Cu3As: Uncommon Crystallographic Features, Low-Temperature Phase Transitions, Thermodynamic and Physical Properties
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis
2.2. Sample Characterization
2.3. Structural Characterization
2.4. Thermal Analysis
2.5. Transport and Physical Property Measurements
3. Results and Discussion
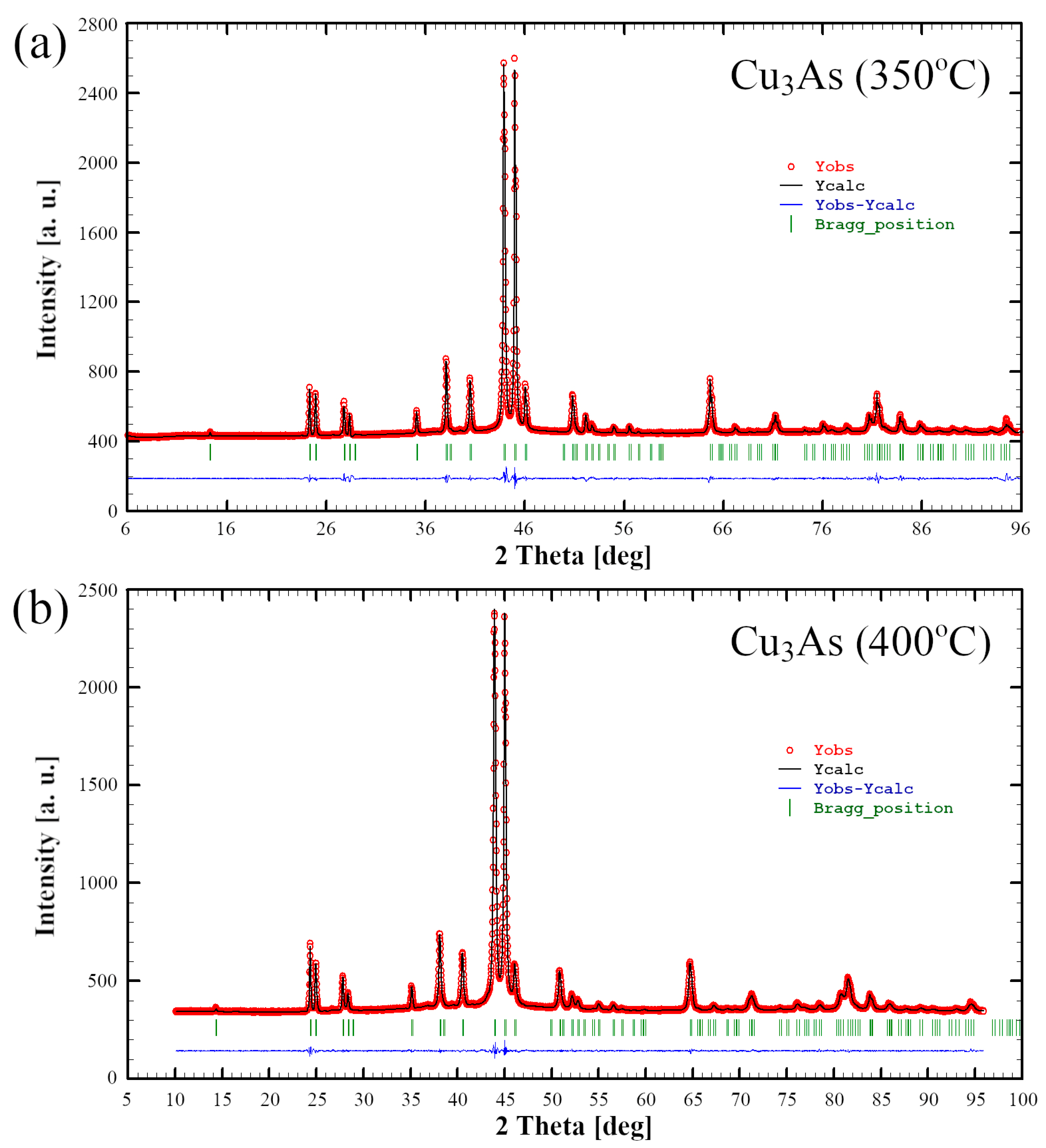
3.1. Phase Analysis
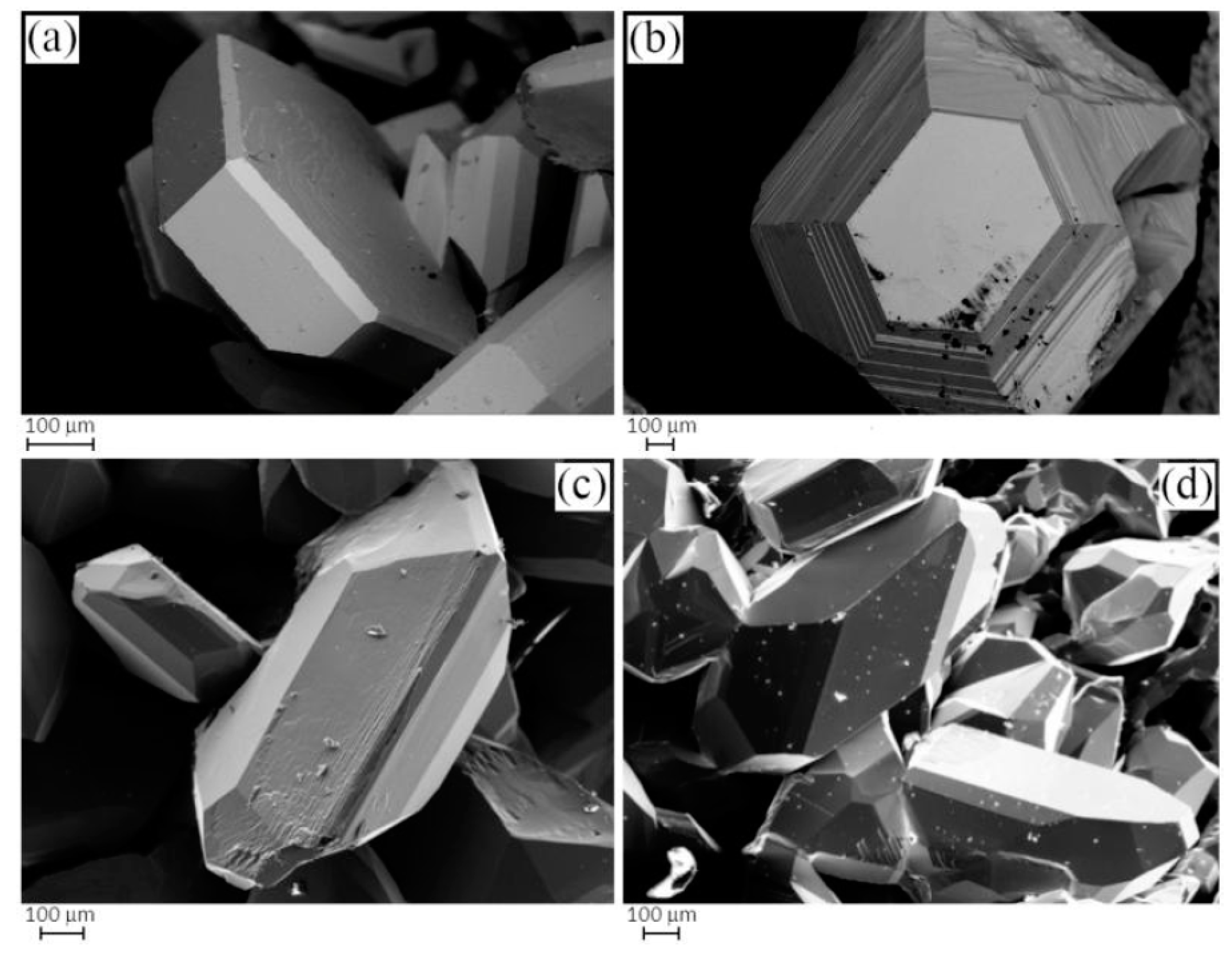
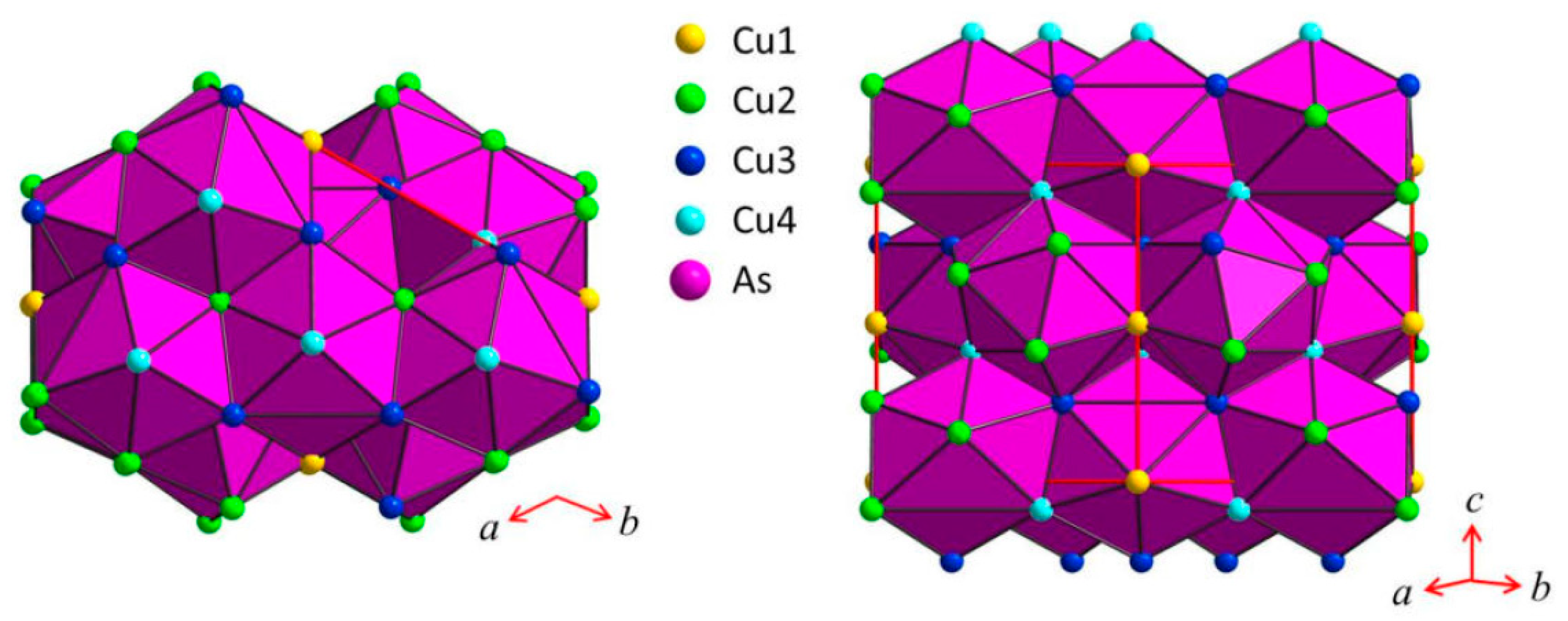
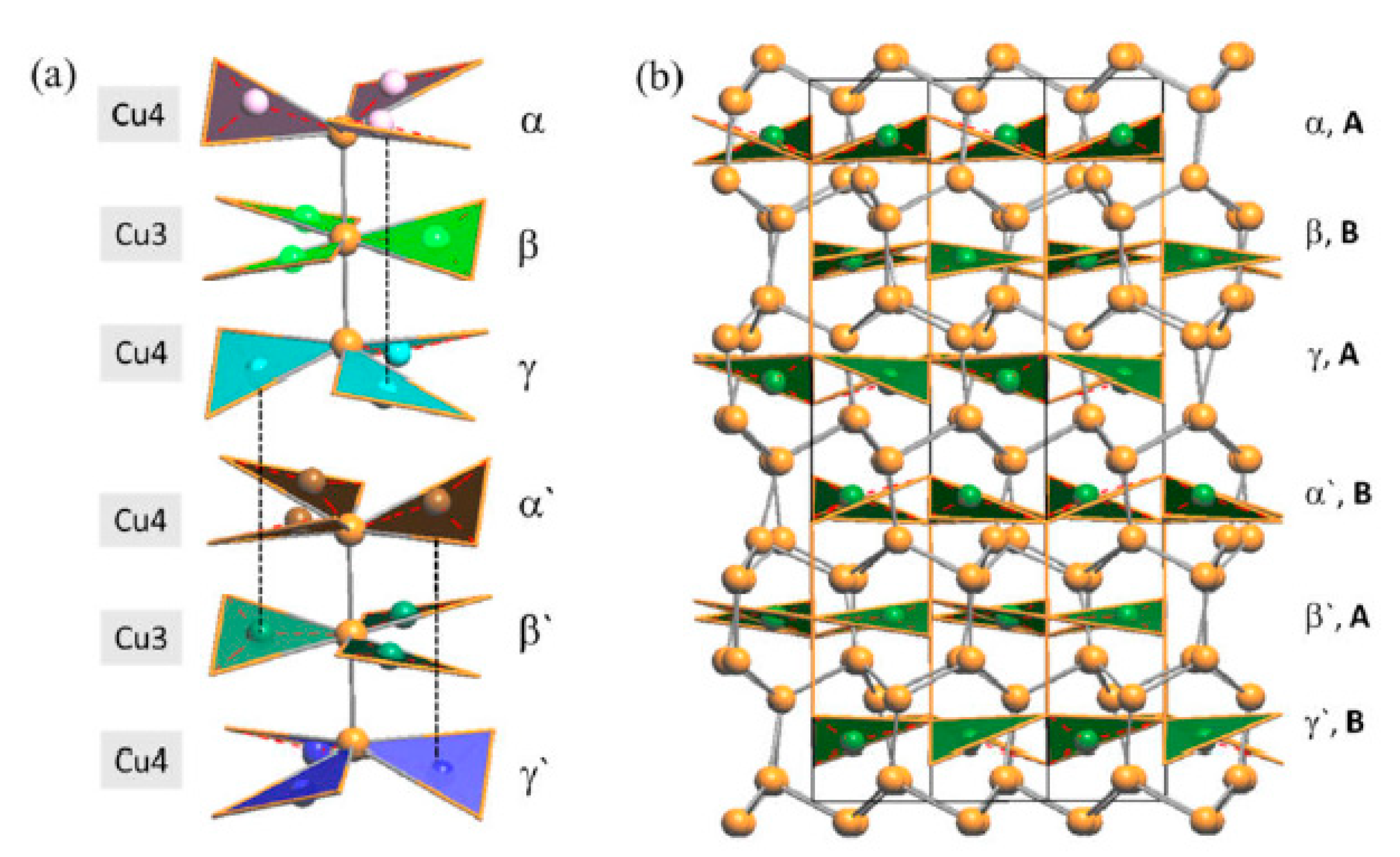
3.2. Single-Crystal Structural Study of the Cu3As Compound
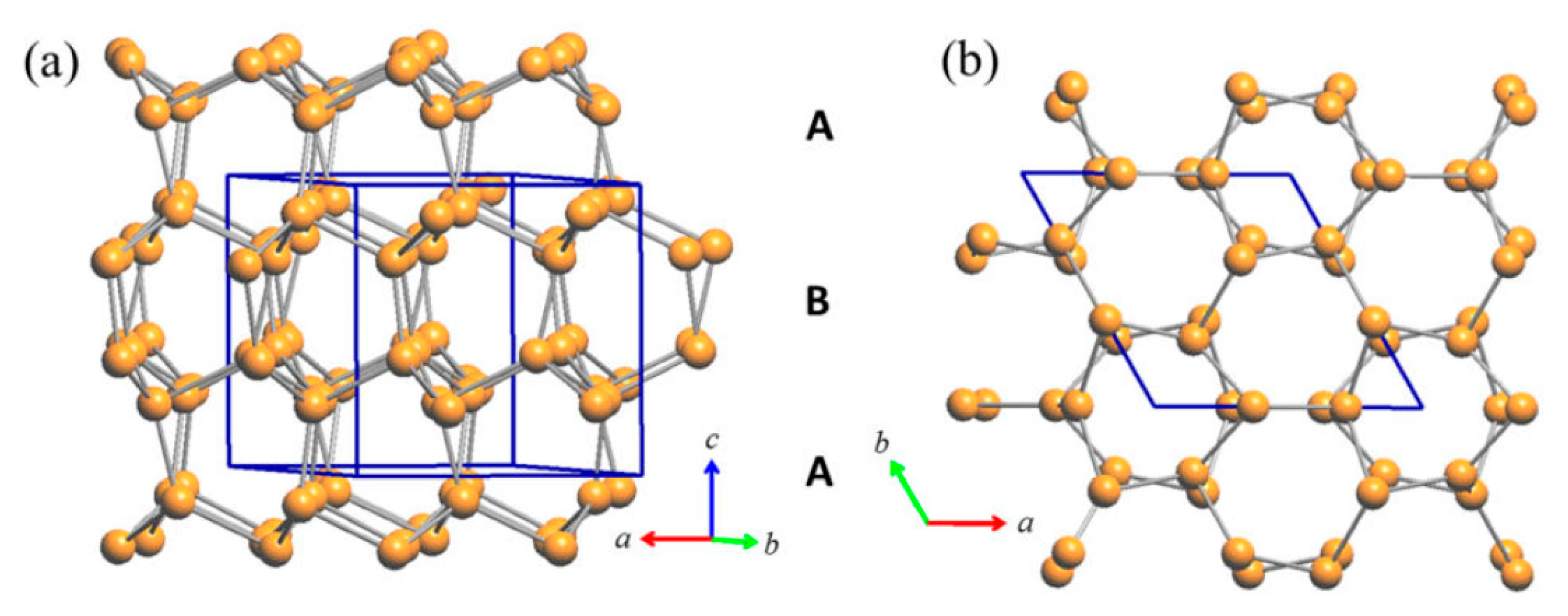
