Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution
Abstract
1. Introduction
2. Materials
3. Methodology
3.1. Mix Design
3.2. Alkali-Alkaline Activator and Test Sample Preparation
3.3. Preparation of Geopolymer Concrete Specimens and Testing Methods
4. Results and Discussions
4.1. Consistency of Fresh Concrete
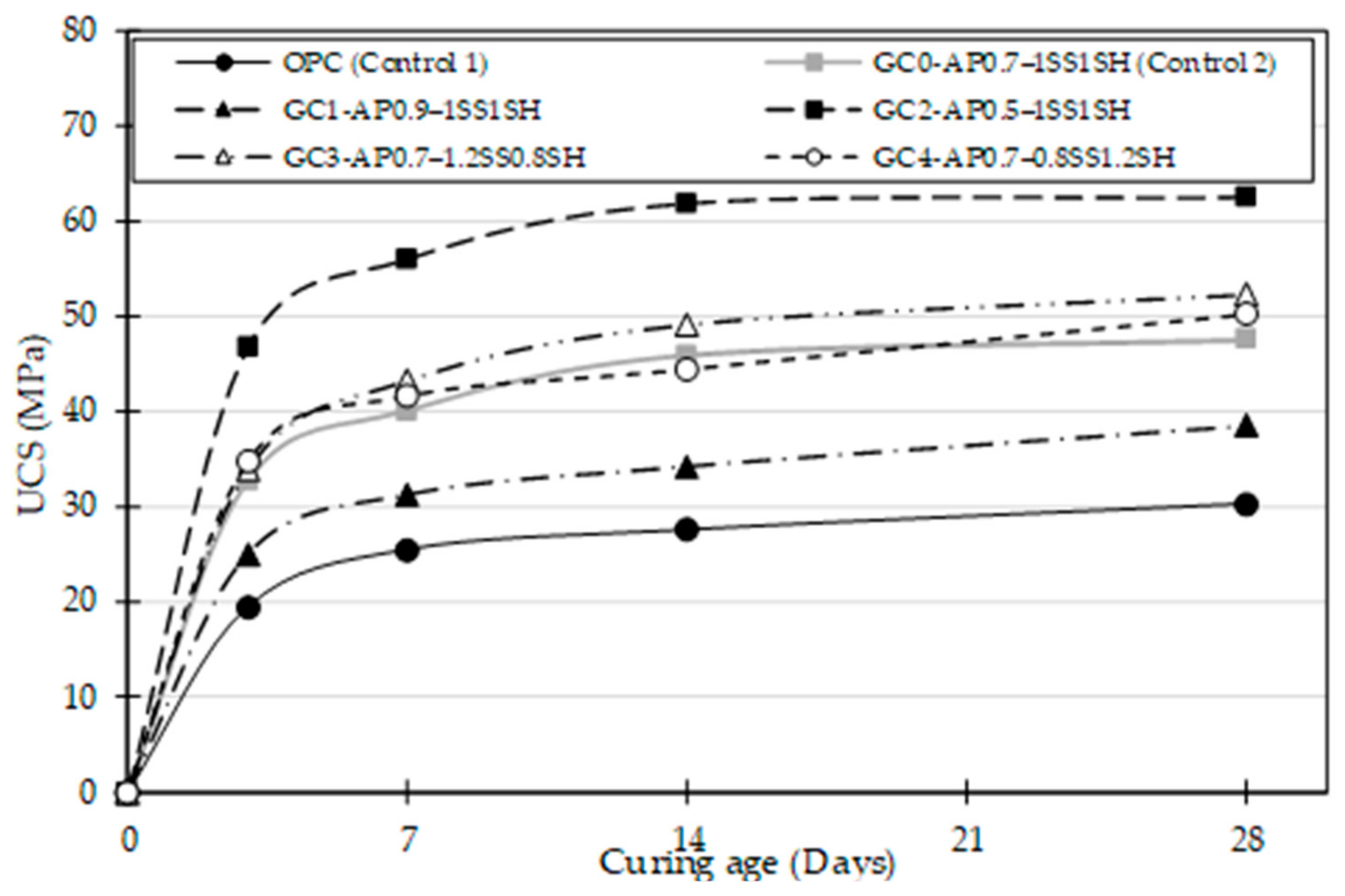
4.2. Concrete Strength Development
4.2.1. Unconfined Compressive Strength (UCS)
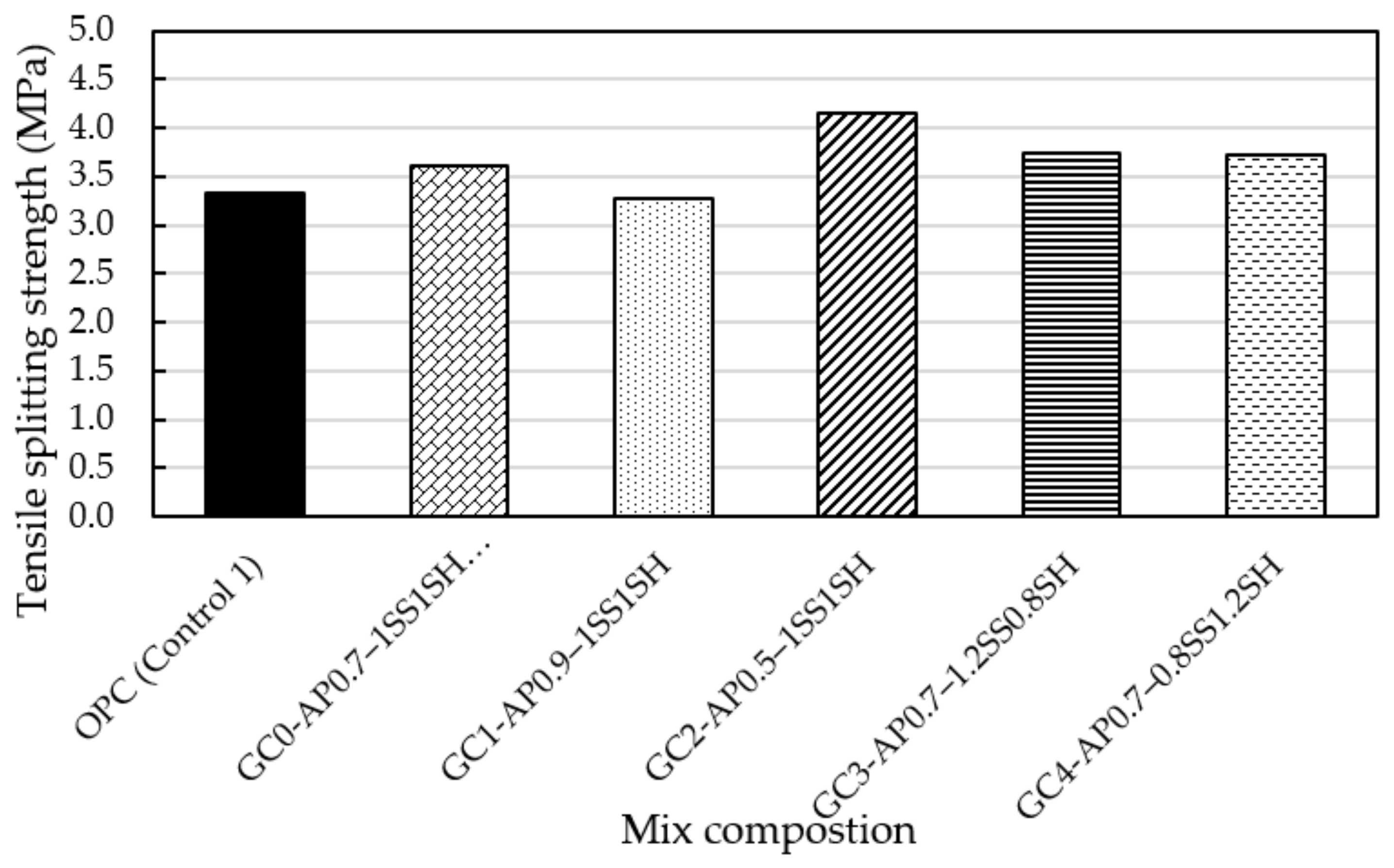
4.2.2. Tensile Splitting (TS) Test
5. Conclusions
- The impact of A/P ratio variations in the geopolymer mix GC0, GC1, and GC2 in terms of consistency indicated a reduction in slump values as the A/P ratio decreased from 0.5 to 0.9, while the compatibility degree showed a reversal of the identified trend in slump. Moreover, the variation in the SS/SH ratio (SS increase) at a fixed A/P ratio of 0.7 negatively impacted the consistency of the developed geopolymer concrete (slump reduction) due to the high viscosity of the SS solution;
- The formulated geopolymer concrete mixes (GC0–GC4) achieved higher UCS at all curing ages than the OPC (Control 1) mix, with mix GC2 attaining the highest UCS value of 62.6 MPa, thereby displaying the superiority of the alkali activation and polymerisation process over the CSH gel formation in ordinary concrete production;
- The effect of the A/P ratio observed in GC0, GC1, and GC2 mixes in terms of strength showed a UCS value increase at all curing ages (3, 7, 14, and 28 days) for every reduction in the A/P ratio. Therefore, in contrast to the decrease in UCS trend observed with an increase in the A/P ratio, efflorescence, which impacts the aesthetics of the concrete application, is likely to develop on the geopolymer concrete due to the excess alkaline compound in the concrete system;
- In terms of SS/SH variations, the UCS gain is minimal compared with the A/P ratio variations. This suggests that the A/P ratio has a more pronounced impact on strength gain, which is attributed to the geopolymerisation process;
