Preparation of In Situ Growth Multiscale β-Sialon Grain-Reinforced Al2O3-Based Composite Ceramic Tool Materials
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Preparation Procedures
2.2. Characterization
3. Results and Discussion
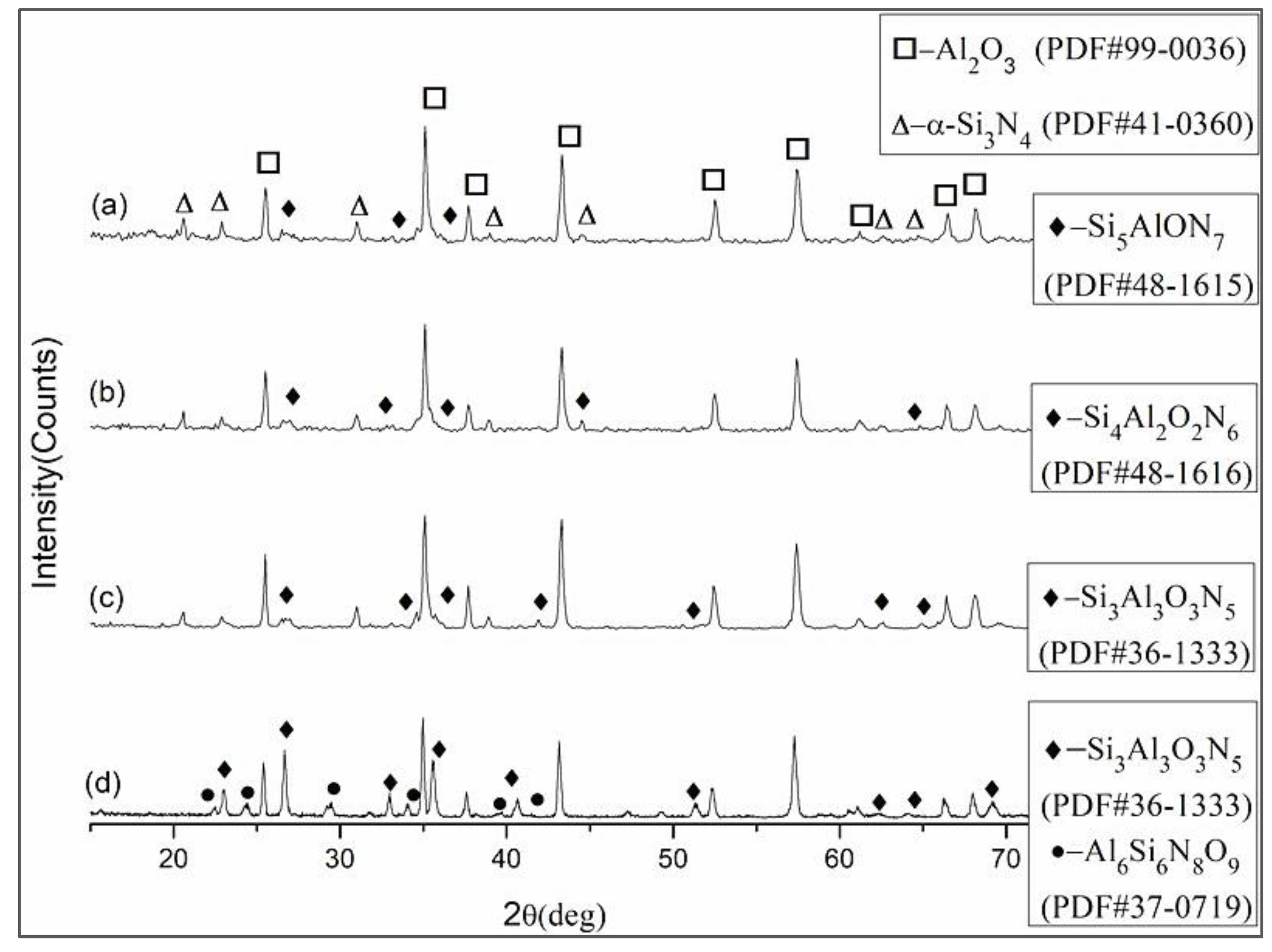
3.1. Phase Composition, Mechanical Properties and Microstructure
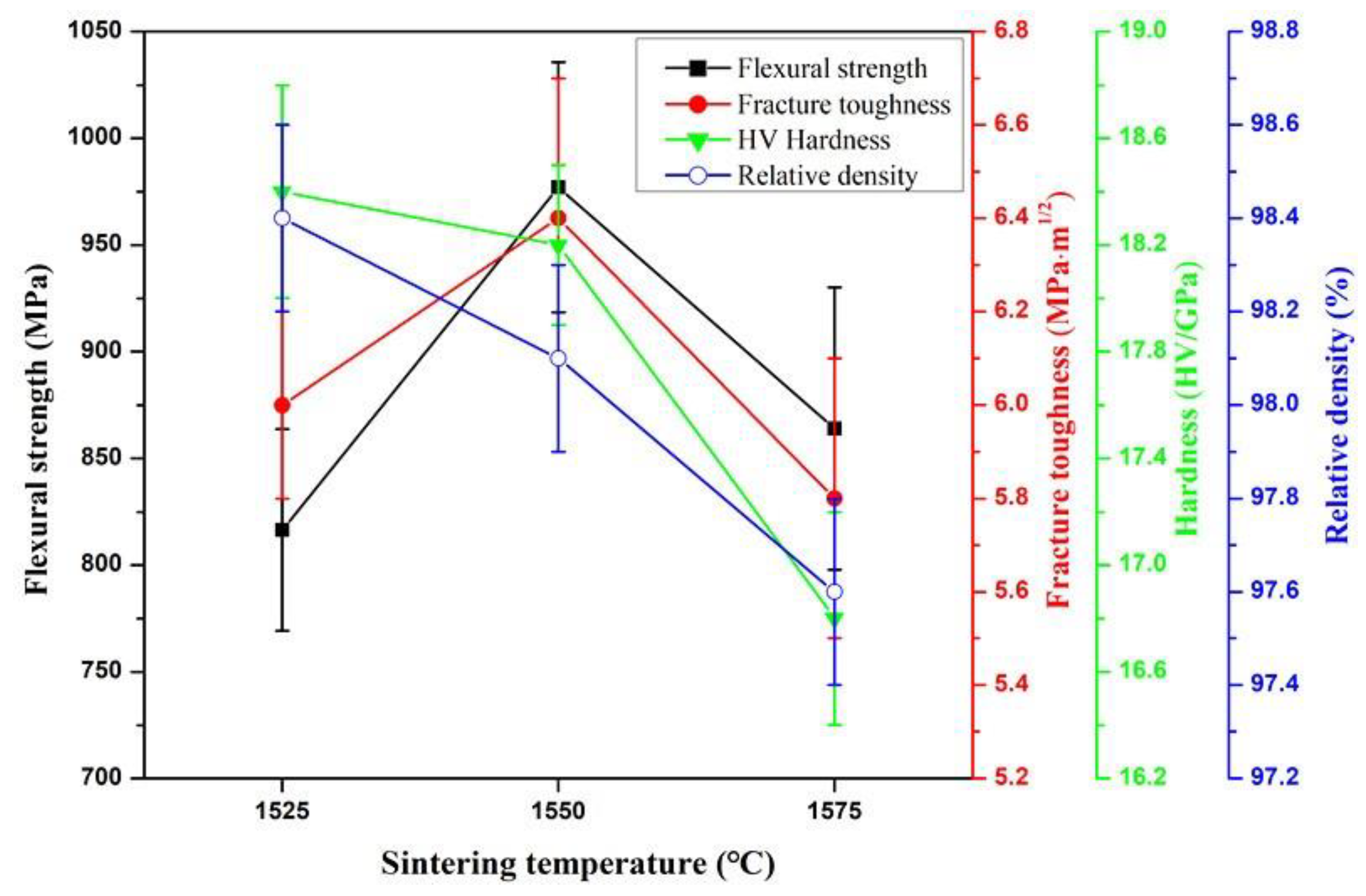
3.1.1. Effect of Sintering Temperature
