Application of an Eco-Friendly Adhesive and Electrochemical Nanostructuring for Joining of Aluminum A1050 Plates
Abstract
1. Introduction
2. Materials and Methods
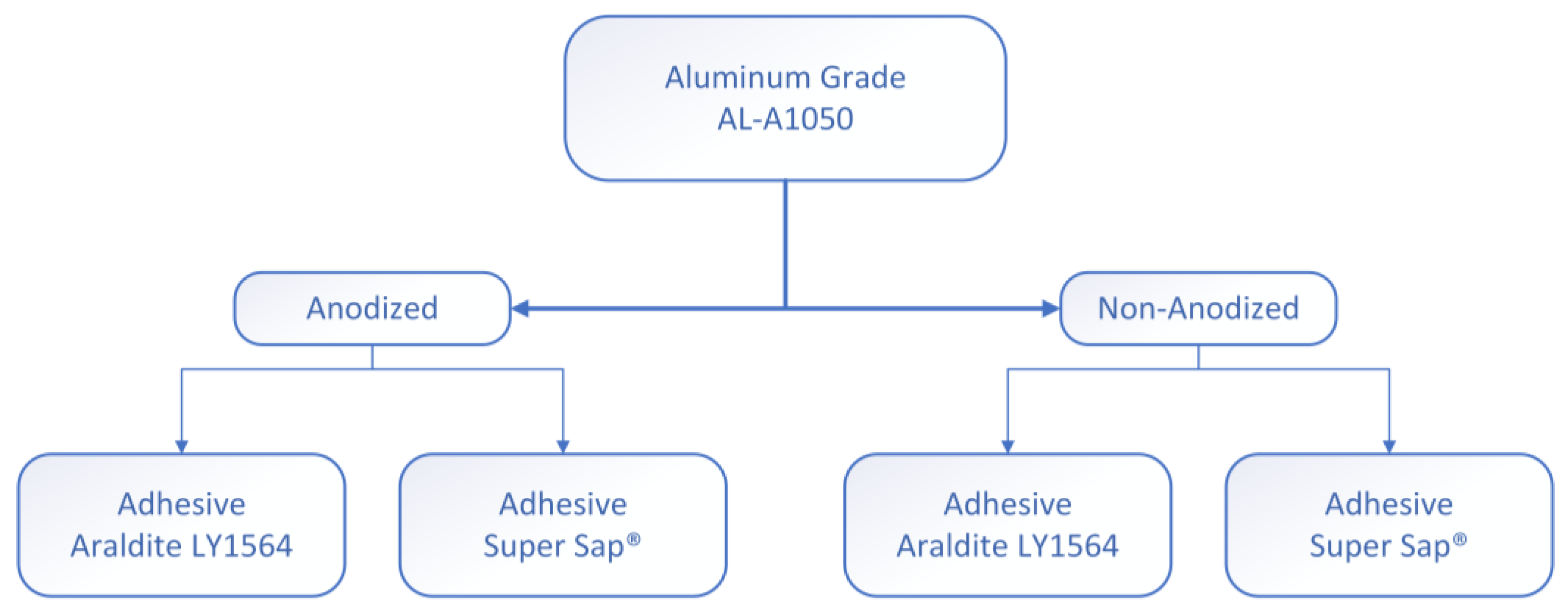
2.1. Materials
2.2. Electrochemical Anodization of the Adherends
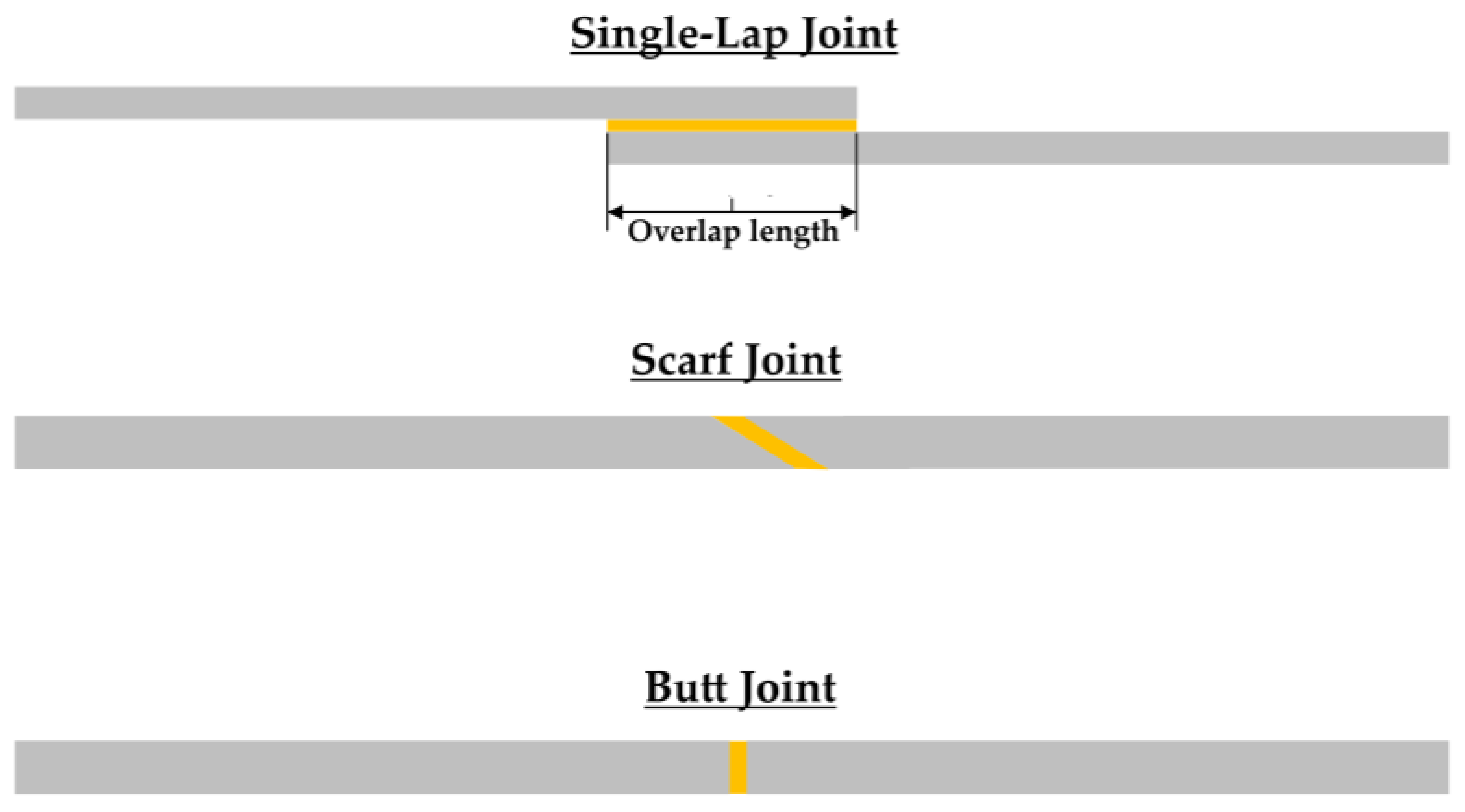
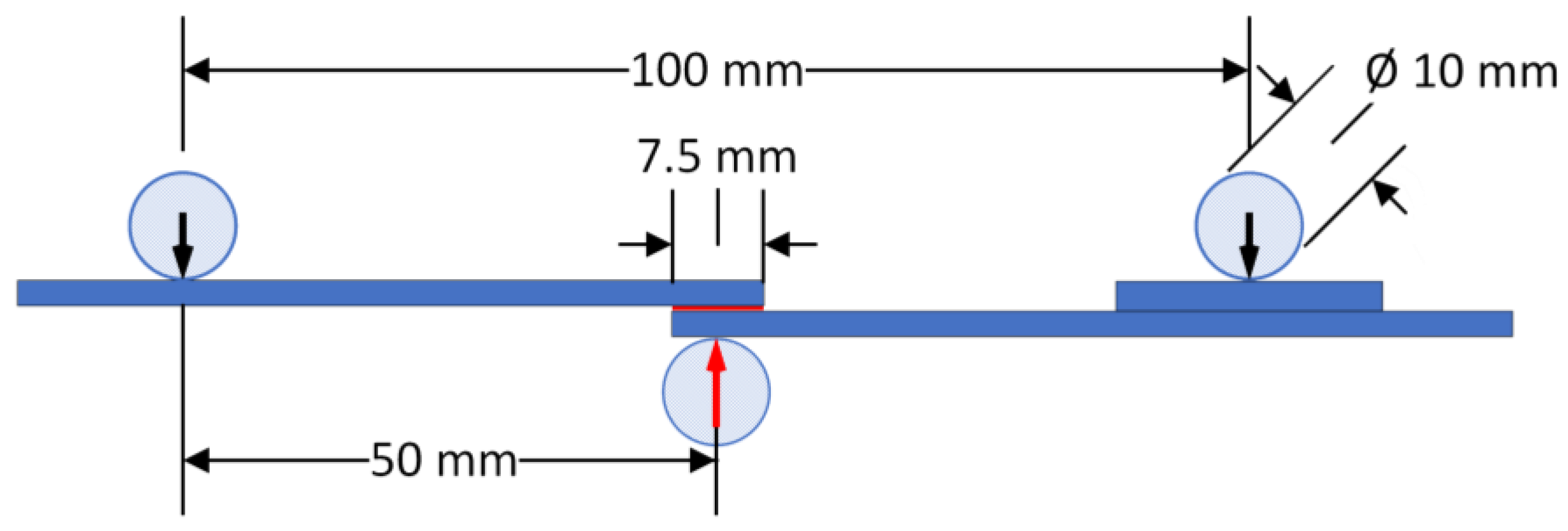
