Electrochemical Atomic Force Microscopy Study on the Dynamic Evolution of Lithium Deposition
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Electrolytes
2.2. In Situ Electrochemical Atomic Force Microscopy
2.3. Battery Preparation and Electrochemical Test
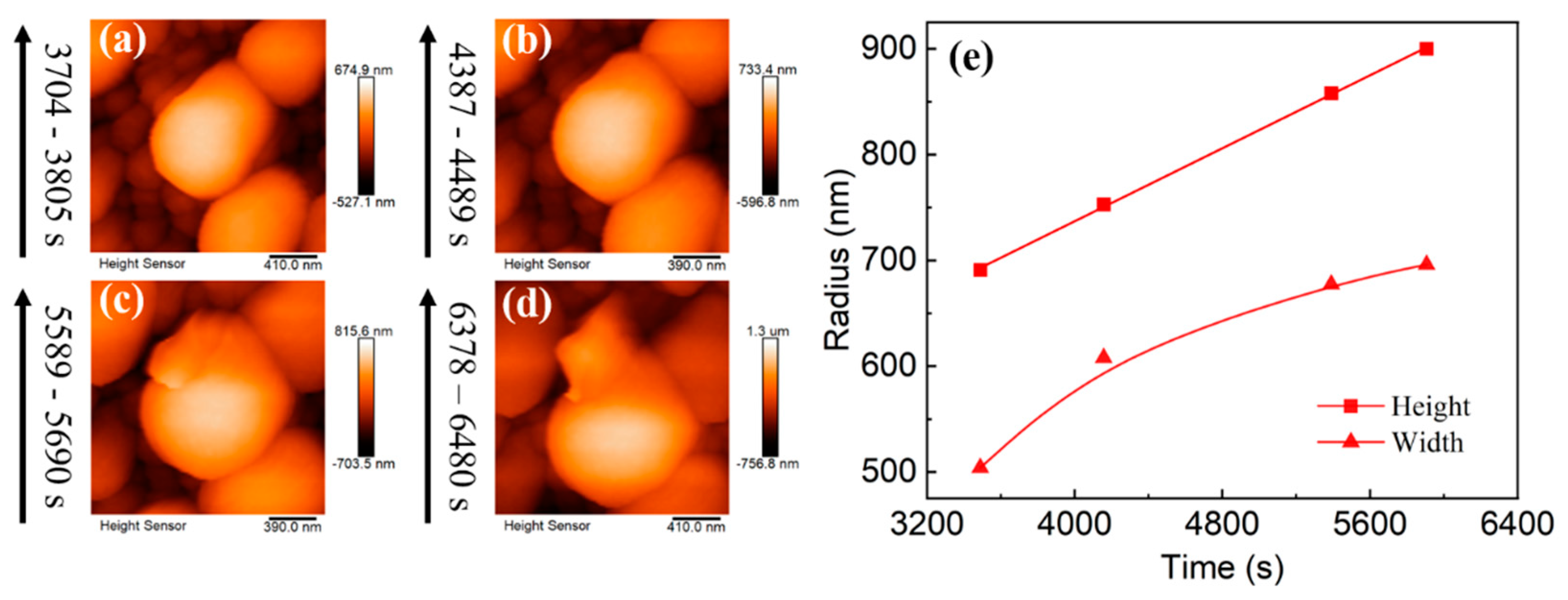
3. Results and Discussion
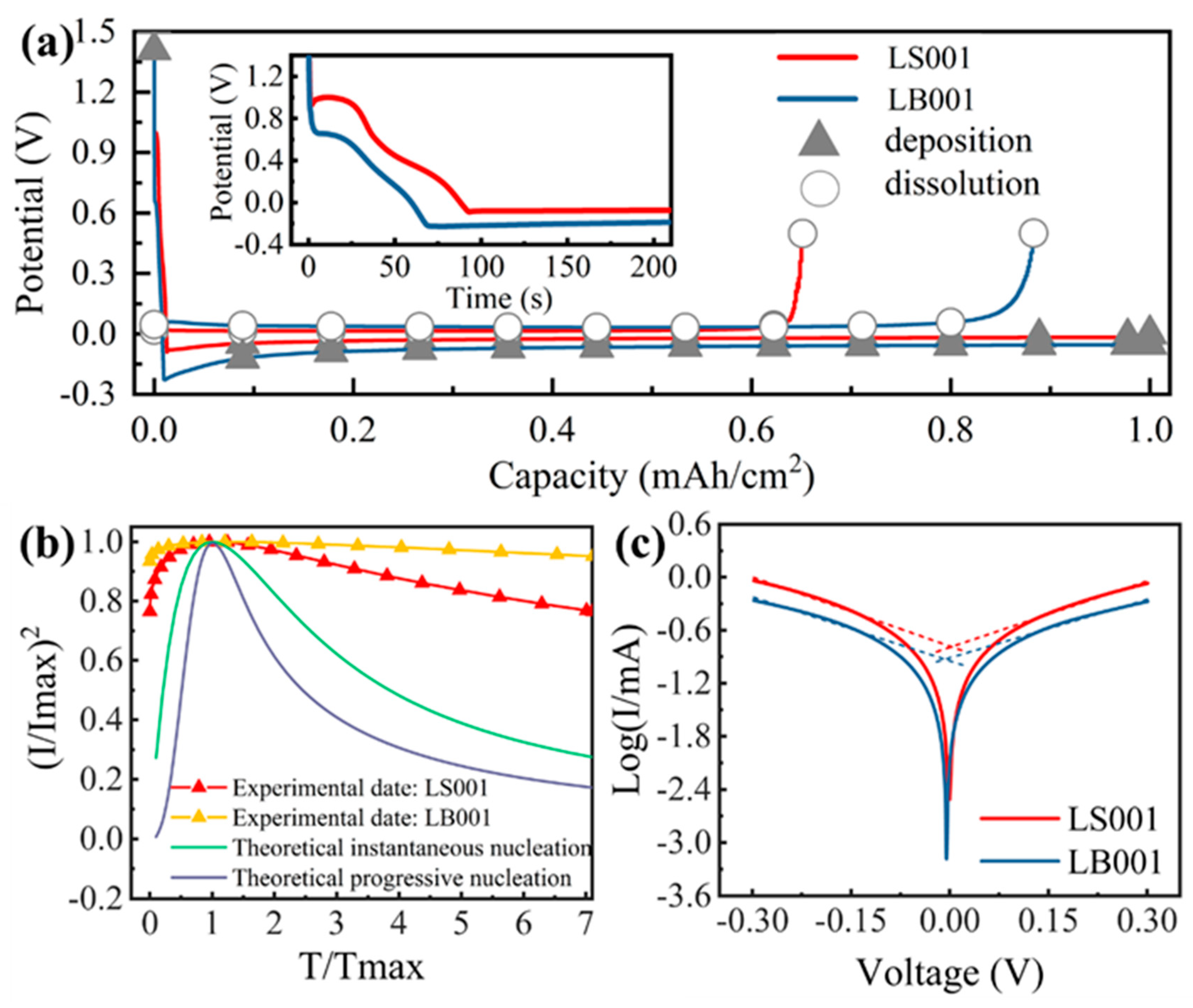
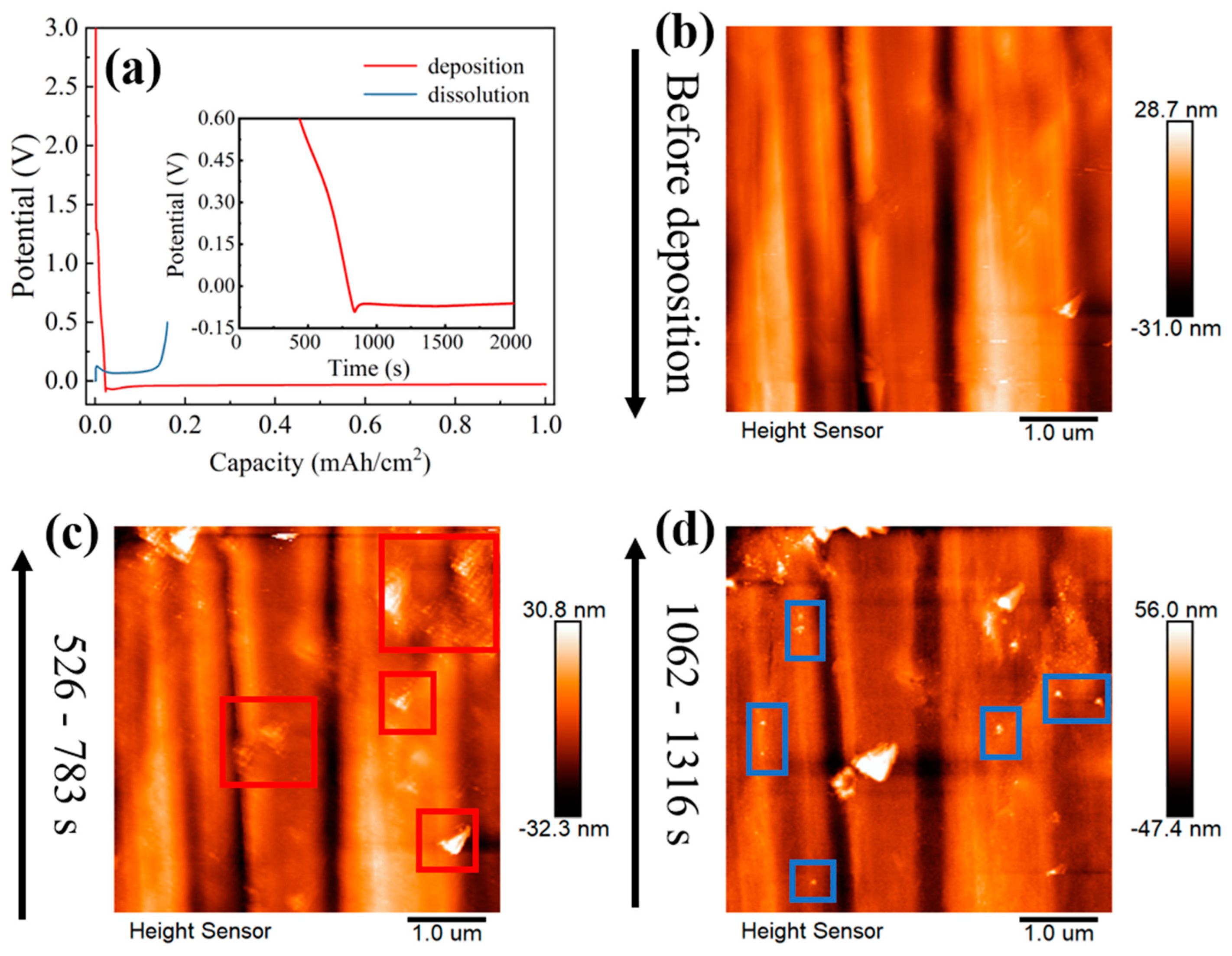
3.1. Nucleation Process
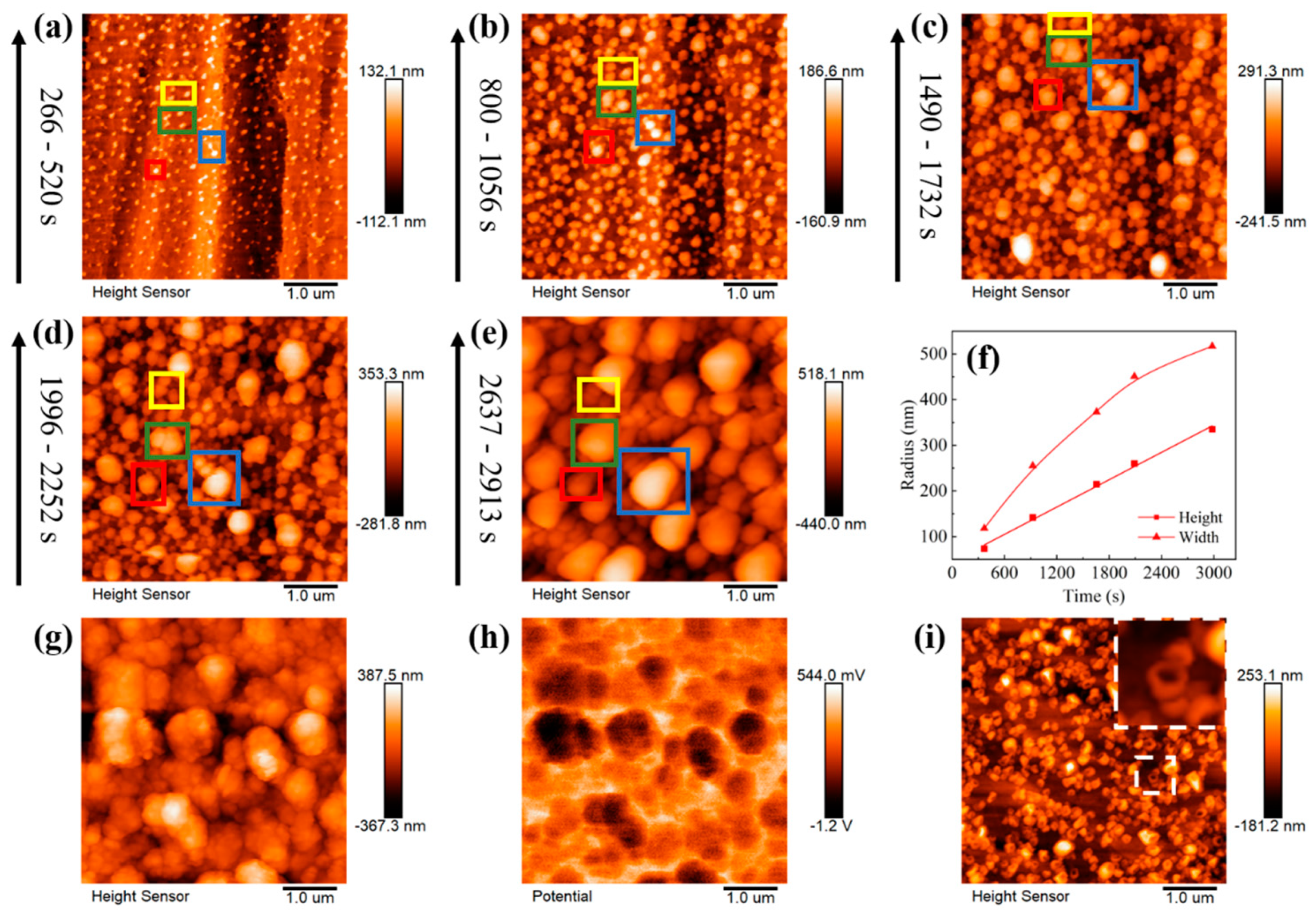
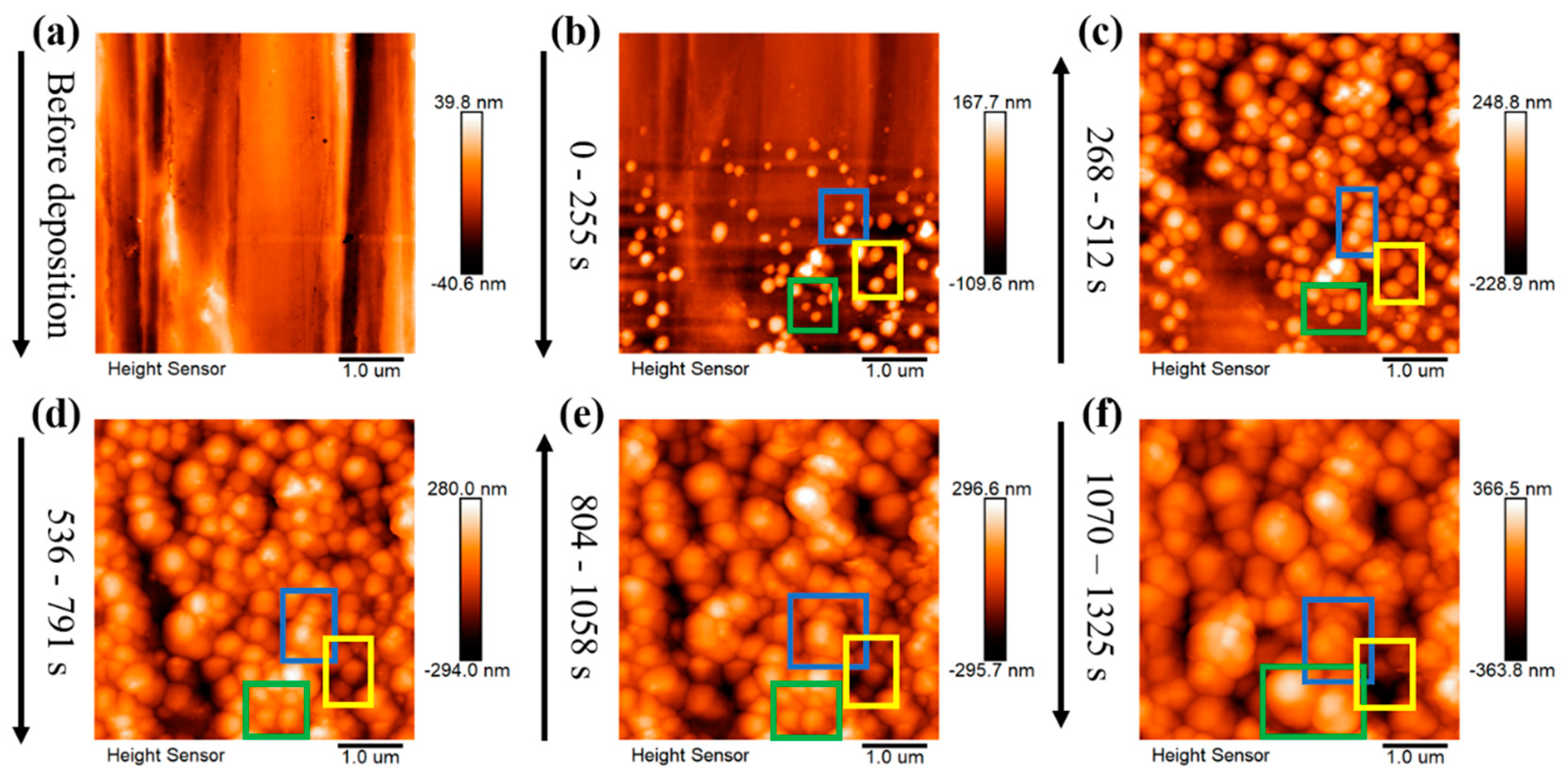
3.2. Lithium Nuclei Growth Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar]
- Hu, X.; Cao, Y.; Deng, Y.; Deng, J.; Lu, H. Graphene film with folds for a stable lithium metal anode. Ionics 2020, 26, 5357–5365. [Google Scholar]
- Liu, S.; Xia, J.; Zhang, W.; Wan, H.; Zhang, J.; Xu, J.; Rao, J.; Deng, T.; Hou, S.; Nan, B.; et al. Salt-in-Salt Reinforced Carbonate Electrolyte for Li Metal Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210522. [Google Scholar]
- Zhao, C.-Z.; Zhang, X.-Q.; Cheng, X.-B.; Zhang, R.; Xu, R.; Chen, P.-Y.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl. Acad. Sci. USA 2017, 114, 11069–11074. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Q.; Cheng, X.-B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar]
- Ebadi, M.; Lacey, M.J.; Brandell, D.; Araujo, C.M. Density Functional Theory Modeling the Interfacial Chemistry of the LiNO3 Additive for Lithium–Sulfur Batteries by Means of Simulated Photoelectron Spectroscopy. J. Phys. Chem. C 2017, 121, 23324–23332. [Google Scholar] [CrossRef]
