Enhancing Wettability of Cu3P/Cu Systems through Doping with Si, Sn, and Zr Elements: Insights from First Principles Analysis
Abstract
:1. Introduction
2. Computation Methods and Models
2.1. Computation Methods
2.2. Computation Methods
3. Results
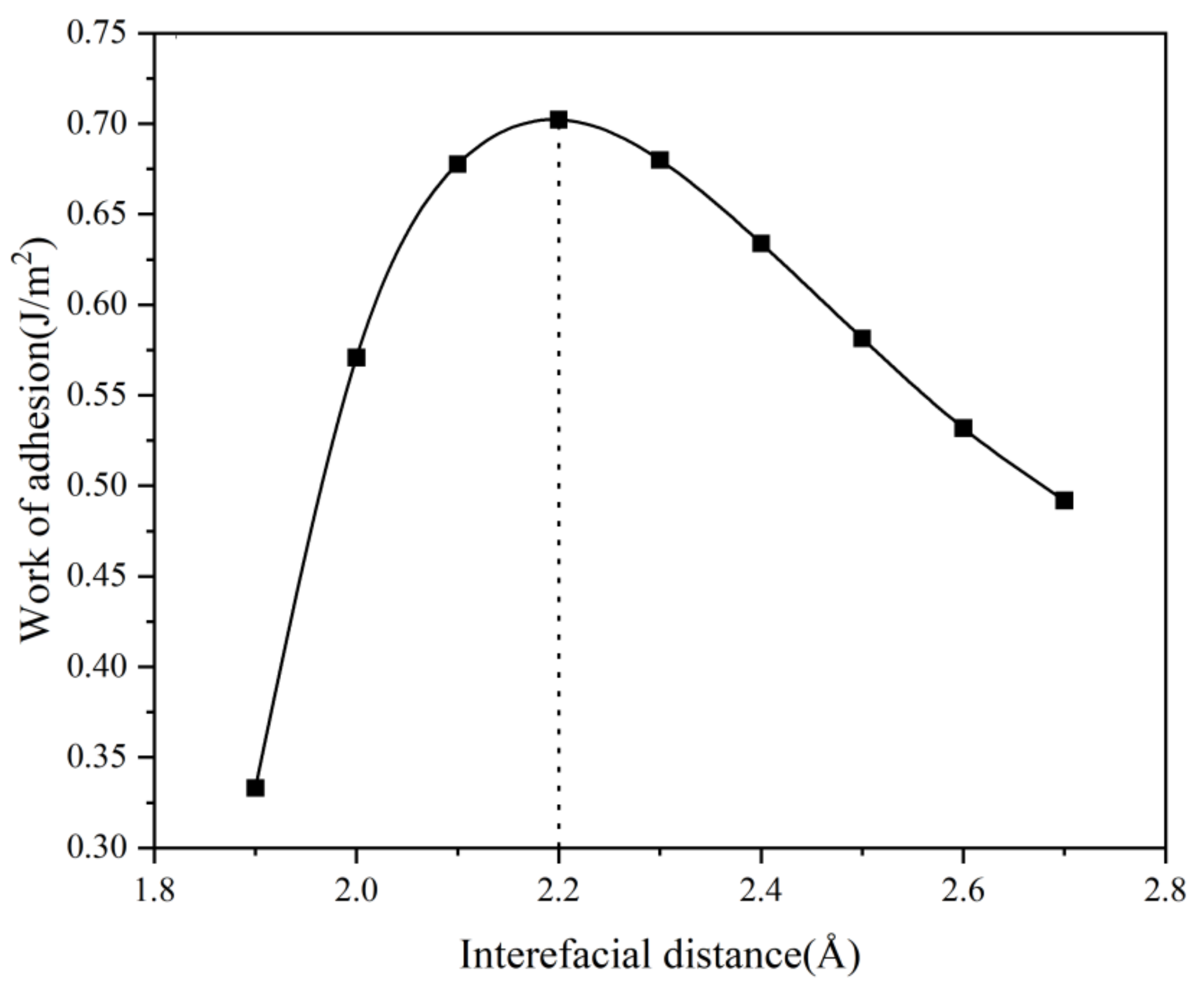
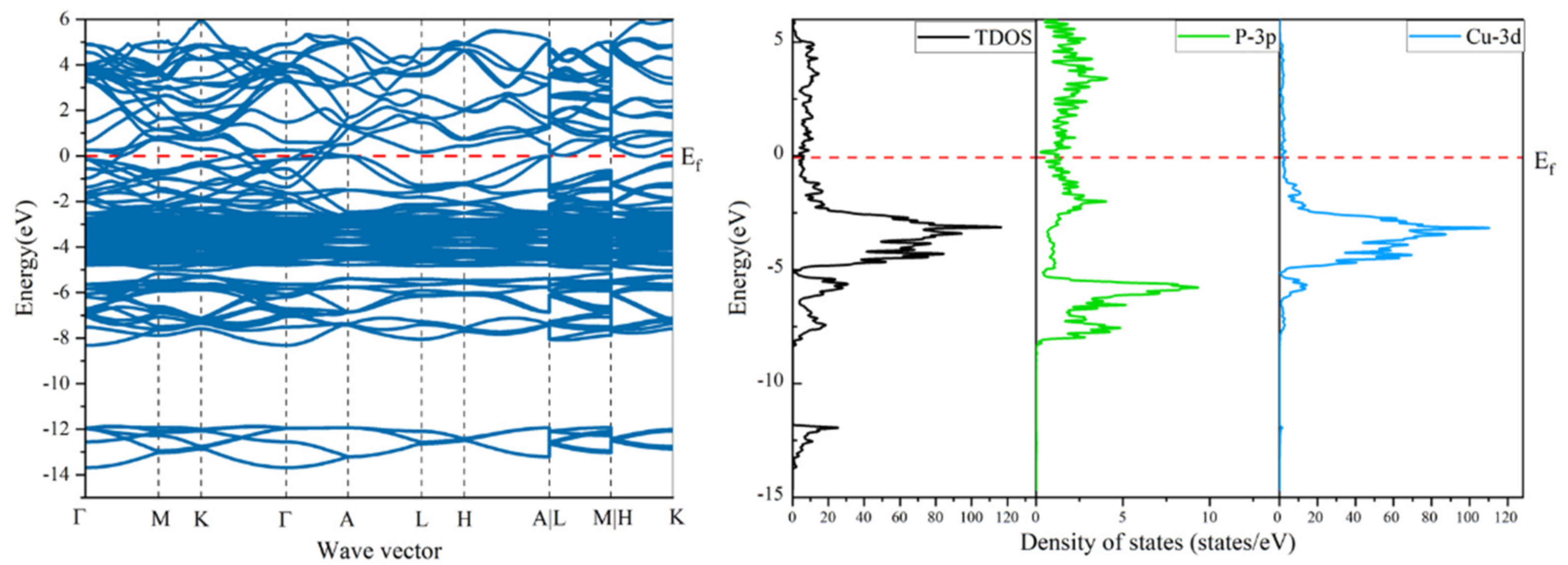
3.1. Work of Adhesion and Interface Stability of Cu3P/Cu Interface
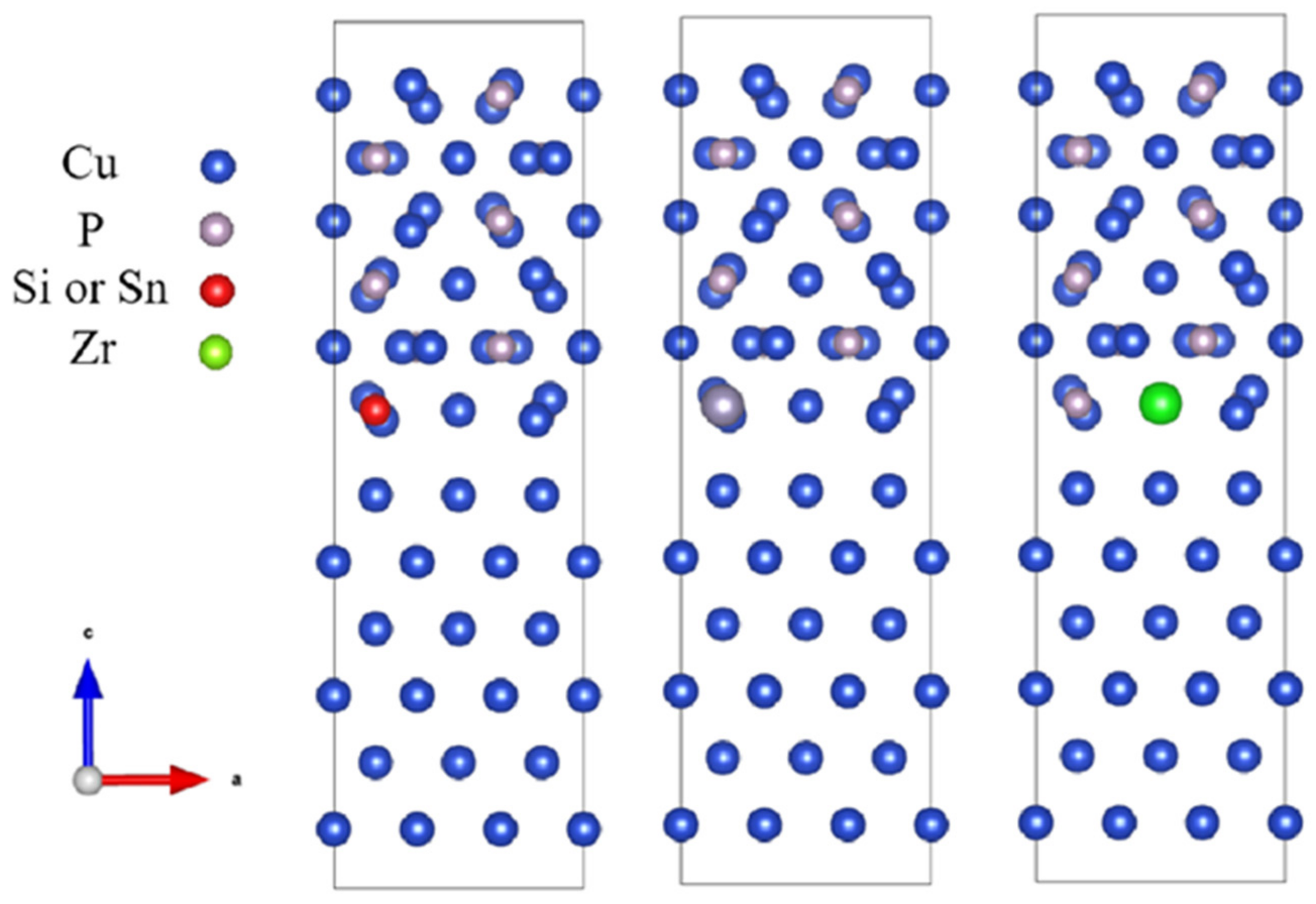
3.2. CuP–(Si, Sn, and Zr)/Cu Interfaces
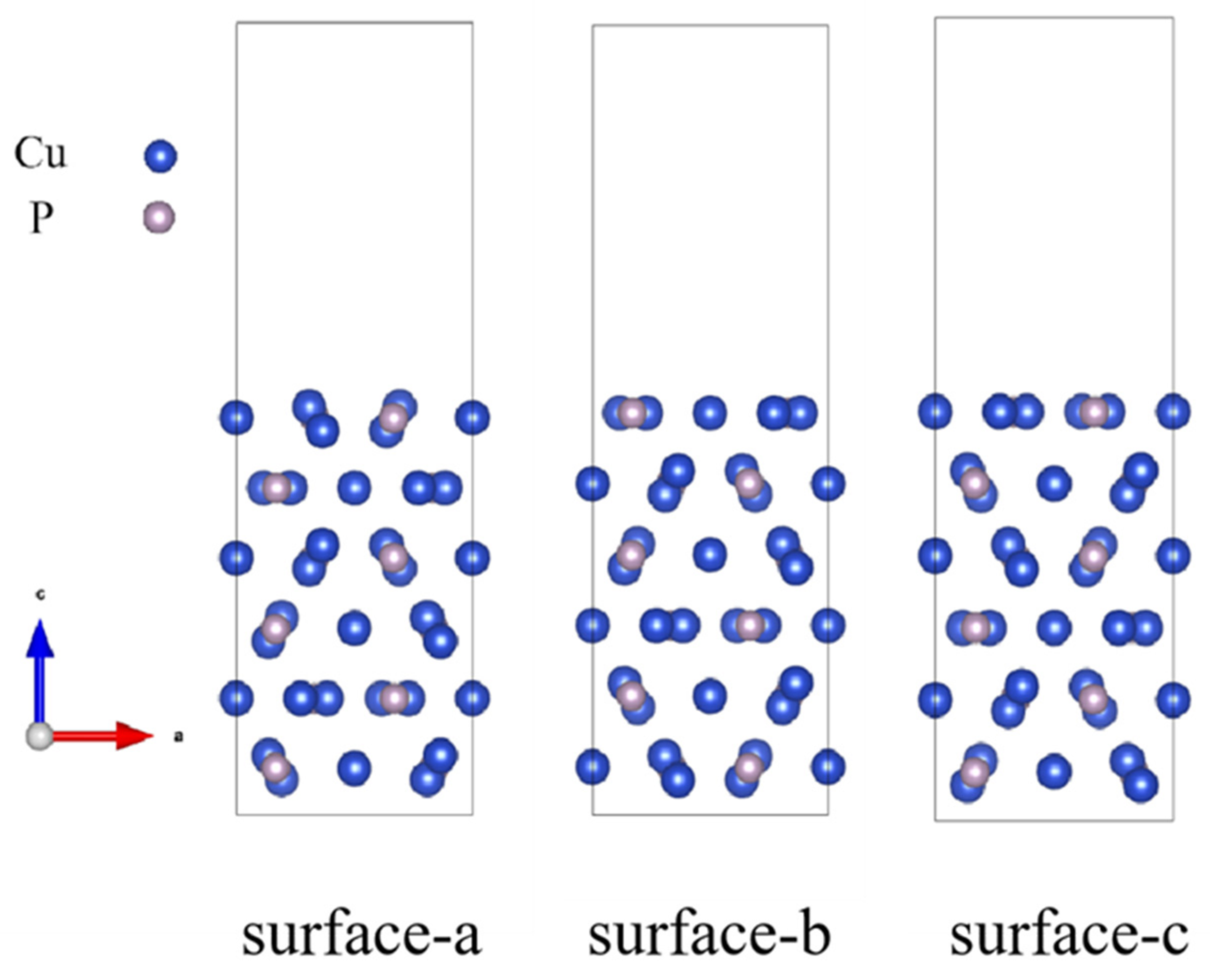
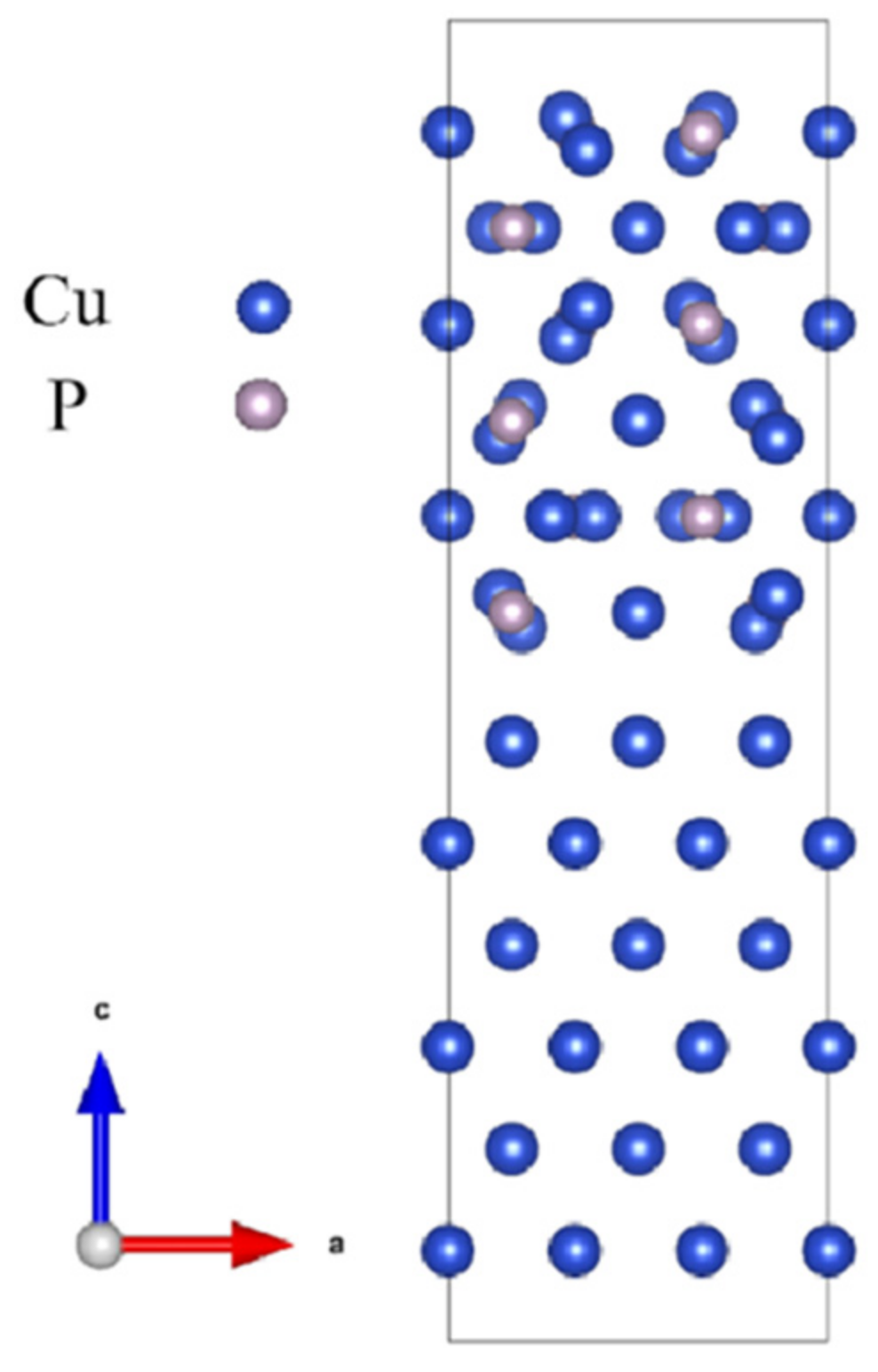
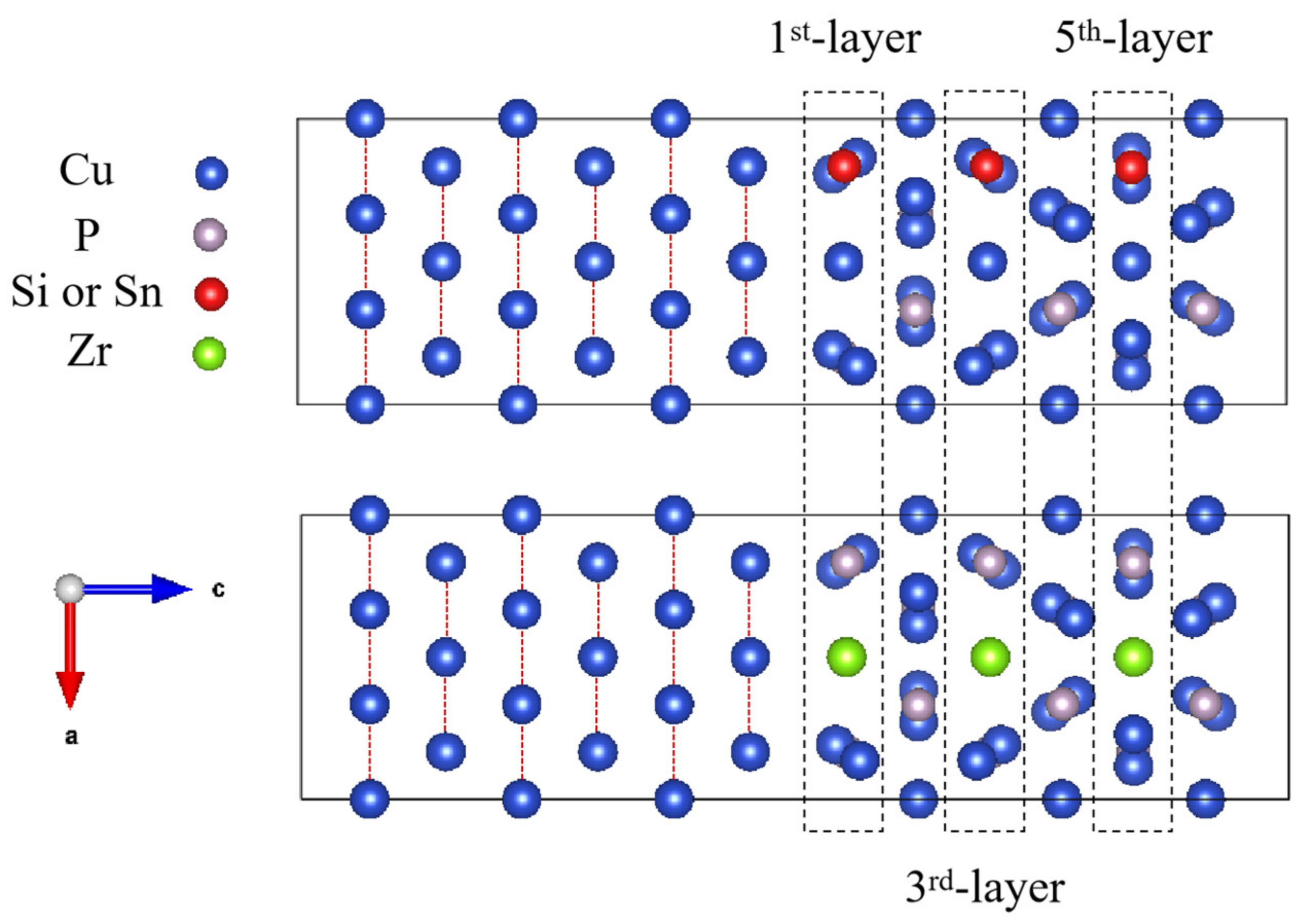
3.2.1. Interfacial Atomic Structure
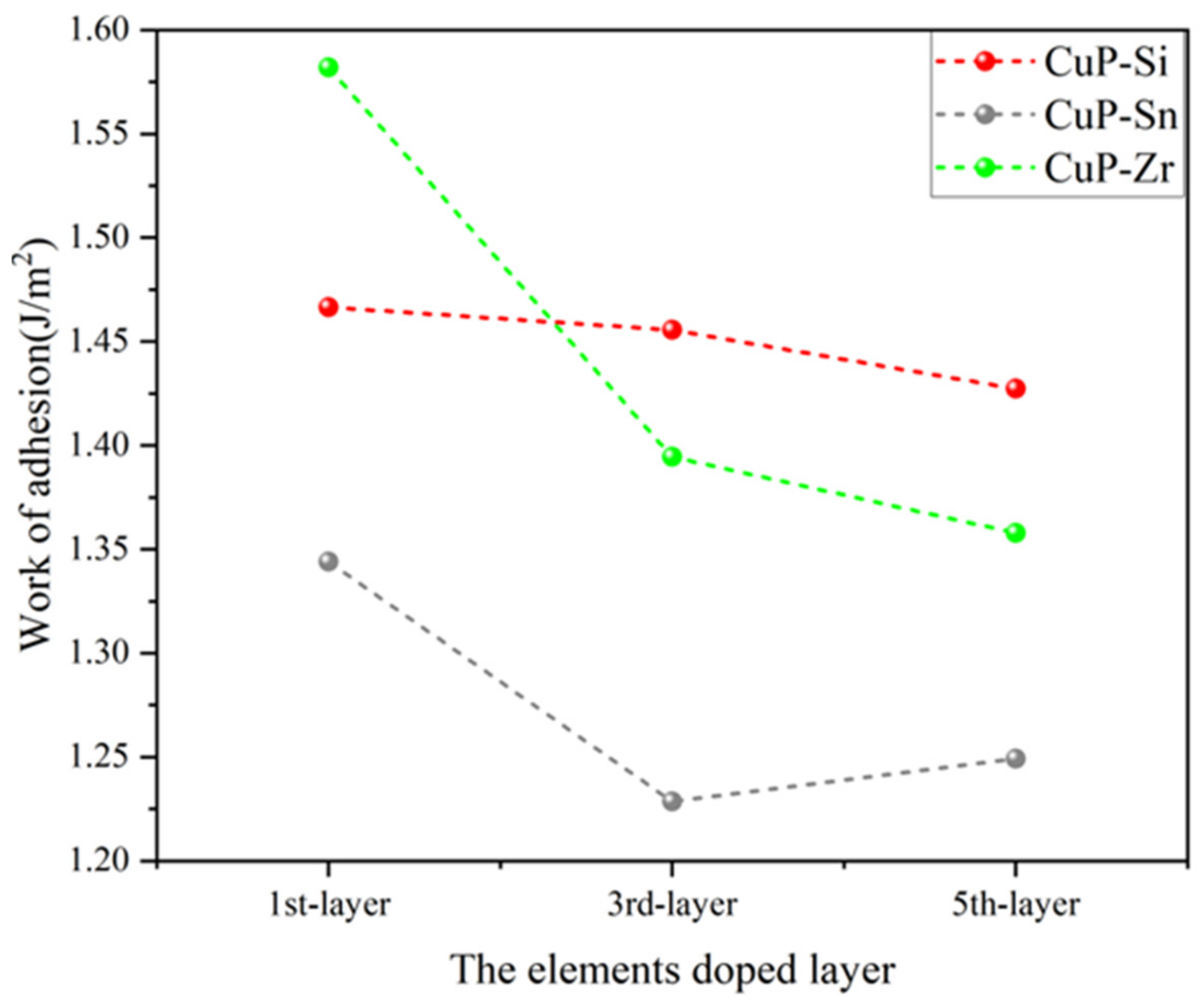
3.2.2. Work of Adhesion
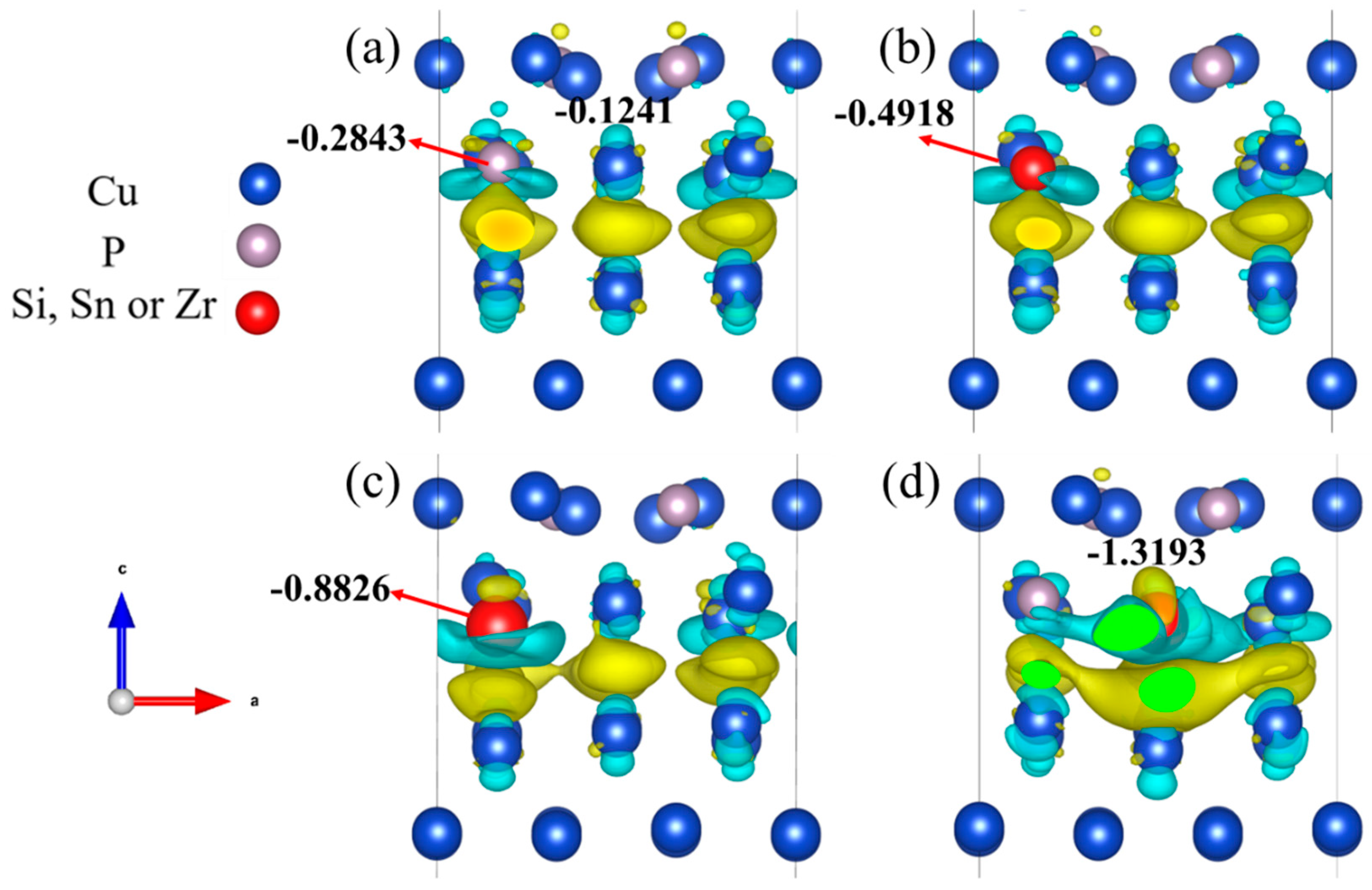
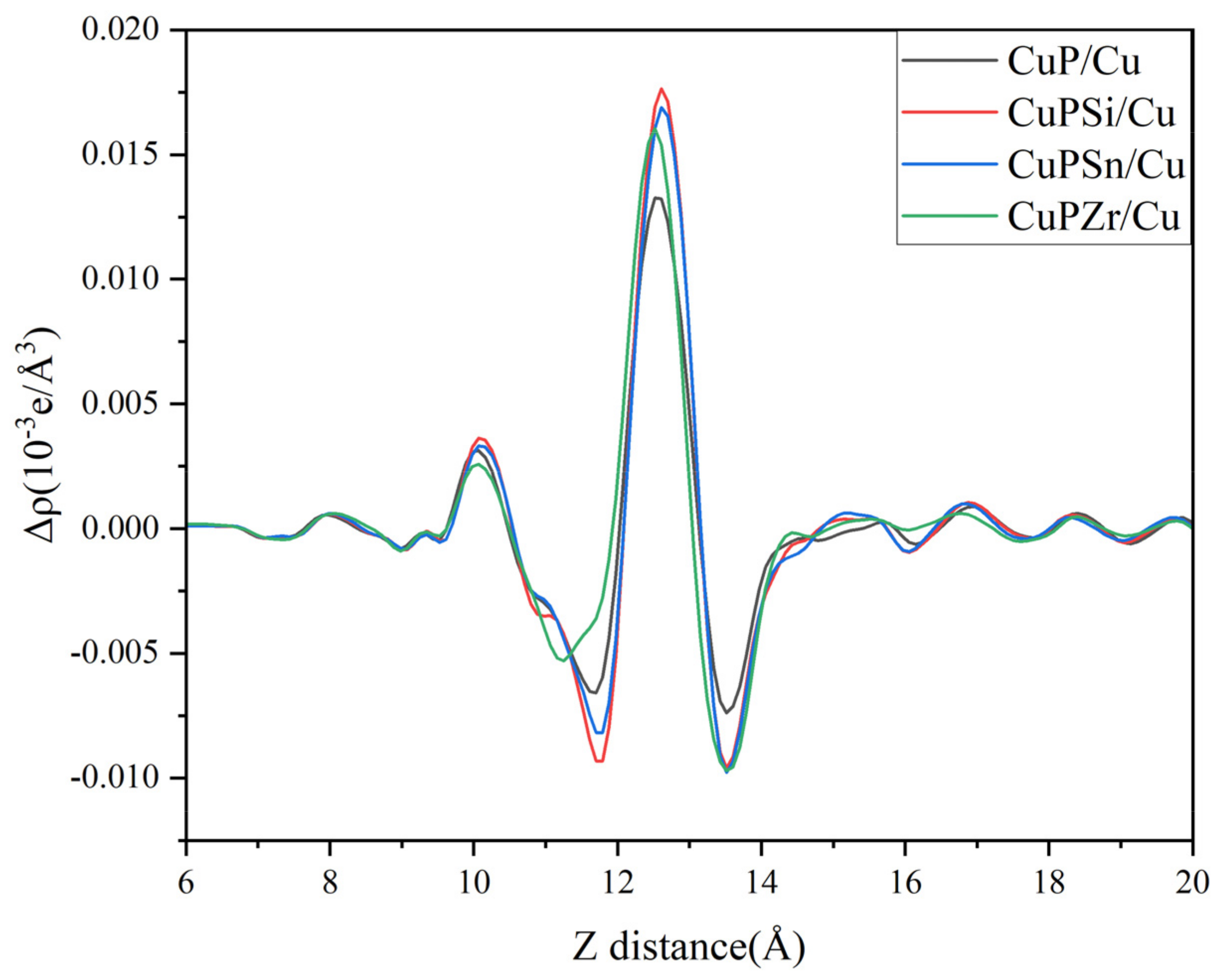