- Depending on the concrete design strength or construction applications, a 0.5–0.7 A/P ratio with a SS/SH ratio of 1:1 or a slight SS increase of not more than 2% is advised.
- Although a laboratory-synthesised SF-derived geopolymer product was successful in this research, certain criteria should be considered in large-scale production on a construction site. As such, for properly storing activators and precursor materials under the required conditions, only machine mixing or ready-mix concrete should be employed because it replicates the mixing regime in the laboratory, and bath mixing operators must have adequate knowledge of geopolymer concrete.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warburton, R. Global Warming Has Concrete Problem When It Comes to CO2. EcoRi News. 2019. Available online: https://www.ecori.org/climate-change/2019/10/4/global-warming-has-a-co2ncrete-problem (accessed on 10 November 2021).
- Nishanth, L.; Patil, D.N.N. Experimental evaluation on workability and strength characteristics of self-consolidating geopolymer concrete based on GGBFS, flyash and alccofine. Mater. Today Proc. 2022, 59, 51–57. [Google Scholar] [CrossRef]
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.E. Impacts of MgO waste: GGBS formulations on the performance of a stabilised natural high sulphate bearing soil. Constr. Build. Mater. 2022, 315, 125745. [Google Scholar] [CrossRef]
- Abdulrahman, H.; Muhamad, R.; Visintin, P.; Azim Shukri, A. Mechanical properties and bond stress-slip behaviour of fly ash geopolymer concrete. Constr. Build. Mater. 2022, 327, 126909. [Google Scholar] [CrossRef]
- Raza, M.H.; Zhong, R.Y. A sustainable roadmap for additive manufacturing using geopolymers in construction industry. Resour. Conserv. Recycl. 2022, 186, 106592. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Simão, L.; Fernandes, E.; Hotza, D.; Ribeiro, M.J.; Montedo, O.R.K.; Raupp-Pereira, F. Controlling efflorescence in geopolymers: A new approach. Case Stud. Constr. Mater. 2021, 15, e00740. [Google Scholar] [CrossRef]
