Biodegradable Biopolymeric Nanoparticles for Biomedical Applications-Challenges and Future Outlook
Abstract
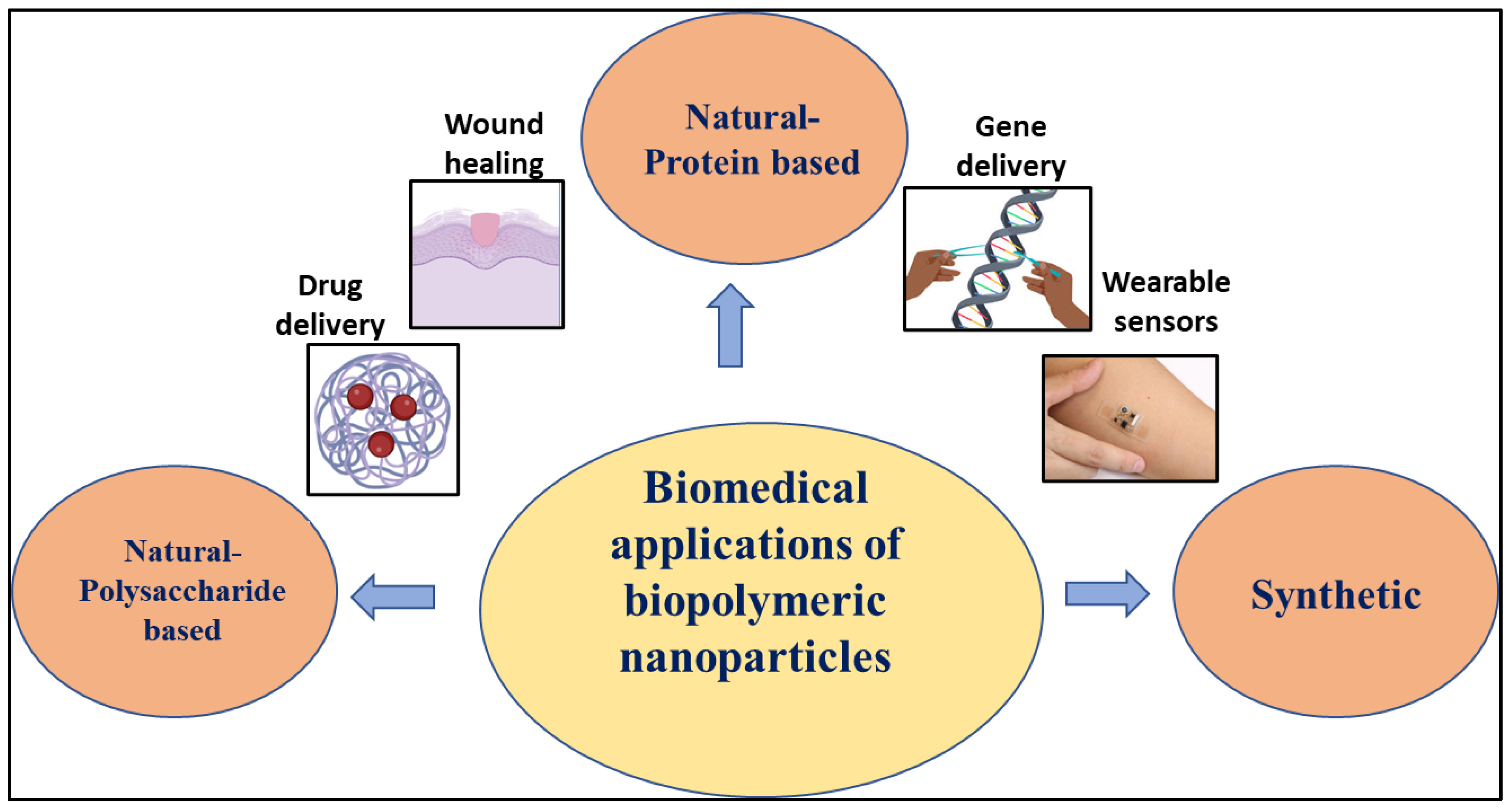
:1. Introduction
2. Protein Based Biopolymeric Nanoparticles
2.1. Albumin
2.2. Gelatin
2.3. Silk Fibroin
2.4. Collagen
2.5. Elastin
3. Polysaccharide Based Polymeric Nanoparticles
3.1. Chitosan
3.2. Alginate
3.3. Starch
3.4. Dextran
4. Synthetic Biopolymeric Nanoparticles
4.1. Polycaprolactone Nanoparticles
4.2. Polylactic Acid Nanoparticles
4.3. Poly Vinyl Alcohol Nanoparticles
5. Fabrication of Biopolymeric Nanoparticles
5.1. Emulsification
5.2. Desolvation
5.3. Coacervation
5.4. Spray Deposition
5.5. Microfluidics
6. Challenges and Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, G.A. Introduction to nanotechnology and its applications to medicine. Surg. Neurol. 2004, 61, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Kundu, J.; Kundu, S.C. Biopolymeric nanoparticles. Sci. Technol. Adv. Mater. 2010, 11, 014104. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Morrin, A.; Killard, A.; Smyth, M.R. Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Chen, J.; Chen, X. Nanoparticles for Gene Delivery. Small 2013, 9, 2034–2044. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Wilhelm, C.; Clément, O.; Gazeau, F. Cell labeling with magnetic nanoparticles: Opportunity for magnetic cell imaging and cell manipulation. J. Nanobiotechnology 2013, 11, S7. [Google Scholar] [CrossRef] [Green Version]
- Bhirde, A.; Xie, J.; Swierczewska, M.; Chen, X. Nanoparticles for cell labeling. Nanoscale 2011, 3, 142–153. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [Green Version]
- Nitta, S.K.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric Nanoparticles–Multifunctional Materials of the Future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21. [Google Scholar] [CrossRef]
- Verma, M.L.; Dhanya, B.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Mizrahi, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Yoha, K.S.; Priyadarshini, S.R.; Moses, J.A.; Anandharamakrishnan, C. Surface Modification of Bio-polymeric Nanoparticles and Its Applications. In Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2020; pp. 261–282. [Google Scholar] [CrossRef]
- Meng, R.; Zhu, H.; Wang, Z.; Hao, S.; Wang, B. Preparation of Drug-Loaded Albumin Nanoparticles and Its Application in Cancer Therapy. J. Nanomater. 2022, 2022, 3052175. [Google Scholar] [CrossRef]
- Pulimood, T.B.; Park, G.R. Debate: Albumin administration should be avoided in the critically ill. Crit. Care 2000, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Liu, J.; Ge, X.; Liu, L.; Xu, W.; Shao, R. Challenges and opportunities of silk protein hydrogels in biomedical applications. Mater. Adv. 2022, 3, 2291–2308. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the Most Important Methods of Improving the Processing Properties of Starch toward Non-Food Applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef]
- Das, M.; Shukla, F.; Thakore, S. Carbohydrate-derived functionalized nanomaterials for drug delivery and environment remediation. In Handbook of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 339–364. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Lu, A.; Chen, X.; Yang, Z. Natural Ingredient-Based Polymeric Nanoparticles for Cancer Treatment. Molecules 2020, 25, 3620. [Google Scholar] [CrossRef]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein−Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef]
- Stein, N.C.; Mulac, D.; Fabian, J.; Herrmann, F.C.; Langer, K. Nanoparticle albumin-bound mTHPC for photodynamic therapy: Preparation and comprehensive characterization of a promising drug delivery system. Int. J. Pharm. 2020, 582, 119347. [Google Scholar] [CrossRef] [PubMed]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Du, Y.; Lei, L.; Xia, X.; Wang, X.; Tong, F.; Li, Y.; Gao, H. Co-delivery of ibrutinib and hydroxychloroquine by albumin nanoparticles for enhanced chemotherapy of glioma. Int. J. Pharm. 2023, 630, 122436. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, W.; Chen, D.; Yang, Y.; Sun, T.; Chen, H.; Zhang, J. Electrospun nanofibers containing chitosan-stabilized bovine serum albumin nanoparticles for bone regeneration. Colloids Surf. B Biointerfaces 2022, 217, 112680. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, K.; Lakshmikuttyamma, A.; Thangavel, C.; Shoyele, S.A. CD44-targeted, indocyanine green-paclitaxel-loaded human serum albumin nanoparticles for potential image-guided drug delivery. Colloids Surf. B Biointerfaces 2022, 209, 112162. [Google Scholar] [CrossRef]
- Khella, K.F.; El Maksoud, A.I.A.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic Acid Encapsulated in Albumin Nanoparticles Induces Apoptosis in Breast and Colorectal Cancer Cells. Molecules 2022, 27, 4102. [Google Scholar] [CrossRef] [PubMed]
- El Tokhy, S.S.; Elgizawy, S.A.; Osman, M.A.; Goda, A.E.; Unsworth, L.D. Tailoring dexamethasone loaded albumin nanoparticles: A full factorial design with enhanced anti-inflammatory activity In vivo. J. Drug Deliv. Sci. Technol. 2022, 72, 103411. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 833–841. [Google Scholar] [CrossRef]