3.1.2. Effect of Sintering Time
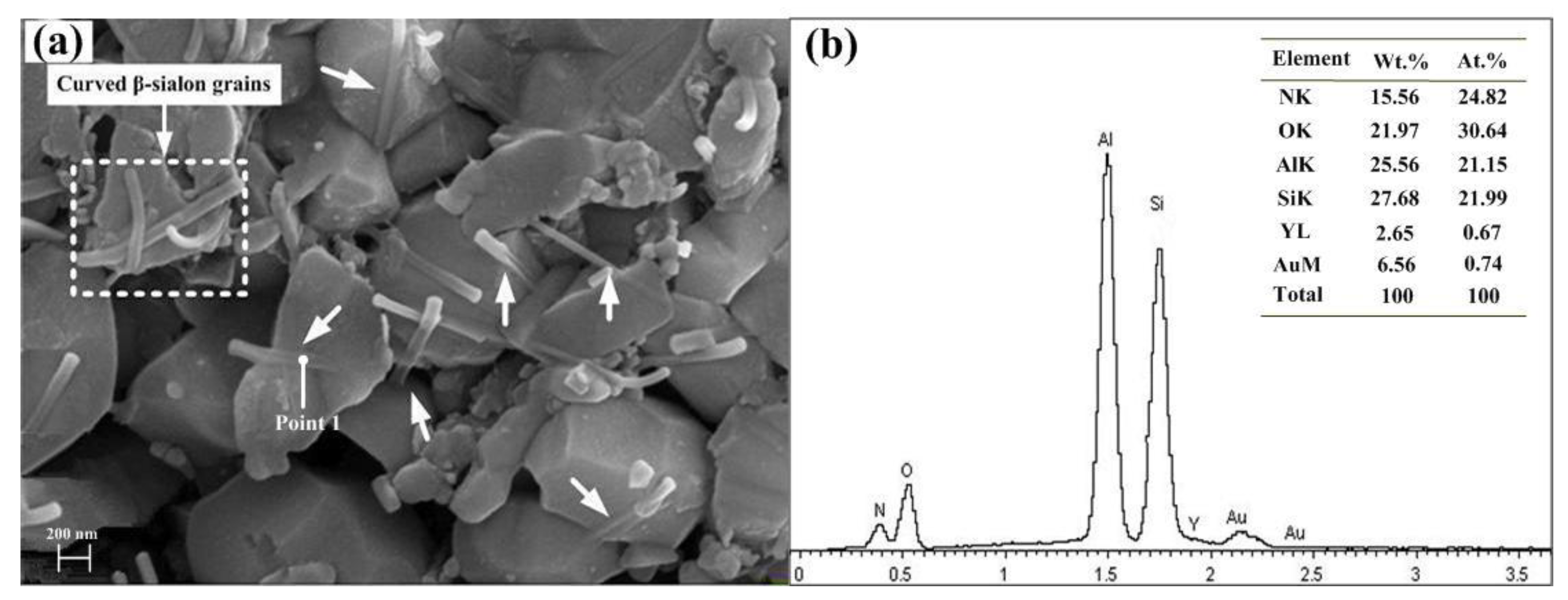
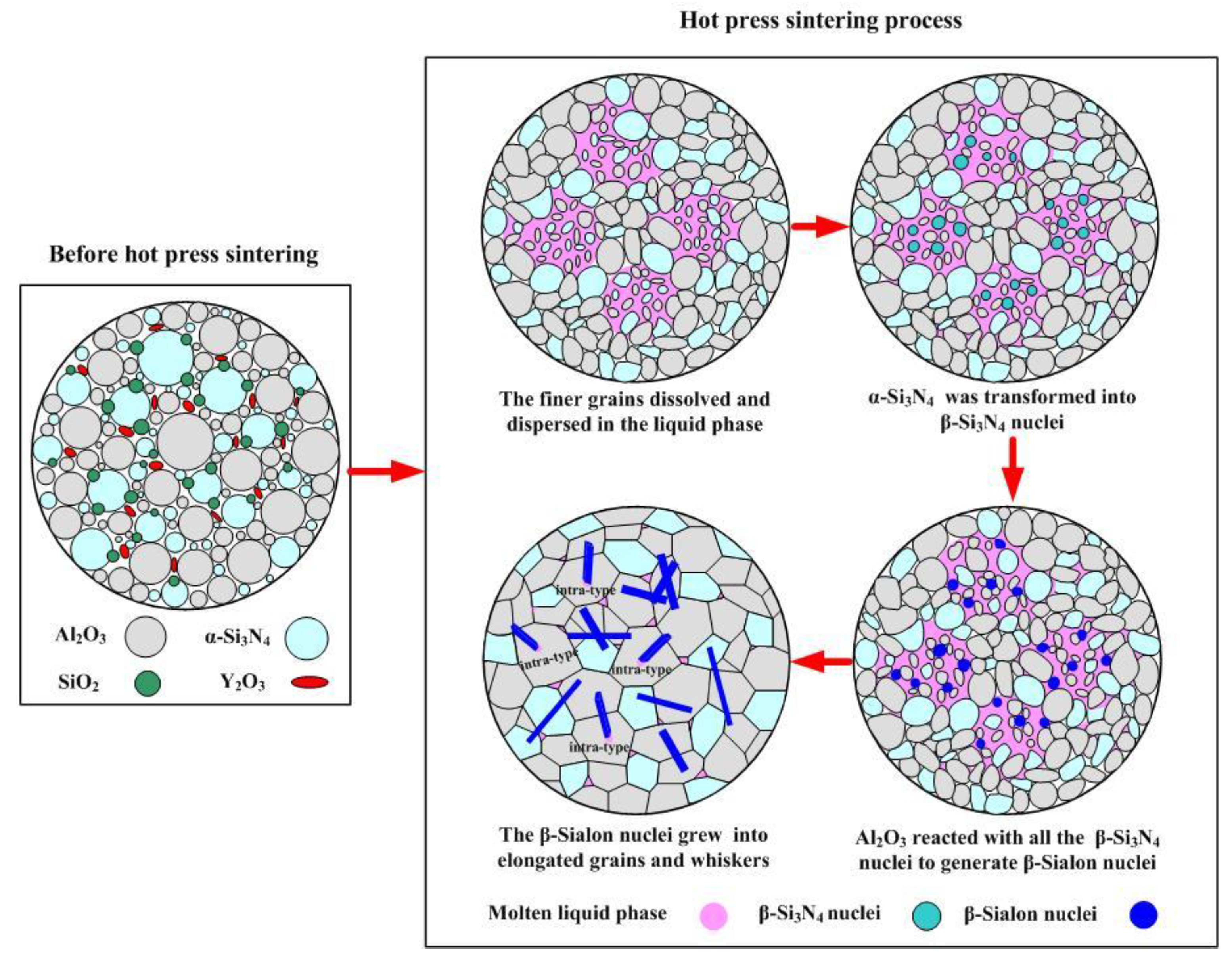
3.2. Solid Solution Reaction Mechanism of β-Sialon Formation
3.3. Strengthening and Toughening Mechanisms
4. Conclusions
- (1)
- A kind of multiscale β-sialon grain-reinforced Al2O3 matrix composite ceramic tool material was named ASN. In ASN, β-sialon (molecular formula: Si4Al2O2N6) was in situ synthesized by hot pressing