- Skariah Thomas, S.; Yang, J.; Bahurudeen, A.; Chinnu, S.N.; Abdalla, J.A.; Hawileh, R.A.; Hamada, H.M. Geopolymer concrete incorporating recycled aggregates: A comprehensive review. Clean. Mater. 2022, 3, 100056. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Wang, S.; Zeng, L.; Ran, P.; Ji, G. Effect of nano-SiO2 on the efflorescence of an alkali-activated metakaolin mortar. Constr. Build. Mater. 2020, 253, 118952. [Google Scholar] [CrossRef]
- Sun, Y.; Ghorbani, S.; Dai, X.; Ye, G.; De Schutter, G. Evaluation of rheology and strength development of alkali-activated slag with different silicates sources. Cem. Concr. Compos. 2022, 128, 104415. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials: State-Of-The-Art Report (RILEM TC 224-AAM); Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Duxson, P.; Fern’andez-Jim’enez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Cheng, Y.; Cong, P.; Zhao, Q.; Hao, H.; Mei, L.; Zhang, A.; Han, Z.; Hu, M. Study on the effectiveness of silica fume-derived activator as a substitute for water glass in fly ash-based geopolymer. J. Build. Eng. 2022, 51, 104228. [Google Scholar] [CrossRef]
- Liu, S.; Ott, W.K. Sodium silicate applications in oil, gas & geothermal well operations. J. Pet. Sci. Eng. 2020, 195, 107693. [Google Scholar] [CrossRef]
- Nordström, J.; Nilsson, E.; Jarvol, P.; Nayeri, M.; Palmqvist, A.; Bergenholtz, J.; Matic, A. Concentration- and pH-dependence of highly alkaline sodium silicate solutions. J. Colloid Interface Sci. 2011, 356, 37–45. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A. Geopolymer additive manufacturing: A review. Addit. Manuf. 2022, 55, 102782. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Dawood Abdul Khadar, S. Molarity activity effect on mechanical and microstructure properties of geopolymer concrete: A review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Cheah, C.B.; Tan, L.E.; Ramli, M. The engineering properties and microstructure of sodium carbonate activated fly ash/slag blended mortars with silica fume. Compos. Part B Eng. 2019, 160, 558–572. [Google Scholar] [CrossRef]
