Local Antibiotic Delivery Ceramic Bone Substitutes for the Treatment of Infected Bone Cavities and Bone Regeneration: A Systematic Review on What We Have Learned from Animal Models
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Systematic Review
3.2. Sample and Methods of the Selected Studies
3.2.1. Experimental Groups
3.2.2. Article Quality
3.2.3. Animal Model
3.2.4. Type of Bone Defect
3.2.5. Pathogenic Strain
3.2.6. Osteomyelitis Induction
3.2.7. Implanted Biomaterial
3.2.8. Antimicrobial Agent
3.3. General Results on Infection Evolution and Bone Remodeling
3.3.1. Time to Cure
3.3.2. Gross Observation
3.3.3. Blood Tests
3.3.4. Radiological Evaluation
3.3.5. Histological Evaluation
3.3.6. Microbiological Evaluation
4. Discussion
4.1. Quality of Selected Papers
4.2. Infection Model
4.3. Osteomyelitis Agent
4.4. Antibiotics
4.5. Bioactive Ceramics
4.6. Evaluation Methods
4.7. Infection Treatment and Biomaterial Osteointegration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpson, A.H.; Deakin, M.; Latham, J.M. Chronic osteomyelitis. The effect of the extent of surgical resection on infection-free survival. J. Bone Jt. Surg. Br. 2001, 83, 403–407. [Google Scholar] [CrossRef]
- Parsons, B.; Strauss, E. Surgical management of chronic osteomyelitis. Am. J. Surg. 2004, 188, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Tetsworth, K.; Cierny, G., 3rd. Osteomyelitis debridement techniques. Clin. Orthop. Relat. Res. 1999, 360, 87–96. [Google Scholar] [CrossRef]
- Holtom, P.D.; Patzakis, M.J. Newer methods of antimicrobial delivery for bone and joint infections. Instr. Course Lect. 2003, 52, 745–749. [Google Scholar]
- Neut, D.; van de Belt, H.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials 2003, 24, 1829–1831. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Kotoura, Y.; Oka, M.; Yamamuro, T.; Wada, R.; Hyon, S.H.; Ikada, Y. A bioabsorbable delivery system for antibiotic treatment of osteomyelitis. The use of lactic acid oligomer as a carrier. J. Bone Jt. Surg. Br. 1991, 73, 246–252. [Google Scholar] [CrossRef]
- Garvin, K.L.; Miyano, J.A.; Robinson, D.; Giger, D.; Novak, J.; Radio, S. Polylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine model. J. Bone Jt. Surg. Am. 1994, 76, 1500–1506. [Google Scholar] [CrossRef]
- Nie, L.; Nicolau, D.P.; Nightingale, C.H.; Browner, B.D.; Quintiliani, R. In vitro elution of ofloxacin from a bioabsorbable polymer. Acta Orthop. Scand. 1995, 66, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Mader, J.T.; Calhoun, J.; Cobos, J. In vitro evaluation of antibiotic diffusion from antibiotic-impregnated biodegradable beads and polymethylmethacrylate beads. Antimicrob. Agents Chemother. 1997, 41, 415–418. [Google Scholar] [CrossRef] [Green Version]
- Kanellakopoulou, K.; Kolia, M.; Anastassiadis, A.; Korakis, T.; Giamarellos-Bourboulis, E.J.; Andreopoulos, A.; Dounis, E.; Giamarellou, H. Lactic acid polymers as biodegradable carriers of fluoroquinolones: An in vitro study. Antimicrob. Agents Chemother. 1999, 43, 714–716. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, C.G.; Gogola, G.R.; Clyburn, T.A.; Raymond, A.K.; Peng, A.S.; Mikos, A.G. Antibiotic microspheres: Preliminary testing for potential treatment of osteomyelitis. Clin. Orthop. Relat. Res. 2003, 415, 279–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanakis, N.; Giamarellou, H.; Moussas, T.; Dounis, E. Chronic osteomyelitis caused by multi-resistant Gram-negative bacteria: Evaluation of treatment with newer quinolones after prolonged follow-up. J. Antimicrob. Chemother. 1997, 39, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Bucholz, R.W.; Carlton, A.; Holmes, R.E. Hydroxyapatite and tricalcium phosphate bone graft substitutes. Orthop. Clin. N. Am. 1987, 18, 323–334. [Google Scholar] [CrossRef]
- Daculsi, G.; Passuti, N.; Martin, S.; Deudon, C.; Legeros, R.Z.; Raher, S. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J. Biomed. Mater. Res. 1990, 24, 379–396. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Weiss, P.; Bosco, J.; Daculsi, G.; Aguado, E. Kinetic study of bone ingrowth and ceramic resorption associated with the implantation of different injectable calcium-phosphate bone substitutes. J. Biomed. Mater. Res. 1999, 47, 28–35. [Google Scholar] [CrossRef]
- Rey, C. Calcium phosphate biomaterials and bone mineral. Differences in composition, structures and properties. Biomaterials 1990, 11, 13–15. [Google Scholar] [PubMed]
- Yuan, H.; Kurashina, K.; de Bruijn, J.D.; Li, Y.; de Groot, K.; Zhang, X. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 1999, 20, 1799–1806. [Google Scholar] [CrossRef]