- Yang, X.-J.; Wang, F.-Q.; Lu, C.-B.; Zou, J.-W.; Hu, J.-B.; Yang, Z.; Sang, H.-X.; Zhang, Y. Modulation of bone formation and resorption using a novel zoledronic acid loaded gelatin nanoparticles integrated porous titanium scaffold: An in vitro and in vivo study. Biomed. Mater. 2020, 15, 055013. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, N.; Trouillet, V.; Clasen, A.; Jung, G.; Hollemeyer, K.; Schneider, M. NIR-Emitting Gold Nanoclusters–Modified Gelatin Nanoparticles as a Bioimaging Agent in Tissue. Adv. Healthc. Mater. 2019, 8, 1900993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.; Ansari, M.; Mishra, R.K.; Kumar, A.; Vyawahare, A.; Verma, R.K.; Raza, S.S.; Khan, R. Enteric-coated gelatin nanoparticles mediated oral delivery of 5-aminosalicylic acid alleviates severity of DSS-induced ulcerative colitis. Mater. Sci. Eng. C 2021, 119, 111582. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, A.; Li, Y.; Hu, J.; Liu, X.; Li, J.; Zhang, Y.; Li, G.; Zheng, Z. Fabrication of silk fibroin nanoparticles for controlled drug delivery. J. Nanoparticle Res. 2012, 14, 736. [Google Scholar] [CrossRef]
- Lujerdean, C.; Baci, G.-M.; Cucu, A.-A.; Dezmirean, D.S. The Contribution of Silk Fibroin in Biomedical Engineering. Insects 2022, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef]
- Mathur, A.B.; Gupta, V. Silk fibroin-derived nanoparticles for biomedical applications. Nanomedicine 2010, 5, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Yang, M.; Zhu, L. Preparation and biomedical applications of silk fibroin-nanoparticles composites with enhanced properties—A review. Mater. Sci. Eng. C 2019, 95, 302–311. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Wang, X.; Li, B.; Guo, Y.; Dong, K. Development of silk fibroin-sodium alginate scaffold loaded silk fibroin nanoparticles for hemostasis and cell adhesion. Int. J. Biol. Macromol. 2022, 211, 514–523. [Google Scholar] [CrossRef]
- Fatemeh, M.; Hamidreza, M.; Ali, S.M.; Shahrokh, S.; Mehdi, F. Fabri-cation of Silk Scaffold Containing Simvastatin-Loaded Silk Fibroin Nanoparticles for Regenerating Bone Defects. Iran. Biomed. J. 2022, 26, 116–123. [Google Scholar]
- Rahmani, H.; Fattahi, A.; Sadrjavadi, K.; Khaledian, S.; Shokoohinia, Y. Preparation and Characterization of Silk Fibroin Nanoparticles as a Potential Drug Delivery System for 5-Fluorouracil. Adv. Pharm. Bull. 2019, 9, 601–608. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, G.; Zhang, Q.; Wu, W.; Zhang, Y.; Wu, B.; Liu, Z.; Tong, X.; Xiao, B.; Cheng, L.; et al. PX478-loaded silk fibroin nanoparticles reverse multidrug resistance by inhibiting the hypoxia-inducible factor. Int. J. Biol. Macromol. 2022, 222, 2309–2317. [Google Scholar] [CrossRef]
- Nidhin, M.; Vedhanayagam, M.; Sangeetha, S.; Kiran, M.S.; Nazeer, S.S.; Jayasree, R.S.; Sreeram, K.J.; Nair, B.U. Fluorescent nanonetworks: A novel bioalley for collagen scaffolds and Tissue Engineering. Sci. Rep. 2014, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.Z.; Czernuszka, J.T. Development of biodegradable scaffolds for tissue engineering: A perspective on emerging technology. Mater. Sci. Technol. 2007, 23, 379–391. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Grigore, M.E. Hydrogels for Cardiac Tissue Repair and Regeneration. J. Cardiovasc. Med. Cardiol. 2017, 4, 049–057. [Google Scholar] [CrossRef]
- Lo, S.; Fauzi, M. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef]
- Arun, A.; Malrautu, P.; Laha, A.; Luo, H.; Ramakrishna, S. Collagen Nanoparticles in Drug Delivery Systems and Tissue Engineering. Appl. Sci. 2021, 11, 11369. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Udekwu, K.; Skog, M.; Pacioni, N.L.; Stamplecoskie, K.G.; González-Béjar, M.; Polisetti, N.; Wickham, A.; Richter-Dahlfors, A.; Griffith, M.; et al. The biocompatibility and antibacterial properties of collagen-stabilized, photochemically prepared silver nanoparticles. Biomaterials 2012, 33, 4947–4956. [Google Scholar] [CrossRef]
- Nicklas, M.; Schatton, W.; Heinemann, S.; Hanke, T.; Kreuter, J. Preparation and characterization of marine sponge collagen nanoparticles and employment for the transdermal delivery of 17β-estradiol-hemihydrate. Drug Dev. Ind. Pharm. 2009, 35, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Mecham, R.; Imberman, M.; Faris, B.; Franzblau, C. A High Molecular Weight Species of Soluble Elastin-Proelastin. Adv. Exp. Med. Biol. 1977, 79, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Seyoung, H.; Wook, C.D.; Nam, K.H.; Gwon, P.C. Protein-Based Nanoparticles as Drug Delivery Sys-tems. Pharmaceutics 2020, 12, 604. [Google Scholar]
- Almine, J.F.; Bax, D.V.; Mithieux, S.M.; Nivison-Smith, L.; Rnjak, J.; Waterhouse, A.; Wise, S.G.; Weiss, A.S. Elastin-based materials. Chem. Soc. Rev. 2010, 39, 3371–3379. [Google Scholar] [CrossRef]
- Wu, Y.; Mackay, J.A.; McDaniel, J.R.; Chilkoti, A.; Clark, R.L. Fabrication of Elastin-Like Polypeptide Nanoparticles for Drug Delivery by Electrospraying. Biomacromolecules 2008, 10, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, R.; Bessa, P.C.; Reis, R.L.; Rodriguez-Cabello, J.C.; Casal, M. Elastin-Based Nanoparticles for Delivery of Bone Morphogenetic Proteins. In Nanoparticles in Biology and Medicine; Humana Press: Totowa, NJ, USA, 2012; pp. 353–363. [Google Scholar] [CrossRef]