3.2.3. Electronic Properties of the Interface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Ma, Q.; Xia, C. Micromorphology change and microstructure of Cu-P based amorphous filler during heating process. High Temp. Mater. Process. 2019, 38, 651–661. [Google Scholar] [CrossRef]
- Xue, S.; Wang, B.; Zhang, L.; Long, W. Development of green welding technology in China during the past decade. Mater. Rep. 2019, 33, 2813–2830. [Google Scholar]
- Luo, Q.; Xue, S.; Wu, J. Influences of Sn on properties of Ag-based and Cu-based brazing filler metals. Crystals 2021, 11, 1403. [Google Scholar] [CrossRef]
- Li, Y.-N.; Wang, C.-W.; Peng, Z.-L.; Yan, J.-C.; Liu, X.-S. Dissolution behavior of Cu in Cu-Ag and Cu-P brazing alloys using weld brazing. Trans. Nonferrous Met. Soc. China 2011, 21, s394–s399. [Google Scholar] [CrossRef]
- Wu, J.; Xue, S.; Luo, Q. Development of a Novel Low-Silver Cu-P Brazing Filler Metal Bearing Sn. Crystals 2022, 12, 66. [Google Scholar] [CrossRef]
- Lu, J.; Qi, W.; Zhang, W.; Li, Y.; Qi, F.; Zhong, S.; Xu, S. Effect of Cu-P-Sn on brazing diamond with Ni-based filler alloy. Int. J. Adv. Manuf. Technol. 2017, 90, 649–656. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, P.; Zhang, M.; Liu, G.; Xu, Z.; Yang, J.; Shao, H.; Qiao, G. Improving the wettability of Ag/ZrB2 system by Ti, Zr and Hf addition: An insight from first-principle calculations. Appl. Surf. Sci. 2020, 517, 146201. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Huang, J.; Ye, Z.; Sun, X.; Chen, S.; Zhao, Y. First-principles calculations on Ni/W interfaces in Steel/Ni/W hot isostatic pressure diffusion bonding layer. Appl. Surf. Sci. 2019, 475, 906–916. [Google Scholar] [CrossRef]
