Recycling of Contaminated Marine Sediment and Industrial By-Products through Combined Stabilization/Solidification and Granulation Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Framework of the Experimentation
2.2. Contaminated Marine Sediments
2.3. Geopolymers Precursors and Activator Solutions
2.4. Composition and Microstructural Analysis
2.5. S/S Testing and Granule Characterization
3. Results
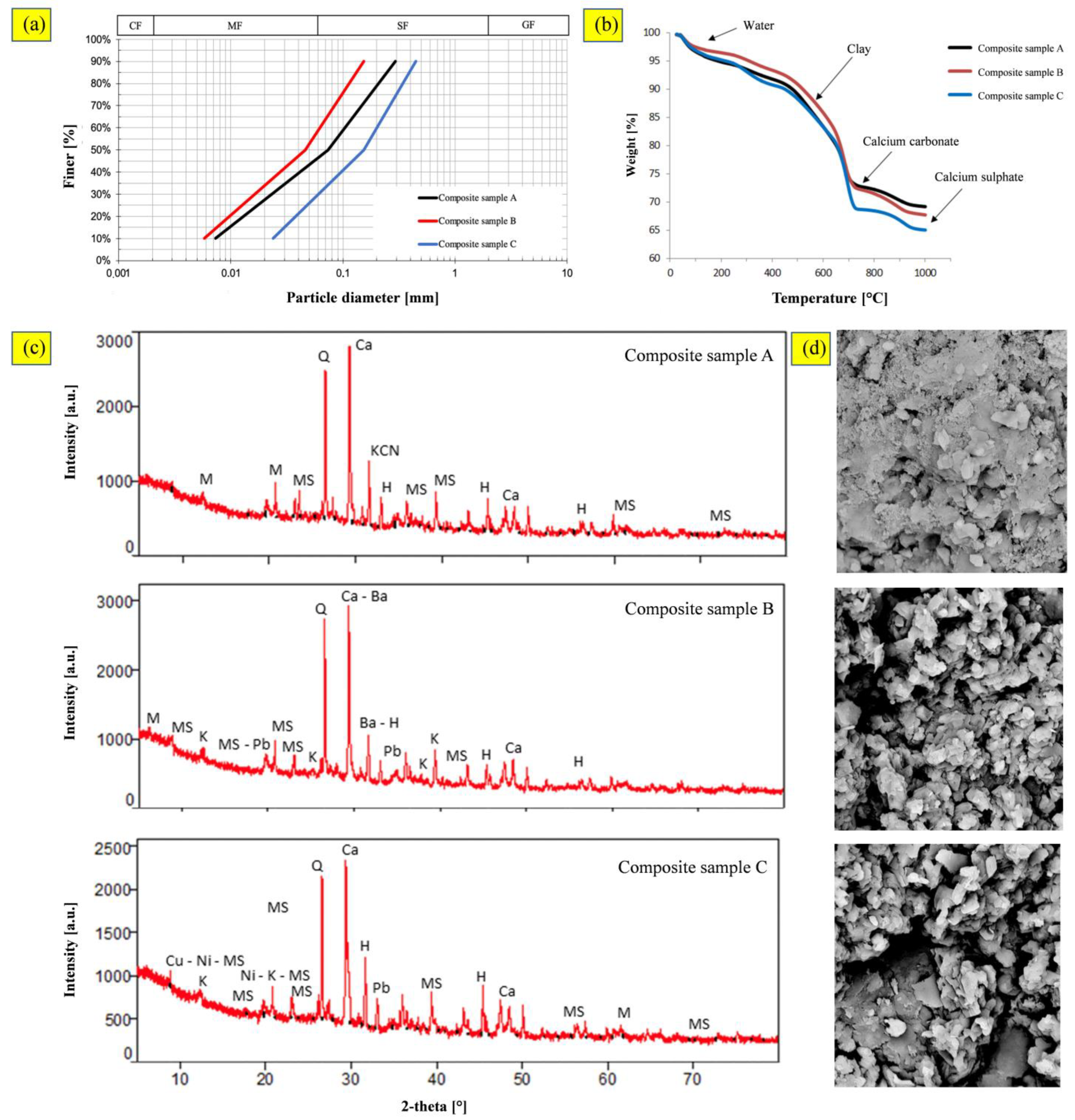
3.1. Contaminated-Marine-Sediment Characterization
3.2. Characterization of Geopolymers’ Precursors
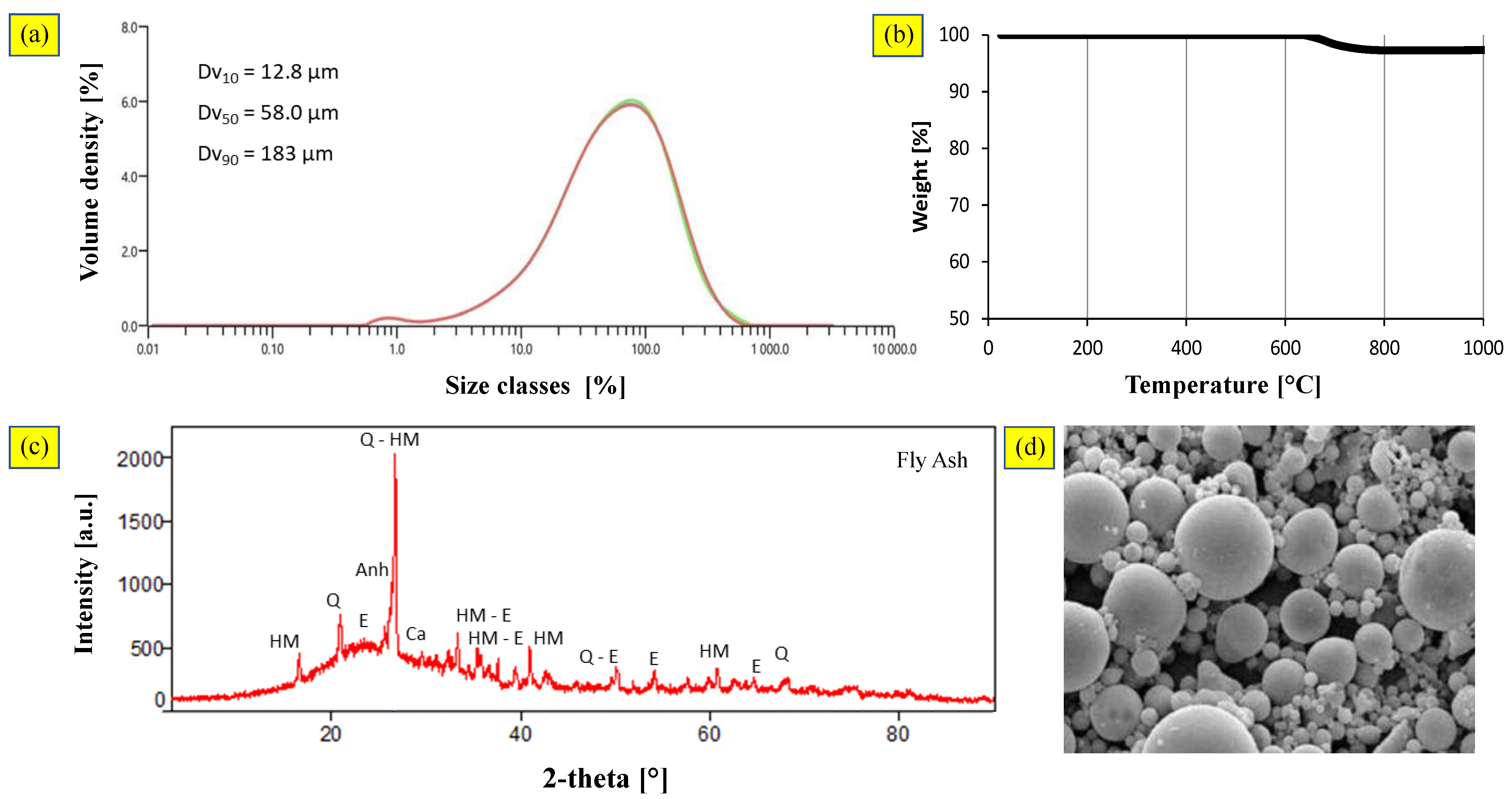
3.2.1. Coal Fly Ash
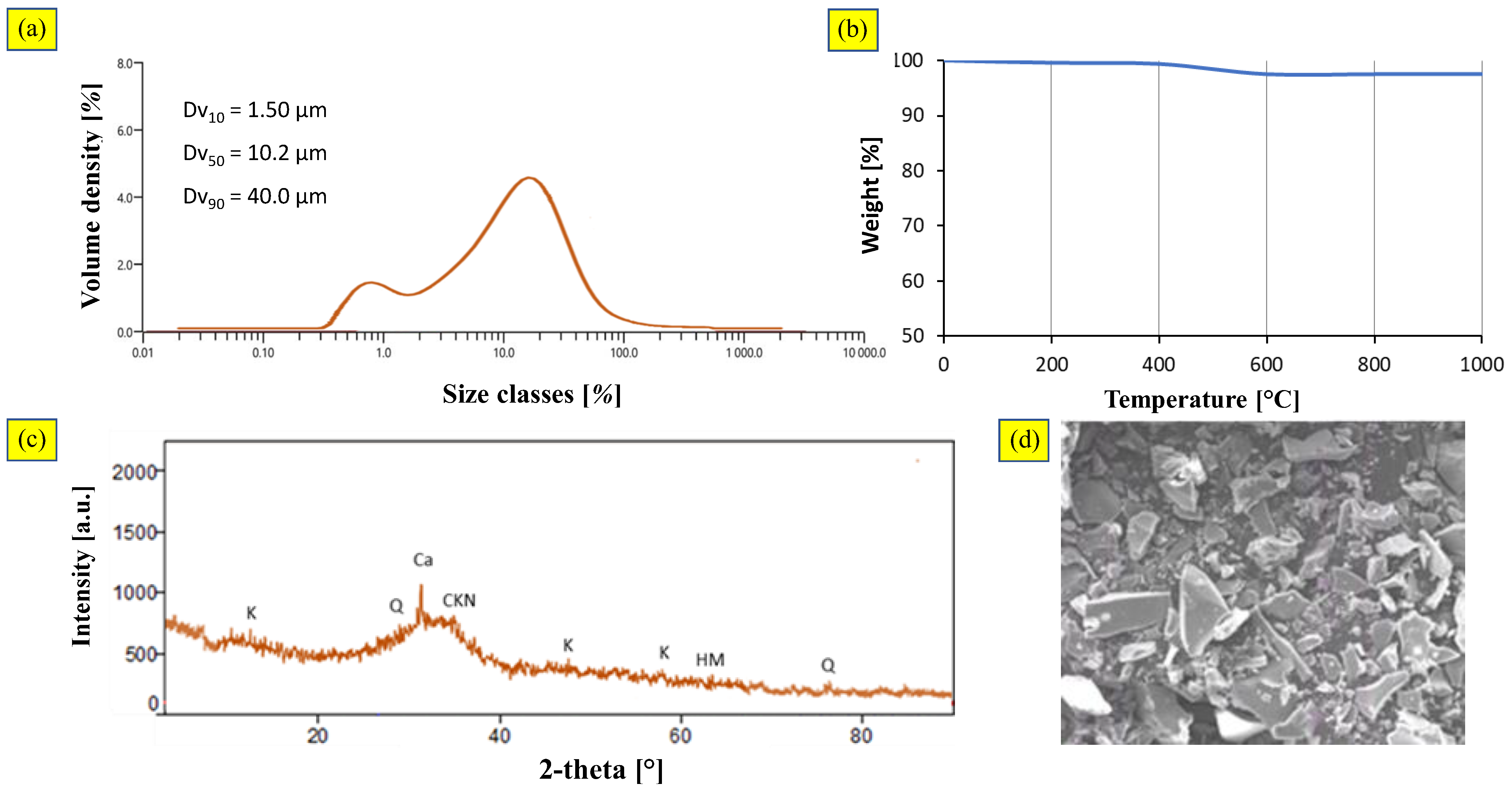
3.2.2. Blast Furnace Slag
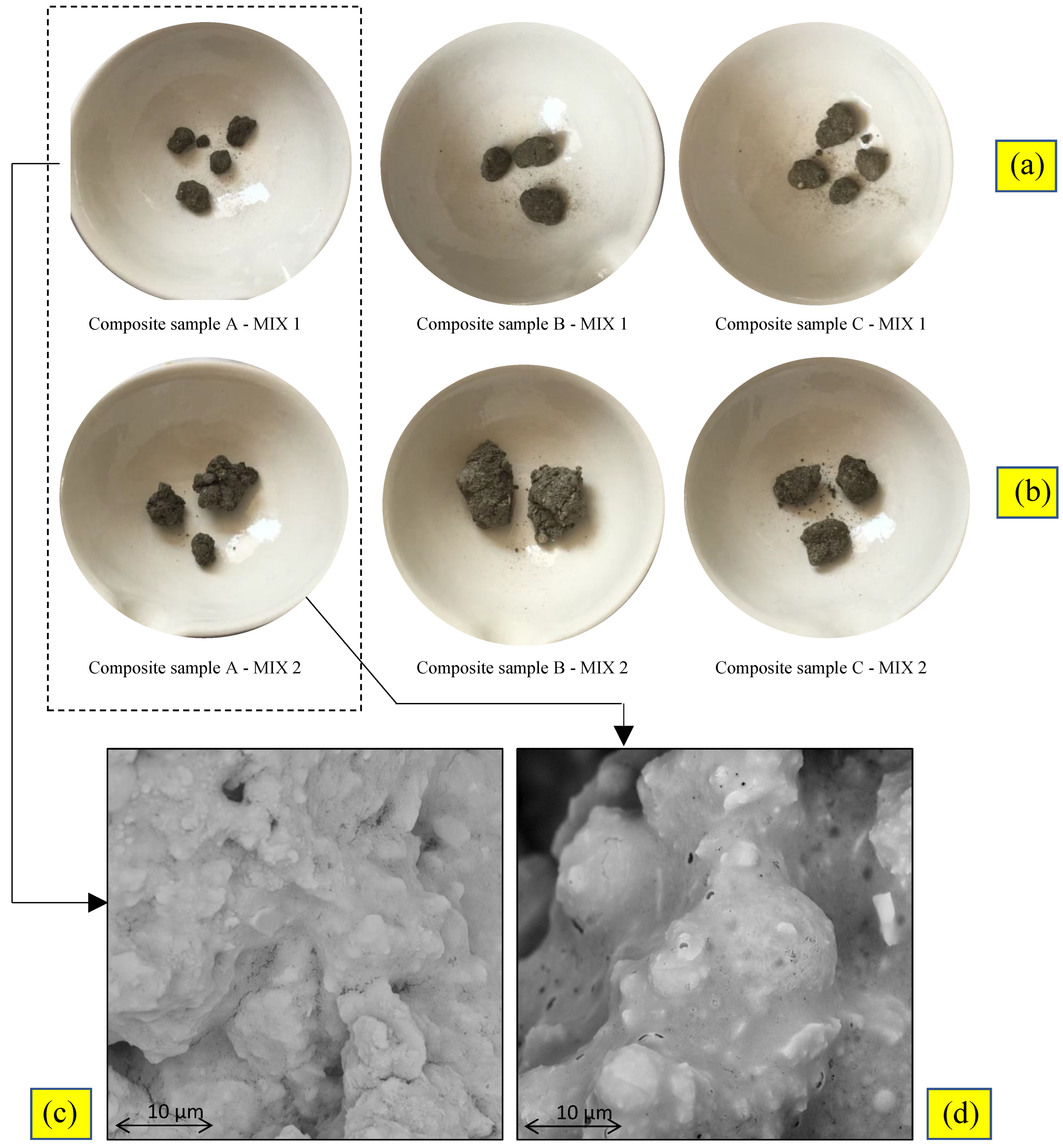
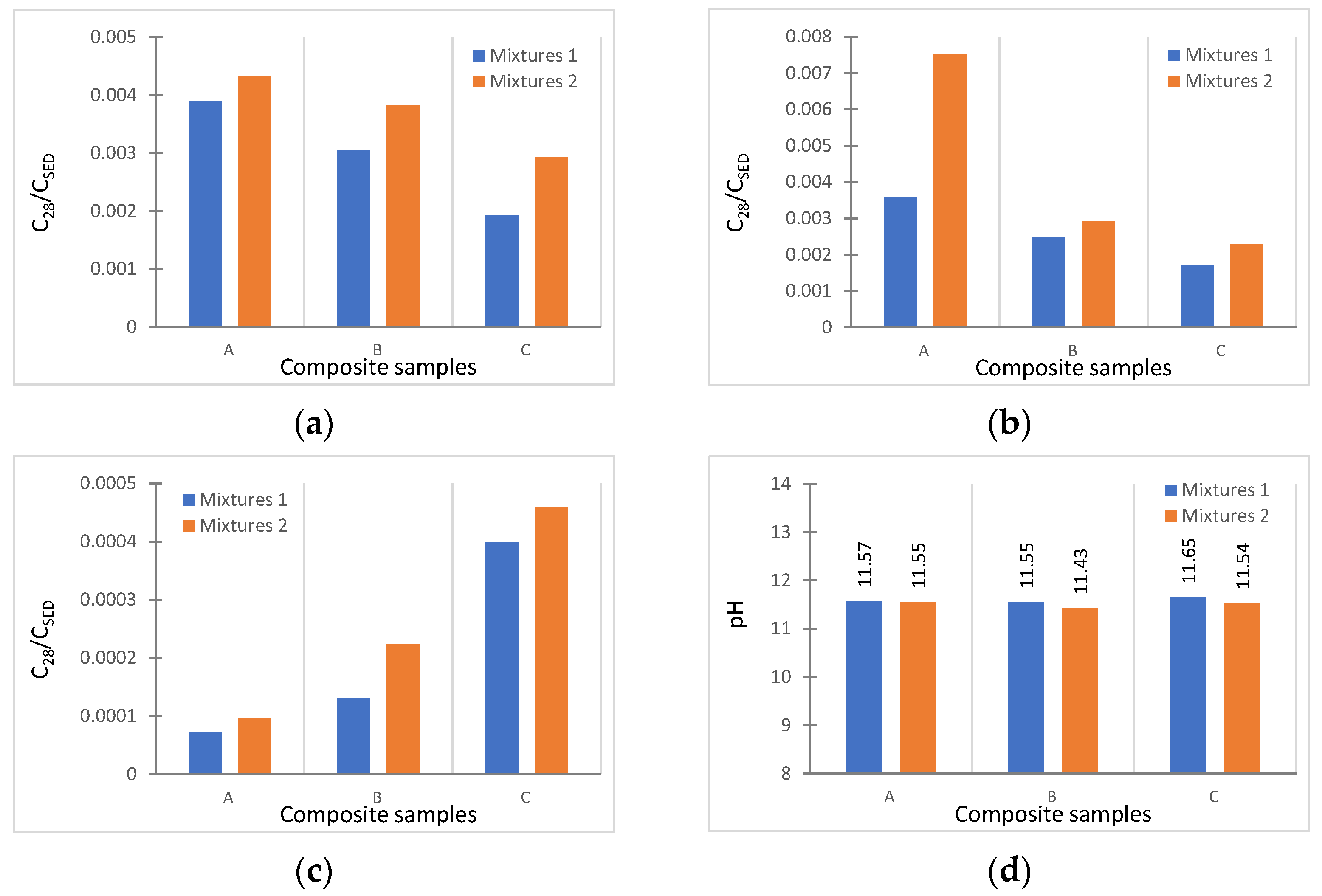
3.3. S/S Performance and Granule Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todaro, F.; Barjoveanu, G.; De Gisi, S.; Teodosiu, C.; Notarnicola, M. Sustainability assessment of reactive capping alternatives for the remediation of contaminated marine sediments. J. Clean. Prod. 2021, 286, 124946. [Google Scholar] [CrossRef]
- Abdel-shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Kujlu, R.; Moslemzadeh, M.; Rahimi, S.; Aghayani, E.; Ghanbari, F.; Mahdavianpour, M. Selecting the best stabilization/solidification method for the treatment of oil-contaminated soils using simple and applied best-worst multi-criteria decision-making method. Environ. Pollut. 2020, 263, 114447. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kwok, J.S.H.; Tsang, D.C.W.; Poon, C. Mixture design and treatment methods for recycling contaminated sediment. J. Hazard. Mater. 2015, 283, 623–632. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Yang, F. Binders alternative to Portland cement and waste management for sustainable construction Part 2. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar]
- Petrella, A.; Spasiano, D.; Rizzi, V.; Cosma, P.; Race, M.; De Vietro, N. Lead ion sorption by perlite and reuse of the exhausted material in the construction field. Appl. Sci. 2018, 8, 1882. [Google Scholar] [CrossRef]