- Lin, X.; Yu, J.; Effat, M.B.; Zhou, G.; Robson, M.J.; Kwok, S.C.T.; Li, H.; Zhan, S.; Shang, Y.; Ciucci, F. Ultrathin and Non-Flammable Dual-Salt Polymer Electrolyte for High-Energy-Density Lithium-Metal Battery. Adv. Funct. Mater. 2021, 31, 2010261. [Google Scholar]
- Ren, X.; Zou, L.; Jiao, S.; Mei, D.; Engelhard, M.H.; Li, Q.; Lee, H.; Niu, C.; Adams, B.D.; Wang, C.; et al. High-Concentration Ether Electrolytes for Stable High-Voltage Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 896–902. [Google Scholar]
- Yu, L.; Chen, S.; Lee, H.; Zhang, L.; Engelhard, M.H.; Li, Q.; Jiao, S.; Liu, J.; Xu, W.; Zhang, J.-G. A Localized High-Concentration Electrolyte with Optimized Solvents and Lithium Difluoro(oxalate)borate Additive for Stable Lithium Metal Batteries. ACS Energy Lett. 2018, 3, 2059–2067. [Google Scholar]
- Chen, S.; Zheng, J.; Mei, D.; Han, K.S.; Engelhard, M.H.; Zhao, W.; Xu, W.; Liu, J.; Zhang, J.G. High-Voltage Lithium-Metal Batteries Enabled by Localized High-Concentration Electrolytes. Adv. Mater. 2018, 30, e1706102. [Google Scholar]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.; Yin, Y.-X.; Zhang, S.-F.; Shi, Y.; Liu, L.; Zeng, X.-X.; Wen, R.; Guo, Y.-G.; Wan, L.-J. Synergism of Al-containing solid electrolyte interphase layer and Al-based colloidal particles for stable lithium anode. Nano Energy 2017, 36, 411–417. [Google Scholar]
- Xu, Y.; Wu, H.; He, Y.; Chen, Q.; Zhang, J.-G.; Xu, W.; Wang, C. Atomic to Nanoscale Origin of Vinylene Carbonate Enhanced Cycling Stability of Lithium Metal Anode Revealed by Cryo-Transmission Electron Microscopy. Nano Lett. 2020, 20, 418–425. [Google Scholar] [PubMed]
- Liu, Y.; Qin, Y.; Peng, Z.; Zhou, J.; Wan, C.; Wang, D. Hexamethylene diisocyanate as an electrolyte additive for high-energy density lithium ion batteries. J. Mater. Chem. A 2015, 3, 8246–8249. [Google Scholar]