- Nelson, C.L.; McLaren, S.G.; Skinner, R.A.; Smeltzer, M.S.; Thomas, J.R.; Olsen, K.M. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J. Orthop. Res. 2002, 20, 643–647. [Google Scholar] [CrossRef]
- Trecant, M.; Guicheux, J.; Grimandi, G.; Leroy, M.; Daculsi, G. Dynamic compaction: A new process to compact therapeutic agent-loaded calcium phosphates. Biomaterials 1997, 18, 141–145. [Google Scholar] [CrossRef]
- Kawanabe, K.; Okada, Y.; Matsusue, Y.; Iida, H.; Nakamura, T. Treatment of osteomyelitis with antibiotic-soaked porous glass ceramic. J. Bone Jt. Surg. Br. 1998, 80, 527–530. [Google Scholar] [CrossRef]
- Solberg, B.D.; Gutow, A.P.; Baumgaertner, M.R. Efficacy of gentamycin-impregnated resorbable hydroxyapatite cement in treating osteomyelitis in a rat model. J. Orthop. Trauma 1999, 13, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P.; Mateus, A.Y.; Sousa, J.C.; Monteiro, F.J. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. A 2007, 81, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- McLaren, A.C. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin. Orthop. Relat. Res. 2004, 427, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Beenken, K.E.; Smith, J.K.; Skinner, R.A.; McLaren, S.G.; Bellamy, W.; Gruenwald, M.J.; Spencer, H.J.; Jennings, J.A.; Haggard, W.O.; Smeltzer, M.S. Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J. Biomater. Appl. 2014, 29, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Buxton, T.B.; Walsh, D.S.; Harvey, S.B.; McPherson, J.C., 3rd; Hartmann, J.F.; Plowman, K.M. Bisphosphonate-ciprofloxacin bound to Skelite is a prototype for enhancing experimental local antibiotic delivery to injured bone. Br. J. Surg. 2004, 91, 1192–1196. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, D.; Yan, L.; Wu, J. In vitro and in vivo osteogenic activity of the novel vancomycin-loaded bone-like hydroxyapatite/poly(amino acid) scaffold. J. Biomater. Appl. 2016, 30, 1566–1577. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, D.; Yan, L.; Wu, J. In vitro and in vivo drug release and antibacterial properties of the novel vancomycin-loaded bone-like hydroxyapatite/poly amino acid scaffold. Int. J. Nanomed. 2017, 12, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.F.; Chia, W.T.; Liu, H.Y.; Hsiao, C.W.; Hsiao, H.C.; Yang, C.M.; Sung, H.W. Inflammation-induced drug release by using a pH-responsive gas-generating hollow-microsphere system for the treatment of osteomyelitis. Adv. Healthc. Mater. 2014, 3, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.N.; Tyndall, D.; Waller, S.; Lane, J.M.; Brause, B.D. Treatment of experimental osteomyelitis with antibiotic-impregnated bone graft substitute. J. Orthop. Res. 1993, 11, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Faber, C.; Stallmann, H.P.; Lyaruu, D.M.; Joosten, U.; von Eiff, C.; van Nieuw Amerongen, A.; Wuisman, P.I. Comparable efficacies of the antimicrobial peptide human lactoferrin 1–11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob. Agents Chemother. 2005, 49, 2438–2444. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.G.; Pang, L.; Chen, Z.R.; Tan, X.P. Dual-delivery of vancomycin and icariin from an injectable calcium phosphate cement-release system for controlling infection and improving bone healing. Mol. Med. Rep. 2013, 8, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, T.; Yongqing, X.; Tiane, Z.; Gang, L.; Yonggang, Y.; Muyao, J.; Jun, L.; Jing, D. Treatment of osteomyelitis by liposomal gentamicin-impregnated calcium sulfate. Arch. Orthop. Trauma Surg. 2009, 129, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Itokazu, M.; Ohno, T.; Tanemori, T.; Wada, E.; Kato, N.; Watanabe, K. Antibiotic-loaded hydroxyapatite blocks in the treatment of experimental osteomyelitis in rats. J. Med. Microbiol. 1997, 46, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.T.; Luo, S.H.; Zhang, C.Q.; Wang, J.Q. In vitro and in vivo efficacies of teicoplanin-loaded calcium sulfate for treatment of chronic methicillin-resistant Staphylococcus aureus osteomyelitis. Antimicrob. Agents Chemother. 2010, 54, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.T.; Zhang, X.; Luo, S.H.; Liu, X.; Huang, W.H.; Rahaman, M.N.; Day, D.E.; Zhang, C.Q.; Xie, Z.P.; Wang, J.Q. Novel borate glass/chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis. Acta Biomater. 2010, 6, 812–819. [Google Scholar] [CrossRef]
- Jiang, J.L.; Li, Y.F.; Fang, T.L.; Zhou, J.; Li, X.L.; Wang, Y.C.; Dong, J. Vancomycin-loaded nano-hydroxyapatite pellets to treat MRSA-induced chronic osteomyelitis with bone defect in rabbits. Inflamm. Res. 2012, 61, 207–215. [Google Scholar] [CrossRef]