- Wang, D.; Abriak, N.E.; Zentar, R. Strength and deformation properties of Dunkirk marine sediments solidified with cement, lime and fly ash. Eng. Geol. 2013, 166, 90–99. [Google Scholar] [CrossRef]
- Couvidat, J.; Benzaazoua, M.; Chatain, V.; Bouamrane, A.; Bouzahzah, H. Feasibility of the reuse of total and processed contaminated marine sediments as fine aggregates in cemented mortars. Construct. Build. Mater. 2016, 112, 892–902. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Molino, A.; Roviello, G.; Colangelo, F.; Molino, B.; Cioffi, R. Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: Upscaling from binders to precast paving cement-free bricks. Constr. Build. Mater. 2017, 133, 14–26. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Gubitosa, J.; Fini, P.; Petrella, A.; Agostiano, A.; Semeraro, P.; Cosma, P. An alternative use of olive pomace as a wide-ranging bioremediation strategy to adsorb and recover disperse orange and disperse red industrial dyes from wastewater. Separations 2017, 4, 29. [Google Scholar] [CrossRef]
- Tsang, D.C.; Olds, W.E.; Weber, P.A.; Yip, A.C. Soil stabilization using AMD sludge, compost and lignite: TCLP leachability and continuous acid leaching. Chemosphere 2013, 93, 2839–2847. [Google Scholar] [CrossRef]
- De Gisi, S.; Todaro, F.; Mesto, E.; Schingaro, E.; Notarnicola, M. Recycling contaminated marine sediments as filling materials by pilot scale stabilization/solidification with lime, organoclay and activated carbon. J. Clean. Prod. 2020, 269, 122416. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.; Kua, H.W.; Yang, J.; Ok, Y.S.; Ding, S.; Hou, D.; Poon, C.S. The roles of biochar as green admixture for sediment-based construction products. Cement. Concrete. Comp. 2019, 104, 103348. [Google Scholar] [CrossRef]
- Shubbar, A.A.; Jafer, H.; Dulaimi, A.; Hashim, K.; Atherton, W.; Sadique, M. The development of a low carbon binder produced from the ternary blending of cement, ground granulated blast furnace slag and high calcium fly ash: An experimental and statistical approach. Constr. Build. Mater. 2018, 187, 1051–1060. [Google Scholar] [CrossRef]
