Dielectric Anisotropy. Materials 2020, 13, 746. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Tondiglia, V.P.; White, T.J. Electrically Reconfigurable Liquid Crystalline Mirrors. ACS Omega 2018, 3, 4453. [Google Scholar] [CrossRef]
- Broer, D.J.; Lub, J.; Mol, G.N. Wide-band reflective polarizers from cholesteric polymer networks with a pitch gradient. Nature 1995, 378, 467–469. [Google Scholar] [CrossRef]
- White, T.J.; McConney, M.E.; Bunning, T.J. Dynamic color in stimuli-responsive cholesteric liquid crystals. J. Mater. Chem. 2010, 20, 9832–9847. [Google Scholar] [CrossRef]
- Zhang, L.; Nie, Q.; Jiang, X.-F.; Zhao, W.; Hu, X.; Shui, L.; Zhou, G. Enhanced Bandwidth Broadening of Infrared Reflector Based on Polymer Stabilized Cholesteric Liquid Crystals with Poly(N-vinylcarbazole) Used as Alignment Layer. Polymers 2021, 13, 2238. [Google Scholar] [CrossRef]
- Hu, X.; de Haan, L.T.; Khandelwal, H.; Schenning, A.P.H.J.; Nian, L.; Zhou, G. Cell thickness dependence of electrically tunable infrared reflectors based on polymer stabilized cholesteric liquid crystals. Sci. China Mater. 2018, 61, 745–751. [Google Scholar] [CrossRef]
- Khandelwal, H.; Debije, M.G.; White, T.J.; Schenning, A.P.H.J. Electrically tunable infrared reflector with adjustable bandwidth broadening up to 1100 nm. J. Mater. Chem. A 2016, 4, 6064–6069. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, W.; Yang, W.; Xiao, L.; De Haan, L.T.; Zhao, W.; Li, N.; Shui, L.; Zhou, G. Effective electrically tunable infrared reflectors based on polymer stabilised cholesteric liquid crystals. Liq. Cryst. 2019, 46, 185–192. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Nemati, H.; Yang, H.; Bunning, T.J.; Yang, D.-K. Effects of polymer network on electrically induced reflection band broadening of cholesteric liquid crystals. J. Polym. Sci. Part B 2017, 55, 835–846. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Kim, S.; Choi, S.; Jang, W.; Kim, J.; Lee, J.-H. Broadening the reflection bandwidth of polymer-stabilized cholesteric liquid crystal via a reactive surface coating layer. Appl. Opt. 2017, 56, 5731–5735. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; He, W.; Yang, Z.; Cao, H.; Wang, D.; Li, Y. Research Progress of Cholesteric Liquid Crystals with Broadband Reflection. Molecules 2022, 27, 4427. [Google Scholar] [CrossRef]
- Nemati, H.; Liu, S.; Moheghi, A.; Tondiglia, V.P.; Lee, K.M.; Bunning, T.J.; Yang, D.-K. Enhanced reflection band broadening in polymer stabilized cholesteric liquid crystals with negative dielectric anisotropy. J. Mol. Liq. 2018, 267, 120–126. [Google Scholar] [CrossRef]
- Duan, M.-y.; Cao, H.; Wu, Y.; Li, E.-l.; Wang, H.H.; Wang, D.; Yang, Z.; He, W.-I.; Yang, H. Broadband reflection in polymer stabilized cholesteric liquid crystal films with stepwise photo-polymerization. Phys. Chem. Chem. Phys. 2017, 19, 2353–2358. [Google Scholar] [CrossRef]
- Lu, L.; Sergan, V.; Bos, P.J. Mechanism of electric-field-induced segregation of additives in a liquid-crystal host. Phys. Rev. E 2012, 86, 051706. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-C.; Tseng, H.-Y.; Chen, C.-W.; Wang, C.-T.; Jau, H.-C.; Wu, Y.-C.; Hsu, W.-H.; Lin, T.-H. Versatile Energy-Saving Smart Glass Based on Tristable Cholesteric Liquid Crystals. ACS Appl. Energy Mater. 2020, 3, 7601–7609. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Guo, S.; Zhang, L.; Li, F.; Yang, H. Dual-Band Modulation of Visible and Near-Infrared Light Transmittance in an All-Solution-Processed Hybrid Micro-Nano Composite Film. ACS Appl. Mater. Interfaces 2017, 9, 40810–40819. [Google Scholar] [CrossRef]
- Liang, X.; Guo, S.; Chen, M.; Li, C.; Wang, Q.; Zou, C.; Zhang, C.; Zhang, L.; Guo, S.; Yang, H. A temperature and electric field-responsive flexible smart film with a full broadband optical modulation. Mater. Horiz. 2017, 4, 878–884. [Google Scholar] [CrossRef]