- Jiang, D.; Carter, E.A. Prediction of strong adhesion at the MoSi2/Fe interface. Acta Mater. 2005, 53, 4489–4496. [Google Scholar] [CrossRef]
- Zhang, K.; Pang, M.; Zhan, Y. Atomic structure and electronic properties of Ag (111)/TiC (111) interface: Insights from first-principles simulations. J. Phys. Chem. Solids 2019, 124, 212–220. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, O. The crystal structure of Cu3P. Acta Chem. Scand. 1972, 26, 2777–2787. [Google Scholar] [CrossRef]
- Kittel, C.; McEuen, P. Introduction to Solid State Physics; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Geng, Q.; Chang, L.; Wei, P.; Luo, Y.; Shi, X.; Asiri, A.M.; Lu, S.; Wang, Z.; Sun, X. Cu3P nanoparticle-reduced graphene oxide hybrid: An efficient electrocatalyst to realize N2-to-NH3 conversion under ambient conditions. Chem. Commun. 2020, 56, 9328–9331. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Klaver, T.; Zhu, S.-E.; Sluiter, M.; Janssen, G. Molecular dynamics simulation of graphene on Cu (1 0 0) and (1 1 1) surfaces. Carbon 2015, 82, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Tyson, W.; Miller, W. Surface free energies of solid metals: Estimation from liquid surface tension measurements. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- Liang, Z.; Zhong, X.; Li, T.; Chen, M.; Feng, G. DFT study on the hydrogen evolution reaction for different facets of Co2P. Chem. Electro. Chem. 2019, 6, 260–267. [Google Scholar] [CrossRef]