- Capobianco, O.; Costa, G.; Baciocchi, R. Assessment of the operating windows of a combined solidification/stabilization and granulation treatment applied to industrial soil in the context of brownfield regeneration. WIT Trans. Eco. Environ. 2014, 181, 577–588. [Google Scholar]
- Nakhaei, A.; Marandi, S.M.; Kermani, S.S.; Bagheripour, M.H. Dynamic properties of granular soils mixed with granulated rubber. Soil Dyn. Earthq. 2012, 43, 124–132. [Google Scholar] [CrossRef]
- Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Manufacture of artificial aggregate using MSWI bottom ash. J. Waste Manag. 2011, 31, 281–288. [Google Scholar] [CrossRef]
- Di Palma, L.; Medici, F.; Vilardi, G. Artificial aggregate from non metallic automotive shredder residue. Chem. Eng. Trans. 2015, 43, 1723–1728. [Google Scholar]
- Ren, P.; Ling, T.C.; Mo, K.H. Recent Advances in Artificial Aggregate Production. J. Clean. Prod. 2020, 291, 125215. [Google Scholar] [CrossRef]
- Vasugi, V.; Ramamurthy, K. Identification of design parameters influencing manufacture and properties of cold-bonded pond ash aggregate. Mater. Design 2014, 54, 264–278. [Google Scholar] [CrossRef]
- Kang, X.; Bate, B.; Chen, R.P.; Yang, W.; Wang, F. Physicochemical and mechanical properties of polymer-amended kaolinite and fly ash–kaolinite mixtures. J. Mater. Civ. Eng. 2019, 31, 04019064. [Google Scholar] [CrossRef]
- Lirer, S.; Liguori, B.; Capasso, I.; Flora, A.; Caputo, D. Mechanical and chemical properties of composite materials made of dredged sediments in a fly-ash based geopolymer. J. Environ. Manage 2017, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.A.; Solanki, C.H.; Kumar, S.; Reddy, K.R.; Du, Y.J. New ternary blend limestone calcined clay cement for solidification/stabilization of zinc contaminated soil. Chemosphere 2019, 235, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Cioffi, R. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Design 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Molino, B.; De Vincenzo, A.; Ferone, C.; Messina, F.; Colangelo, F.; Cioffi, R. Recycling of clay sediments for geopolymer binder production. A new perspective for reservoir management in the framework of Italian legislation: The Occhito reservoir case study. Materials 2014, 7, 5603–5616. [Google Scholar] [CrossRef]
- El-Eswed, B.I.; Yousef, R.I.; Alshaaer, M.; Hamadneh, I.; Al-Gharabli, S.I.; Khalili, F. Stabilization/solidification of heavy metals in kaolin/zeolite based geopolymers. Int. J. Miner. Process. 2015, 137, 34–42. [Google Scholar] [CrossRef]
- Jin, M.; Zheng, Z.; Sun, Y.; Chen, L.; Jin, Z. Resistance of metakaolin-MSWI fly ash based geopolymer to acid and alkaline environments. J. Non-Cryst. Solids 2016, 450, 116–122. [Google Scholar] [CrossRef]
- Colangelo, F.; Farina, I.; Travaglioni, M.; Salzano, C.; Cioffi, R.; Petrillo, A. Innovative Materials in Italy for Eco-Friendly and Sustainable Buildings. Materials 2021, 14, 2048. [Google Scholar] [CrossRef]
- Almadani, M.; Razak, R.A.; Abdullah, M.M.A.B.; Mohamed, R. Geopolymer-Based Artificial Aggregates: A Review on Methods of Producing, Properties, and Improving Techniques. Materials 2022, 15, 5516. [Google Scholar] [CrossRef]
- Takaluoma, E.; Samarina, T. Granulation techniques of geopolymers and alkali-activated materials. In Alkali-Activated Materials in Environmental Technology Applications; Woodhead Publishing: Sawston, UK, 2022; pp. 97–111. [Google Scholar]
- Todaro, F.; De Gisi, S.; Notarnicola, M. Sustainable remediation technologies for contaminated marine sediments: Preliminary results of an experimental investigation. Environ. Eng. Manag. J. 2018, 17, 2465–2471. [Google Scholar] [CrossRef]
- Todaro, F.; De Gisi, S.; Notarnicola, M. Contaminated marine sediment stabilization/solidification treatment with cement/lime: Leaching behaviour investigation. Environ. Sci. Pollut. Res. Int. 2020, 27, 21407–21415. [Google Scholar] [CrossRef]