- Wu, F.; Quan, H.; Han, J.; Peng, X.; Yan, Z.; Zhang, X.; Xiang, Y. Free-standing lithiophilic Ag-nanoparticle-decorated 3D porous carbon nanotube films for enhanced lithium storage. RSC Adv. 2020, 10, 30880–30886. [Google Scholar] [PubMed]
- Zhang, R.; Chen, X.R.; Chen, X.; Cheng, X.B.; Zhang, X.Q.; Yan, C.; Zhang, Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. Int. Ed. Engl. 2017, 56, 7764–7768. [Google Scholar]
- Li, B.Q.; Chen, X.R.; Chen, X.; Zhao, C.X.; Zhang, R.; Cheng, X.B.; Zhang, Q. Favorable Lithium Nucleation on Lithiophilic Framework Porphyrin for Dendrite-Free Lithium Metal Anodes. Research 2019, 2019, 4608940. [Google Scholar]
- He, X.; Ji, X.; Zhang, B.; Rodrigo, N.D.; Hou, S.; Gaskell, K.; Deng, T.; Wan, H.; Liu, S.; Xu, J.; et al. Tuning Interface Lithiophobicity for Lithium Metal Solid-State Batteries. ACS Energy Lett. 2021, 7, 131–139. [Google Scholar]
- Yang, C.P.; Yin, Y.X.; Zhang, S.F.; Li, N.W.; Guo, Y.G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar]
- Gao, X.; Yang, X.; Adair, K.; Li, X.; Liang, J.; Sun, Q.; Zhao, Y.; Li, R.; Sham, T.-K.; Sun, X. 3D Vertically Aligned Li Metal Anodes with Ultrahigh Cycling Currents and Capacities of 10 mA cm−2/20 mAh cm−2 Realized by Selective Nucleation within Microchannel Walls. Adv. Energy Mater. 2020, 10, 1903753. [Google Scholar]
- Zhai, P.; Wang, T.; Jiang, H.; Wan, J.; Wei, Y.; Wang, L.; Liu, W.; Chen, Q.; Yang, W.; Cui, Y.; et al. 3D Artificial Solid-Electrolyte Interphase for Lithium Metal Anodes Enabled by Insulator–Metal–Insulator Layered Heterostructures. Adv. Mater. 2021, 33, 2006247. [Google Scholar]