- Khandelwal, H.; Loonen, R.C.G.M.; Hensen, J.L.M.; Debije, M.G.; Schenning, A.P.H.J. Electrically switchable polymer stabilised broadband infrared reflectors and their potential as smart windows for energy saving in buildings. Sci. Rep. 2015, 5, 11773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nava, G.; Ciciulla, F.; Simoni, F.; Iadlovska, O.; Lavrentovich, O.D.; Lucchetti, L. Heliconical cholesteric liquid crystals as electrically tunable optical filters in notch and bandpass configurations. Liquid Cryst. 2021, 48, 1534–1543. [Google Scholar] [CrossRef]
- Xianyu, H.; Faris, S.; Crawford, G.P. In-plane switching of cholesteric liquid crystals for visible and near-infrared applications. Appl. Opt. 2004, 43, 5006–5015. [Google Scholar] [CrossRef] [PubMed]
- Escuti, M.J.; Bowley, C.C.; Crawford, G.P.; Žumer, S. Enhanced dynamic response of the in-plane switching liquid crystal display mode through polymer stabilization. Appl. Phys. Lett. 1999, 75, 3264–3266. [Google Scholar] [CrossRef]
- Lin, T.H.; Jau, H.-C.; Chen, C.H.; Chen, Y.J.; Wei, T.H.; Chen, C.-W.; Fuh, A.Y.G. Electrically controllable laser based on cholesteric liquid crystal with negative dielectric anisotropy. Appl. Phys. Lett. 2006, 88, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Scaramuzza, N.; Ferrero, C.; Carbone, V.; Versace, C. Dynamics of selective reflections of cholesteric liquid crystals subject to electric fields. J. Appl. Phys. 1995, 77, 572–576. [Google Scholar] [CrossRef]
- Umeton, C.; Bartolino, R.; Cipparone, G.; Simoni, F. Liquid crystal laser tuner. Appl. Opt. 1988, 27, 210–211. [Google Scholar] [CrossRef]
- Umeton, C.; Bartolino, R.; Cipparrone, G.; Xu, F.; Ye, F.; Simoni, F. Wavelength Modulation Using Cholesteric Liquid Crystals. Mol. Cryst. Liq. Cryst. 1987, 144, 323–336. [Google Scholar] [CrossRef]
- Bailey, C.A.; Tondiglia, V.P.; Natarajan, L.V.; Duning, M.M.; Bricker, R.L.; Sutherland, R.L.; White, T.J.; Durstock, M.F.; Bunning, T.J. Electromechanical tuning of cholesteric liquid crystals. J. Appl. Phys. 2010, 107, 013105. [Google Scholar] [CrossRef]
- Yu, H.; Tang, B.Y.; Li, J.; Li, L. Electrically tunable lasers made from electro-optically active photonics band gap materials. Opt. Express 2005, 13, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Worth, B.; Lee, K.M.; Tondiglia, V.P.; Myers, J.; Mou, S.; White, T.J. Dynamic, infrared bandpass filters prepared from polymer-stabilized cholesteric liquid crystals. Appl. Opt. 2016, 55, 7134–7137. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Tondiglia, V.P.; White, T.J. Photosensitivity of reflection notch tuning and broadening in polymer stabilized cholesteric liquid crystals. Soft Matter 2016, 12, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Bunning, T.J.; White, T.J.; McConney, M.E.; Godman, N.P. Effect of Ion Concentration on the Electro-Optic Response in Polymer-Stabilized Cholesteric Liquid Crystals. Crystals 2021, 11, 7. [Google Scholar] [CrossRef]
- Lee, K.M.; Ware, T.H.; Tondiglia, V.P.; Mcbride, M.K.; Zhang, X.; Bowman, C.N.; White, T.J. Initiatorless Photopolymerization of Liquid Crystal Monomers. ACS Appl. Mater. Interfaces 2016, 8, 28040–28046. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Peng, F.; Chen, H.; Yuan, J.; Wu, S.-T.; Li, M.-C.; Lee, S.-L.; Tsai, W.-C. Image sticking in liquid crystal displays with lateral electric fields. J. Appl. Phys. 2014, 116, 193102. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Wang, M.; Tang, Z.; Gao, L.; Xing, H.; Meng, X.; He, Z.; Li, J.; Cai, M.; Wang, X.; et al. Influence of γ-Fe2O3 nanoparticles doping on the image sticking in VAN-LCD. Chin. Opt. Lett. 2020, 18, 033501. [Google Scholar] [CrossRef]