- Dybeł, P.; Furtak, K. Influence of silica fume content on the quality of bond conditions in high-performance concrete specimens. Arch. Civ. Mech. Eng. 2017, 17, 795–805. [Google Scholar] [CrossRef]
- Billong, N.; Oti, J.; Kinuthia, J. Using silica fume based activator in sustainable geopolymer binder for building application. Constr. Build. Mater. 2021, 275, 122177. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.C.M.; Setunge, S.; Dissanayake, R. Life cycle assessment and cost analysis of fly ash–rice husk ash blended alkali-activated. J. Environ. Manag. 2021, 295, 113140. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- BS EN 197-1:2011; Cement. Part 1: Composition, Specifications and Conformity Criteria for Common Cements. BSI Standard Limited: London, UK, 2011.
- BS EN 15167-1:2006; Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout. Part 1: Definitions, Specifications and Conformity Criteria. BSI Standard Limited: London, UK, 2006.
- BS EN 12620:2002+A1:2008; Aggregates for ConcreteBSI Standard Limited: London, UK, 2008.
- Tale Masoule, M.S.; Bahrami, N.; Karimzadeh, M.; Mohasanati, B.; Shoaei, P.; Ameri, F.; Ozbakkaloglu, T. Lightweight geopolymer concrete: A critical review on the feasibility, mixture design, durability properties, and microstructure. Ceram. Int. 2022, 48, 10347–10371. [Google Scholar] [CrossRef]
- BS EN 12350-2:2019; Testing Fresh Concrete. Part 1: Slump Test. BSI Standard Limited: London, UK, 2019.
- BS EN 12350-4:2019; Testing Fresh Concrete. Part 4: Degree of Compactability. BSI Standard Limited: London, UK, 2019.
- BS EN 206:2013+A2:2021; Concrete. Specification, Performance, Production and Conformity. BSI Standard Limited: London, UK, 2021.
- BS EN 12390-2:2019; Testing Hardened Concrete. Part 2: Making and Curing Specimens for Strength Tests. BSI Standard Limited: London, UK, 2019.