- Kim, J.D.; Jung, Y.J.; Woo, C.H.; Choi, Y.C.; Choi, J.S.; Cho, Y.W. Thermo-responsive human α-elastin self-assembled nanoparticles for protein delivery. Colloids Surf. B Biointerfaces 2017, 149, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; El-Kemary, M.A. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef]
- Liu, F.; Xue, L.; Xu, L.; Liu, J.; Xie, C.; Chen, C.; Liu, Y. Preparation and characterization of bovine serum albumin nanoparticles modified by Poly-l-lysine functionalized graphene oxide for BMP-2 delivery. Mater. Des. 2022, 215, 110479. [Google Scholar] [CrossRef]
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Mater. Sci. Eng. C 2021, 123, 112027. [Google Scholar] [CrossRef]
- Xue, Y.; Lee, J.; Kim, H.-J.; Cho, H.-J.; Zhou, X.; Liu, Y.; Tebon, P.; Hoffman, T.; Qu, M.; Ling, H.; et al. Rhodamine Conjugated Gelatin Methacryloyl Nanoparticles for Stable Cell Imaging. ACS Appl. Bio Mater. 2020, 3, 6908–6918. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmad, A.; Kumar, A.; Alam, P.; Khan, T.H.; Jayamurugan, G.; Raza, S.S.; Khan, R. Aminocellulose-grafted-polycaprolactone coated gelatin nanoparticles alleviate inflammation in rheumatoid arthritis: A combinational therapeutic approach. Carbohydr. Polym. 2021, 258, 117600. [Google Scholar] [CrossRef]
- Chen, X.; Zou, J.; Zhang, K.; Zhu, J.; Zhang, Y.; Zhu, Z.; Zheng, H.; Li, F.; Piao, J.-G. Photothermal/matrix metalloproteinase-2 dual-responsive gelatin nanoparticles for breast cancer treatment. Acta Pharm. Sin. B 2021, 11, 271–282. [Google Scholar] [CrossRef]
- Moin, A.; Wani, S.U.D.; Osmani, R.A.; Abu Lila, A.S.; Khafagy, E.-S.; Arab, H.H.; Gangadharappa, H.V.; Allam, A.N. Formulation, characterization, and cellular toxicity assessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer. Drug Deliv. 2021, 28, 1626–1636. [Google Scholar] [CrossRef]
- Vallejo, R.; Gonzalez-Valdivieso, J.; Santos, M.; Rodriguez-Rojo, S.; Arias, F. Production of elastin-like recombinamer-based nanoparticles for docetaxel encapsulation and use as smart drug-delivery systems using a supercritical anti-solvent process. J. Ind. Eng. Chem. 2021, 93, 361–374. [Google Scholar] [CrossRef]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 1–21. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dev, A.; Binulal, N.; Anitha, A.; Nair, S.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-HIV drug delivery applications. Carbohydr. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, Y.; Peng, Z.; She, F.; Kong, L. Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr. Polym. 2011, 85, 698–704. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2. Arthritis Res. Therapy. 2021, 23, 50. [Google Scholar] [CrossRef]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Farahani, M.S.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of curcumin loaded chitosan nanoparticle within poly (ε-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Almutairi, F.M. Biopolymer Nanoparticles: A Review of Prospects for Application as Carrier for Therapeutics and Diagnostics. Int. J. Pharm. Res. Allied Sci. 2019, 8, 25–35. [Google Scholar]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.N.; Wang, A.-Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedi-pine. Int. J. Biomed. Sci. 2008, 4, 221. [Google Scholar] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Zohri, M.; Arefian, E.; Javar, H.A.; Gazori, T.; Aghaee-Bakhtiari, S.H.; Taheri, M.; Fatahi, Y.; Azadi, A.; Khoshayand, M.R.; Ghahremani, M.H. Potential of chitosan/alginate nanoparticles as a non-viral vector for gene delivery: Formulation and optimization using D-optimal design. Mater. Sci. Eng. C 2021, 128, 112262. [Google Scholar] [CrossRef]
- Zhu, Z.; He, F.; Shao, H.; Shao, J.; Li, Q.; Wang, X.; Ren, H.; You, C.; Zhang, Z.; Han, C. Chitosan/Alginate Nanoparticles with Sustained Release of Esculentoside A for Burn Wound Healing. ACS Appl. Nano Mater. 2023, 6, 573–587. [Google Scholar] [CrossRef]
- Caldonazo, A.; Almeida, S.L.; Bonetti, A.F.; Lazo, R.E.L.; Mengarda, M.; Murakami, F.S. Pharmaceutical applications of starch nanoparticles: A scoping review. Int. J. Biol. Macromol. 2021, 181, 697–704. [Google Scholar] [CrossRef]
- Campelo, P.H.; Sant’Ana, A.S.; Clerici, M.T.P.S. Starch nanoparticles: Production methods, structure, and properties for food applications. Curr. Opin. Food Sci. 2020, 33, 136–140. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and application of starch nanoparticles for nanocomposites: A review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch Nanoparticles: A Review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Alp, E.; Damkaci, F.; Güven, E.; Tenniswood, M. Starch nanoparticles for delivery of the histone deacetylase inhibitor CG-1521 in breast cancer treatment. Int. J. Nanomed. 2019, 14, 1335–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, S.; Yu, L.; Elshazly, E.H.; Wang, H.; Chen, K.; Zhang, S.; Ke, L.; Gong, R. Codelivery of DOX and siRNA by folate-biotin-quaternized starch nanoparticles for promoting synergistic suppression of human lung cancer cells. Drug Deliv. 2019, 26, 499–508. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.-H. Smart drug delivery of p-Coumaric acid loaded aptamer conjugated starch nanoparticles for effective triple-negative breast cancer therapy. Int. J. Biol. Macromol. 2022, 195, 22–29. [Google Scholar] [CrossRef]