- Temesgen, N.T.; Tegegne, W.A.; Shitaw, K.N.; Fenta, F.W.; Nikodimos, Y.; Taklu, B.W.; Jiang, S.-K.; Huang, C.-J.; Wu, S.-H.; Su, W.-N.; et al. Mitigating dendrite formation and electrolyte decomposition via functional double layers coating on copper current collector in anode-free lithium metal battery. J. Taiwan Inst. Chem. Eng. 2021, 128, 87–97. [Google Scholar]
- Yi, R.; Mao, Y.; Shen, Y.; Chen, L. Self-Assembled Monolayers for Batteries. J. Am. Chem. Soc. 2021, 143, 12897–12912. [Google Scholar]
- Zhang, H.; Shen, C.; Huang, Y.; Liu, Z. Spontaneously formation of SEI layers on lithium metal from LiFSI/DME and LiTFSI/DME electrolytes. Appl. Surf. Sci. 2021, 537, 147983. [Google Scholar]
- Chen, X.R.; Zhao, B.C.; Yan, C.; Zhang, Q. Review on Li Deposition in Working Batteries: From Nucleation to Early Growth. Adv. Mater. 2021, 33, e2004128. [Google Scholar]
- Chen, X.; Chen, J.; Zhou, X.; You, M.; Zhang, C.; Yue, W. Two-dimensional graphene-based Li4Ti5O12 with hierarchical pore structure and large pseudocapacitive effect as high-rate and long-cycle anode material for lithium-ion batteries. Electrochim. Acta 2022, 405, 139814. [Google Scholar]
- Yu, H.; Lan, H.; Yan, L.; Qian, S.; Cheng, X.; Zhu, H.; Long, N.; Shui, M.; Shu, J. TiNb2O7 hollow nanofiber anode with superior electrochemical performance in rechargeable lithium ion batteries. Nano Energy 2017, 38, 109–117. [Google Scholar]
- Fu, X.; Wang, T.; Shen, W.; Jiang, M.; Wang, Y.; Dai, Q.; Wang, D.; Qiu, Z.; Zhang, Y.; Deng, K.; et al. A High-Performance Carbonate-Free Lithium|Garnet Interface Enabled by a Trace Amount of Sodium. Adv. Mater. 2020, 32, e2000575. [Google Scholar]