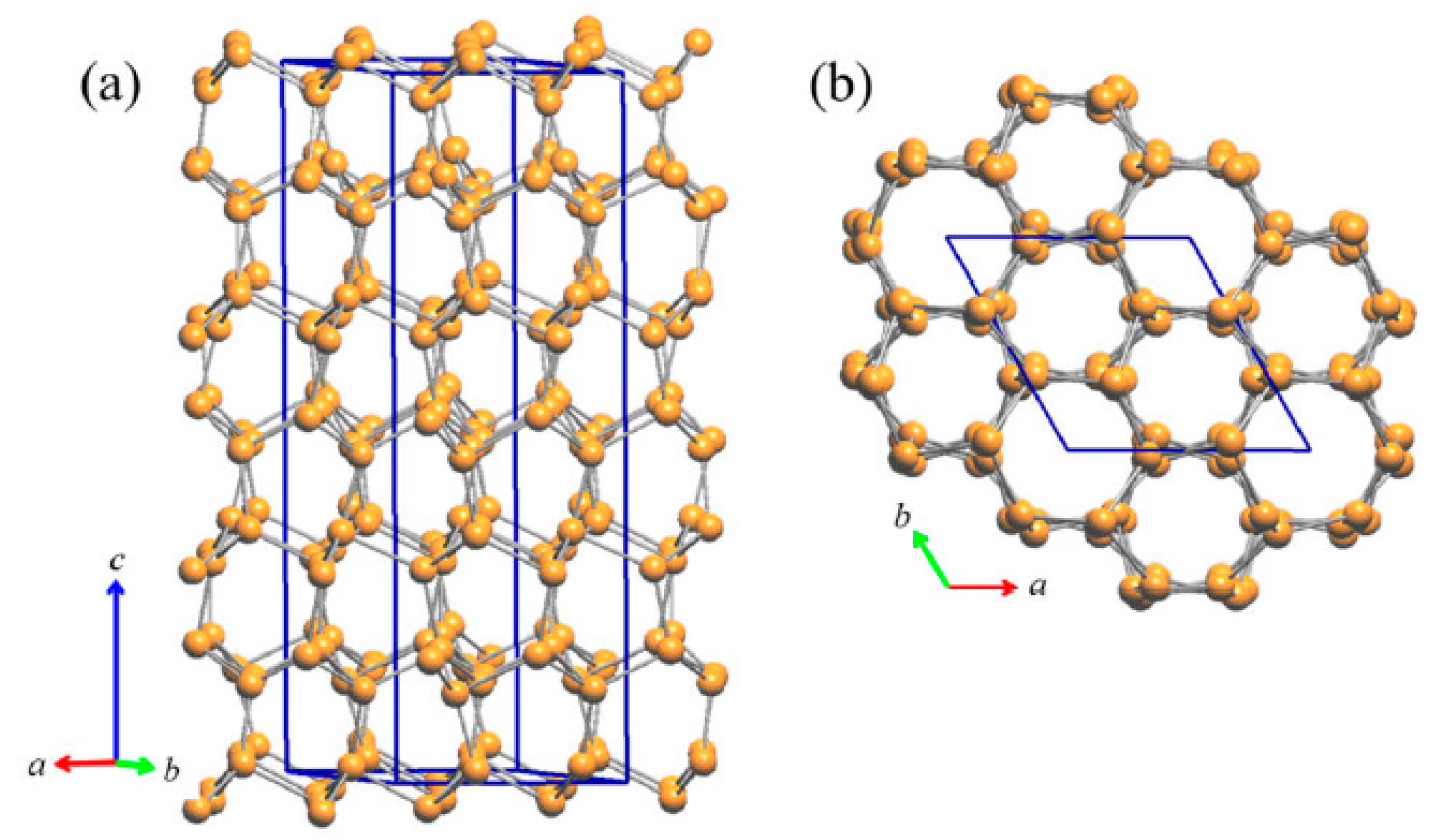
3.2.1. RT-Cu3As Crystal Structure
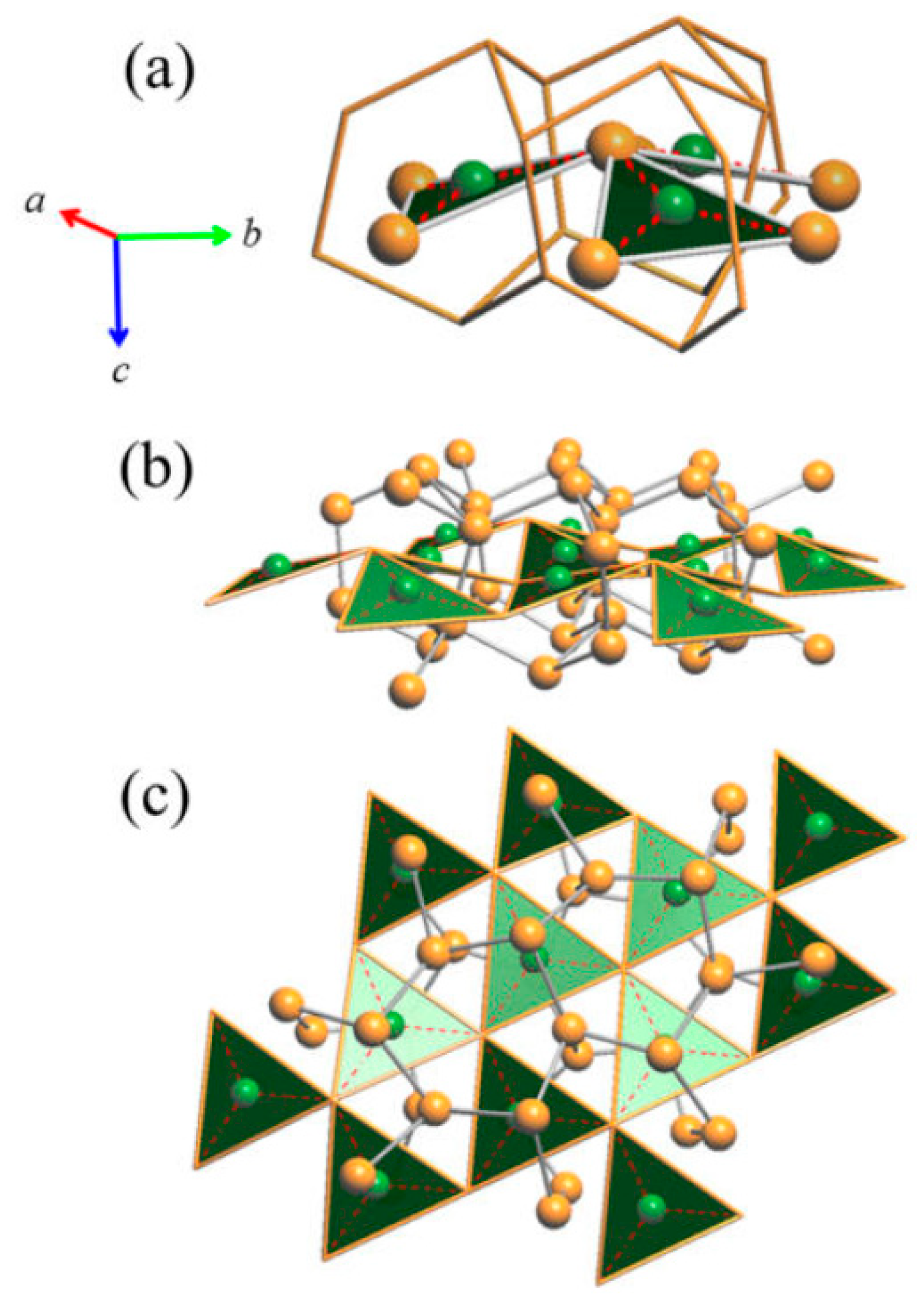
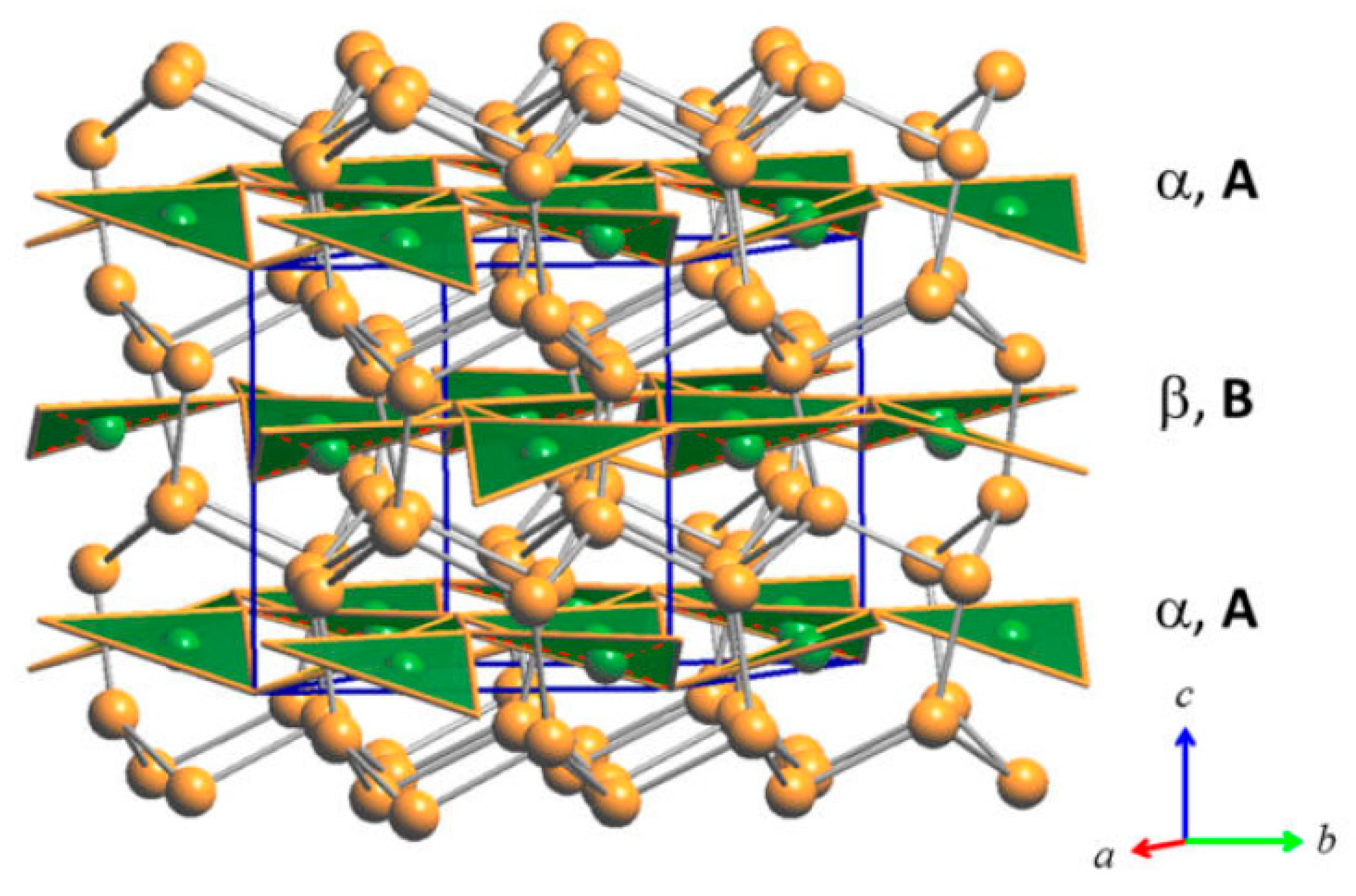
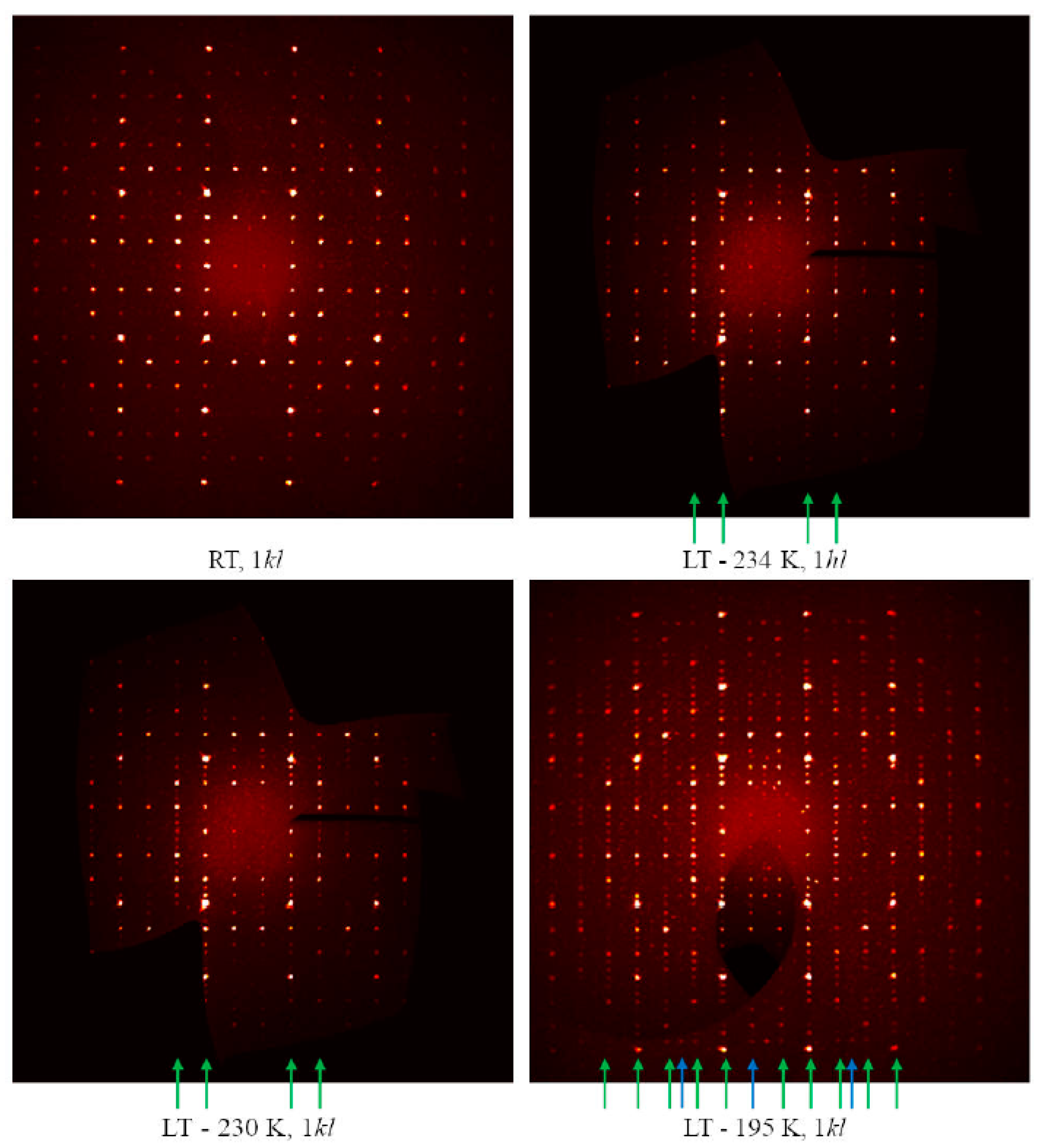
3.2.2. LT-Cu3As Crystal Structure
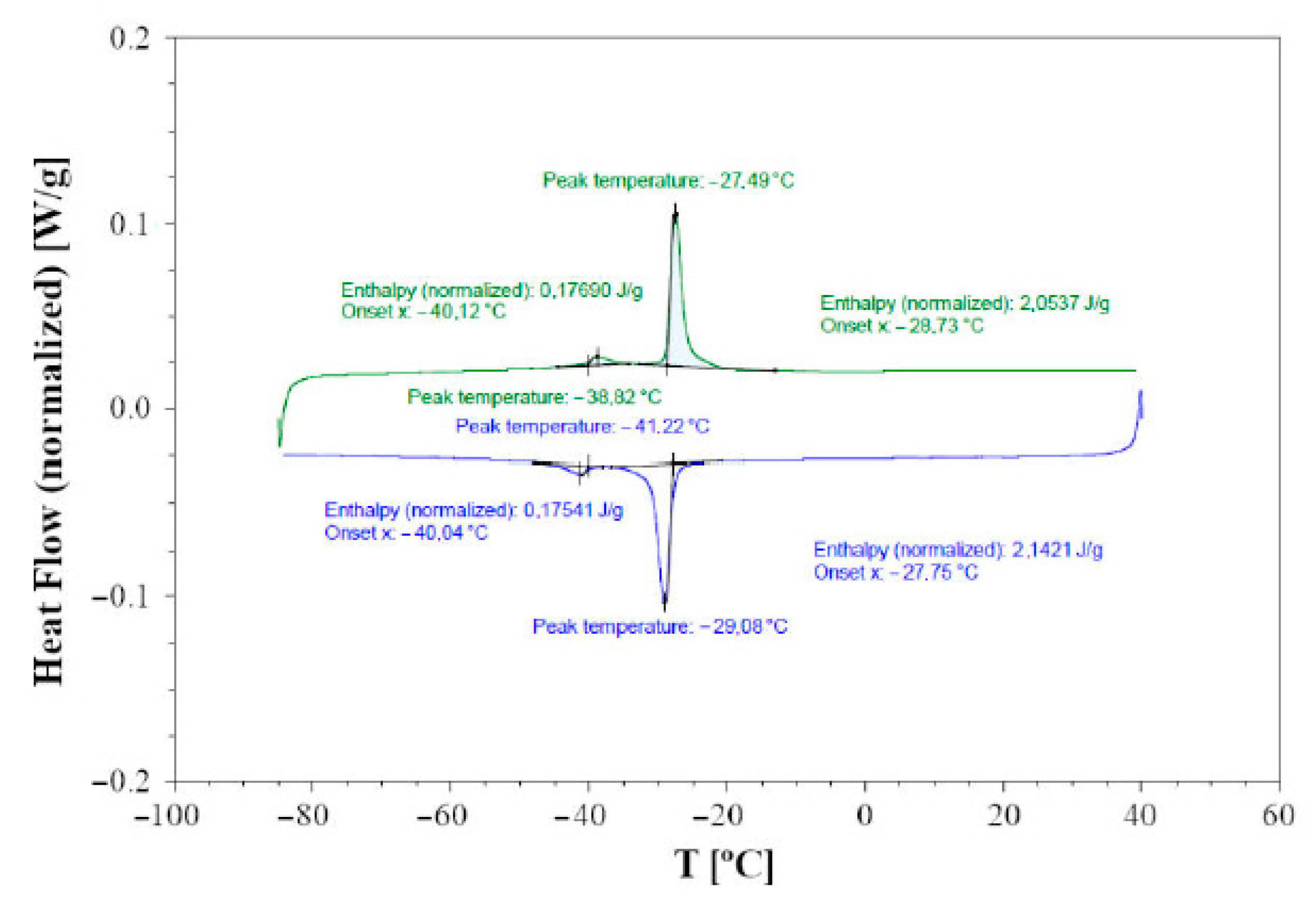
3.3. DTA and DSC Calorimetry
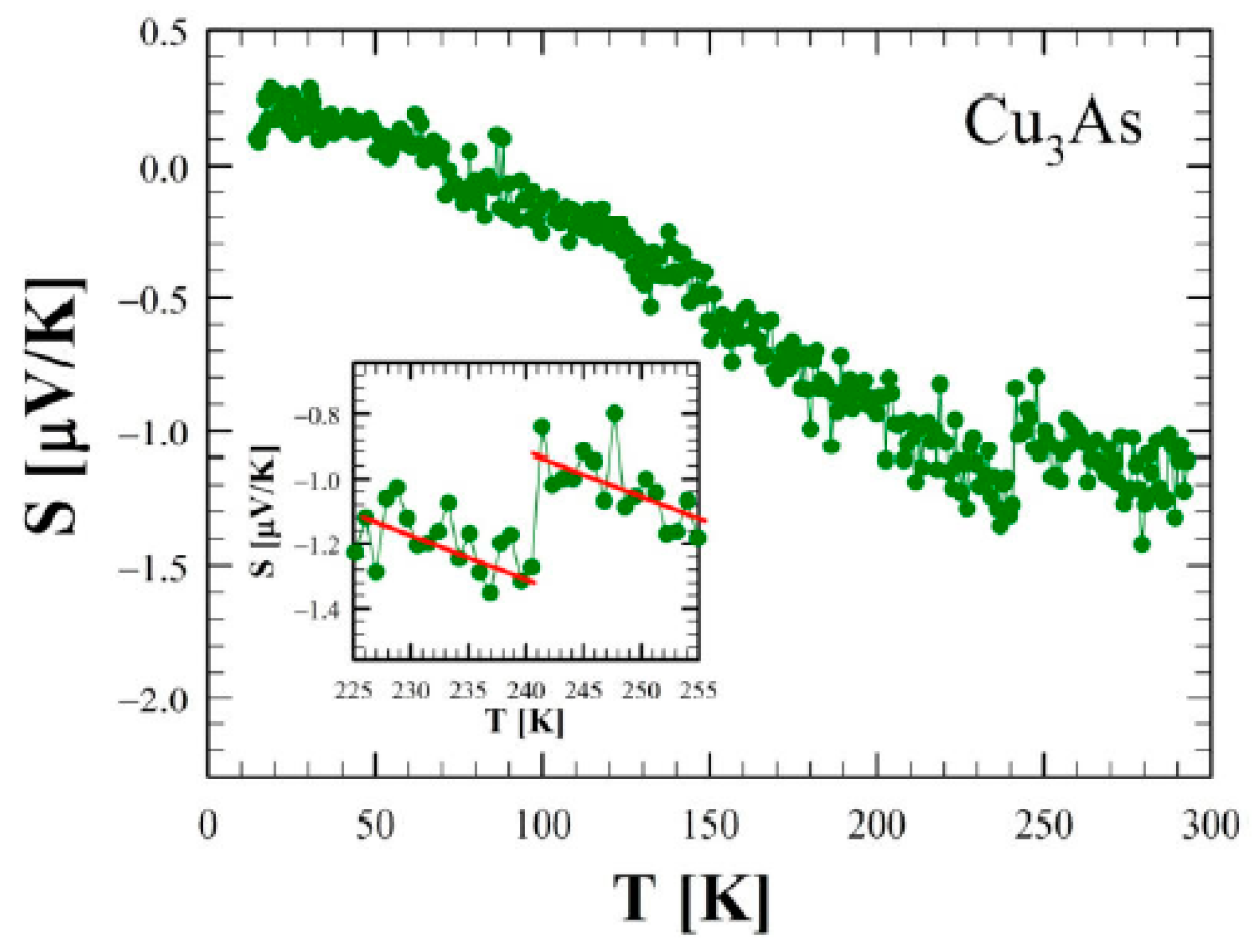
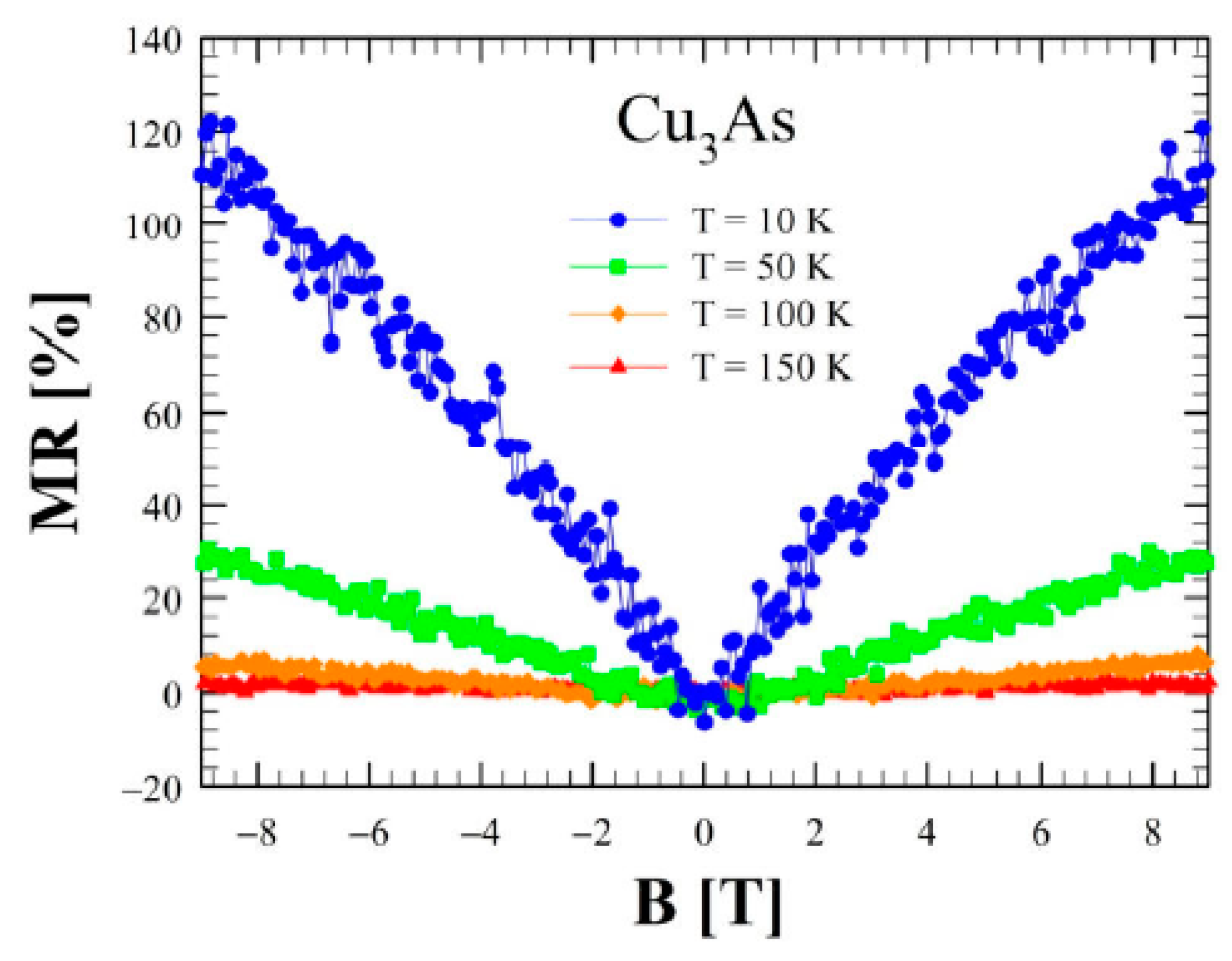
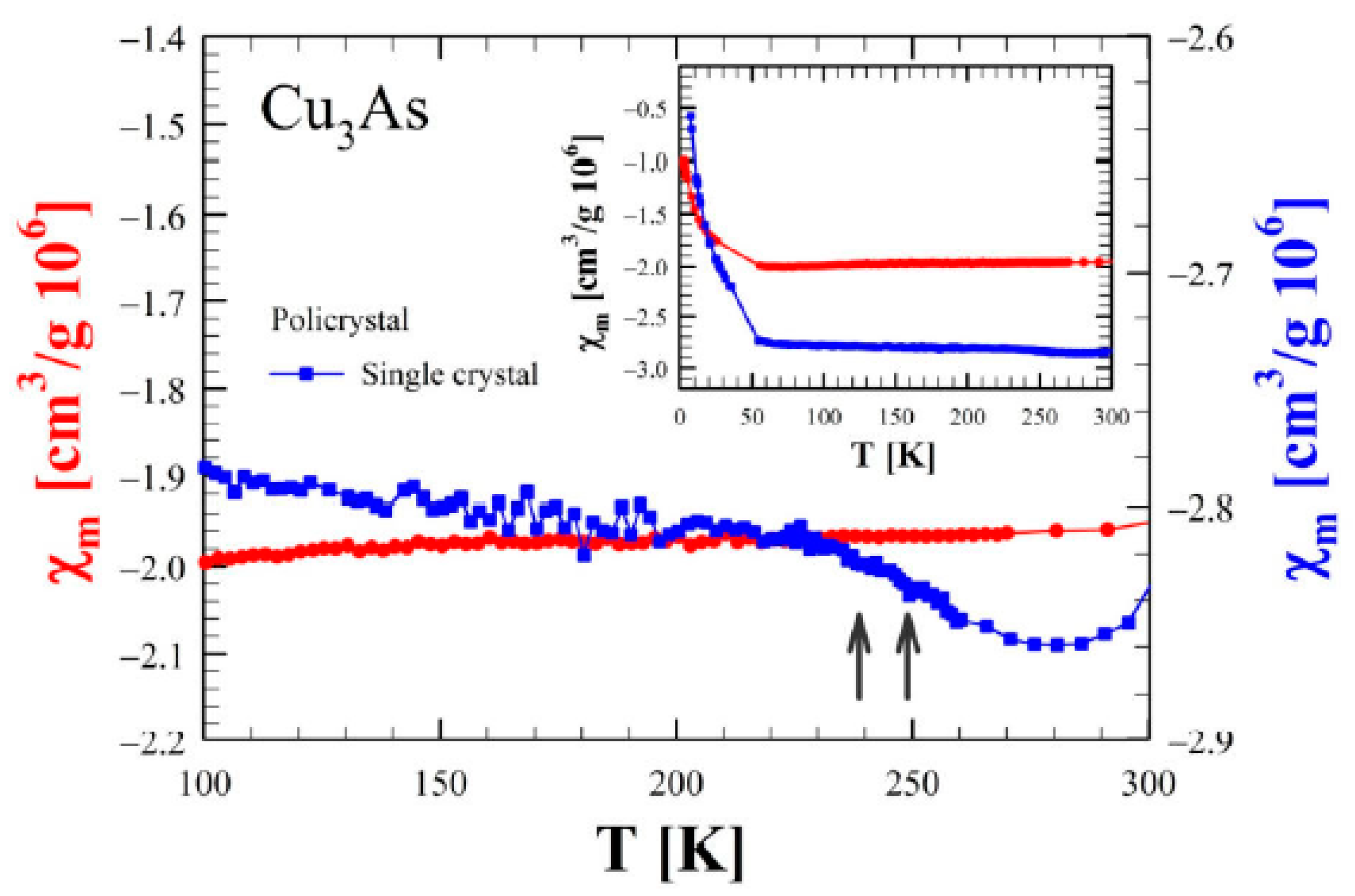
3.4. Transport and Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Heyding, R.D.; Despault, G.J.G. The copper/arsenic system and the copper arsenide minerals. Can. J. Chem. 1960, 38, 2477–2481. [Google Scholar] [CrossRef]