- Joosten, U.; Joist, A.; Frebel, T.; Brandt, B.; Diederichs, S.; von Eiff, C. Evaluation of an in situ setting injectable calcium phosphate as a new carrier material for gentamicin in the treatment of chronic osteomyelitis: Studies in vitro and in vivo. Biomaterials 2004, 25, 4287–4295. [Google Scholar] [CrossRef]
- Joosten, U.; Joist, A.; Gosheger, G.; Liljenqvist, U.; Brandt, B.; von Eiff, C. Effectiveness of hydroxyapatite-vancomycin bone cement in the treatment of Staphylococcus aureus induced chronic osteomyelitis. Biomaterials 2005, 26, 5251–5258. [Google Scholar] [CrossRef] [PubMed]
- Kanellakopoulou, K.; Galanopoulos, I.; Soranoglou, V.; Tsaganos, T.; Tziortzioti, V.; Maris, I.; Papalois, A.; Giamarellou, H.; Giamarellos-Bourboulis, E.J. Treatment of experimental osteomyelitis caused by methicillin-resistant Staphylococcus aureus with a synthetic carrier of calcium sulphate (Stimulan) releasing moxifloxacin. Int. J. Antimicrob. Agents 2009, 33, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kankilic, B.; Bilgic, E.; Korkusuz, P.; Korkusuz, F. Vancomycin containing PLLA/beta-TCP controls experimental osteomyelitis in vivo. J. Orthop. Surg. Res. 2014, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, M.; Simsek-Kaya, G.; Gursan, N.; Kirecci, E.; Dayi, E.; Gundogdu, B. Local treatment of chronic osteomyelitis with surgical debridement and tigecycline-impregnated calcium hydroxyapatite: An experimental study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 340–347. [Google Scholar] [CrossRef]
- Koort, J.K.; Makinen, T.J.; Suokas, E.; Veiranto, M.; Jalava, J.; Knuuti, J.; Tormala, P.; Aro, H.T. Efficacy of ciprofloxacin-releasing bioabsorbable osteoconductive bone defect filler for treatment of experimental osteomyelitis due to Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 1502–1508. [Google Scholar] [CrossRef] [Green Version]
- Korkusuz, F.; Uchida, A.; Shinto, Y.; Araki, N.; Inoue, K.; Ono, K. Experimental implant-related osteomyelitis treated by antibiotic-calcium hydroxyapatite ceramic composites. J. Bone Jt. Surg. Br. 1993, 75, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Lulu, G.A.; Karunanidhi, A.; Mohamad Yusof, L.; Abba, Y.; Mohd Fauzi, F.; Othman, F. In vivo efficacy of tobramycin-loaded synthetic calcium phosphate beads in a rabbit model of staphylococcal osteomyelitis. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- Melichercik, P.; Cerovsky, V.; Nesuta, O.; Jahoda, D.; Landor, I.; Ballay, R.; Fulin, P. Testing the efficacy of antimicrobial peptides in the topical treatment of induced osteomyelitis in rats. Folia Microbiol. 2018, 63, 97–104. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Calhoun, J.H.; Mader, J.T. Experimental osteomyelitis treatment with antibiotic-impregnated hydroxyapatite. Clin. Orthop. Relat. Res. 2002, 401, 239–247. [Google Scholar] [CrossRef]
- Stallmann, H.P.; Faber, C.; Bronckers, A.L.; Nieuw Amerongen, A.V.; Wuisman, P.I. Osteomyelitis prevention in rabbits using antimicrobial peptide hLF1-11- or gentamicin-containing calcium phosphate cement. J. Antimicrob. Chemother. 2004, 54, 472–476. [Google Scholar] [CrossRef]
- Thomas, D.B.; Brooks, D.E.; Bice, T.G.; DeJong, E.S.; Lonergan, K.T.; Wenke, J.C. Tobramycin-impregnated calcium sulfate prevents infection in contaminated wounds. Clin. Orthop. Relat. Res. 2005, 441, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, X.; Jia, W.; Zhang, C.; Huang, W.; Wang, J. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. J. Control. Release 2009, 139, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Jiang, D.M.; Cao, Z.D.; Wu, J.; Wang, X.; Wang, Z.L.; Li, Y.J.; Yi, Y.F. Treatment of Staphylococcus aureus-induced chronic osteomyelitis with bone-like hydroxyapatite/poly amino acid loaded with rifapentine microspheres. Drug Des. Dev. Ther. 2015, 9, 3665–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egawa, S.; Hirai, K.; Matsumoto, R.; Yoshii, T.; Yuasa, M.; Okawa, A.; Sugo, K.; Sotome, S. Efficacy of Antibiotic-Loaded Hydroxyapatite/Collagen Composites Is Dependent on Adsorbability for Treating Staphylococcus aureus Osteomyelitis in Rats. J. Orthop. Res. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, R.; Schaner, K.; Schroeder, M.; Wohlers, A.; Shreffler, J.; Schaper, C.; Subramanian, H.; Brooks, A. Extended Release Combination Antibiotic Therapy from a Bone Void Filling Putty for Treatment of Osteomyelitis. Pharmaceutics 2019, 11, 592. [Google Scholar] [CrossRef] [Green Version]