- Boyle, D.T.; Li, Y.; Pei, A.; Vila, R.A.; Zhang, Z.; Sayavong, P.; Kim, M.S.; Huang, W.; Wang, H.; Liu, Y.; et al. Resolving Current-Dependent Regimes of Electroplating Mechanisms for Fast Charging Lithium Metal Anodes. Nano Lett. 2022, 22, 8224–8232. [Google Scholar] [PubMed]
- Wang, W.-W.; Gu, Y.; Wang, J.-H.; Chen, Z.-B.; Yin, X.-T.; Wu, Q.-H.; Yan, J.-W.; Mao, B.-W. Probing Mechanical Properties of Solid-Electrolyte Interphases on Li Nuclei by In Situ AFM. J. Electrochem. Soc. 2022, 169, 020563. [Google Scholar]
- Luchkin, S.Y.; Lipovskikh, S.A.; Katorova, N.S.; Savina, A.A.; Stevenson, K.J. Solid-electrolyte interphase nucleation and growth on carbonaceous negative electrodes for Li-ion batteries visualized with in situ atomic force microscopy. Sci. Rep. 2020, 10, 8550. [Google Scholar] [PubMed]
- Ning, Z.; Jolly, D.S.; Li, G.; De Meyere, R.; Pu, S.D.; Chen, Y.; Kasemchainan, J.; Ihli, J.; Gong, C.; Liu, B.; et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells. Nat. Mater. 2021, 20, 1121–1129. [Google Scholar] [PubMed]
- Shadike, Z.; Lee, H.; Borodin, O.; Cao, X.; Fan, X.; Wang, X.; Lin, R.; Bak, S.M.; Ghose, S.; Xu, K.; et al. Identification of LiH and nanocrystalline LiF in the solid-electrolyte interphase of lithium metal anodes. Nat. Nanotechnol. 2021, 16, 549–554. [Google Scholar]
- Niu, C.J.; Liu, D.Y.; Lochala, J.A.; Anderson, C.S.; Cao, X.; Gross, M.E.; Xu, W.; Zhang, J.G.; Whittingham, M.S.; Xiao, J.; et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 2021, 6, 723–732. [Google Scholar]
- Zhang, Z.; Luo, H.; Liu, Z.; Wang, S.; Zhou, X.; Liu, Z. A chemical lithiation induced Li4.4Sn lithiophilic layer for anode-free lithium metal batteries. J. Mater. Chem. A 2022, 10, 9670–9679. [Google Scholar]