and a solid solution reaction process. The solid solution reaction mechanism of β-sialon in the Al2O3 matrix was a double mechanism of unequivalence (or hetero-valence) and interstitial filling.
- (2)
- By optimizing the sintering temperature and the holding time, the optimal comprehensive mechanical properties and higher relative density of the ASN tool material were obtained under a pressure of 32 MPa at 1550 °C for 20min. The flexural strength, fracture toughness, Vickers hardness and relative density were 997 ± 59 MPa, 6.4 ± 0.3 MPa·m1/2, 18.2 ± 0.4 GPa and 98.1 ± 0.2%, respectively.
- (3)
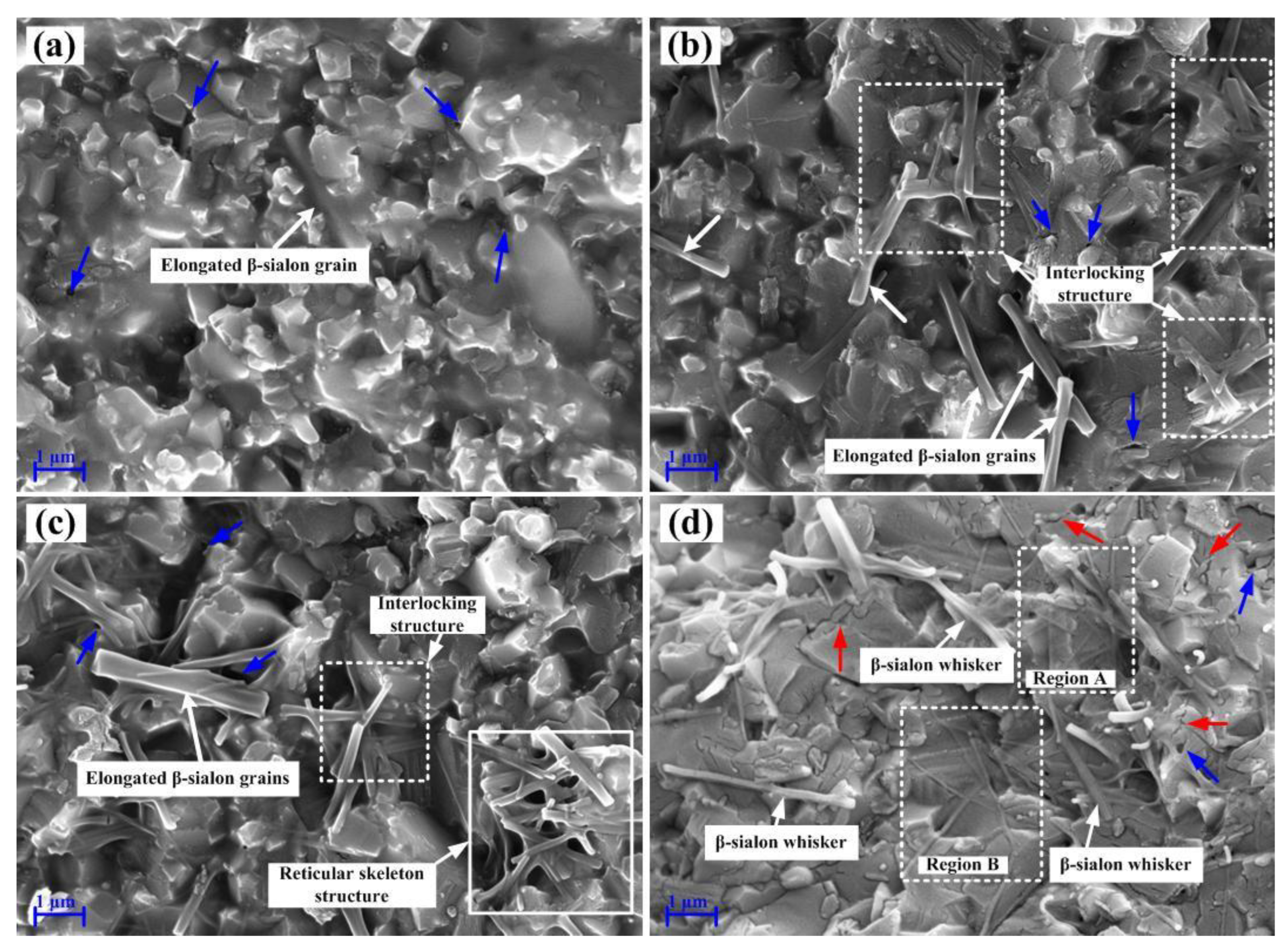
- The multiscale β-sialon grains mainly consisted of elongated β-sialon grains with a diameter of 0.3–0.4 μm and an aspect ratio of 6–9, elongated β-sialon grains with a diameter of 70 nm and an aspect ratio of 