- Son, S.-R.; An, J.; Choi, J.-W.; Lee, J.H. Fabrication of TiO2-Embedded Polyimide Layer with High Transmittance and Improved Reliability for Liquid Crystal Displays. Polymers 2021, 13, 376. [Google Scholar] [CrossRef]
- Mitov, M.; Dessaud, N. Cholesteric liquid crystalline materials reflecting more than 50% of unpolarized incident light intensity. Liq. Cryst. 2007, 34, 183. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Li, J.; Ye, S.; Wei, J.; Guo, J. A bio-inspired cellulose nanocrystal-based nanocomposite photonic film with hyper-reflection and humidity-responsive actuator properties. J. Mater. Chem. C 2016, 4, 9687. [Google Scholar] [CrossRef]
- Agez, G.; Mitov, M. Cholesteric Liquid Crystalline Materials with a Dual Circularly Polarized Light Reflection Band Fixed at Room Temperature. J. Phys. Chem. B 2011, 115, 6421–6426. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.J.; Dai, H.T.; Zhang, X.H.; Luo, D.; Sun, X.W. Flexible cholesteric films with super-reflectivity and high stability based on a multi-layer helical structure. J. Mater. Chem. C 2017, 5, 10828. [Google Scholar] [CrossRef]
- Guo, J.; Chen, F.; Qu, Z.; Yang, H.; Wei, J. Electrothermal Switching Characteristics from a Hydrogen-Bonded Polymer Network Structure in Cholesteric Liquid Crystals with a Double-Handed Circularly Polarized Light Reflection Band. J. Phys. Chem. B 2011, 115, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Makov, D.M. Peak reflectance and color gamut of superimposed left- and right-handed cholesteric liquid crystals. Appl. Opt. 1980, 19, 1274–1277. [Google Scholar]
- Schadt, M.; Fünfschilling, J. New Liquid Crystal Polarized Color Projection Principle. Jpn. J. Appl. Phys. 1990, 29, 1974. [Google Scholar] [CrossRef]
- Tondiglia, V.P.; Rumi, M.; Idehenre, I.U.; Lee, K.M.; Binzer, J.F.; Banerjee, P.P.; Evans, D.R.; McConney, M.E.; Bunning, T.J.; White, T.J. Electrical Control of Unpolarized Reflectivity in Polymer Stabilized Cholesteric Liquid Crystals at Oblique Incidence. Adv. Opt. Mater. 2018, 6, 1800957. [Google Scholar] [CrossRef]
- Lee, W.; Wang, C.-T.; Lin, C.-H. Recovery of the electrically resistive properties of a degraded liquid crystal. Displays 2010, 31, 160–163. [Google Scholar] [CrossRef]
- Gosse, B.; Gosse, J.P. Degradation of liquid crystal devices under d.c. excitation and their electrochemistry. J. Appl. Electrochem. 1976, 6, 515–519. [Google Scholar] [CrossRef]
- Wen, C.-H.; Gauza, S.; Wu, S.T. Ultraviolet stability of liquid crystals containing cyano and isothiocyanato terminal groups. Liq. Cryst. 2004, 31, 1479–1485. [Google Scholar] [CrossRef]
- Lin, P.-T.; Wu, S.T.; Shang, C.Y.; Hsu, C.S. UV Stability of High Birefirngence Liquid Crystals. Mol. Cryst. Liq. Cryst. 2004, 411, 243–253. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.M.; Marsh, Z.M.; Crenshaw, E.P.; Tohgha, U.N.; Ambulo, C.P.; Wolf, S.M.; Carothers, K.J.; Limburg, H.N.; McConney, M.E.; Godman, N.P. Recent Advances in Electro-Optic Response of Polymer-Stabilized Cholesteric Liquid Crystals. Materials 2023, 16, 2248. https://doi.org/10.3390/ma16062248
Lee KM, Marsh ZM, Crenshaw EP, Tohgha UN, Ambulo CP, Wolf SM, Carothers KJ, Limburg HN, McConney ME, Godman NP. Recent Advances in Electro-Optic Response of Polymer-Stabilized Cholesteric Liquid Crystals. Materials. 2023; 16(6):2248. https://doi.org/10.3390/ma16062248
Chicago/Turabian StyleLee, Kyung Min, Zachary M. Marsh, Ecklin P. Crenshaw, Urice N. Tohgha, Cedric P. Ambulo, Steven M. Wolf, Kyle J. Carothers, Hannah N. Limburg, Michael E. McConney, and Nicholas P. Godman. 2023. "Recent Advances in Electro-Optic Response of Polymer-Stabilized Cholesteric Liquid Crystals" Materials 16, no. 6: 2248. https://doi.org/10.3390/ma16062248