- Zhao, Q.; Deng, Y.; Utomo, N.W.; Zheng, J.; Biswal, P.; Yin, J.; Archer, L.A. On the crystallography and reversibility of lithium electrodeposits at ultrahigh capacity. Nat. Commun. 2021, 12, 6034. [Google Scholar]
- Zhao, Y.; Chen, B.; Xia, S.; Yu, J.; Yan, J.; Ding, B. Selective nucleation and targeted deposition effect of lithium in a lithium-metal host anode. J. Mater. Chem. A 2021, 9, 5381–5389. [Google Scholar]
- Wang, S.; Yin, X.; Liu, D.; Liu, Y.; Qin, X.; Wang, W.; Zhao, R.; Zeng, X.; Li, B. Nanoscale observation of the solid electrolyte interface and lithium dendrite nucleation–growth process during the initial lithium electrodeposition. J. Mater. Chem. A 2020, 8, 18348–18357. [Google Scholar]
- Chen, X.R.; Yao, Y.X.; Yan, C.; Zhang, R.; Cheng, X.B.; Zhang, Q. A Diffusion--Reaction Competition Mechanism to Tailor Lithium Deposition for Lithium-Metal Batteries. Angew. Chem. Int. Ed. Engl. 2020, 59, 7743–7747. [Google Scholar]
- Shen, X.; Zhang, R.; Wang, S.; Chen, X.; Zhao, C.; Kuzmina, E.; Karaseva, E.; Kolosnitsyn, V.; Zhang, Q. The dynamic evolution of aggregated lithium dendrites in lithium metal batteries. Chin. J. Chem. Eng. 2021, 37, 137–143. [Google Scholar]
- Chen, X.-R.; Yan, C.; Ding, J.-F.; Peng, H.-J.; Zhang, Q. New insights into “dead lithium” during stripping in lithium metal batteries. J. Energy Chem. 2021, 62, 289–294. [Google Scholar]
- Shen, C. Atomic Force Microscopy for Energy Research; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2022. [Google Scholar]