2.3. Specimens’ Manufacturing
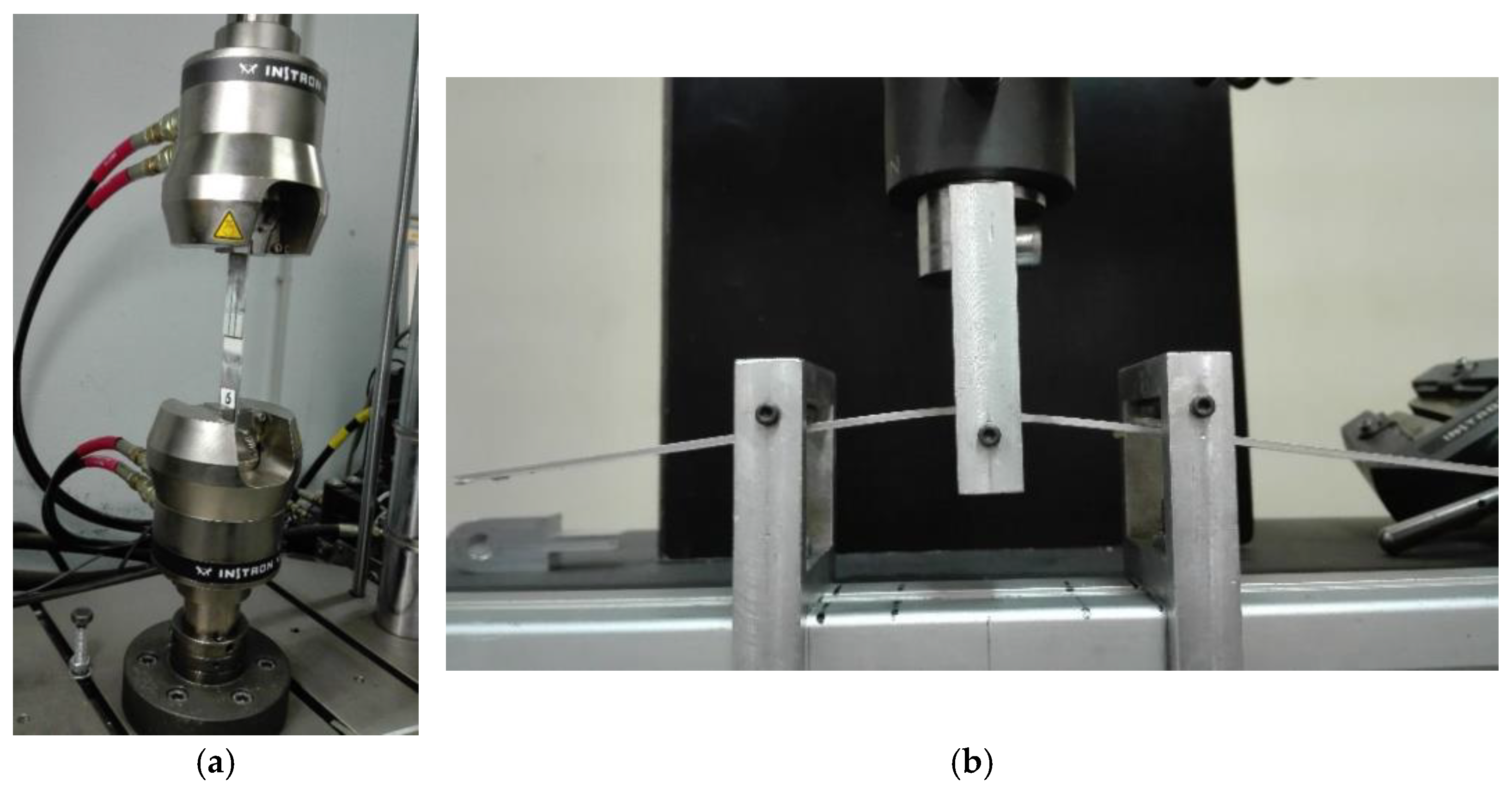
2.4. Surface Analysis and Mechanical Characterization
2.5. Data Analysis
3. Results and Discussion
3.1. Scanning Electron Microscopy
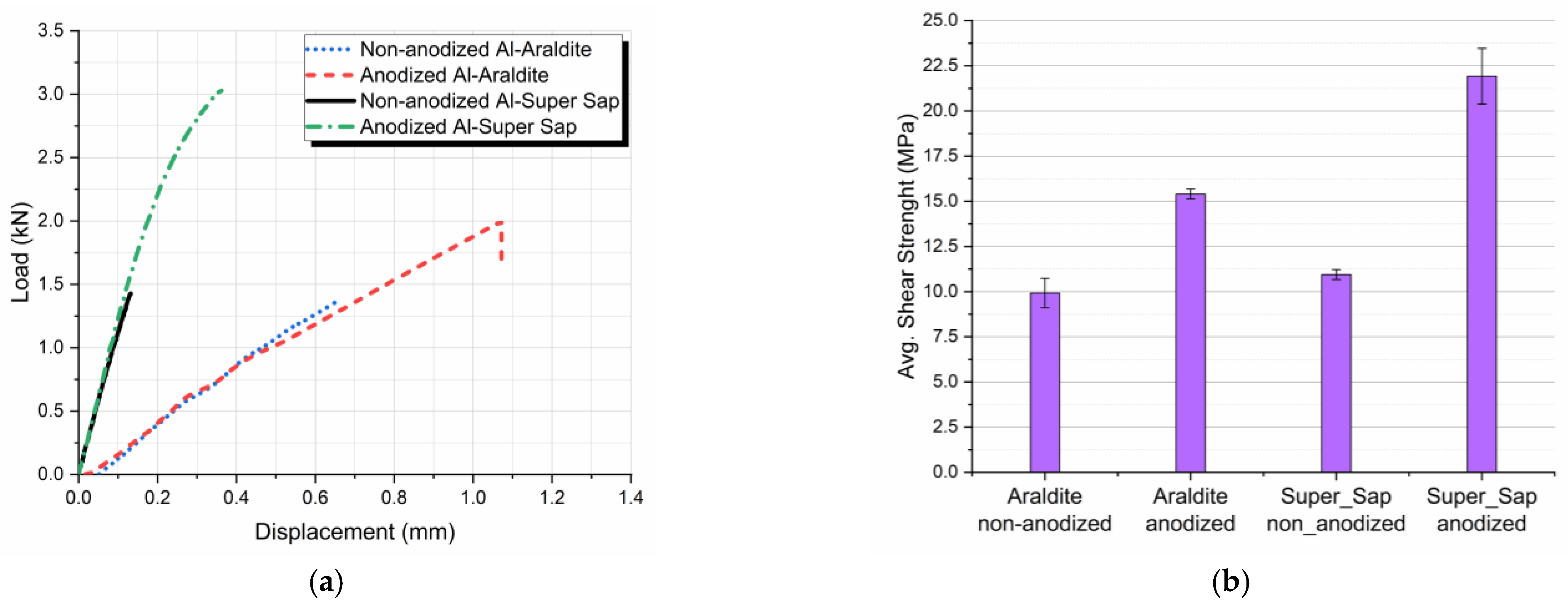
3.2. Tension-Shear Testing
- For both types of adhesives applied, aluminum anodization resulted in shear strength enhancement.
- The use of the eco-friendly adhesive resulted in a superior shear behavior of the joints as compared with the joints where Araldite adhesive was used.
- The maximum tensile-shear strength was achieved when using both anodized aluminum adherends and the eco-friendly epoxy adhesive.