- Mödlinger, M.; Cziegler, A.; Macció, D.; Schnideritsch, H.; Sabatini, B. Archaeological Arsenical Bronzes and Equilibrium in the As-Cu System. Met. Mater. Trans. B 2018, 49, 2505–2513. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Laughlin, D.E. The As−Cu (Arsenic-Copper) system. Bull. Alloy Phase Diagr. 1988, 9, 605–618. [Google Scholar] [CrossRef]
- Naud, J.; Priest, P. Contribution a l’etude du systeme cuivre-arsenic. Mater. Res. Bull. 1972, 7, 783–792. [Google Scholar] [CrossRef]
- Brauer, G.; Zintl, E. Konstitution von Phosphiden, Arseniden, Antimoniden und Wismutiden des Lithiums, Natriums und Kaliums. Z. Phys. Chem. Abstr. B 1937, 37, 323–352. [Google Scholar] [CrossRef]
- Benda, K.V.; Juza, R. On the Ternary Phases in the System Lithium-Copper-Arsenic. Z. Anorg. Allg. Chem. 1969, 371, 172–192. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data—Crystal Structure Database for Inorganic Compounds; ASM International: Materials Park, OH, USA, 2020. [Google Scholar]
- Iglesias, J.E.; Nowacki, W. Refinement of the Crystal Structure of α Domeykite, Structure Related to the A15 type. Z. Kristallogr. 1977, 145, 334–345. [Google Scholar] [CrossRef]