10, β-sialon whiskers with a diameter of 0.2 μm and an aspect ratio of 12–15, and intragranular β-sialon whiskers with a diameter of 70 nm.
- (4)
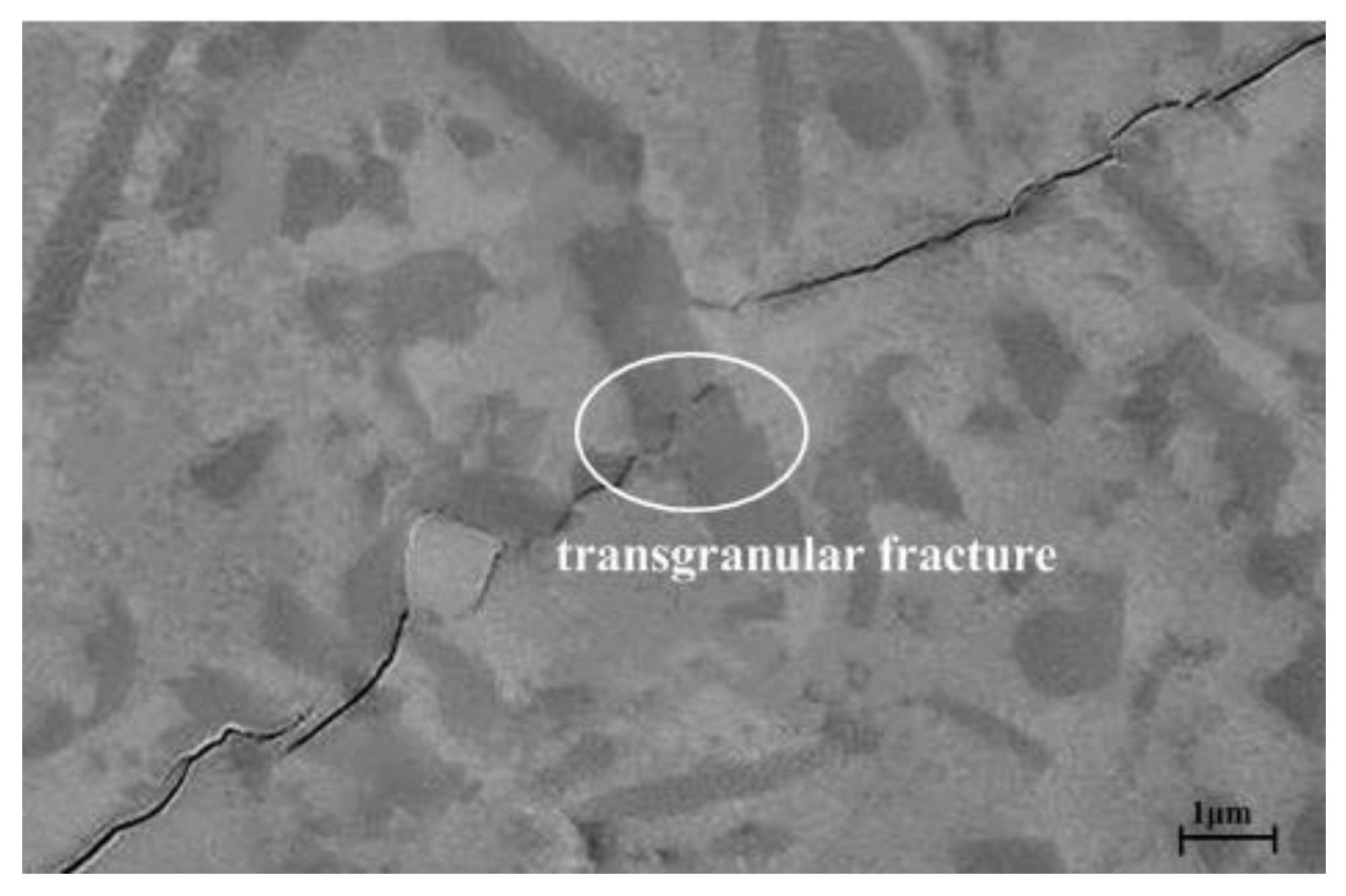
- Several strengthening and toughening mechanisms acted simultaneously in the composite ASN, such as mixed structure mode (intergranular and transgranular), elongated grain pullout, interface bonding, crack reflection, pinning and bridging.