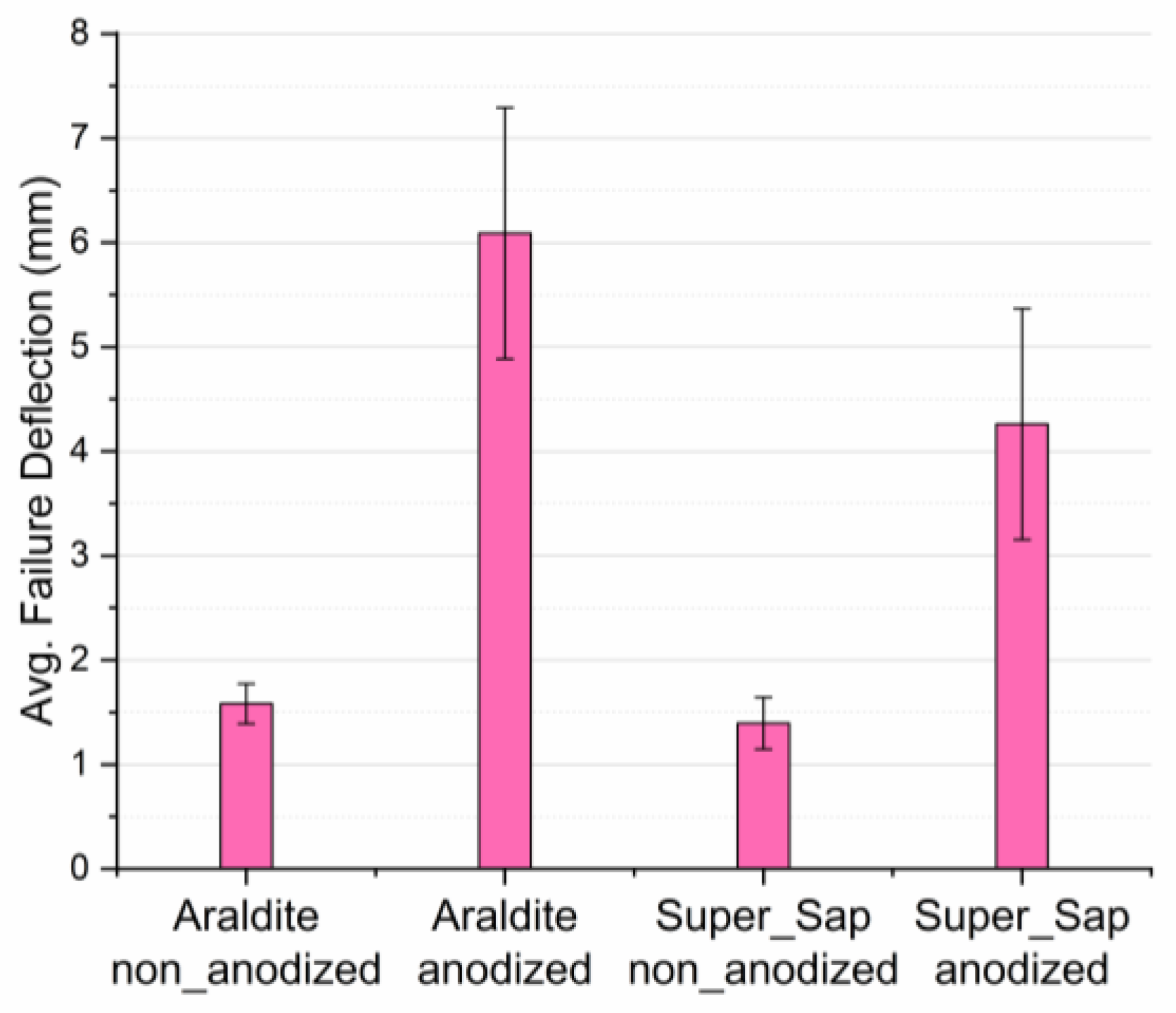
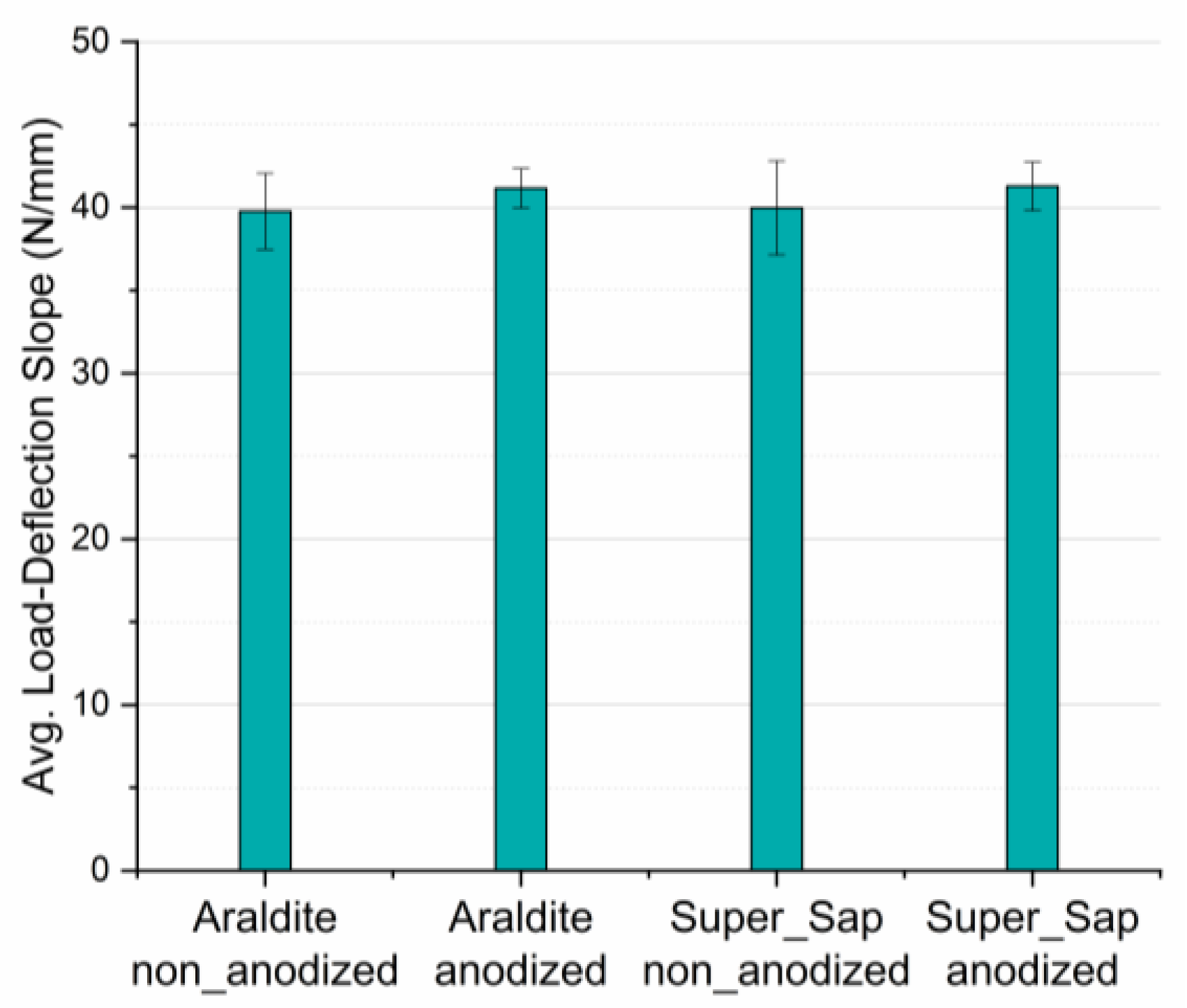
3.3. Three-Point Bending
4. Conclusions
- For both types of adhesives applied, aluminum anodization resulted in shear strength enhancement,
- The use of the eco-friendly adhesive resulted in a superior shear behavior of the joints as compared with the joints where Araldite adhesive was used.
- A maximum tensile-shear strength enhancement of 42.2% was achieved when using anodized aluminum adherends bonded with the eco-friendly epoxy.
- In joints bonded with Araldite adhesive, anodization of aluminum adherends resulted to a considerable increase of 92.9% in the flexural failure load as compared with the non-anodized ones, while the respective increase for the joints bonded with Super Sap adhesive was 73.5%.
- The findings clearly showed that using anodized aluminum adherends bonded with the eco-friendly Super Sap adhesive we can achieve better mechanical performance of the aluminum single-lap joints as compared with the harmful for the environment aluminum single-lap joints bonded with Araldite epoxy adhesive, thus decreasing the environmental footprint.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papanicolaou, G.C.; Karagiannis, D.; Kousiatza, C.; Kontaxis, L.C.; Portan, D.V. Flexural behavior of single-lap joints of similar and dissimilar adherends. J. Adhes. Sci. Technol. 2022, 37, 624–648. [Google Scholar] [CrossRef]
- Grant, L.D.R.; Adams, R.D.; da Silva, L.F.M. Experimental and numerical analysis of single-lap joints for the automotive industry. Int. J. Adhes. Adhes. 2009, 29, 405–413. [Google Scholar] [CrossRef]
- PlasticsEurope Epoxy Resins Committee (ERC). Epoxy Resins and Curing Agents: Toxicology, Health, Safety and Environmental Aspects; Plastics Europe Epoxy Resins Committee: Brussels, Belgium, 2017. [Google Scholar]
- Khoon Poh, A.; Choy Sin, L.; Sit Foon, C.; Cheng Hock, C. Polyurethane wood adhesive from palm oil-based polyester polyol. J. Adhes. Sci. Technol. 2014, 28, 1020–1033. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Kumar Yadav, S.; Palmese, G.R.; Stanzione, J.F., III. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Ma, S.; Li, T.; Liu, X.; Zhu, J. Research progress on bio-based thermosetting resins. Polym. Int. 2016, 65, 164–173. [Google Scholar] [CrossRef]
- Celikbag, Y.; Nuruddin, M.; Biswas, M.; Asafu-Adjaye, O.; Via, B.K. Bio-oil-based phenol–formaldehyde resin: Comparison of weight- and molar-based substitution of phenol with bio-oil. J. Adhes. Sci. Technol. 2020, 34, 2743–2754. [Google Scholar] [CrossRef]
- Bertomeu, D.; García-Sanoguera, D.; Fenollar, O.; Boronat, T.; Balart, R. Use of eco-friendly epoxy resins from renewable resources as potential substitutes of petrochemical epoxy resins for ambient cured composites with flax reinforcements. Polym. Compos. 2012, 33, 683–692. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Development of Biobased Epoxy Resins: A Review. Polym.-Plast. Technol. Eng. 2017, 57, 133–155. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Valasek, P.; Muller, M. Recycling of waste rubber powder and micro-particles as filler of thermosets–abrasive wear. Eng. Rural Develop. 2014, 13, 396–400. [Google Scholar]