- Nallasamy, P.; Ramalingam, T.; Nooruddin, T.; Shanmuganathan, R.; Arivalagan, P.; Natarajan, S. Polyherbal drug loaded starch nanoparticles as promising drug delivery system: Antimicrobial, antibiofilm and neuroprotective studies. Process. Biochem. 2020, 92, 355–364. [Google Scholar] [CrossRef]
- Majcher, M.J.; McInnis, C.L.; Himbert, S.; Alsop, R.J.; Kinio, D.; Bleuel, M.; Rheinstadter, M.; Smeets, N.M.; Hoare, T. Photopolymerized Starchstarch Nanoparticle (SNP) network hydrogels. Carbohydr. Polym. 2020, 236, 115998. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Wasiak, I.; Kulikowska, A.; Janczewska, M.; Michalak, M.; Cymerman, I.A.; Nagalski, A.; Kallinger, P.; Szymanski, W.W.; Ciach, T. Dextran Nanoparticle Synthesis and Properties. PLoS ONE 2016, 11, e0146237. [Google Scholar] [CrossRef] [Green Version]
- Thambi, T.; Gil You, D.; Han, H.S.; Deepagan, V.G.; Jeon, S.M.; Suh, Y.D.; Choi, K.Y.; Kim, K.; Kwon, I.C.; Yi, G.-R.; et al. Bioreducible Carboxymethyl Dextran Nanoparticles for Tumor-Targeted Drug Delivery. Adv. Heal. Mater. 2014, 3, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Keliher, E.J.; Yoo, J.; Nahrendorf, M.; Lewis, J.S.; Marinelli, B.; Newton, A.; Pittet, M.J.; Weissleder, R. 89Zr-Labeled Dextran Nanoparticles Allow in Vivo Macrophage Imaging. Bioconjugate Chem. 2011, 22, 2383–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamwal, S.; Ram, B.; Ranote, S.; Dharela, R.; Chauhan, G.S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int. J. Biol. Macromol. 2019, 123, 968–978. [Google Scholar] [CrossRef]
- Butzbach, K.; Konhäuser, M.; Fach, M.; Bamberger, D.N.; Breitenbach, B.; Epe, B.; Wich, P.R. Receptor-mediated Uptake of Folic Acid-functionalized Dextran Nanoparticles for Applications in Photodynamic Therapy. Polymers 2019, 11, 896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, J.; Li, Y.; Wen, J.; Chen, Y.; Yang, J.; Zhao, L.; Xia, Y.; Du, H.; Tao, J.; Li, Y.; et al. Acitretin-Conjugated Dextran Nanoparticles Ameliorate Psoriasis-like Skin Disease at Low Dosages. Front. Bioeng. Biotechnol. 2022, 9, 1430. [Google Scholar] [CrossRef]
- Naha, P.C.; Hsu, J.C.; Kim, J.; Shah, S.; Bouché, M.; Si-Mohamed, S.; Rosario-Berrios, D.N.; Douek, P.; Hajfathalian, M.; Yasini, P.; et al. Dextran-Coated Cerium Oxide Nanoparticles: A Computed Tomography Contrast Agent for Imaging the Gastrointestinal Tract and Inflammatory Bowel Disease. ACS Nano 2020, 14, 10187–10197. [Google Scholar] [CrossRef]
- Abid, M.; Naveed, M.; Azeem, I.; Faisal, A.; Nazar, M.F.; Yameen, B. Colon specific enzyme responsive oligoester crosslinked dextran nanoparticles for controlled release of 5-fluorouracil. Int. J. Pharm. 2020, 586, 119605. [Google Scholar] [CrossRef] [PubMed]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerfaces 2013, 110, 313–320. [Google Scholar] [CrossRef]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.; Morsali, A.; Bozorgmehr, M.R.; Beyramabadi, S.A. Quantum chemical studies of chitosan nanoparticles as effective drug delivery systems for 5-fluorouracil anticancer drug. J. Mol. Liq. 2020, 302, 112495. [Google Scholar] [CrossRef]
- Yu, A.; Shi, H.; Liu, H.; Bao, Z.; Dai, M.; Lin, D.; Lin, D.; Xu, X.; Li, X.; Wang, Y. Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int. J. Pharm. 2020, 575, 118943. [Google Scholar] [CrossRef]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Zahoor, A.; Rajesh, P.; Sadhna, S.; Khuller, G.K. Alginate Nanoparticles as Antituberculosis Drug Carriers: Formulation Development, Pharmacokinetics and Therapeutic Potential. Indian J. Chest. Dis. Allied. Sci. 2005, 48, 171–176. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Ma, X.; Jian, R.; Chang, P.R.; Yu, J. Fabrication and Characterization of Citric Acid-Modified Starch Nanoparticles/Plasticized-Starch Composites. Biomacromolecules 2008, 9, 3314–3320. [Google Scholar] [CrossRef] [PubMed]
- Delrish, E.; Ghassemi, F.; Jabbarvand, M.; Lashay, A.; Atyabi, F.; Soleimani, M.; Dinarvand, R. Biodistribution of Cy5-labeled Thiolated and Methylated Chitosan-Carboxymethyl Dextran Nanoparticles in an Animal Model of Retinoblastoma. J. Ophthalmic Vis. Res. 2022, 17, 58. [Google Scholar] [CrossRef]
- Jafar, M.M.A.; Heather, S.; Al, A.A. Functional Biopolymers; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Vert, M.; Li, S.M.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Joseph, A.; Shavi, G.; Rao, J.V.; Udupa, N. Development and evaluation of carboplatin-loaded PCL nanoparticles for intranasal delivery. Drug Deliv. 2016, 23, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zeng, X.; Chen, H.; Zheng, Y.; Song; Huang, L.; Ma, Y. Novel docetaxel-loaded nanoparticles based on PCL-Tween 80 copolymer for cancer treatment. Int. J. Nanomed. 2011, 6, 2679–2688. [Google Scholar] [CrossRef] [Green Version]
- Abriata, J.P.; Turatti, R.C.; Luiz, M.T.; Raspantini, G.L.; Tofani, L.B.; Amaral, R.L.F.D.; Swiech, K.; Marcato, P.D.; Marchetti, J.M. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater. Sci. Eng. C 2019, 96, 347–355. [Google Scholar] [CrossRef]
- El-Habashy, S.E.; Eltaher, H.M.; Gaballah, A.; Zaki, E.I.; Mehanna, R.A.; El-Kamel, A.H. Hybrid bioactive hydroxyapatite/polycaprolactone nanoparticles for enhanced osteogenesis. Mater. Sci. Eng. C 2021, 119, 111599. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Hu, D.; Peng, J.; Xiao, Y.; Hao, Y.; Pan, M.; Yuan, L.; Qian, Z. Cabazitaxel-loaded MPEG-PCL copolymeric nanoparticles for enhanced colorectal cancer therapy. Appl. Mater. Today 2021, 25, 101210. [Google Scholar] [CrossRef]