- (5)
- The nucleation mechanism of β-sialon and cutting experiments will be further investigated. The ASN ceramic cutting tool prepared in this study expanded the varieties of ceramic cutting tools and their application range and proportion in machining.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Chen, Z.; Tian, C.; Wu, J.; Xiao, G.; Guo, N.; Yi, M.; Zhang, J.; Xu, C. Addition of Nano CaF2@SiO2 and SiC Whiskers in Ceramic Tools for Wear Reduction and Improved Machinability. Materials 2022, 15, 5430. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wang, D.; Zhang, J. Wear Mechanisms and Notch Formation of Whisker-Reinforced Alumina and Sialon Ceramic Tools during High-Speed Turning of Inconel 718. Materials 2022, 15, 3860. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Wang, Z.T.; Yu, H.; Ning, Y.Q. Microstructural origin and control mechanism of the mixed grain structure in Ni-based superalloys. J. Alloys Compd. 2022, 900, 163515. [Google Scholar] [CrossRef]
- Chen, Y.H.; Mao, Y.Q.; Lu, W.W.; He, P. Investigation of welding crack in micro laser welded NiTiNb shape memory alloy and Ti6Al4V alloy dissimilar metals joints. Opt. Laser Technol. 2017, 91, 197–202. [Google Scholar] [CrossRef]
- Yin, Z.B.; Huang, C.Z.; Yuan, J.T.; Zou, B.; Liu, H.L.; Zhu, H.T. Cutting performance and life prediction of of an Al2O3/TiC micro–nano-composite ceramic tool when machining austenitic stainless steel. Ceram. Int. 2015, 41, 7059–7065. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, H.L.; Huang, C.Z.; Wang, J.; Cheng, B.L.; Zhan, Q.P. Evolution mechanisms of high temperature mechanical properties and microstructures of Al2O3/SiCw/TiCn nanocomposite materials. J. Alloys Compd. 2018, 737, 46–52. [Google Scholar] [CrossRef]
- Lian, X.; Hua, X.; Wang, X.; Deng, L. In Situ Growth Behavior of SiC Whiskers with High Aspect Ratio in the Synthesis of ZrB2-SiC Composite Powders. Materials 2020, 13, 3502. [Google Scholar] [CrossRef]
- Xu, L.; Huang, C.Z.; Liu, H.L.; Zou, B.; Zhu, H.T.; Zhao, G.L.; Wang, J. Study on in-situ synthesis of ZrB2 whiskers in ZrB2–ZrC matrix powder for ceramic cutting tools. Int. J. Refract. Met. Hard Mater. 2013, 37, 98–105. [Google Scholar] [CrossRef]
- Xu, L.; Huang, C.Z.; Liu, H.L.; Zou, B.; Zhu, H.T.; Zhao, G.L.; Wang, J. In situ synthesis of ZrB2–ZrC x ceramic tool materials toughened by elongated ZrB2. Mater. Des. 2013, 49, 226–233. [Google Scholar] [CrossRef]
- Zhao, G.L.; Huang, C.Z.; Liu, H.L.; Zou, B.; Zhu, H.T.; Wang, J. Preparation of in-situ growth TaC whiskers toughening Al2O3 ceramic matrix composite. Int. J. Refract. Met. Hard Mater. 2013, 36, 122–125. [Google Scholar] [CrossRef]
- Bai, X.L.; Huang, C.Z.; Wang, J.; Zou, B.; Liu, H.L. Sintering mechanisms of Al2O3-based composite ceramic tools having 25% Si3N4 additions. Int. J. Refract. Met. Hard Mater. 2018, 73, 132–138. [Google Scholar] [CrossRef]
- Li, J.M.; Li, X.Q.; Qiu, H.; Cao, T.; Qu, S.G. Fabrication of in situ elongated β-Sialon grains bonded to tungsten carbide via two-step spark plasma sintering. Ceram. Int. 2021, 47, 27324–27333. [Google Scholar] [CrossRef]
- Lima Filho, V.X.; Davim, J.P.; Cairo, C.A.; Ferreira, J.M.F. Preparation and characterization of SiAlON matrix composites reinforced with combustion synthesis rod-like SiAlON particles. Int. J. Refract. Met. Hard Mater. 2009, 27, 647–652. [Google Scholar] [CrossRef]