- Xie, J.; Kayali, O. Effect of initial water content and curing moisture conditions on the development of fly ash-based geopolymers in heat and ambient temperature. Constr. Build. Mater. 2014, 67, 20–28. [Google Scholar] [CrossRef]
- BS EN 12390-3:2019; Testing Hardened Concrete. Part 3: Compressive Strength of Test Specimens. BSI Standard Limited: London, UK, 2019.
- BS EN 12390-6:2009; Testing Hardened Concrete. Part 6: Tensile Splitting Strength of Test Specimens. BSI Standard Limited: London, UK, 2009.
- Provis, J.L.; Duxson, P.; van Deventer, J.S.J. The role of particle technology in developing sustainable construction materials. Adv. Powder Technol. 2010, 21, 2–7. [Google Scholar] [CrossRef]
- Meesala, C.R.; Verma, N.K.; Kumar, S. Critical review on fly-ash based geopolymer concrete. Struct. Concr. 2019, 21, 1013–1028. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilisation and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Yaseri, S.; Hajiaghaei, G.; Mohammadi, F.; Mahdikhani, M.; Farokhzad, R. The role of synthesis parameters on the workability, setting and strength properties of binary binder based geopolymer paste. Constr. Build. Mater. 2017, 157, 534–545. [Google Scholar] [CrossRef]
- Sathonsaowaphak, A.; Chindaprasirt, P.; Pimraksa, K. Workability and strength of lignite bottom ash geopolymer mortar. J. Hazard. Mater. 2009, 168, 44–50. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Qiu, J.; Wu, P.; Zhao, Y. Alkali activation of blast furnace slag using a carbonate-calcium carbide residue alkaline mixture to prepare cemented paste backfill. Constr. Build. Mater. 