- Pan, Q.; Tian, J.; Zhu, H.; Hong, L.; Mao, Z.; Oliveira, J.M.; Reis, R.L.; Li, X. Tumor-Targeting Polycaprolactone Nanoparticles with Codelivery of Paclitaxel and IR780 for Combinational Therapy of Drug-Resistant Ovarian Cancer. ACS Biomater. Sci. Eng. 2020, 6, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Kumar, V.; Singh, B.; Chaudhary, A.; Yadav, S.C. Nanoencapsulation and characterization of Albizia chinensis isolated antioxidant quercitrin on PLA nanoparticles. Colloids Surf. B Biointerfaces 2011, 82, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnology 2021, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Božik, M.; Bravo, I.; Clemente-Casares, P.; Sánchez, A.L.; Juan, A.; Klouček, P.; Alonso-Moreno, C. PEI-coated PLA nanoparticles to enhance the antimicrobial activity of carvacrol. Food Chem. 2020, 328, 127131. [Google Scholar] [CrossRef]
- Ghaffarzadegan, R.; Khoee, S.; Rezazadeh, S. Fabrication, characterization and optimization of berberine-loaded PLA nanoparticles using coaxial electrospray for sustained drug release. DARU J. Pharm. Sci. 2020, 28, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, H.; Gu, Y.; Wang, X.; Wu, P. Dual drug-loaded PLA nanoparticles bypassing drug resistance for improved leukemia therapy. J. Nanoparticle Res. 2019, 21, 83. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Su, Y.; Kim, G.B.; Selvi, E.; Ma, C.; Aragon-Sanabria, V.; Hsieh, J.; Dong, C.; Yang, J. Immune Cell-Mediated Biodegradable Theranostic Nanoparticles for Melanoma Targeting and Drug Delivery. Small 2017, 13, 1603121. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Microstructural and mechanical properties of porous biocomposite scaffolds based on polyvinyl alcohol, nano-hydroxyapatite and cellulose nanocrystals. Cellulose 2014, 21, 3409–3426. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Antunes-Ricardo, M.; Martínez-Morales, P.; Sánchez, M.L. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int. J. Pharm. 2021, 600, 120478. [Google Scholar] [CrossRef]
- El-Dafrawy, S.M.; Tarek, M.; Samra, S.; Hassan, S.M. Synthesis, photocatalytic and antidiabetic properties of ZnO/PVA nanoparticles. Sci. Rep. 2021, 11, 11404. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Wang, N.; Wu, X.S. Poly(vinyl alcohol) nanoparticles prepared by freezing–thawing process for protein/peptide drug delivery. J. Control. Release 1998, 56, 117–126. [Google Scholar] [CrossRef]
- Byun, Y.; Hwang, J.B.; Bang, S.H.; Darby, D.; Cooksey, K.; Dawson, P.L.; Park, H.J.; Whiteside, S. Formulation and characterization of α-tocopherol loaded poly ε-caprolactone (PCL) nanoparticles. LWT-Food Sci. Technol. 2011, 44, 24–28. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Kakran, M.; Antipina, M.N. Emulsion-based techniques for encapsulation in biomedicine, food and personal care. Curr. Opin. Pharmacol. 2014, 18, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Y.; Xia, F.; Zhao, Y.P. Preparation of hydroxypropyl methyl cellulose phthalate nanoparticles with mixed solvent using supercritical antisolvent process and its application in co-precipitation of insulin. Adv. Powder Technol. 2012, 23, 157–163. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Biopolymer Nanoparticles from Heat-Treated Electrostatic Protein-Polysaccharide Complexes: Factors Affecting Particle Characteristics. J. Food Sci. 2010, 75, N36–N43. [Google Scholar] [CrossRef]
- Vecchione, D.; Grimaldi, A.M.; Forte, E.; Bevilacqua, P.; Netti, P.A.; Torino, E. Hybrid Core-Shell (HyCoS) Nanoparticles produced by Complex Coacervation for Multimodal Applications. Sci. Rep. 2017, 7, 45121. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a Reproducible Method for Production of Polymeric Microspheres for Biomedical Applications. Polymers 2011, 3, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of Monodisperse Particles by Using Microfluidics: Control over Size, Shape, and Composition. Angew. Chem. Int. Ed. 2005, 44, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Krishnadasan, S.; Brown, R.J.C.; Demello, A.J.; Demello, J.C. Intelligent routes to the controlled synthesis of nanoparticles. Lab Chip 2007, 7, 1434–1441. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: A review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Strategies for Tissue Engineering in Regenerative Medicine: A Biofabrication and Biopolymer Perspective. Molecules 2021, 26, 2518. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef]





| Polymer | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Albumin | Highly abundant, biodegradable, biocompatible, non-cytotoxic. | Immunogenic effects, very expensive, lack of efficacy. | [18,19] |
| Gelatin | Enhanced cell adhesion, proliferation and cell infiltration in the scaffolds, good stability and biodegradability, osteoconductive, non-immunogenic | Low stability in normal physiological conditions, poor bioactivity, brittle, fast degradation rate under physiological conditions | [16,20] |
| Silk fibroin | Biocompatible, osteoconductive, improves cell migration and angiogenesis, good elastic properties, moderate degradation rate. | Low mechanical strength, degradation of silk releases by-products that can cause immunogenic reactions, inability to induce osteogenesis. | [16,20,21] |