- Cotecchia, F.; Vitone, C.; Sollecito, F.; Mali, M.; Miccoli, D.; Petti, R.; Milella, D.; Ruggieri, G.; Bottiglieri, O.; Corbelli, V.; et al. A geo-chemo-mechanical study of a highly polluted marine system (Taranto, Italy) for the enhancement of the conceptual site model. Sci. Rep. 2021, 11, 4017. [Google Scholar] [CrossRef]
- Vitone, C.; Federico, A.; Puzrin, A.M.; Ploetze, M.; Carrassi, E.; Todaro, F. On the geotechnical characterisation of the polluted submarine sediments from Taranto. Environ. Sci. Pollut. Res. 2016, 23, 12535–12553. [Google Scholar] [CrossRef]
- Petrillo, A.; Colangelo, F.; Farina, I.; Travaglioni, M.; Salzano, C.; Cioffi, R. Multi-criteria analysis for Life Cycle Assessment and Life Cycle Costing of lightweight artificial aggregates from industrial waste by double-step cold bonding palletization. J. Clean. Prod. 2022, 351, 131395. [Google Scholar] [CrossRef]
- Perumal, P.; Hasnain, A.; Luukkonen, T.; Kinnunen, P.; Illikainen, M. Role of Surfactants on the Synthesis of Impure Kaolin-Based Alkali-Activated, Low-Temperature Porous Ceramics. Open Ceram. 2021, 6, 10009. [Google Scholar] [CrossRef]
- Provis, J.L.; Arbi, K.; Bernal, S.A.; Bondar, D.; Buchwald, A.; Castel, A.; Chithiraputhiran, S.; Cyr, M.; Dehghan, A.; Dombrowski-Daube, K.; et al. RILEM TC 247-DTA round robin test: Mix design and reproducibility of compressive strength of alkali-activated concretes. Mater. Struct. 2019, 52, 1–13. [Google Scholar] [CrossRef]
- EN 13055; Lightweight Aggregates. iTeh: Toronto, ON, Canada, 2016.
- EN 12457-2; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges. iTeh: Toronto, ON, Canada, 2002.
- EPA. EPA Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma—Mass Spectrometry; EPA: Washington, DC, USA, 1994. [Google Scholar]
- UNI 11060; Cultural Heritage—Natural and Artificial Stones. European Committee for Standardization: Brussels, Belgium, 2003.
- BS 812; Methods for Sampling and Testing of Mineral Aggregates Sands and Filters. British Standard Institution: London, UK, 1960.