- Dvorzhinskiy, A.; Perino, G.; Chojnowski, R.; van der Meulen, M.C.H.; Bostrom, M.P.G.; Yang, X. Ceramic composite with gentamicin decreases persistent infection and increases bone formation in a rat model of debrided osteomyelitis. J. Bone Jt. Infect. 2021, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Wang, W.T.; Wang, X.P.; Yu, D.M.; Wang, Z.L.; Wang, W.B. PL/Vancomycin/Nano-hydroxyapatite Sustained-release Material to Treat Infectious Bone Defect. Open Life Sci. 2020, 15, 92–107. [Google Scholar] [CrossRef] [Green Version]
- Dahners, L.E.; Funderburk, C.H. Gentamicin-loaded plaster of paris as a treatment of experimental osteomyelitis in rabbits. Clin. Orthop. Relat. Res. 1987, 29, 278–282. [Google Scholar] [CrossRef]
- Eitenmuller, J.; Schmidt, K.H.; Peters, G.; Gellissen, G.; Weltin, R.; Reichmann, W. Experimental and preliminary clinical-experience with absorbable calcium-phosphate granules containing an antibiotic or antiseptic for the local treatment of osteomyelitis. J. Hosp. Infect. 1985, 6, 177–184. [Google Scholar] [CrossRef]
- Norden, C.W.; Myerowitz, R.L.; Keleti, E. Experimental osteomyelitis due to Staphylococcus aureus or Pseudomonas aeruginosa: A radiographic-pathological correlative analysis. Br. J. Exp. Pathol. 1980, 61, 451–460. [Google Scholar]
- Smeltzer, M.S.; Thomas, J.R.; Hickmon, S.G.; Skinner, R.A.; Nelson, C.L.; Griffith, D.; Parr, T.R., Jr.; Evans, R.P. Characterization of a rabbit model of staphylococcal osteomyelitis. J. Orthop. Res. 1997, 15, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Beenken, K.E.; Bradney, L.; Bellamy, W.; Skinner, R.A.; McLaren, S.G.; Gruenwald, M.J.; Spencer, H.J.; Smith, J.K.; Haggard, W.O.; Smeltzer, M.S. Use of xylitol to enhance the therapeutic efficacy of polymethylmethacrylate-based antibiotic therapy in treatment of chronic osteomyelitis. Antimicrob. Agents Chemother. 2012, 56, 5839–5844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Translat. 2015, 3, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell. Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, D.J.T. Modelo animal de doença: Critérios de escolha e espéciesde animais de uso corrente. Acta Cir. Bras. 2004, 19, 59–65. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Norden, C.W. Experimental osteomyelitis. I. A description of the model. J. Infect. Dis. 1970, 122, 410–418. [Google Scholar] [CrossRef]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur. Cell. Mater. 2014, 27, 196–212. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [Green Version]
- Nibbering, P.H.; Ravensbergen, E.; Welling, M.M.; van Berkel, L.A.; van Berkel, P.H.; Pauwels, E.K.; Nuijens, J.H. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brady, R.A.; Leid, J.G.; Camper, A.K.; Costerton, J.W.; Shirtliff, M.E. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect. Immun. 2006, 74, 3415–3426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.J., Jr.; Ward, C.L.; Romano, D.R.; Hurtgen, B.J.; Hardy, S.K.; Woodbury, R.L.; Trevino, A.V.; Rathbone, C.R.; Wenke, J.C. Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro. BMC Musculoskelet. Disord. 2013, 14, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, N.P.; Skovdal, S.M.; Meyer, R.L.; Dagnaes-Hansen, F.; Fuursted, K.; Petersen, E. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog. Dis. 2016, 74, ftw019. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhao, D.; Dangaria, S.J.; Luan, X.; Diekwisch, T.G.; Jiang, G.; Saiz, E.; Liu, G.; Tomsia, A.P. Combinatorial Design of Hydrolytically Degradable, Bone-like Biocomposites Based on PHEMA and Hydroxyapatite. Polymer 2013, 54, 909–919. [Google Scholar] [CrossRef] [Green Version]
- Kankilic, B.; Bayramli, E.; Kilic, E.; Dagdeviren, S.; Korkusuz, F. Vancomycin containing PLLA/beta-TCP controls MRSA in vitro. Clin. Orthop. Relat. Res. 2011, 469, 3222–3228. [Google Scholar] [CrossRef] [Green Version]
- Lopezberestein, G. Liposomes as Carriers of Antimicrobial Agents. Antimicrob. Agents Chemother. 1987, 31, 675–678. [Google Scholar] [CrossRef] [Green Version]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Wei, X.; Egawa, S.; Matsumoto, R.; Yasuda, H.; Hirai, K.; Yoshii, T.; Okawa, A.; Nakajima, T.; Sotome, S. Augmentation of fracture healing by hydroxyapatite/collagen paste and bone morphogenetic protein-2 evaluated using a rat femur osteotomy model. J. Orthop. Res. 2018, 36, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Aktekin, C.N.; Ozturk, A.M.; Tabak, A.Y.; Altay, M.; Korkusuz, F. A different perspective for radiological evaluation of experimental osteomyelitis. Skelet. Radiol. 2007, 36, 945–950. [Google Scholar] [CrossRef]