| Collagen | Low immunogenicity, enhanced permeability properties, excellent cell adhesion, proliferation and differentiation properties, biodegradable, biocompatible. | Low mechanical strength, low structure stability, variability in different collagen sources. | [16,20,22] |
| Chitosan | Mucoadhesive nature, enhanced biocompatibility, osteoconductive, non-toxic, promotes cell adhesion, hemostatic potential, biodegradable, anti-bacterial activity. | In vivo degradation rate is very high, low mechanical strength, cross-linkers are required to maintain stability, solubility is less and viscosity is high at neutral pH, control of nanoparticle size is difficult. | [16,20,23] |
| Alginate | Biocompatible, biodegradable, cell compatible, gel-forming capability, low immunogenicity, mimics the extracellular matrix, low cost, ability of encapsulation. | Low mechanical properties, degradation is questionable sometimes, poor cell adhesion, sterilization is difficult. | [16,20,24] |
| Starch | Biodegradable, low cost, biocompatible, easily available, good cell adhesion. | Very high viscosity, low Mechanical properties, fragile, stability issues, water uptake is very high, modifying chemically can release toxic by-products. | [16,25] |
| Dextran | Biocompatible, anti-thrombotic property, good water solubility, functionalization can be carried out easily. | High cost, non-availability, very high permeability, encapsulated drugs are released very fast. | [26,27] |
| Poly- caprolactone | Compatible with cells, non-toxic, cell proliferation and angiogenesis can be controlled, good mechanical properties, improved cellular proliferation. | Bioactivity is less, poor cellular adhesion due to hydrophobic surface, use of toxic solvents. | [16] |
| Polyvinyl alcohol | Biocompatible, good elastic nature, water-soluble polymer, good tensile strength, improved flexibility, stability to various temperatures, low cost. | Lacks cell adhesion property, in growth of bone cells is significantly less, very high water uptake. | [16,28,29] |
| Polylactic acid | Biocompatible, cell compatible, degradation rate is good, by-products are non-toxic, properties can be easily tailored, eco-friendly. | Lack of cell adhesion and proliferation property, expensive, brittle (elongation at break is less than 10%), chemically inert. | [16,30] |
| Protein | Overall Composition | Application | Key Findings of the Study | Reference |
|---|---|---|---|---|
| Albumin | Human serum albumin + ibrutinib and hydroxychloroquine (nanoparticles) | Co-drug delivery system for treatment of glioma | Improved bioavailability Prolonged survival time in in vivo treated mice High cytotoxicity against C6 cells | [36] |
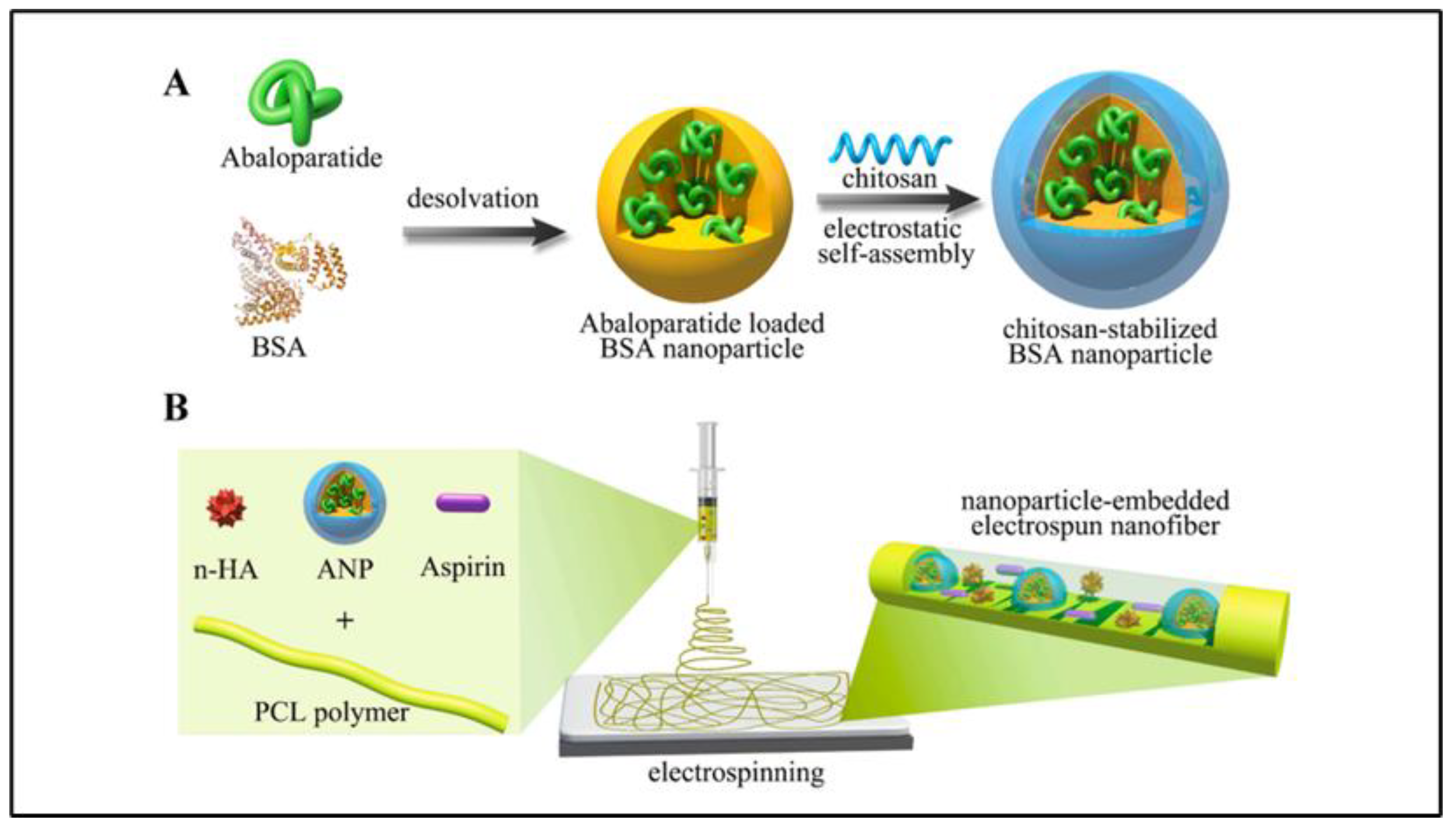
| Albumin | Bovine serum albumin + abaloparatide + aspirin + polycaprolactone + hydroxyapatite (nanofibrous scaffold) | Bone regeneration | Improved degradation rate Slow drug release Enhanced compatibility Improved bone regeneration | [37] |
| Albumin | Human serum albumin (HSA) + indocyanine green (ICG) + paclitaxel (PTX) + hyaluronic acid (nanoparticles) | Image-guided drug delivery | Efficient drug release in the tumor environment Efficient anti-cancer activity | [38] |
| Albumin | Bovine serum albumin + carnosic acid | Anti-tumor activity of breast cancer and colon cancer. | Enhanced loading activity Improved release profile of the drug Enhanced anti-tumor activity Upregulation of GCLC gene and downregulation of BCL-2 and COX-2 gene. | [39] |
| Albumin | Bovine serum albumin + silymarin + curcumin + chitosan | Muco-inhalable drug delivery system | Significant reduction of interleukin-6 and c-reactive protein Efficient anti-viral activity in in vitro COVID-19 experiment | [71] |
| Albumin | Bovine serum albumin + poly-L-lysine + graphene oxide | Bone regeneration | Controlled release of BMP-2 (14 days) Improved matrix mineralization Enhanced Alkaline phosphatase (ALP) activity | [72] |
| Gelatin | Gelatin + concanavalin-A + cisplatin | Drug delivery for cancer therapy | Enhanced cellular uptake of nanoparticles Enhanced reactive oxygen species and apoptosis in cancer cells | [73] |
| Gelatin | Gelatin methacrylol nanoparticles + rhodamine | Cell imaging | Improved cell viability and cell proliferation in vitro Superior cell compatibility Enhanced cellular uptake Improved fluorescent properties | [74] |
| Gelatin | Amino cellulose + polycaprolactone + gelatin nanoparticles | Rheumatoid arthritis | Reduction in swelling and inflammation in rats. Maintaining cartilage and bone tissue architecture. Reduction of inflammatory markers | [75] |
| Gelatin | Gelatin + indocyanine + doxorubicin | Breast cancer treatment | Improved drug release Suppressed the tumor growth in vivo Enhanced degradation of matrix metalloproteinase-2 | [76] |
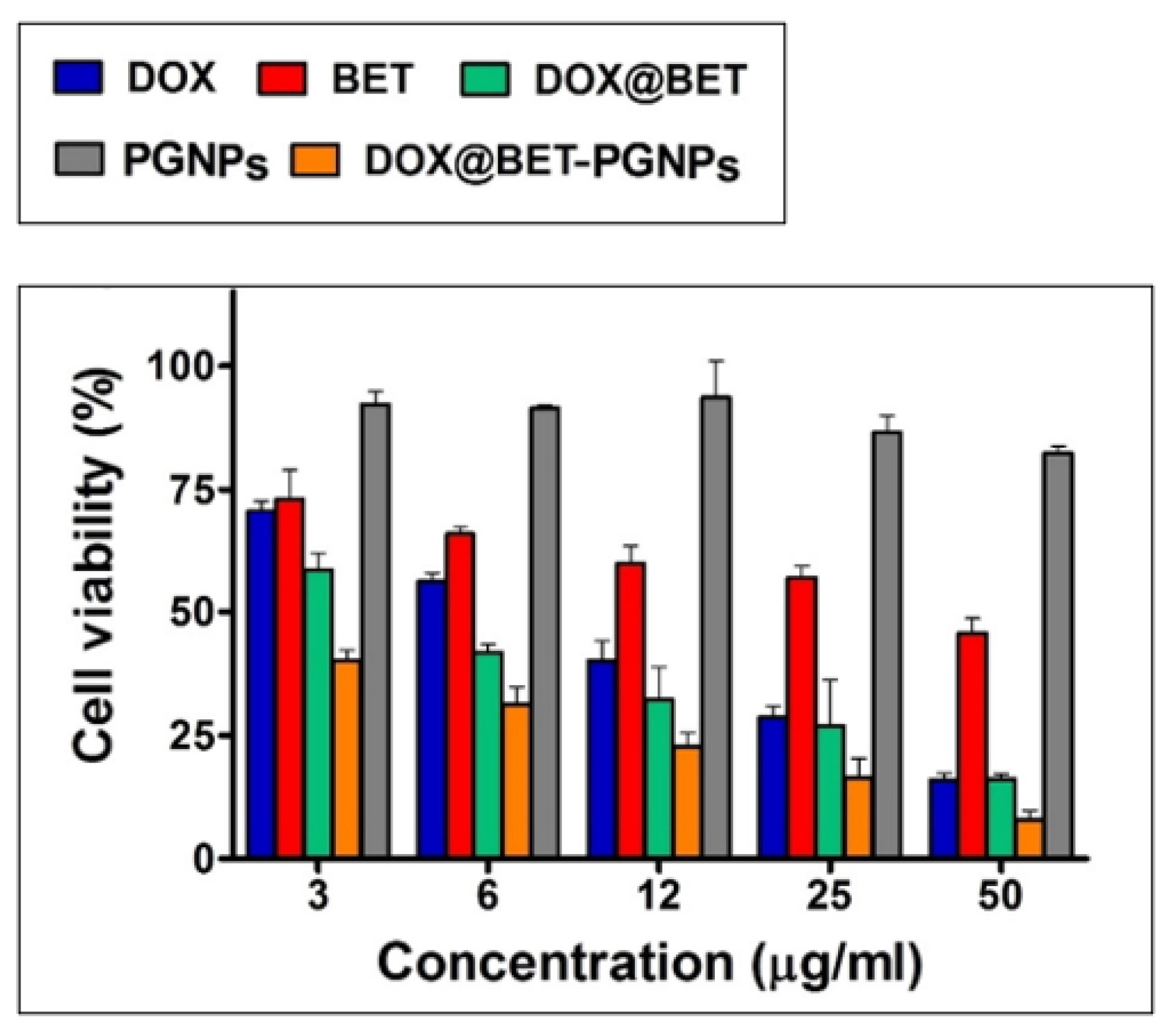
| Gelatin | Polyethylene glycol grafted gelatin nanoparticles + doxorubicin + betanin | Cancer therapy | Enhanced cellular uptake Cell apoptosis induced in MCF cells; Controlled drug release observed | [43] |
| Silk fibroin | Curcumin + silk fibroin nanoparticles | Cancer therapy | Enhance anti-tumor activity Improved anti-oxidant activity Curcumin was found to be fluorescent when encapsulated | [52] |