- Nair, S.S.; Dartiailh, C.; Levin, D.B.; Yan, N. Highly Toughened and Transparent Biobased Epoxy Composites Reinforced with Cellulose Nanofibrils. Polymers 2019, 11, 612. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2012, 20, 829–846. [Google Scholar] [CrossRef]
- Cohen, E.; Binshtok, O.; Dotan, A.; Dodiuk, H. Prospective materials for biodegradable and/or biobased pressure-sensitive adhesives: A review. J. Adhes. Sci. Technol. 2013, 27, 1998–2013. [Google Scholar] [CrossRef]
- Imam, S.H.; Bilbao-Sainz, C.; Chiou, B.S.; Glenn, G.M.; Orts, W.J. Biobased adhesives, gums, emulsions, and binders: Current trends and future prospects. J. Adhes. Sci. Technol. 2013, 27, 1972–1997. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives–advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef]
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M.; Se, M.N. Brief Overview on Bio-Based Adhesives and Sealants. Polymers 2019, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, Y.; Chen, M.; Zhang, Y.; Li, J.; Gao, Q.; Shi, S.Q. Soy protein adhesive with bio-based epoxidized daidzein for high strength and mildew resistance. Chem. Eng. J. 2020, 390, 124622. [Google Scholar] [CrossRef]
- Adams, R.D.; Comyn, J.; Wake, W.C. Structural Adhesive Joints in Engineering, 2nd ed.; Springer: Dordrecht, The Netherlands, 1997; ISBN 978-0-412-70920-3. [Google Scholar]
- Wake, W.C. Adhesion and Adhesives: Science and Technology; Kinloch, A.J., Ed.; British Polymer Journal; Chapman and Hall: London, UK, 1987; Volume 20, p. xii + 441. ISBN 0-412-27440-X. [Google Scholar]
- Adams, R.D. Adhesive Bonding: Science, Technology and Applications, 1st ed.; Adams, R., Ed.; Woodhead Publishing: Cambridge, UK, 2005; ISBN 978-1-85573-741-9. [Google Scholar]
- Volkersen, O. Rivet strength distribution in tensile-stressed rivet joints with constant cross-section. Luftfahrforschung 1938, 15, 41–47. [Google Scholar]
- Goland, M.; Reissner, E. The Stresses in Cemented Joints. J. Appl. Mech. 1944, 11, A17–A27. [Google Scholar] [CrossRef]
- Hart-Smith, L.J. Adhesive-Bonded Double-Lap Joints; NASA CR-112235; NASA: Washington, DC, USA, January 1973. [Google Scholar]
- Pinto, A.M.G.; Magalhães, A.G.; Campilho, R.D.S.G.; de Moura, M.F.S.F.; Baptista, A.P.M. Single-Lap Joints of Similar and Dissimilar Adherends Bonded with an Acrylic Adhesive. J. Adhes. 2009, 85, 351–376. [Google Scholar] [CrossRef]
- Wu, X.-F.; Jenson, R.A. Interfacial stresses of bonded single-lap joints under mechanical and thermomechani-cal loads. Int. J. Solid Mater. 2019, 1, 79–115. [Google Scholar]