- Odekerken, J.C.; Arts, J.J.; Surtel, D.A.; Walenkamp, G.H.; Welting, T.J. A rabbit osteomyelitis model for the longitudinal assessment of early post-operative implant infections. J. Orthop. Surg. Res. 2013, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mader, J.T.; Wilson, K.J. Comparative evaluation of cefamandole and cephalothin in the treatment of experimental Staphylococcus aureus osteomyelitis in rabbits. J. Bone Jt. Surg. Am. 1983, 65, 507–513. [Google Scholar] [CrossRef]
- Lane, J.M.; Sandhu, H.S. Current approaches to experimental bone grafting. Orthop. Clin. N. Am. 1987, 18, 213–225. [Google Scholar] [CrossRef]
- Tadrous, P.J. On the concept of objectivity in digital image analysis in pathology. Pathology 2010, 42, 207–211. [Google Scholar] [CrossRef]

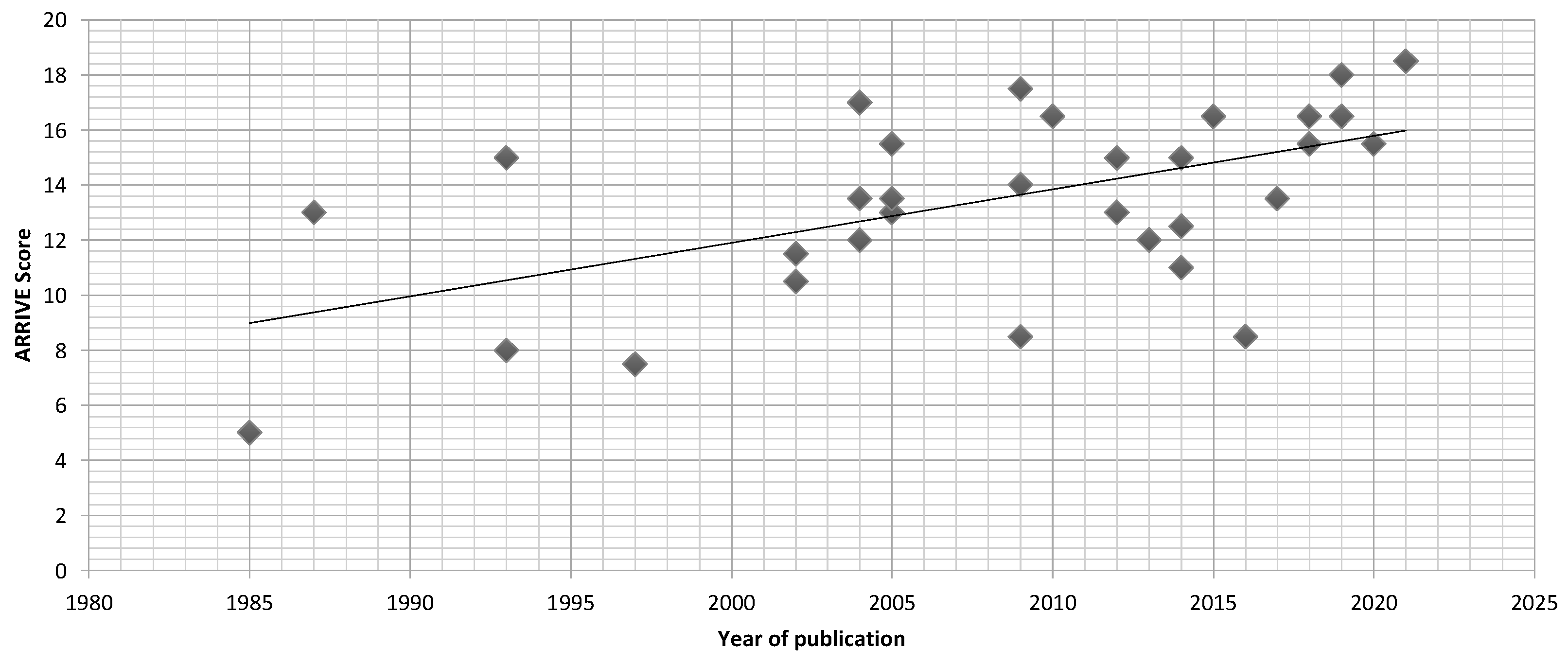


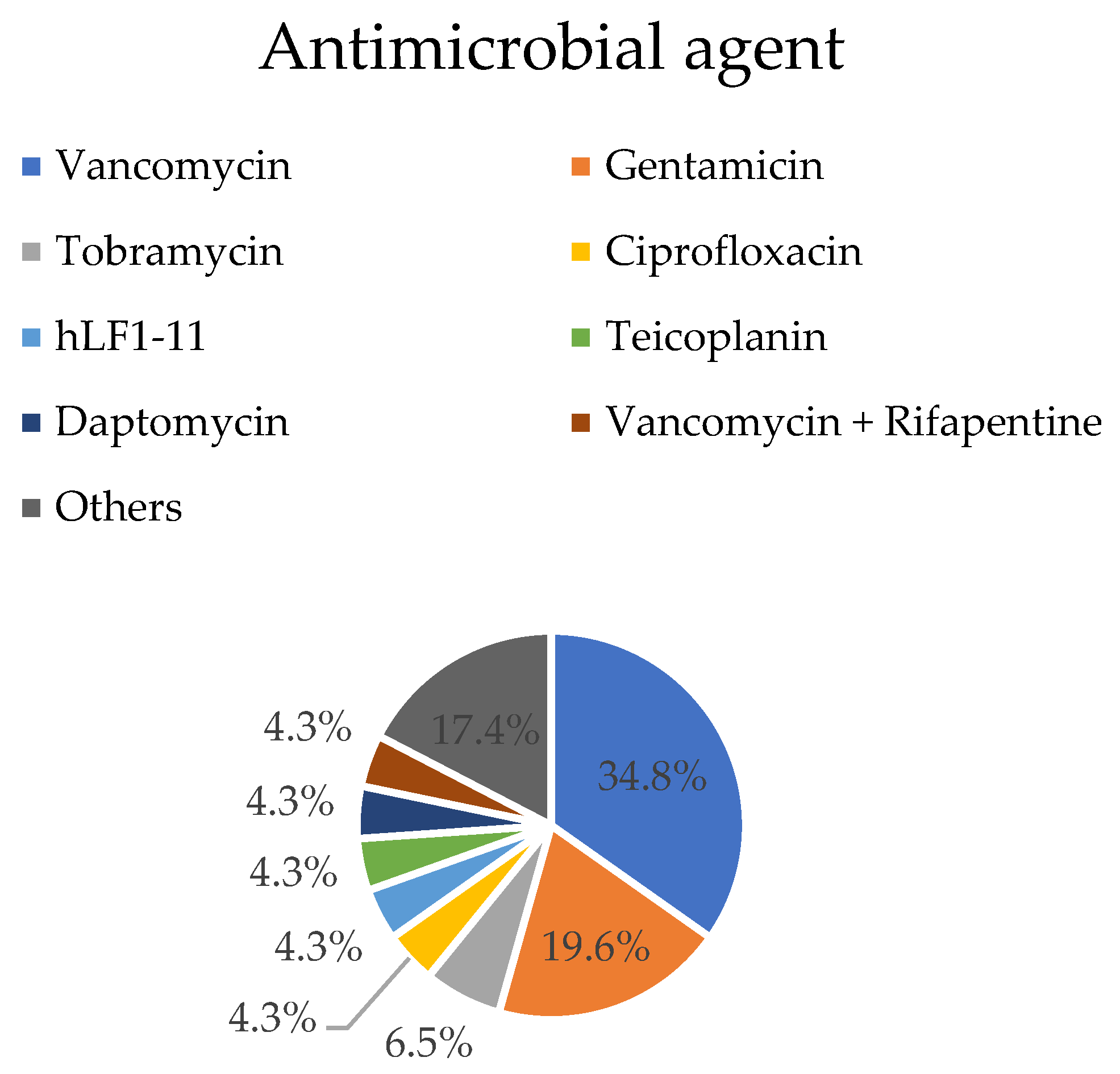
| Author | ARRIVE | Animal | nE | nC | Agent | Defect Location | T1 (w) | Antibiotic | Material | T2 (w) | Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eitenmuller [59] | 5 | Mongrel dogs | 6 * | 6 * | S. aureus | Proximal tibial metaphysis | 3 | Povidone-iodine | Hydroxyapatite granules | 2, 4, 9, and 10 | GO; Hist; X-ray |
| Mongrel dogs | 6 * | 6 * | S. aureus | Proximal tibial metaphysis | 3 | Flucloxacillin | Hydroxyapatite granules | 2, 4, 9, and 10 | GO; Hist; X-ray | ||
| Dahners [58] | 13 | NZWR | 10 | 8 | S. aureus | Proximal tibial metaphysis | 2 | Gentamicin | Calcium sulfate | 5 | GO; Hist; Microb; X-ray |
| Cornell [32] | 15 | NZWR | 22 | 9 | S. aureus | Proximal tibial metaphysis | 3 | Gentamicin | Hydroxyapatite beads | 6 and 17 | GO; Microb; X-ray |
| Korkusuz [46] | 8 | S-D rats | 25 | 25 | S. aureus | Proximal tibial metaphysis | 7 | Gentamicin | Hydroxyapatite | 1, 2, 3, 4, and 5 | Hist; Microb; X-ray |
| Itokazu [36] | 7.5 | Wistar rats | 21 | 21 | S. aureus | Proximal tibial metaphysis | 4 | Arbekacin | Hydroxyapatite blocks | 1, 3, 5, and 7 | Hist; X-ray |
| Nelson [19] | 11.5 | NZWR | 13 | 13 | S. aureus | Radial diaphysis | 4 | Tobramycin | Calcium sulfate pellets | 4 | Hist; Lab; Microb; X-ray |
| Shirtliff [49] | 10.5 | NZWR | 12 | 10 | MRSA | Proximal tibial metaphysis | 2 | Vancomycin | Hydroxyapatite cement | 4 | Microb; X-ray |
| Buxton [28] | 12 | S-D rats | 6 | 6 | S. aureus | Tibia diaphysis | 0 | Ciprofloxacin (1) | Calcium phosphate cement | 2 | GO; Lab |
| Joosten [40] | 13.5 | NZWR | 11 | 6 | MSSA | Proximal tibial metaphysis | 3 | Gentamicin | Hydroxyapatite cement | 3 | GO; Hist; Lab; Microb; X-ray |
| Stallmann [50] | 17 | NZWR | 7 | 8 | MSSA | Proximal femur | 0 | hLF1-11 | Hydroxyapatite cement | 3 | Hist; Lab; X-ray |
| NZWR | 6 | 8 | MSSA | Proximal femur | 0 | Gentamicin | Hydroxyapatite cement | 3 | Hist; Lab; X-ray | ||
| Faber [33] | 15.5 | NZWR | 8 | 6 | MRSA | Proximal tibial metaphysis | 3 | Gentamicin | Calcium phosphate cement | 3 | GO; Hist; Lab; Microb; X-ray |
| NZWR | 8 | 6 | MRSA | Proximal tibial metaphysis | 3 | hLF1-11 | Calcium phosphate cement | 3 | GO; Hist; Lab; Microb; X-ray | ||
| Joosten [41] | 13.5 | NZWR | 6 | 5 | S. aureus SCV | Proximal tibial metaphysis | 3 | Vancomycin | Hydroxyapatite cement | 3 and 6 | GO; Hist; Lab; Microb |
| NZWR | 7 | 6 | MRSA | Proximal tibial metaphysis | 3 | Vancomycin | Hydroxyapatite cement | 3 and 6 | GO; Hist; Lab; Microb | ||
| Koort [45] | 13.5 | NZWR | 9 | 5 | MSSA | Proximal tibial metaphysis | 2 | Ciprofloxacin | Microspheres of bioactive glass (2) | 6 | Hist; Microb; PET; pQCT; SEM; X-ray |
| Thomas [51] | 13 | Spanish goats | 12 | 12 | MSSA (3) | Proximal tibial metaphysis | 0 | Tobramycin | Calcium sulfate pellets | 3 | GO; Microb; X-ray |
| Hui [35] | 8.5 | NZWR | 6 | 6 | MSSA | Proximal tibial metaphysis | 2 | Gentamicin (4) | Calcium sulfate | 2 | GO; Microb; X-ray |
| NZWR | 6 | 6 | MSSA | Proximal tibial metaphysis | 2 | Gentamicin | Calcium sulfate | 2 | GO; Microb; X-ray | ||
| Kanellakopoulou [42] | 14 | NZWR | 36 | 18 | MRSA | Proximal tibial metaphysis | 3 | Moxifloxacin | Calcium sulfate (5) | 1, 2, 3, 4, 5, and 6 | Hist; Microb |
| Xie [52] | 17.5 | NZWR | 16 | 11 | MRSA | Proximal tibial metaphysis | 3 | Vancomycin | Borate glass pellets | 8 | GO; Hist; Lab; Microb; X-ray |
| Jia [37] | 16.5 | NZWR | 12 | 12 | MRSA | Proximal tibial metaphysis | 3 | Teicoplanin | Calcium sulfate paste | 6 | GO; Hist; Lab; Microb; X-ray |
| Jia [38] | 16.5 | NZWR | 14 | 14 | MRSA | Proximal tibial metaphysis | 4 | Teicoplanin | Borate glass pellets | 12 | GO; Hist; Lab; Microb; X-ray |
| Jiang [39] | 15 | NZWR | 20 | 20 | MRSA | Proximal tibial metaphysis | 3 | Vancomycin | Nanohydroxyapatite pellets | 1, 2, 3, 6, and 12 | GO; Hist; Microb; X-ray |
| Kaya [44] | 13 | NZWR | 7 | 7 | MRSA | Proximal tibial metaphysis | 3 | Tigecycline | Calcium hydroxyapatite cement | 3 | GO; Hist; Microb; X-ray |