- Liebisch, W.; Schubert, K. The Structure of Copper-Arsenic Alloys. J. Less-Common Met. 1971, 23, 231–236. [Google Scholar] [CrossRef]
- Roush, R.A.; Mazzola, M.S.; Stoudt, D.C. Infrared photoconductivity via deep copper acceptors in silicon-doped, copper-compensated gallium arsenide photoconductive switches. IEEE Trans. Electron. Devices 1993, 40, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Yvon, K.; Jeitschko, W.; Parthé, E. LAZY PULVERIX, a computer program, for calculating X-ray and neutron diffraction powder patterns. J. Appl. Crystallogr. 1977, 10, 73–74. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Bruker. APEX4; V2021.10-0; Bruker AXS Inc.: Madison, WI, USA, 2021. [Google Scholar]
- Bruker. SAINT; v8.30A; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker. XPREP; V2014/2; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Bruker. SADABS; V2016/2; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SHELXL-2019/1; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Olofsson, O. The crystal structure of Cu3P. Acta Chem. Scand. 1972, 26, 2777–2787. [Google Scholar] [CrossRef]
- Xie, Y.; Su, H.L.; Qian, X.F.; Liu, X.M.; Qian, Y.T. A Mild One-Step Solvothermal Route to Metal Phosphides (Metal=Co, Ni, Cu). J. Solid State Chem. 2000, 149, 88–91. [Google Scholar] [CrossRef]