2022, 320, 126234. [Google Scholar] [CrossRef]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix design and mechanical properties of geopolymer and alkali activated concrete: Review of the state-of-the-art and the development of a new unified approach. Constr. Build. Mater. 2020, 256, 119380. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Gomaa, E.; Sargon, S.; Kashosi, C.; ElGawady, M. Fresh properties and compressive strength of high calcium alkali activated fly ash mortar. J. King Saud Univ. Eng. Sci. 2017, 29, 356–364. [Google Scholar] [CrossRef]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Siyal, A.A.; Azizli, K.A.; Man, Z.; Ullah, H. Effects of Parameters on the Setting Time of Fly Ash Based Geopolymers Using Taguchi Method. Procedia Eng. 2016, 148, 302–307. [Google Scholar] [CrossRef]
- Upadhyay, H.; Mungule, M.; Iyer, K.K.R. Issues and challenges for development of geopolymer concrete. Mater. Today Proc. 2022, 65, 1567–1574. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Phoo-ngernkham, T.; Hanjitsuwan, S.; Horpibulsuk, S.; Poowancum, A.; Injorhor, B. Effect of calcium-rich compounds on setting time and strength development of alkali-activated fly ash cured at ambient temperature. Case Stud. Constr. Mater. 2018, 9, e00198. [Google Scholar] [CrossRef]
- Morsy, A.M.; Ragheb, A.M.; Shalan, A.H.; Mohamed, O.H. Mechanical Characteristics of GGBFS/FA-Based Geopolymer Concrete and Its Environmental Impact. Pract. Period. Struct. Des. Constr. 2022, 27, 04022017. [Google Scholar] [CrossRef]
- Gomes Silveira, N.C.; Figueiredo Martins, M.L.; Bezerra, A.C.d.S.; Gabriel da Silva Araújo, F. Ecological geopolymer produced with a ternary system of red mud, glass waste, and Portland cement. Clean. Eng. Technol. 2022, 6, 100379. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Fu, D.; Santini, T.C.; Jiang, J.; Hartley, W.; Xue, S. Simulation study for the formation of alkaline efflorescence on bauxite residue disposal areas following the phosphogypsum addition. J. Clean. Prod. 2020, 262, 121266. [Google Scholar] [CrossRef]
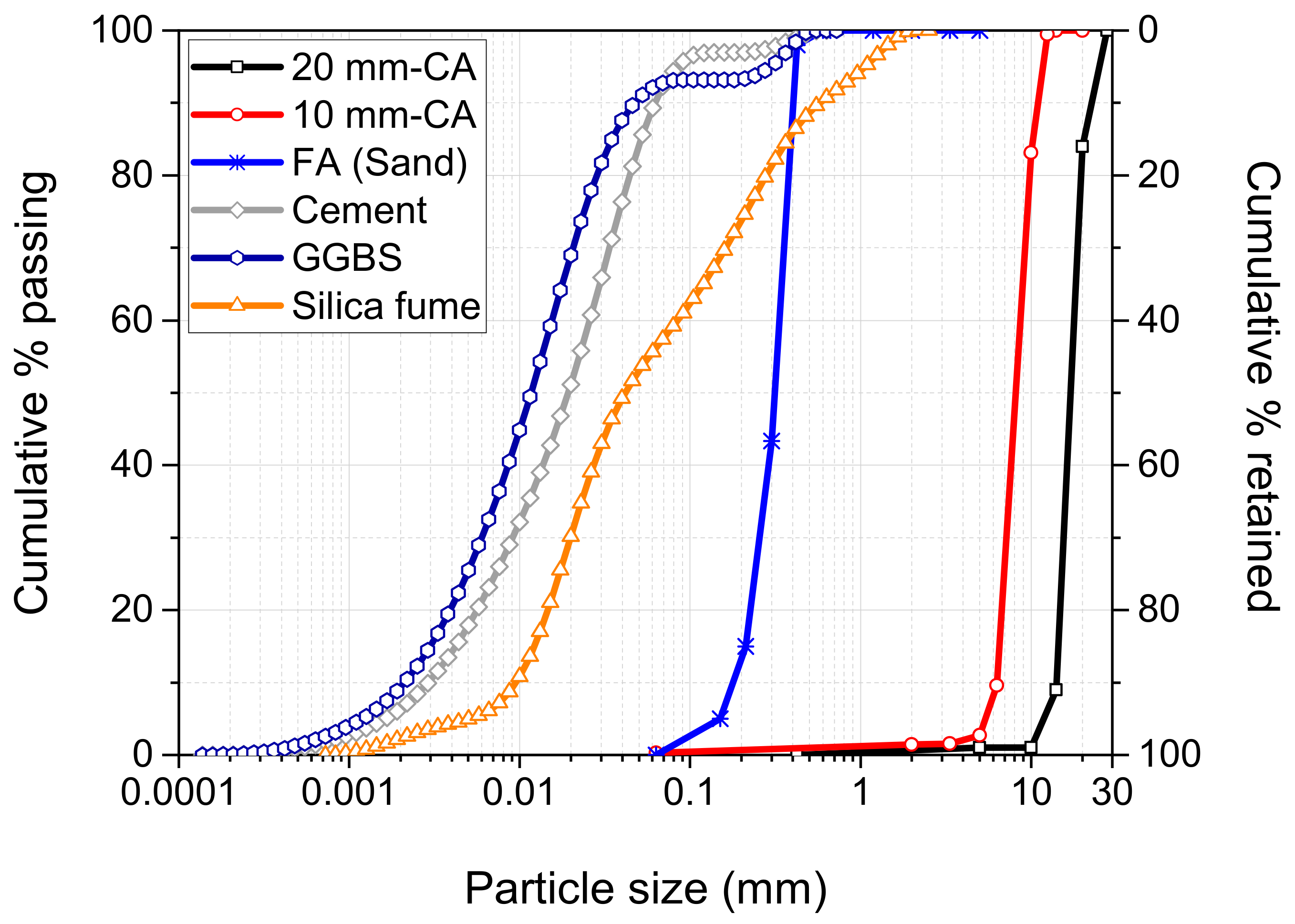
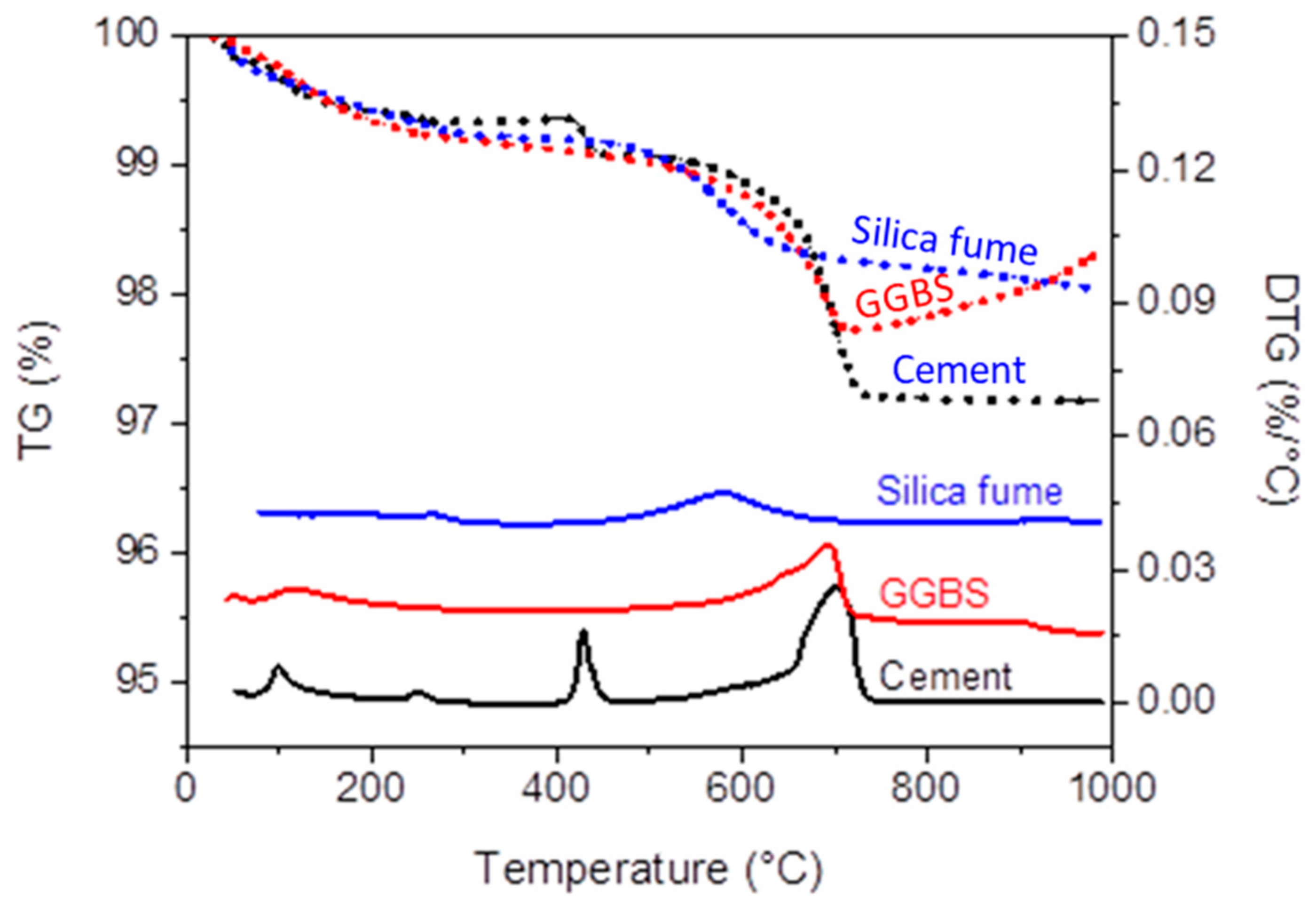


| Oxides | Compositions (%) | ||
|---|---|---|---|
| Cement | GGBS | SF | |
| CaO | 61.49 | 37.99 | 0.2 |
| MgO | 3.54 | 8.78 | 0.1 |
| SiO2 | 18.84 | 35.54 | 97.1 |
| Al2O3 | 4.77 | 11.46 | 0.2 |
| Na2O | 0.02 | 0.37 | - |
| P2O5 | 0.1 | 0.02 | 0.03 |
| Fe2O3 | 2.87 | 0.42 | 0.01 |
| Mn2O3 | 0.05 | 0.43 | - |
| K2O | 0.57 | 0.43 | 0.2 |
| TiO2 | 0.26 | 0.7 | - |
| V2O5 | 0.06 | 0.04 | - |
| BaO | 0.05 | 0.09 | - |
| SO3 | 3.12 | 1.54 | 0.1 |
| Loss on ignition | 4.3 | 2 | 0.5 |
| Other Properties | Cement | GGBS | SF |
|---|---|---|---|
| Insoluble residue | 0.5 | 0.3 | - |
| Bulk density (kg/m3) | 1400 | 1200 | 300 |
| Specific gravity (Mg/m3) | 3.15 | 2.9 | 3.15 |
| Glass content | - | 90 | - |
| Blaine fineness (m2/kg) | 365 | 450 | - |
| Alkalinity value (pH) | 13.41 | 10.4 | 7 |
| Colour | Grey | Off-white | Grey |
| Physical form | Fine powder | Fine powder | Powder |
| Physical Properties | Coarse Aggregates | Fine Aggregates (Sand) | |
|---|---|---|---|
| 20 mm | 10 mm | ||
| Uniformity coefficient (CU) | 1.3 | 3.3 | 0.11 |
| Curvature coefficient (CC) | 7.5 | 1.5 | 1.75 |
| Flakiness index (%) | 23 | 30–35 | - |
| Elongation index (%) | 12 | 17–22 | - |
| Shape index (%) | 7 | 12 | - |
| Impact value | 15 | 23 | - |
| Fineness modulus (mm) | - | 4 | 1.54 |
| Uncompacted bulk density (Mg/m3) | 2.57 | 1.35 | 1.5 |