| Huang [34] | 12 | NZWR | 12 | 12 | S. aureus (6) | Radial diaphysis | 0 | Vancomycin | Calcium phosphate cement (7) | 4, 8, and 12 | GO; Hist; X-ray |
| Beenken [27] | 11 | NZWR | 6 | 6 | MSSA | Radial diaphysis | 3 | Daptomycin | Calcium sulfate hemihydrate | 3 | Hist; Microb; X-ray |
| NZWR | 6 | 6 | MSSA | Radial diaphysis | 3 | Daptomycin | Calcium sulfate (8) | 3 | Hist; Microb; X-ray | ||
| Chung [31] | 12.5 | NZWR | 6 | 6 | MRSA | Tibia | 3 | Vancomycin (9) | Calcium phosphate cement | 3 | GO; Hist; Lab |
| NZWR | 6 | 6 | MRSA | Tibia | 3 | Vancomycin | Calcium phosphate cement | 3 | GO; Hist; Lab | ||
| Kankilic [43] | 15 | S-D rats | 10 | 12 | MRSA | Proximal tibial metaphysis | 3 | Vancomycin | β-tricalcium phosphate (10) | 1 and 6 | Hist; Microb; X-ray |
| S-D rats | 10 | 12 | MRSA | Proximal tibial metaphysis | 3 | Vancomycin (coated) | β-tricalcium phosphate (10) | 1 and 6 | Hist; Microb; X-ray | ||
| Yan [53] | 16.5 | NZWR | 8 | 8 | MSSA | Proximal tibial metaphysis | 4 | Rifapentine (11) | Bone-like hydroxyapatite scaffold (12) | 4 and 12 | GO; Hist; Lab; Microb; X-ray |
| Cao [29] | 8.5 | NZWR | 5 | 5 | MSSA | Proximal tibial metaphysis | (13) | Vancomycin (11) | Hydroxyapatite scaffold (12) | 4, 8, and 12 | GO; Hist; Lab; Microb; X-ray |
| NZWR | 5 | 5 | MRSA | Proximal tibial metaphysis | (13) | Vancomycin (11) | Bone-like hydroxyapatite (12)(14) | 4, 8, and 12 | GO; Hist; Lab; Microb; X-ray | ||
| Cao [30] | 13.5 | NZWR | 12 | 12 | MSSA | Proximal tibial metaphysis | (13) | Vancomycin | Bone-like hydroxyapatite (12) | NA | GO; Hist; Lab; Microb; X-ray |
| NZWR | 12 | 12 | MRSA | Proximal tibial metaphysis | (13) | Vancomycin | Bone-like hydroxyapatite (12) | NA | GO; Hist; Lab; Microb; X-ray | ||
| Lulu [47] | 15.5 | NZWR | 4 | 4 | S. aureus | Tibia midshaft | 0 | Tobramycin | Calcium phosphate beads | 4 | GO; Hist; Lab; Microb; X-ray |
| Melicherčík [48] | 16.5 | Wistar rats | 8 | 8 | MRSA | Femoral cavities | 1 | AMP | Calcium phosphate | 1 | X-ray |
| Egawa [54] | 18.0 | Wistar rats | 18 | 18 | MSSA | Distal femur | 1 | Cefazolin | Hydroxyapatite/collagen sponge | 1, 2, and 4 | GO; Hist; Micro-CT, Microb |
| Wistar rats | 18 | 18 | MSSA | Distal femur | 1 | Vancomycin | Hydroxyapatite/collagen sponge | 1, 2, and 4 | GO; Hist; Micro-CT, Microb | ||
| Hasan [55] | 16.5 | S-D rats | 5 | 12 | S. aureus | Proximal tibial metaphysis | 0 | Vancomycin and rifampicin | Hydroxyapatite (15) | 10 | GO; Hist; Microb; X-ray |
| S-D rats | 3 | 3 | S. aureus | Proximal tibial metaphysis | 0 | Vancomycin and rifampicin | Hydroxyapatite (15) | 6 | GO; Hist; Microb; X-ray | ||
| Liu [57] | 15.5 | NZWR | 10 | 10 | MSSA | Proximal tibial metaphysis | (13) | Vancomycin | Platelet-lysate/nano-hydroxiapatite | 1, 2, 3, 6, and 12 | Hist; X-ray |
| Dvorzhinskiy [56] | 18.5 | S-D Rats | 32 | 20 | S. aureus | Proximal tibial metaphysis | 3 | Gentamicin | Hydroxyapatite/CaS | 6 and 26 | GO; Hist; Micro-CT; Microb |
| Author | Antibiotic Characteristics | Ceramic Used | Discussion | |||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic Used | Formulation | Amount | Material | Form | Amount | Infection | Bone Formation | |
| Buxton [28] | Ciprofloxacin | E41 (1) | 0.35% wt | Calcium phosphate | Granules | 0.1 mL (2) | Fewer bacteria; no gross signs of osteomyelitis | Histological evidence of bone healing |
| Stallmann [50] | hLF1-11 (3) | NI | 5% wt | Calcium phosphate | Injectable cement | NI | Significant decrease in viable bacteria | Not different from the control group |
| Gentamicin | NI | 5% wt | Calcium phosphate | Injectable cement | NI | Significant decrease in viable bacteria | Better remodeling by ingrowing bone | |
| Thomas [51] | Tobramycin | Powder | 10% wt | Calcium sulfate | Pellets | Fifteen pellets/animal | Prevented infection in 10/12 animals | NI |
| Huang [34] | Vancomycin | Solution (4) | 2 mg IC and 20 mg vancomycin per cylinder | Calcium phosphate | Cylinders (4 mm × 15 mm) | One cylinder/animal | No bacteria were detected; all controls showed signs of infection | Defects were completely repaired by the 12th week; all controls showed progression of bone destruction |
| Lulu [47] | Tobramycin | Solution | NI (5) | Calcium phosphate | 0.2 g beads | One bead/animal | Inhibition of the S. aureus growth | Repaired bone defect and recanalization of the medullary cavity |
| Hasan [55] | Vancomycin and rifampicin | Solution | NI (6) | Hydroxyapatite over a calcium carbonate core | Cylinders (4 mm diameter × 3.5 mm height) | One cylinder/animal | Disappearance of all clinical, imagological, and microbiological signs of infection | Healed bone with cortical bridging, new bone growth, and osseointegration |