| Silk fibroin | Silk fibroin + sodium alginate + silk fibroin nanoparticles (scaffold) | Wound healing | Improved cell adhesion Enhanced hemostasis Improved platelet adhesion Excellent biocompatibility and improved cell adhesion and proliferation | [53] |
| Silk fibroin | Silk fibroin + simvastatin (nanoparticles) | Bone regeneration | Sustained release profile Improved ALP production Enhanced production of osteoblast cells | [54] |
| Silk fibroin | Silk fibroin + 5 fluorouracil (nanoparticles) | Drug delivery | Improved loading efficiency Slower release of the drug | [55] |
| Silk fibroin | Silk fibroin nanoparticles + PX478 + doxorubicin | Reverse multi-drug resistance | Increased cellular uptake Downregulation of genes-MDR1, VEGF and GLUT-1 | [56] |
| Silk fibroin | Silk fibroin + tamoxifen (nanoparticles) | Breast cancer | The particle size was found to be 186.1 nm Encapsulation efficiency was found to be 79.08% Biphasic release profile was observed | [77] |
| Collagen | Collagen + estradiol–hemihydrate | Transdermal drug delivery | Enhanced drug loading capacity Increased sustained drug release Improved drug absorption | [64] |
| Elastin | Elastin-like polymeric nanoparticles + bone morphogenic protein | Drug delivery system | Improved encapsulation efficiency Compatible with C2C12 cells | [69] |
| Elastin | α-elastin + methoxy polyethylene glycol + BSA/Insulin | Protein delivery | Encapsulation at low temperatures with simple mixing Sustained release for 72 h The nanoparticles are of normal size distribution | [70] |
| Elastin | Elastin-like recombinamers + docetaxel + RGD peptide | Drug delivery system | High yield of 70% Monodispersed nanoparticles-40 nm Very much effective against breast cancer cell line | [78] |
| Polysaccharide | Overall Composition | Application | Key Findings of the Study | References |
|---|---|---|---|---|
| Chitosan | Chitosan + polylactic acid + lamivudine | Drug delivery | Drug release was found to be higher when higher percentage was loading The nanoparticles were found to be non-toxic to the L929 cell line The degradation rate increases with respect to pH | [84] |
| Chitosan | Chitosan + 5-fluorouracil and leucovorin | Drug delivery | Improved encapsulation efficiency and drug loading capacity Release profile can be modulated by changing the parameters | [85] |
| Chitosan | Chitosan + ellagic acid | Oral cancer therapy | Particle size was found to be 176 nm; Encapsulation efficiency was found to be 94 ± 1.03%. Sustained release of the drug was observed. Cytotoxicity was observed in KB cell line | [115] |
| Chitosan | Chitosan + tetracycline+ gentamycin + ciproflaxin | Drug delivery | Superior antibacterial properties; improved physiochemical and mechanical properties; greater penetration of nanoparticles observed in the fiber | [116] |
| Chitosan | Chitosan + 5-fluorouracil | Drug delivery | Negative binding energy makes it energetically suitable; high drug loading capacity; reduced toxicity and increased reactivity | [117] |
| Chitosan | Chitosan + dexamethasone | Drug delivery | Particle size ranged from 277 to 289 nm; Drug release increased up to 8 h and was constant upto 48 h. Mild cytotoxicity was observed against L929, HCEC and RAW 264.7 cells. Effective anti-inflammatory activity against RAW macrophages | [118] |
| Chitosan | Chitosan + sodium alginate + polyvinyl alcohol + rosuvastatin | Drug delivery | Enhanced mechanical properties of the hydrogel film. The size of the nanoparticles ranged between 100–150 nm. Encapsulated drug was released within 24 h. High cell viability of fibroblast cells observed after 72 h of incubation | [119] |
| Alginate | Alginate + rifampicin/isoniazid/pyrazinamide/ethambutol | Anti-tuberculosis drug carrier | High drug encapsulation ranging from 70 to 90%. Improved bioavailability of the drugs Promising in vivo results | [120] |
| Alginate | Chitosan + alginate nanoparticles + curcumin diethyl disuccinate | Drug delivery | Enhanced stability; good bioavailability; improved cellular uptake; cytotoxicity against Hep G2 cell line. | [93] |
| Alginate | Chitosan oligosaccharide + alginate nanoparticles + astaxanthin | Drug delivery | Encapsulation efficiency and drug loading capacity were found to be 71.3% and 6.9%. Exhibited stability in acidic, alkaline and ultraviolet light. Sustained drug release was observed. Improved bioavailability and anti-oxidant activity. | [121] |
| Alginate | Chitosan + alginate nanoparticles | Gene delivery | Particle size of 111 nm; no toxicity observed; transfection efficiency of 29.9% | [94] |
| Alginate | Chitosan + alginate nanoparticles + esculentoside | Wound healing | Enhanced healing rate; improved anti-inflammatory activity; Sustained drug release rate | [95] |
| Starch | Starch nanoparticles + citric acid (nanocomposite) | - | The size of the nanoparticles ranged from 50 to 100 nm. Improved storage modulus and glass transition temperature. Decrease in water vapor permeability | [122] |
| Starch | Starch + CG-1521 | Breast cancer treatment | Slow release of the drug; Improved cytotoxicity towards MCF-7 cell line. Cell cycle arrest was induced and apoptosis was seen in MCF-7 cells | [100] |
| Starch | Starch nanoparticles + curcumin | Drug delivery | Enhanced encapsulation efficiency (80%) Controlled release observed | [101] |