- Oyama, Y.; Kamigiato, O. Solid solubility of some oxides in Si3N4. Jpn. J. Appl. Phys. 1971, 10, 1637–1642. [Google Scholar] [CrossRef]
- Jack, K.H.; Wilson, W.I. Ceramics based on the Si-Al-O-N and relates systems. Nat. Phys. Sci. 1972, 238, 28–29. [Google Scholar] [CrossRef]
- Loong, C.K.; Richardson, J.W.; Sukuzi, S.; Ozawa, M. Crystal Phase and Phonon Densities of States of β′-SiAION Ceramics, Si6−zAlzOzN8−z (0 ≤ z ≤ 4). J. Am. Ceram. Soc. 1996, 79, 3250–3256. [Google Scholar] [CrossRef]
- Jack, K.H. Review: Sialons and Related Nitrogen ceramics. J. Mater. Sci. 1976, 11, 1135–1158. [Google Scholar] [CrossRef]
- Ekström, T.; Nygren, M. SiAlON Ceramics. J. Am. Ceram. Soc. 1992, 75, 259–276. [Google Scholar] [CrossRef]
- Riley, F.L. Silicon Nitride and Related Materials. J. Am. Ceram. Soc. 2000, 83, 245–265. [Google Scholar] [CrossRef]
- Hwang, S.L.; Chen, I.-W. Reaction Hot-pressing of α’-SiAlON and β’-SiAlON ceramics. J. Am. Ceram. Soc. 1994, 77, 165–171. [Google Scholar] [CrossRef]
- Ekström, T.; Kall, P.O.; Nygren, M.; Olsson, P.O. Dense Single-phase Beta-SiAlON ceramics by Glass-Encapsulated Hot Isostatic Pressing. J. Mater. Sci. 1989, 24, 1853–1861. [Google Scholar] [CrossRef]
- Lv, X.; Li, X.; Huang, J.; Ge, C.; Yu, Q. Effect of Ultra-High Pressure Sintering and Spark Plasma Sintering and Subsequent Heat Treatment on the Properties of Si3N4 Ceramics. Materials 2022, 15, 7309. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.S.; Khan, M.; Ahmed, B.A.; Ghanim, A.A.; Patel, F.; Ehsan, M.A.; Ali, S.; Laoui, T.; Ali, S. Synthesis and characterization of alkaline earth and rare earth doped sialon Ceramics by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2021, 97, 105500. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, D.X.; Zuo, K.H.; Xia, Y.F.; Yin, J.W.; Liang, H.Q.; Zeng, Y.P. The synthesis of single-phase β-Sialon porous ceramics using self-propagating high-temperature processing. Ceram. Int. 2022, 48, 4371–4375. [Google Scholar] [CrossRef]
- Wang, P.L.; Zhang, J.H.; He, J.B.; Yan, D.S. Formation behavior and microstructure of multi-cation containing neodymium and ytterbium or yttrium. J. Eur. Ceram. Soc. 2000, 20, 1987–1995. [Google Scholar] [CrossRef]
- Xiong, Q.M.; Chen, Z.; Huang, J.T.; Zhang, M.; Song, H.; Hou, X.F.; Li, X.B.; Feng, Z.J. Preparation, structure and mechanical properties of Sialon ceramics by transition metal-catalyzed nitriding reaction. Rare Met. 2020, 39, 589–596. [Google Scholar] [CrossRef]
- Ukyo, Y.; Suda, A. Effect of a Small Amount of SiO2 on the Reaction during Sintering in the Si3N4-Y2O3-AlN System. J. Ceram. Soc. Jpn. 1996, 104, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Izhevskiy, V.A.; Genova, L.A.; Bressiani, J.C.; Aldinge, F. Progress in SiAlON ceramics. J. Eur. Ceram. Soc. 2000, 20, 2275–2295. [Google Scholar] [CrossRef]
- Ghosh, G.; Vaynman, S.; Fine, M.E. Microstructure of a sialon composite prepared by hot pressing and reactive sintering of β-Si3N4 coated with amorphous Al2O3. Ceram. Int. 1999, 25, 649–659. [Google Scholar] [CrossRef]
- Song, J.H.; Hao, C.Y.; Yang, H.T. Reinforcement mechanisms of Si3N4/Al2O3 nano composite cramics fabricated by the spark plasma sintering (Eng). J. Chin. Ceram. Soc. 2007, 35, 930–933. [Google Scholar] [CrossRef]
- Lange, F.F. Fracture toughness of Si3N4 as a function of the initial α-phase content. J. Am. Ceram. Soc. 1979, 62, 428–430. [Google Scholar] [CrossRef]