| Vancomycin and rifampicin | Solution | NI (6) | Hydroxyapatite over a calcium carbonate core | Cylinders (4 mm diameter × 3.5 mm height) | One cylinder/animal | Healing without any signs of infection | New bone formation, ongoing bridging of newly formed bone, and limited mature collagen structure | |
| Author | Antibiotic Characteristics | Ceramic Used | Discussion | |||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic Used | Formulation | Amount | Material | Form | Amount/Animal | Infection | Bone Formation | |
| Dahners [58] | Gentamicin | Powder | 50 mg per cm3 of calcium sulfate | Calcium sulfate | Powder | 1 cm3 | Cure rate of 2/10; clinical and radiographic improvement in all other animals | NI |
| Eitenmuller [59] | Povidone-iodine | NI | 10% wt | Hydroxyapatite | Granules | NI | Resolution of clinical and radiological signs of infection | Good osteointegration of material at 10 weeks |
| Flucloxacillin | NI | 10% wt | Hydroxyapatite | Granules | NI | Resolution of clinical and radiological signs of infection | Only peripheral osteointegration of material at 10 weeks | |
| Cornell [32] | Gentamicin | Gentamicin sulfate and gentamicin crobefat | NI | Hydroxyapatite | Beads | 40 mg | Infection eradication in 16/22 animals | NI |
| Korkusuz [46] | Gentamicin | Powder | 5 mg/block | Hydroxyapatite | Blocks (4 × 3 × 3 mm) | One block | Eradication of infection without removal of the metal implants in all animals | NI |
| Itokazu [36] | Arbekacin | Powder | 0.84 mg/block | Hydroxyapatite | Blocks (2 × 2 × 3 mm) | One block | Cure in 5/7 rats | New bone formation was visible at the surface of the block and complete contact without fibrous tissue was evident at the interface between the bone and implant at 7 weeks |
| Nelson [19] | Tobramycin | Powder | 10% wt | Calcium sulfate | Pellets (3.4 mm diameter × 4.7 mm length, average weight of 100 mg) | Six pellets (average) | Infection cure in 11/13 animals | Rabbits showed 96% of the pellets resorbed and 51% bone formation in the original defect |
| Shirtliff [49] | Vancomycin | Powder | 10% wt | Hydroxyapatite | Powder | 2–7 g | Infection cure rate of 81.8% | NI |
| Joosten [40] | Gentamicin | Powder | 3.2% wt | Hydroxyapatite (1) | Paste | 1.4–2.5 g (average 2.0 g) | No evidence of infection in all animals | Little evidence of resorption |
| Faber [33] | Gentamicin | Powder | 5% wt | Calcium phosphate (2) | Paste | 2.4 ± 0.3 g | Absence of bacteria in 6/8 animals; imagiological signs of infection present in 5/8 | NI |
| hLF1-11 | NI | 5% wt | Calcium phosphate (2) | Paste | 2.2 ± 0.2 g | Infection cure in 5/8 animals; significantly reduced bacterial load in 2/8 | NI | |
| Joosten [41] | Vancomycin | Powder | 16% wt | Hydroxyapatite (1) | Cylinders (6 mm diameter × 12 mm length) | NI | No evidence of infection in all animals | Little evidence of resorption |
| Vancomycin | Powder | 16% wt | Hydroxyapatite (1) | Cylinders (6 mm diameter × 12 mm length) | NI | No evidence of infection in all animals | Little evidence of resorption | |
| Koort [45] | Ciprofloxacin | Powder | 7.6% wt | Bioactive glass (3) | Pellets (1 mm diameter × 0.9 mm length) | NI | Successful for eradication of the bone pathogen; soft tissue infections need systemic antimicrobial treatment | Need to perform a long-term follow-up of the osteoconductive response |
| Hui [35] | Gentamicin | Loaded liposomes | NI | Calcium sulfate | Powder | NI | Complete sterilization of bone (100% cure) | NI |
| Gentamicin | Powder | NI | Calcium sulfate | Powder | NI | More effective than controls, but did not sterilize all bone tissues | NI | |
| Kanellakopoulou [42] | Moxifloxacin | Powder | 10% wt | Calcium sulfate | Cylinder (50 mg) | One cylinder | Complete eradication of infection | NI |
| Xie [52] | Vancomycin | Powder | 8% wt | Borate glass | Pellets (6 mm × 6 mm) | NI | Treatment rate of 73.3% | Borate glass mostly reabsorbed and replaced by new bone |
| Jia [37] | Teicoplanin | Powder | 10% wt | Calcium sulfate | Pellets (4.7 mm diameter × 3.5 mm length) | NI | Lower radiological and histological scores and lower rate of MRSA culture, but did not resolve bone infection in all animals | Newly formed bone remodeled and restored to its original structural integrity |
| Jia [38] | Teicoplanin | Powder | 8% wt | Borate glass (4) | Pellets (4.7 mm diameter × 3.5 mm length) | NI | Lower rate of MRSA culture | Degradation of pellets and new bone formation |
| Jiang [39] | Vancomycin | Powder | 16% wt | Nanohydroxyapatite | Cylinders (3.2 mm diameter × 10 mm length) | NI | Bacteria count decreased significantly | Normal bone after 12 weeks |