- Sayman, O. Elasto-plastic stress analysis in an adhesively bonded single-lap joint. Compos. Part B Eng. 2012, 43, 204–209. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Morton, J. The effect of a spew fillet on adhesive stress distributions in laminated composite single-lap joints. Compos. Struct. 1995, 32, 123–131. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Xia, H.; Li, X.; Zhang, X.; Yang, H. Effects of surface treatment and adhesive thickness on the shear strength of precision bonded joints. Polym. Test. 2021, 94, 107063. [Google Scholar] [CrossRef]
- Salamat, A.; Islam, T. Fabrication of an anodized porous alumina relative humidity sensor with improved sensitivity. Instrum. Sci. Technol. 2020, 48, 128–145. [Google Scholar] [CrossRef]
- Batista-Grau, P.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. Formation of ZnO nanowires by anodization under hydrodynamic conditions for photoelectrochemical water splitting. Surf. Coat. Technol. 2020, 381, 125197. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Murugananthan, M.; Zhang, Y.; Zhang, L. Electrochemically self-doped WO3/TiO2 nanotubes for photocatalytic degradation of volatile organic compounds. Appl. Catal. B Environ. 2020, 260, 118205. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Portan, D.V.; Petropoulos, G.N.; Kontaxis, L.C. Effect of TiO2 nanotubes developed on pure titanium substrates on the mechanical performance of titanium-titanium single-lap adhesive joints. Cienc. Tecnol. Mater. 2016, 28, 130–137. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Kontaxis, L.C.; Portan, D.V.; Petropoulos, G.N.; Valeriou, E.; Alexandropoulos, D. Mechanical Performance Enhancement of Aluminum Single-Lap Adhesive Joints Due to Organized Alumina Nanotubes Layer Formation on the Aluminum Adherends. Appl. Nano 2021, 2, 206–221. [Google Scholar] [CrossRef]
- Özel, A.; Kadioglu, F.; Sen, S.; Sadeler, R. Finite element analysis of adhesive joints in four-point bending load. J. Adhes. 2003, 79, 683–697. [Google Scholar] [CrossRef]
- Özel, A.; Aydin, M.D.; Temiz, Ş. The effects of overlap length and adherend thickness on the strength of adhesively bonded joints subjected to bending moment. J. Adhes. Sci. Technol. 2004, 18, 313–325. [Google Scholar] [CrossRef]
- 1050A Aluminium Properties Technical Information. Available online: https://www.metals4u.co.uk/blog/1050a-aluminium (accessed on 25 February 2023).
- Super Sap INR System. Available online: https://www.pecepoxy.co.uk/data-sheets/TDS_INR_v2.pdf (accessed on 25 February 2023).
- Araldite LY 1564*/Aradur 2954*-Mouldlife. Available online: https://www.yumpu.com/en/document/read/15623592/araldite-ly-1564-aradur-2954-mouldlife (accessed on 25 February 2023).
- Zaraska, L.; Sulka, G.D.; Szeremeta, J.; Jaskuła, M. Porous anodic alumina formed by anodization of aluminum alloy (AA1050) and high purity aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Preparation of anodized aluminium oxide at high temperatures using low purity aluminium (Al6082). Surf. Coat. Technol. 2019, 378, 124970. [Google Scholar] [CrossRef]
- Fredericks, P. The Complete Guide to Anodizing Aluminum Parts-Aerospace Metals. Available online: https://aerospacemetalsllc.com/the-complete-guide-to-anodizing-aluminum-parts/ (accessed on 25 February 2023).