| Pre-dried particle density (Mg/m3) | - | 2.69 | 2.6 |
| Water absorption (%) | 1.1 | 2 | 21 |
| Mix Code | Elaborated Abbreviation | Concrete Binder | W (L) | Aggregates (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC (kg) | Geopolymer Binder | FA | 10 mm | 20 mm | |||||||
| GGBS (kg) | A/P Ratio | SS:SH | Activator (mL) | ||||||||
| SS | SH | ||||||||||
| C | OPC (Control 1) | 7.3 | - | - | - | - | - | 4 | 14.6 | 7.3 | 14.6 |
| GC0 | GC0–AP0.7–1SS1SH (Control 2) | - | 4.3 | 0.7 | 1:1 | 1031 | 1031 | 1.5 | 14.6 | 7.3 | 14.6 |
| GC1 | GC1–AP0.9–1SS1SH | - | 3.85 | 0.9 | 1:1 | 1186 | 1186 | 1.5 | 14.6 | 7.3 | 14.6 |
| GC2 | GC2–AP0.5–1SS1SH | - | 4.88 | 0.5 | 1:1 | 835 | 835 | 1.5 | 14.6 | 7.3 | 14.6 |
| GC3 | GC3–AP0.7–1.2SS0.8SH | - | 4.3 | 0.7 | 0.8:1.2 | 1237 | 825 | 1.5 | 14.6 | 7.3 | 14.6 |
| GC4 | GC4–AP0.7–0.8SS1.2SH | - | 4.3 | 0.7 | 1.2:0.8 | 825 | 1237 | 1.5 | 14.6 | 7.3 | 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeleke, B.O.; Kinuthia, J.M.; Oti, J.; Ebailila, M. Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution. Materials 2023, 16, 2400. https://doi.org/10.3390/ma16062400
Adeleke BO, Kinuthia JM, Oti J, Ebailila M. Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution. Materials. 2023; 16(6):2400. https://doi.org/10.3390/ma16062400
Chicago/Turabian StyleAdeleke, Blessing O., John M. Kinuthia, Jonathan Oti, and Mansour Ebailila. 2023. "Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution" Materials 16, no. 6: 2400. https://doi.org/10.3390/ma16062400
APA StyleAdeleke, B. O., Kinuthia, J. M., Oti, J., & Ebailila, M. (2023). Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution. Materials, 16(6), 2400. https://doi.org/10.3390/ma16062400