| Starch | Starch nanoparticles + doxorubicin + siRNA | Cancer therapy | Low cell proliferation; enhanced cytotoxicity against A549 cell line; decreased expression of IGFR 1 protein | [102] |
| Starch | Starch nanoparticles + para coumaric acid | Breast cancer | Increased cytotoxicity towards MDA-MB-231 cells; burst release observed initially; enhanced encapsulation efficiency | [103] |
| Starch | Starch nanoparticles + triphala churna | Drug delivery system | Enhanced encapsulation efficiency Improved anti-bacterial and anti-oxidant activity; initial drug release was found to be very fast | [104] |
| Dextran | Dextran nanoparticles + doxorubicin | Cancer therapy | Enhanced anti-tumor effect; high cytotoxicity towards SCC7 cancer cell line; improved encapsulation efficiency | [108] |
| Dextran | Zirconium-89 labeled dextran nanoparticles | In vivo imaging | Enhanced tumor uptake; half-life of 3.9 h. Targets only tissue macrophages | [109] |
| Dextran | Dextran nanoparticles + glucose oxidase | Insulin delivery | Controlled release of insulin -90% under artificial intestinal fluid conditions; mechanism—Non-Fickian diffusion | [110] |
| Dextran | Dextran nanoparticles + acitretin | Treatment of psoriasis skin disease | Average size of 100 nm; sustained release of 80%. Enhanced proliferation level of keratinocytes; improved inhibition of STAT-3 phosphorylation | [112] |
| Dextran | Carboxymethyl dextran nanoparticles + Cy-5 labeling | Retinoblastoma | Enhanced ocular bioavailability; more affinity toward ocular tumor | [123] |
| Dextran | Dextran nanoparticles + Cerium oxide nanoparticles | CT contrast imaging agent | Oxidative stress protection; no toxicity observed; majority of the drug released in 24 h | [113] |
| Synthetic Biopolymer | Overall Composition | Application | Key Findings of the Study | References |
|---|---|---|---|---|
| Polycaprolactone | Polycaprolactone nanoparticles + carboplatin | Intra nasal delivery | Size- 311.6 ± 4.7 nm; Biphasic pattern of drug release-initial burst release followed by slow and controlled release. Cytotoxic towards human glioblastoma cell line. Better nasal absorption than free drug | [130] |
| Polycaprolactone | Polycaprolactone + Tween 80 + docetaxel | Cancer therapy | Enhanced cellular uptake; Improved cytotoxicity against C6 glioma cells; 35% of the drug released in 28 days. | [131] |
| Polycaprolactone | Polycaprolactone nanoparticles + paclitaxel | Cancer therapy | Enhanced encapsulation efficiency; the size was found to be 140 nm. Cell viability reduced against SKOV-3 cell line | [132] |
| Polycaprolactone | Polycaprolactone nanoparticles + α-tocopherol | - | Decrease in encapsulation efficiency, particle size when the ultrasonication time was increased. | [149] |
| Polycaprolactone | Polycaprolactone + hydroxyapatite | Bone tissue engineering | Enhanced cell proliferation and differentiation; Moderate expression of markers such as Runx-2 and osteopontin. High expression of sialoprotein at the end of 10 days. | [133] |
| Polycaprolactone | Polycaprolactone + chitosan + dorzolamide | Ocular drug delivery | Biphasic pattern of drug release; Enhanced drug permeation rate; Improved mucoadhesion; It was found to be non-cytotoxic and safe to use. | [135] |
| Polylactic acid | Polylactic acid + quercitrin | Therapeutic effect | Size- 250 ± 68 nm; encapsulation efficiency −40%; drug release -burst release followed by sustained release. Enhanced anti-oxidant activity. | [138] |
| Polylactic acid | Polylactic acid + rifampicin | Antibacterial activity | Biphasic drug release; Improved antibiotic efficiency | [139] |
| Polylactic acid | Polylactic acid + polyethylene imine coating + carvacrol | Anti-oxidant and Antibacterial activity | Enhanced anti-oxidant and antimicrobial activity. Improved stability rate | [140] |
| Polylactic acid | Polylactic acid + berberine | Drug delivery system | Technique: coaxial electrospray; high cellular uptake; cell cytotoxicity against HCT116 cell line; slow release profile of the drug was reported | [141] |
| Polylactic acid | Polylactic acid + daunorubicin + glycyrrhizic acid | Leukemia | Inhibited leukemia cells; enhanced drug uptake; improved apoptosis rate | [142] |
| Polyvinyl alcohol | ZnO + polyvinyl alcohol nanoparticles | Treatment of diabetes | Exhibited photocatalytic activity In vivo analysis reported lower glucose level | [147] |
| Polyvinyl alcohol | Bovine serum albumin + polyvinyl alcohol nanoparticles | Delivery of proteins | High drug loading capability; drug release up to 30 h controlled by diffusion process; Enhanced stability of the loaded drug | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreena, R.; Nathanael, A.J. Biodegradable Biopolymeric Nanoparticles for Biomedical Applications-Challenges and Future Outlook. Materials 2023, 16, 2364. https://doi.org/10.3390/ma16062364
Sreena R, Nathanael AJ. Biodegradable Biopolymeric Nanoparticles for Biomedical Applications-Challenges and Future Outlook. Materials. 2023; 16(6):2364. https://doi.org/10.3390/ma16062364
Chicago/Turabian StyleSreena, Radhakrishnan, and Arputharaj Joseph Nathanael. 2023. "Biodegradable Biopolymeric Nanoparticles for Biomedical Applications-Challenges and Future Outlook" Materials 16, no. 6: 2364. https://doi.org/10.3390/ma16062364