- ICRAM. Values of Intervention for Sediments of Areas Strongly Anthropized with Reference to the Site of Reclamation of National Interest in Taranto; Central Institute for Scientific and Technological Research applied to the Sea: Rome, Italy, 2004. (In Italian) [Google Scholar]
- Abora, K.; Beleña, I.; Bernal, S.A.; Dunster, A.; Nixon, P.A.; Provis, J.L.; Tagnit-Hamou, A.; Winnefeld, F. Durability and testing–Chemical matrix degradation processes. In Alkali Activated Materials; Springer: Dordrecht, The Netherlands, 2014; pp. 177–221. [Google Scholar]
- Gaál, F.; Szöllősy, I.; Arnold, M.; Paulik, F. Determination of the organic matter, metal carbonate and mobile water in soils simultaneous TG, DTG, DTA and EGA techniques. J. Therm. Anal. Calorim. 1994, 42, 1007–1016. [Google Scholar] [CrossRef]
- Oudghiri, F.; García-Morales, J.L.; Rodríguez-Barroso, M.R. Evaluation of Sediments Decontamination by Chelating Agents using Thermogravimetric Analysis. Int. J. Environ. Res. 2015, 9, 657–662. [Google Scholar]
- Bensharada, M.; Telford, R.; Stern, B.; Gaffney, V. Loss on ignition vs. thermogravimetric analysis: A comparative study to determine organic matter and carbonate content in sediments. J. Paleolimnol. 2021, 67, 191–197. [Google Scholar] [CrossRef]
- Labianca, C.; De Gisi, S.; Todaro, F.; Notarnicola, M. DPSIR model applied to the remediation of contaminated sites. A case study: Mar Piccolo of Taranto. Appl. Sci. 2020, 10, 5080. [Google Scholar] [CrossRef]
- Sarkar, A.; Rano, R.; Mishra, K.K.; Sinha, I.N. Particle size distribution profile of some Indian fly ash—A comparative study to assess their possible uses. Fuel Process. Technol. 2005, 86, 1221–1238. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Li, W.; Wang, Y.; Luo, G.; Qiao, Y.; Zhang, Y.; Xu, B. Using a material database and data fusion method to accelerate the process model development of high shear wet granulation. Sci. Rep. 2021, 11, 16514. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R.; Cachim, P. Waste and Supplementary Cementitious Materials in Concrete; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- FAO. Italian Ministerial Decree Identification of not-hazardous waste subjected to simplified recovery procedures. Ital. Off. J. 1988, 16, 88. (In Italian) [Google Scholar]
- Colangelo, F.; Messina, F.; Di Palma, L.; Cioffi, R. Recycling of non-metallic automotive shredder residues and coal fly-ash in cold-bonded aggregates for sustainable concrete. Compos. B Eng. 2017, 116, 46–52. [Google Scholar] [CrossRef]
- Hattaf, R.; Benchikhi, M.; Azzouzi, A.; El Ouatib, R.; Gomina, M.; Samdi, A.; Moussa, R. Preparation of Cement Clinker from Geopolymer-Based Wastes. Materials 2021, 14, 6534. [Google Scholar] [CrossRef]
- Pan, Y.; Rossabi, J.; Pan, C.; Xie, X. Stabilization/solidification characteristics of organic clay contaminated by lead when using cement. J. Hazard. Mater. 2019, 362, 132–139. [Google Scholar] [CrossRef]
- Shi, C.; Fernández-Jiménez, A. Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements. J. Hazard. Mater. 2006, 137, 1656–1663. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Al-Tabbaa, A.; Yi, Y.; Stegemann, J.A. pH-dependent leaching behaviour and other performance properties of cement-treated mixed contaminated soil. J. Environ. Sci. 2012, 24, 1630–1638. [Google Scholar] [CrossRef]
- Komonweeraket, K.; Cetin, B.; Aydilek, A.H.; Benson, C.H.; Edil, T.B. Effects of pH on the leaching mechanisms of elements from fly ash mixed soils. Fuel 2015, 140, 788–802. [Google Scholar] [CrossRef]
- De Gisi, S.; Romaniello, L.; Dalessandro, M.; Todaro, F.; Notarnicola, M. Recovery of iron rich residues from integrated steel making process by hydrated lime/molasses pressurised cold agglomeration. J. Clean. Prod. 2019, 233, 830–840. [Google Scholar] [CrossRef]
- Arfania, H.; Asadzadeh, F. Mobility of heavy metals after spiking in relation to sediment and metal properties: Leaching column study. J. Soils Sediment 2015, 15, 2311–2322. [Google Scholar] [CrossRef]
- Tigue, A.A.S.; Malenab, R.A.J.; Dungca, J.R.; Yu, D.E.C.; Promentilla, M.A.B. Chemical Stability and Leaching Behavior of One-Part Geopolymer from Soil and Coal Fly Ash Mixtures. Minerals 2018, 8, 411. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, D.; van Deventer, J.S.J. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef]