- Sulka, G.D.; Stroobants, S.; Moshchalkov, V.; Borghs, G.; Celis, J.-P. Synthesis of Well-Ordered Nanopores by Anodizing Aluminum Foils in Sulfuric Acid. J. Electrochem. Soc. 2002, 149, D97. [Google Scholar] [CrossRef]
- Lim, J.H.; Wiley, J.B. Controlling pore geometries and interpore distances of anodic aluminum oxide templates via three-step anodization. J. Nanosci. Nanotechnol. 2015, 15, 633–641. [Google Scholar] [CrossRef]
- Ozcan, M.; Tunca, B.; Biltas, İ.; Tuken, T. The effect of different pre-surface finishing method on the aluminium anodization of the 6xxx series alloy. ACTA Metall. Slovaca 2021, 27, 185–189. [Google Scholar] [CrossRef]
- Araoyinbo, A.O.; Noor, A.F.M.; Sreekantan, S.; Aziz, A. Voltage effect on electrochemical anodization of aluminum at ambient temperature. Int. J. Mech. Mater. Eng. 2010, 5, 53–58. [Google Scholar]
- Mubashar, A.; Ashcroft, I.A.; Critchlow, G.W.; Crocombe, A.D. Moisture absorption–desorption effects in adhesive joints. Int. J. Adhes. Adhes. 2009, 29, 751–760. [Google Scholar] [CrossRef]
- Ghumatkar, A.; Budhe, S.; Sekhar, R.; Banea, M.D.; De Barros, S. Influence of Adherend Surface Roughness on the Adhesive Bond Strength. Lat. Am. J. Solids Struct. 2016, 13, 2356–2370. [Google Scholar] [CrossRef]
- Cho, T.M.; Choo, Y.S.; Lee, M.J.; Oh, H.C.; Lee, B.C.; Park, T.H.; Shin, Y.S. Effect of Surface Roughness on the Adhesive Strength of the Heat-Resistant Adhesive RTV88. J. Adhes. Sci. Technol. 2012, 23, 1875–1882. [Google Scholar] [CrossRef]
- Pereira, A.M.; Ferreira, J.M.; Antunes, F.V.; Bártolo, P.J. Analysis of manufacturing parameters on the shear strength of aluminium adhesive single-lap joints. J. Mater. Process. Technol. 2010, 210, 610–617. [Google Scholar] [CrossRef]

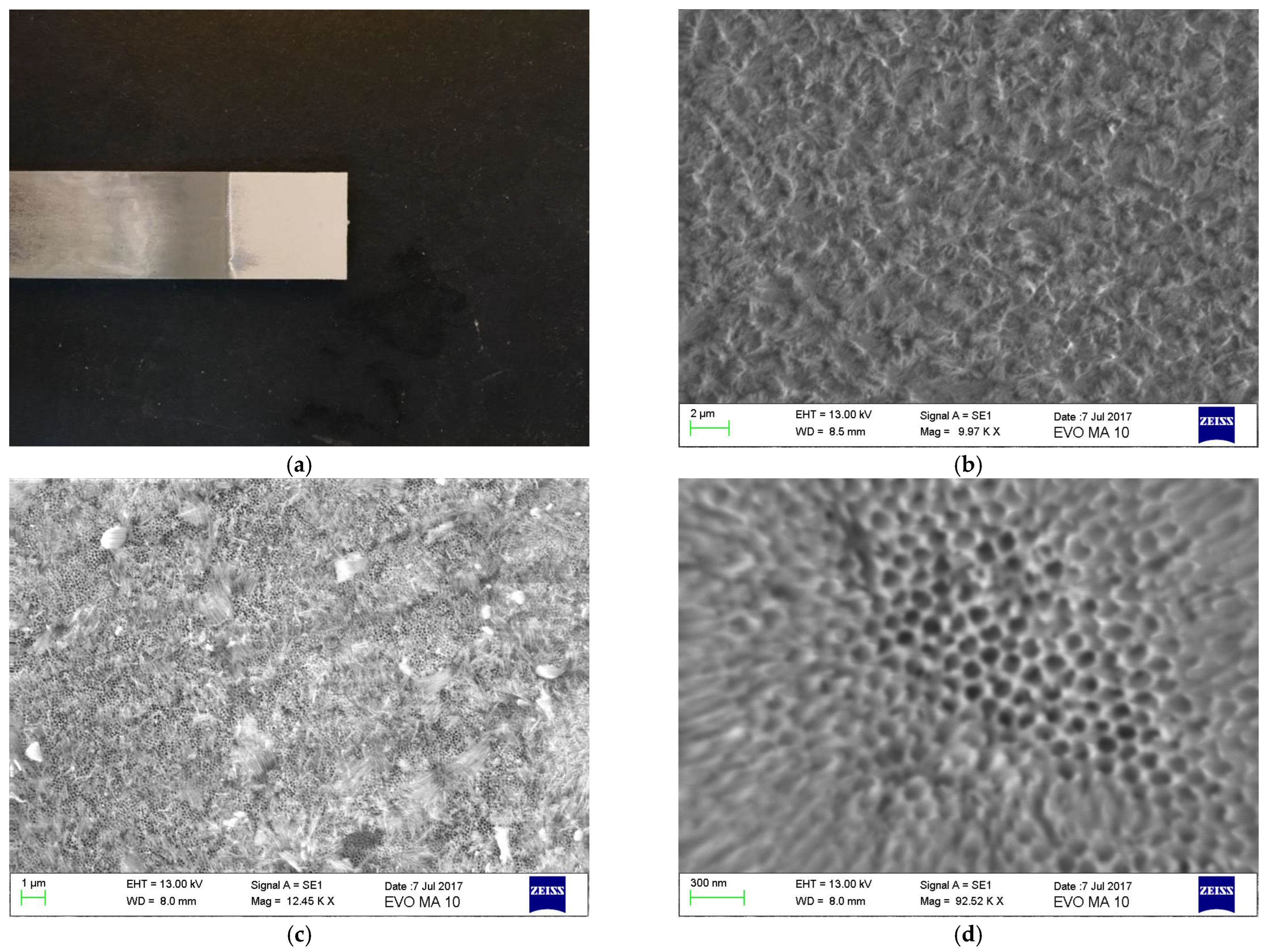

| Property | Value |
|---|---|
| Density (Kg/m3) | 2.79 |
| Modulus of Elasticity (GPa) | 73 |
| Tensile Strength (MPa) | 75 |
| Proof Stress 0.2% (MPa) | 35 |
| Fatigue Strength 50 mil. Cycles (MPa) | 20 |
| Shear Strength (MPa) | 50 |
| Hardness Vickers (HV) | 22 |
| Hardness Brinell | 20 |
| Elongation 50 mm (%) | 32 |
| Melting Point (°C) | 640 |
| Thermal Conductivity (W/m∙K) | 121–193 |
| Thermal Expansion | 23.1 × 10−6 grad−1 |
| Property | Super Sap INR/INS | Araldite LY1564/Aradur 2954 |
|---|---|---|
| Viscosity at 25 °C (mPas) | 2200/25 | 1200–1400/70–120 |
| Density (g/cm3) | 1.1 (mixed) | 1.1–1.2/0.94–0.95 |
| Mix ratio (PBW) | 100:33 | 100:35 |
| Cure cycle | 24 h at 25 °C + 2 h at 120 °C | 1 h at 80 °C + 8 h at 140 °C |
| Tensile Modulus (GPa) | 3.38 1 | 2.55–2.65 4 |
| Tensile Strength (MPa) | 68.9 1 | 71–77 4 |
| Elongation (%) | 3–4 1 | 4.5–5.5 4 |
| Flexural Modulus (GPa) | 2.62 2 | 2.6–2.8 5 |
| Flexural Strength (MPa) | 105.5 2 | 120–124 5 |
| Ultimate Tg by DSC (°C) | 104.4 3 | 148 6 |