- Guo, H.J.; Sun, Y.; Zhao, Y.; Liu, G.X.; Song, Y.X.; Wan, J.; Jiang, K.C.; Guo, Y.G.; Sun, X.; Wen, R. Surface Degradation of Single-crystalline Ni-rich Cathode and Regulation Mechanism by Atomic Layer Deposition in Solid-State Lithium Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202211626. [Google Scholar] [PubMed]
- Liu, G.X.; Tian, J.X.; Wan, J.; Li, Y.; Shen, Z.Z.; Chen, W.P.; Zhao, Y.; Wang, F.; Liu, B.; Xin, S.; et al. Revealing the High Salt Concentration Manipulated Evolution Mechanism on the Lithium Anode in Quasi-Solid-State Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212744. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Zhang, Y.Z.; Zhou, C.; Wen, R.; Wan, L.J. Revealing the Correlations between Morphological Evolution and Surface Reactivity of Catalytic Cathodes in Lithium-Oxygen Batteries. J. Am. Chem. Soc. 2021, 143, 21604–21612. [Google Scholar]
- Shen, C.; Wang, S.; Jin, Y.; Han, W.Q. In Situ AFM Imaging of Solid Electrolyte Interfaces on HOPG with Ethylene Carbonate and Fluoroethylene Carbonate-Based Electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 25441–25447. [Google Scholar] [CrossRef]
- Cao, W.; Lu, J.; Zhou, K.; Sun, G.; Zheng, J.; Geng, Z.; Li, H. Organic-inorganic composite SEI for a stable Li metal anode by in-situ polymerization. Nano Energy 2022, 95, 106983. [Google Scholar]
- Liu, T.; Lin, L.; Bi, X.; Tian, L.; Yang, K.; Liu, J.; Li, M.; Chen, Z.; Lu, J.; Amine, K.; et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat. Nanotechnol. 2018, 14, 50–56. [Google Scholar] [PubMed]
- Kitta, M.; Sano, H. Real-Time Observation of Li Deposition on a Li Electrode with Operand Atomic Force Microscopy and Surface Mechanical Imaging. Langmuir 2017, 33, 1861–1866. [Google Scholar]