- Smith, J.R.; Jiang, Y.; Evans, A.G. Adhesion of the γ-Ni(Al)/α-Al2O3 interface: A first-principles assessment. Int. J. Mater. Res. 2007, 98, 1214–1221. [Google Scholar] [CrossRef]
- Young, T., III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Han, Y.; Dai, Y.; Wang, J.; Shu, D.; Sun, B. First-principles calculations on Al/AlB2 interfaces. Appl. Surf. Sci. 2011, 257, 7831–7836. [Google Scholar]
- Smith, J.R.; Hong, T.; Srolovitz, D.J. Metal-ceramic adhesion and the Harris functional. Phys. Rev. Lett. 1994, 72, 4021. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Medvedeva, J.; Delley, B.; Freeman, A.; Newman, N.; Stampfl, C. Role of embedded clustering in dilute magnetic semiconductors: Cr doped GaN. Phys. Rev. Lett. 2005, 95, 256404. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| This Work | Experiment [12,13] | Previous Calculation [14] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a/Å | b/Å | c/Å | a/Å | b/Å | c/Å | a/Å | b/Å | c/Å | |
| Cu | 3.621 | 3.621 | 3.621 | 3.620 | 3.620 | 3.620 | 3.621 | 3.621 | 3.621 |
| Cu3P | 6.974 | 6.974 | 7.199 | 6.959 | 6.959 | 7.143 | 6.960 | 6.960 | 7.180 |
| Slab Thickness | ||
|---|---|---|
| Cu (100) | 1 | 1.433 |
| 2 | 1.448 | |
| 3 | 1.451 | |
| 4 | 1.454 | |
| 5 | 1.434 | |
| 6 | 1.433 | |
| 7 | 1.432 | |
| 8 | 1.431 |
| The Formation Energy of Doped (eV) | ||
|---|---|---|
| Substituted P | Substituted Cu | |
| CuP–Si | −1.2637 | 1.2642 |
| CuP–Sn | 3.4962 | 5.2153 |
| CuP–Zr | 3.0571 | −1.1363 |
| Cu3P/Cu | 1.2869 |
| CuP–Si/Cu | 1.4273 |
| CuP–Sn/Cu | 1.3440 |
| CuP–Zr/Cu | 1.5819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Cheng, F.; He, L.; Tang, W.; Wang, Y.; Chen, R.; Hu, C.; Ma, X.; Shen, H. Enhancing Wettability of Cu3P/Cu Systems through Doping with Si, Sn, and Zr Elements: Insights from First Principles Analysis. Materials 2023, 16, 2492. https://doi.org/10.3390/ma16062492
Yu S, Cheng F, He L, Tang W, Wang Y, Chen R, Hu C, Ma X, Shen H. Enhancing Wettability of Cu3P/Cu Systems through Doping with Si, Sn, and Zr Elements: Insights from First Principles Analysis. Materials. 2023; 16(6):2492. https://doi.org/10.3390/ma16062492
Chicago/Turabian StyleYu, Shimeng, Fang Cheng, Lian He, Weigang Tang, Yongsheng Wang, Rong Chen, Chenglu Hu, Xiao Ma, and Hangyan Shen. 2023. "Enhancing Wettability of Cu3P/Cu Systems through Doping with Si, Sn, and Zr Elements: Insights from First Principles Analysis" Materials 16, no. 6: 2492. https://doi.org/10.3390/ma16062492