- Lee, D.-D.; Kang, S.-J.L.; Yoon, D.N. Mechanism of Grain Growth and α-β’ Transformation During Liquid-Phase Sintering of β’-Sialon. J. Am. Ceram. Soc. 1988, 71, 803–806. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture Toughness Determinations by Indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Q.; Yu, Z.; Wang, H.; Zhang, T. Influence of Y2O3 addition on the microstructure of TiC reinforced Ti-based composite coating prepared by laser cladding. Mater. Charact. 2022, 189, 111962. [Google Scholar] [CrossRef]
- Tanaka, I.; Nasu, S.; Adachi, H.; Miyamoto, Y.; Niihara, K. Electronic structure behind the mechanical properties of β-Sialon. Acta Metall. Mater. 1992, 40, 1995–2001. [Google Scholar] [CrossRef]
- Einarsrud, M.A.; Mitomo, M. Mechanism of Grain Growth of β-Sialon. J. Am. Ceram. Soc. 1993, 76, 1624–1626. [Google Scholar] [CrossRef]
- Shen, Z.J.; Zhao, Z.; Peng, H.; Nygren, M. Formation of tough interlocking microstructures in silicon nitride ceramics by dynamic ripening. Nature 2002, 417, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, M.; Hirao, K.; Toriyama, M.; Kanzaki, S. Modeling and Simulation of Grain Growth in Si3N4-I. Anisotropic Ostwald Ripening. Acta Mater. 1998, 46, 6541–6550. [Google Scholar] [CrossRef]
- Hwang, S.L.; Chen, I.W. Nucleation and growth of β-Sialon. J. Am. Ceram. Soc. 1994, 77, 1719–1728. [Google Scholar] [CrossRef]
- Tiegs, T.N.; Montgomery, F.C.; Schroeder, J.L.; Barker, D.L.; Menchhofer, P.A. Effect of powder characteristics on the α- to -β Si3N4 transformation kinetics. J. Am. Ceram. Soc. 1997, 18, 437–447. [Google Scholar] [CrossRef]
- Murakami, Y.; Yamamoto, H. Phase relations of Al2−xYxO3-SiO2-Si3N4 system (x = 0.75 and 1.06) and properties of oxynitride glasses. J. Ceram. Soc. Jpn. 1992, 100, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Becher, P.F. Microstructure design of toughened ceramics. J. Am. Ceram. Soc. 1991, 74, 255–269. [Google Scholar] [CrossRef]



| Al2O3-Based Ceramic Tool | Main Ingredient | Flexural Strength (MPa) | Fracture Toughness (MPa·m1/2) | Vickers Hardness (GPa) | Relative Density (%) |
|---|---|---|---|---|---|
| ASN | Al2O3 + β-Sialon | 997 | 6.4 | 18 | 98.1 |
| LT55 | Al2O3 + TiC | 850 | 5.1 | 21 | - |
| ATTC | Al2O3μ + TiCμ + TiC n + Co | 916 | 8.3 | 18 | - |
| AWTC | Al2O3μ + (W,Ti)Cμ + TiCn + Co | 882 | 7.2 | 19 | - |
| WG-300 | Al2O3 + SiCW | 690 | 8.8 | 20 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Xue, Y.; Bai, X.; Shen, X.; He, J.; Zhang, Y.; Li, A. Preparation of In Situ Growth Multiscale β-Sialon Grain-Reinforced Al2O3-Based Composite Ceramic Tool Materials. Materials 2023, 16, 2333. https://doi.org/10.3390/ma16062333
Zhu J, Xue Y, Bai X, Shen X, He J, Zhang Y, Li A. Preparation of In Situ Growth Multiscale β-Sialon Grain-Reinforced Al2O3-Based Composite Ceramic Tool Materials. Materials. 2023; 16(6):2333. https://doi.org/10.3390/ma16062333
Chicago/Turabian StyleZhu, Jian, Yunna Xue, Xiaolan Bai, Xuehui Shen, Jianqun He, Yu Zhang, and Anhai Li. 2023. "Preparation of In Situ Growth Multiscale β-Sialon Grain-Reinforced Al2O3-Based Composite Ceramic Tool Materials" Materials 16, no. 6: 2333. https://doi.org/10.3390/ma16062333
APA StyleZhu, J., Xue, Y., Bai, X., Shen, X., He, J., Zhang, Y., & Li, A. (2023). Preparation of In Situ Growth Multiscale β-Sialon Grain-Reinforced Al2O3-Based Composite Ceramic Tool Materials. Materials, 16(6), 2333. https://doi.org/10.3390/ma16062333