| Kaya [44] | Tigecycline | Powder | 5% wt | Hydroxyapatite | Powder | 0.5–2 g | Decline in all clinical and imagological signs of infection | NI |
| Beenken [27] | Daptomycin | Powder | 15% wt | Calcium sulfate | Cylinders (4 mm diameter × 10 mm length) | One cylinder | Reduction in bacteria count was not different from controls | NI |
| Daptomycin | Powder | 15% wt | Calcium sulfate (5) | Cylinders (4 mm diameter × 10 mm length) | One cylinder | Significant reduction in bacteria count | NI | |
| Chung [31] | Vancomycin | Shells (6) | 20% wt | Calcium phosphate | Paste | NI | Highly effective local antibacterial activity | NI |
| Vancomycin | Powder | 5% wt | Calcium phosphate | Paste | NI | Reduction in inflammation signs | NI | |
| Kankilic [43] | Vancomycin | Beads | 10% wt | Calcium phosphate (7) | 1.5 mm diameter beads | NI | Cure of infection in all animals | Biocompatibility and osteointegration |
| Vancomycin | Coated beads | 10% wt | Calcium phosphate (7) | PLLA-coated 1.5 mm diameter beads | NI | Cure of infection in all animals | Biocompatibility and osteointegration | |
| Yan [53] | Rifapentine | Microspheres (8) | 4% wt | Hydroxyapatite (9) | Cylinders (5 mm diameter × 15 mm length) | One cylinder | Bacterial colony counts were extremely low, suggesting eradication of infection | Most of the material was degraded and new trabecular bone formed; bone shape gradually improved and returned to normal |
| Cao [29] | Vancomycin | Microspheres (10) | 8% wt | Hydroxyapatite (9) | Cylinders (5 mm diameter × 15 mm length) | 3 g | Progressive disappearance of imagological signs of infection | Scaffold almost integrated with complete healing of all bone defects |
| Vancomycin | Microspheres (10) | 8% wt | Hydroxyapatite (9) | Cylinders (5 mm diameter × 15 mm length) | 3 g | Progressive disappearance of imagological signs of infection | Scaffold almost integrated with complete healing of all bone defects | |
| Cao [30] | Vancomycin | Microspheres (10) | 8% wt | Hydroxyapatite (9) | Cylinders (5 mm diameter × 15 mm length) | 3 g | Curative ratio reached 75% | NI |
| Vancomycin | Microspheres (10) | 8% wt | Hydroxyapatite (9) | Cylinders (5 mm diameter × 15 mm length) | 3 g | Curative ratio reached 66.7% | NI | |
| Melicherčík [48] | Antimicrobial peptides (AMP) | NI | 5% wt | Calcium phosphate | Paste | NI | Reduced infection | Minimal signs of the presence of the carrier, probably as a result of its resorption |
| Egawa [54] | Cefazolin | Powder | 2% wt | Hydroxyapatite (11) | Sponges (3 × 3 × 4 mm) | One sponge | MSSA proliferation was prevented at week 2 | Some degradation of ceramic, without complete osteointegration |
| Vancomycin | Powder | 2% wt | Hydroxyapatite (11) | Sponges (3 × 3 × 4 mm) | One sponge | MSSA proliferation was prevented at week 1 | Implanted material was maintained and replaced with new bone at week 4 | |
| Liu [57] | Vancomycin | Powder | 16% wt | Hydroxyapatite | Cylinders (6 mm diameter × 20 mm length) | One cylinder | Progressive disappearance of radiographic and histological signs of infection | Lamellar bone was formed |
| Dvorzhinskiy [56] | Gentamicin | Powder | 0.29 mg (12) | Hydroxyapatite/calcium sulfate | Cylinders (3 mm diameter × 3 mm length) | One cylinder | No infection was detectable at both 6 weeks and 6 months | New bone growth was detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alegrete, N.; Sousa, S.R.; Peleteiro, B.; Monteiro, F.J.; Gutierres, M. Local Antibiotic Delivery Ceramic Bone Substitutes for the Treatment of Infected Bone Cavities and Bone Regeneration: A Systematic Review on What We Have Learned from Animal Models. Materials 2023, 16, 2387. https://doi.org/10.3390/ma16062387
Alegrete N, Sousa SR, Peleteiro B, Monteiro FJ, Gutierres M. Local Antibiotic Delivery Ceramic Bone Substitutes for the Treatment of Infected Bone Cavities and Bone Regeneration: A Systematic Review on What We Have Learned from Animal Models. Materials. 2023; 16(6):2387. https://doi.org/10.3390/ma16062387
Chicago/Turabian StyleAlegrete, Nuno, Susana R. Sousa, Bárbara Peleteiro, Fernando J. Monteiro, and Manuel Gutierres. 2023. "Local Antibiotic Delivery Ceramic Bone Substitutes for the Treatment of Infected Bone Cavities and Bone Regeneration: A Systematic Review on What We Have Learned from Animal Models" Materials 16, no. 6: 2387. https://doi.org/10.3390/ma16062387
APA StyleAlegrete, N., Sousa, S. R., Peleteiro, B., Monteiro, F. J., & Gutierres, M. (2023). Local Antibiotic Delivery Ceramic Bone Substitutes for the Treatment of Infected Bone Cavities and Bone Regeneration: A Systematic Review on What We Have Learned from Animal Models. Materials, 16(6), 2387. https://doi.org/10.3390/ma16062387