| Type of Joint (Anodization Effect) | Shear Strength (MPa) | Difference (%) | Type of Joint (Adhesive Effect) | Shear Strength (MPa) | Difference (%) |
|---|---|---|---|---|---|
| Araldite non-anodized | 9.9 | 55.5 | Araldite non-anodized | 9.9 | 10.1 |
| Araldite anodized | 15.4 | Super Sap non-anodized | 10.9 | ||
| Super Sap non-anodized | 10.9 | 100.9 | Araldite anodized | 15.4 | 42.2 |
| Super Sap anodized | 21.9 | Super Sap anodized | 21.9 |
| Type of Joint (Anodization Effect) | Failure Load (N) | Difference (%) | Type of Joint (Adhesive Effect) | Failure Load (N) | Difference (%) |
|---|---|---|---|---|---|
| Araldite non-anodized | 56.3 | 92.9 | Araldite non-anodized | 56.32 | −9.4 |
| Araldite anodized | 108.6 | Super Sap non-anodized | 51.00 | ||
| Super Sap non-anodized | 51.0 | 73.5 | Araldite anodized | 108.6 | −18.5 |
| Super Sap anodized | 88.5 | Super Sap anodized | 88.5 |
| Type of Joint (Anodization Effect) | Failure Deflection (mm) | Difference (%) | Type of Joint (Adhesive Effect) | Failure Deflection (mm) | Difference (%) |
|---|---|---|---|---|---|
| Araldite non-anodized | 1.59 | 283.0 | Araldite non-anodized | 1.59 | −12.6 |
| Araldite anodized | 6.09 | Super Sap non-anodized | 1.39 | ||
| Super Sap non-anodized | 1.39 | 206.5 | Araldite anodized | 6.09 | −30.0 |
| Super Sap anodized | 4.26 | Super Sap anodized | 4.26 |
| Type of Joint (Anodization Effect) | Load-Deflection Slope (N/mm) | Difference (%) | Type of Joint (Adhesive Effect) | Load-Deflection Slope (N/mm) | Difference (%) |
|---|---|---|---|---|---|
| Araldite non-anodized | 39.8 | 3.5 | Araldite non-anodized | 39.8 | 0.5 |
| Araldite anodized | 41.2 | Super Sap non-anodized | 40 | ||
| Super Sap non-anodized | 40 | 3.3 | Araldite anodized | 41.2 | 0.2 |
| Super Sap anodized | 41.3 | Super Sap anodized | 41.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanicolaou, G.C.; Kontaxis, L.C.; Kouris, N.; Portan, D.V. Application of an Eco-Friendly Adhesive and Electrochemical Nanostructuring for Joining of Aluminum A1050 Plates. Materials 2023, 16, 2428. https://doi.org/10.3390/ma16062428
Papanicolaou GC, Kontaxis LC, Kouris N, Portan DV. Application of an Eco-Friendly Adhesive and Electrochemical Nanostructuring for Joining of Aluminum A1050 Plates. Materials. 2023; 16(6):2428. https://doi.org/10.3390/ma16062428
Chicago/Turabian StylePapanicolaou, George C., Lykourgos C. Kontaxis, Nikolaos Kouris, and Diana V. Portan. 2023. "Application of an Eco-Friendly Adhesive and Electrochemical Nanostructuring for Joining of Aluminum A1050 Plates" Materials 16, no. 6: 2428. https://doi.org/10.3390/ma16062428
APA StylePapanicolaou, G. C., Kontaxis, L. C., Kouris, N., & Portan, D. V. (2023). Application of an Eco-Friendly Adhesive and Electrochemical Nanostructuring for Joining of Aluminum A1050 Plates. Materials, 16(6), 2428. https://doi.org/10.3390/ma16062428