- Xu, H.; Gong, W.; Syltebo, L.; Izzo, K.; Lutze, W.; Pegg, I.L. Effect of blast furnace slag grades on fly ash based geopolymer waste forms. Fuel 2014, 133, 332–340. [Google Scholar] [CrossRef]
- Łach, M.; Korniejenko, K.; Walter, J.; Stefańska, A.; Mikuła, J. Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide. Materials 2020, 13, 495. [Google Scholar] [CrossRef]
- Łach, M.; Hebdowska-Krupa, M.; Komar, N. Strength and leachability of geopolymers with the addition of municipal solid waste ashes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 706, 012010. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Zhang, T.-T.; Jia, S.B.; Liu, J.; Quan, X.-H.; Zheng, W. Mechanical properties and leaching characteristics of geopolymer-solidified/stabilized lead-contaminated soil. Adv. Civ. Eng. 2019, 2019, 6015769. [Google Scholar] [CrossRef]
- Gesoğlu, M.; Güneyisi, E.; Alzeebaree, R.; Mermerdaş, K. Effect of silica fume and steel fiber on the mechanical properties of the concretes produced with cold bonded fly ash aggregates. Constr. Build. Mater. 2013, 40, 982–990. [Google Scholar] [CrossRef]
- Güneyisi, E.; Gesoğlu, M.; Mohamadameen, A.; Alzeebaree, R.; Algın, Z.; Mermerdaş, K. Enhancement of shrinkage behavior of lightweight aggregate concretes by shrinkage reducing admixture and fiber reinforcement. Constr. Build. Mater. 2018, 54, 91–98. [Google Scholar] [CrossRef]

| Mix | Activator Solutions | Geopolymer Precursors | Water | Fluidifier | Sediment | |||
|---|---|---|---|---|---|---|---|---|
| Na2SiO5 | NaOH | H2O | CFA | BFS | ||||
| 1 | 9.8 | 1.0 | 2.0 | 31.2 | 5.2 | 7.2 | 2.0 | 41.6 |
| 2 | 11.4 | 1.8 | 2.6 | 31.2 | 5.2 | 5.2 | 1.0 | 41.6 |
| Unit | Natural Sample | Limit Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP3 | SP4 | SP6 | SP7 | SP9 | SP11 | SP12 | SP14 | SP16 | SP17 | |||
| Chemo-Physical parameters | ||||||||||||
| pH | u. pH | 9.1 | 8.9 | 9.1 | 9.2 | 9.2 | 9.2 | 8.9 | 8.8 | 9.1 | 9.3 | - |
| Eh | mV | −109.5 | −100.9 | −106.5 | −115.8 | −114.6 | −117.8 | −98.2 | −100.9 | −116.9 | −122.7 | - |
| MC | % | 50.83 | 52.0 | 50.6 | 20.2 | 51.8 | 53.8 | 58.4 | 65.1 | 51.8 | 54.2 | - |
| OM | % | 19.90 | 23.4 | 19.4 | 20.2 | 17.1 | 18.8 | 12.9 | 23.7 | 18.5 | 17.5 | - |
| Ash | % | 29.27 | 24.6 | 30.0 | 59.6 | 31.1 | 27.4 | 28.7 | 11.2 | 29.7 | 28.3 | - |
| GF | % | 4.80 | 20.68 | 12.87 | 13.69 | 3.96 | 7.41 | 31.76 | 18.43 | 14.39 | 7.47 | - |
| SF | % | 12.20 | 44.16 | 14.87 | 27.72 | 4.28 | 15.61 | 21.97 | 33.37 | 12.23 | 14.76 | - |
| PF | % | 83.00 | 35.16 | 72.26 | 58.59 | 91.76 | 76.98 | 46.27 | 48.20 | 73.38 | 77.76 | - |
| Inorganic contaminants | ||||||||||||
| As | mg/kg | 11.6 | 31.7 | 48.4 | 65.2 | 9.2 | 13.3 | 13.4 | 19.0 | 11.8 | 18.3 | 20.0 |
| Be | mg/kg | 0.7 | 0.8 | 1.2 | 1.0 | 1.0 | 1.1 | 0.6 | 0.9 | 0.7 | 1.5 | - |
| Cd | mg/kg | 0.4 | 2.5 | 0.9 | 1.0 | 0.5 | 0.5 | 0.5 | 1.3 | 0.4 | 0.6 | 1.00 |
| Cr | mg/kg | 54.5 | 81.8 | 80.8 | 78.0 | 75.2 | 71.6 | 43.3 | 65.3 | 57.1 | 76.7 | 160.0 |
| Co | mg/kg | 6.5 | 8.4 | 9.9 | 11.1 | 8.5 | 7.00 | 4.9 | 7.1 | 5.5 | 7.9 | - |
| Cu | mg/kg | 53.5 | 172.1 | 121.2 | 131.9 | 58.4 | 64.6 | 62.9 | 117.8 | 55.3 | 80.2 | 45.0 |
| Pb | mg/kg | 102.5 | 314.6 | 241.3 | 392.0 | 50.2 | 94.0 | 57.9 | 106.7 | 74.8 | 126.7 | 50.0 |
| Hg | mg/kg | 4.2 | 8.1 | 14.3 | 24.0 | 1.2 | 3.5 | 1.5 | 2.5 | 2.6 | 5.9 | 0.80 |
| Ni | mg/kg | 31.9 | 40.6 | 50.1 | 57.2 | 48.9 | 37.5 | 26.8 | 44.2 | 30.4 | 41.8 | 100.0 |
| V | mg/kg | 56.7 | 64.0 | 105.8 | 75.0 | 73.6 | 77.1 | 62.0 | 103.2 | 59.9 | 84.4 | - |
| Zn | mg/kg | 146.3 | 541.5 | 430.8 | 464.2 | 116.2 | 193.8 | 206.9 | 423.8 | 159.0 | 222.7 | 110.0 |
| Organic contaminants | ||||||||||||
| ΣPAH | µg/kg | 2159 | 11,652 | 6552 | 5810 | 1263 | 922 | 714 | 3172 | 1902 | 4515 | 4000 |
| ΣPCB | µg/kg | 277 | 3163 | 22,253 | 19,283 | 2331 | 794 | 607 | 2345 | 1660 | 3184 | 190 |
| Unit | Composite Sample (Average ± St. Dev) | Limit Value | |||
|---|---|---|---|---|---|
| A | B | C | |||
| Chemo-Physical parameters | |||||
| pH | pH unit | 9.1 ± 0.5 | 9.1 ± 0.3 | 9.0 ± 0.4 | - |
| Eh | mV | −111.5 ± 2.9 | −114.0 ± 2.2 | −105.3 ± 2.6 | - |
| MC | % | 44.2 ± 0.5 | 29.4 ± 0.8 | 58.4 ± 0.9 | - |
| OM | % | 20.3 ± 0.5 | 18.4 ± 0.8 | 18.2 ± 0.9 | - |
| Ash | % | 35.5 ± 0.5 | 42.2 ± 0.8 | 23.4 ± 0.9 | - |
| GF | % | 13.7 ± 1.5 | 5.4 ± 1.5 | 21.5 ± 1.5 | - |