- Shen, C.; Hu, G.; Cheong, L.Z.; Huang, S.; Zhang, J.G.; Wang, D. Direct Observation of the Growth of Lithium Dendrites on Graphite Anodes by Operando EC-AFM. Small Methods 2017, 2, 1700298. [Google Scholar]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar]
- Wang, W.W.; Gu, Y.; Yan, H.; Li, K.X.; Chen, Z.B.; Wu, Q.H.; Kranz, C.; Yan, J.W.; Mao, B.W. Formation sequence of solid electrolyte interphases and impacts on lithium deposition and dissolution on copper: An in situ atomic force microscopic study. Faraday Discuss 2022, 233, 190–205. [Google Scholar] [CrossRef] [PubMed]



| Electrolytes | Marks |
|---|---|
| 1 M LiTFSI DOL:DME (1:1) | LS001 |
| 1 M LiPF6 EC:DMC (1:1) | LB001 |
| 1 M LiTFSI DOL:DME (1:1) + 2% wt. LiNO3 | LS009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Yang, J.; Wang, W.; Liu, Z.; Shen, C. Electrochemical Atomic Force Microscopy Study on the Dynamic Evolution of Lithium Deposition. Materials 2023, 16, 2278. https://doi.org/10.3390/ma16062278
Shi X, Yang J, Wang W, Liu Z, Shen C. Electrochemical Atomic Force Microscopy Study on the Dynamic Evolution of Lithium Deposition. Materials. 2023; 16(6):2278. https://doi.org/10.3390/ma16062278
Chicago/Turabian StyleShi, Xixiu, Jingru Yang, Wenyang Wang, Zhaoping Liu, and Cai Shen. 2023. "Electrochemical Atomic Force Microscopy Study on the Dynamic Evolution of Lithium Deposition" Materials 16, no. 6: 2278. https://doi.org/10.3390/ma16062278
APA StyleShi, X., Yang, J., Wang, W., Liu, Z., & Shen, C. (2023). Electrochemical Atomic Force Microscopy Study on the Dynamic Evolution of Lithium Deposition. Materials, 16(6), 2278. https://doi.org/10.3390/ma16062278