| SF | % | 25.4 ± 1.5 | 10.7 ± 1.5 | 22.5 ± 1.5 | - |
| PF | % | 60.9 ± 1.5 | 83.9 ± 1.5 | 56.0 ± 1.5 | - |
| Inorganic contaminants | |||||
| As | mg/kg | 40.5 ± 0.5 | 11.5 ± 0.4 | 15.0 ± 0.3 | 20.0 |
| Be | mg/kg | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | - |
| Ca | mg/kg | 1.2 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 1.00 |
| Cr | mg/kg | 79.2 ± 2.4 | 65.1 ± 1.6 | 54.3 ± 0.9 | 160.0 |
| Co | mg/kg | 9.3 ± 0.5 | 7.3 ± 0.1 | 5.8 ± 0.4 | - |
| Cu | mg/kg | 127.4 ± 4.6 | 58.9 ± 1.6 | 78.7 ± 2.9 | 45.0 |
| Pb | mg/kg | 268.7 ± 1.8 | 82.4 ± 1.1 | 79.8 ± 1.4 | 50.0 |
| Hg | mg/kg | 13.0 ± 0.2 | 3.0 ± 0.5 | 2.2 ± 0.6 | 0.80 |
| Ni | mg/kg | 47.4 ± 1.3 | 39.4 ± 1.7 | 33.8 ± 1.8 | 100.0 |
| V | mg/kg | 82.3 ± 1.8 | 69.1 ± 1.9 | 75.1 ± 1.5 | - |
| Zn | mg/kg | 664.8 ± 1.6 | 152.1 ± 1.1 | 263.2 ± 1.8 | 110.0 |
| Organic contaminants | |||||
| ΣPAHs | µg/kg | 7130 ± 70 | 1450 ± 65 | 1930 ± 83 | 4000 |
| ΣPCBs | µg/kg | 11,950 ± 55 | 1135 ± 40 | 1535 ± 45 | 190 |
| Leaching Characteristics of Granules | |||||||
|---|---|---|---|---|---|---|---|
| Mix | Sample | Parameter | As (µg/L) | Cu (µg/L) | Hg (µg/L) | Pb (µg/L) | Zn (µg/L) |
| 1 | A | Leachate concentration | 158 ± 6 | 554 ± 26 | <LOD | <LOD | 30 ± 1 |
| Environmental goals (a) | ✗ | ✗ | ✓ | ✓ | ✓ | ||
| B | Leachate concentration | 35 ± 14 | 147 ± 19 | < LOD | <LOD | 20 ± 1 | |
| Environmental goals (a) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| C | Leachate concentration | 29 ± 1 | 136 ± 10 | <LOD | <LOD | 65 ± 1 | |
| Environmental goals (a) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 2 | A | Leachate concentration | 175 ± 18 | 957 ± 29 | <LOD | <LOD | 40 ± 1 |
| Environmental goals (a) | ✗ | ✗ | ✓ | ✓ | ✓ | ||
| B | Leachate concentration | 44 ± 2 | 172 ± 20 | <LOD | <LOD | 34 ± 1 | |
| Environmental goals (a) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| C | Leachate concentration | 44 ± 2 | 181 ± 10 | <LOD | <LOD | 75 ± 1 | |
| Environmental goals (a) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Physical Characteristics of Granules | |||||||
| Mix | Sample | Parameter | Density (kg/m3) | Porosity (%) | WAC (%) | AIV (%) | |
| 1 | A | Test results | 1800 ± 53 | 9.17 ± 0.30 | 5.05 ± 0.05 | 32.75 ± 0.12 | |
| Technical goals (b) | ✓ | ✓ | ✓ | ✓ | |||
| B | Test results | 1830 ± 55 | 4.27 ± 0.15 | 2.27 ± 0.02 | 34.46 ± 0.13 | ||
| Technical goals (b) | ✓ | ✓ | ✓ | ✓ | |||
| C | Test results | 1840 ± 55 | 3.32 ± 0.10 | 1.64 ± 0.01 | 34.83 ± 0.12 | ||
| Technical goals (b) | ✓ | ✓ | ✓ | ✓ | |||
| 2 | A | Test results | 1810 ± 53 | 11.86 ± 0.35 | 6.56 ± 0.05 | 31.43 ± 0.12 | |
| Technical goals (b) | ✓ | ✓ | ✓ | ✓ | |||
| B | Test results | 1837 ± 55 | 7.06 ± 0.20 | 3.85 ± 0.03 | 33.21 ± 0.13 | ||
| Technical goals (b) | ✓ | ✓ | ✓ | ✓ | |||
| C | Test results | 1852 ± 56 | 4.44 ± 0.15 | 2.40 ± 0.02 | 33.56 ± 0.12 | ||
| Technical goals (b) | ✓ | ✓ | ✓ | ✓ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todaro, F.; Colangelo, F.; De Gisi, S.; Farina, I.; Ferone, C.; Labianca, C.; Petrella, A.; Cioffi, R.; Notarnicola, M. Recycling of Contaminated Marine Sediment and Industrial By-Products through Combined Stabilization/Solidification and Granulation Treatment. Materials 2023, 16, 2399. https://doi.org/10.3390/ma16062399
Todaro F, Colangelo F, De Gisi S, Farina I, Ferone C, Labianca C, Petrella A, Cioffi R, Notarnicola M. Recycling of Contaminated Marine Sediment and Industrial By-Products through Combined Stabilization/Solidification and Granulation Treatment. Materials. 2023; 16(6):2399. https://doi.org/10.3390/ma16062399
Chicago/Turabian StyleTodaro, Francesco, Francesco Colangelo, Sabino De Gisi, Ilenia Farina, Claudio Ferone, Claudia Labianca, Andrea Petrella, Raffaele Cioffi, and Michele Notarnicola. 2023. "Recycling of Contaminated Marine Sediment and Industrial By-Products through Combined Stabilization/Solidification and Granulation Treatment" Materials 16, no. 6: 2399. https://doi.org/10.3390/ma16062399
APA StyleTodaro, F., Colangelo, F., De Gisi, S., Farina, I., Ferone, C., Labianca, C., Petrella, A., Cioffi, R., & Notarnicola, M. (2023). Recycling of Contaminated Marine Sediment and Industrial By-Products through Combined Stabilization/Solidification and Granulation Treatment. Materials, 16(6), 2399. https://doi.org/10.3390/ma16062399