- Németh, P.; Garvie, L.A.J.; Aoki, T.; Dubrovinskaia, N.; Dubrovinsky, L.; Buseck, P.R. Lonsdaleite is faulted and twinned cubic diamond and does not exist as a discrete material. Nat. Commun. 2014, 5, 5447. [Google Scholar] [CrossRef] [Green Version]
- Trueb, L.F. An Electron-Microscope Study of Shock-Synthesized Diamond. J. Appl. Phys. 1968, 39, 4707–4708. [Google Scholar] [CrossRef]
- Kurdyumov, A.V.; Britun, V.F.; Yarosh, V.V.; Danilenko, A.I.; Zelyavskii, V.B. The influence of the shock compression conditions on the graphite transformations into lonsdaleite and diamond. J. Superhard. Mater. 2012, 34, 19–27. [Google Scholar] [CrossRef]
- Blank, V.; Kulnitskiy, B.; Nuzhdin, A. Lonsdaleite formation in process of reverse phase transition diamond–graphite. Diam. Relat. Mater. 2011, 20, 1315–1318. [Google Scholar] [CrossRef]
- Britun, V.F.; Kurdyumov, A.V.; Petrusha, I.A. Diffusionless Nucleation of Lonsdaleite and Diamond in Hexagonal Graphite under Static Compression. Powder Met. Met. Ceram. 2004, 43, 87–93. [Google Scholar] [CrossRef]
- El Mendili, Y.; Orberger, B.; Chateigner, D.; Bardeau, J.-F.; Gascoin, S.; Petit, S. Raman investigations and ab initio calculations of natural diamond-lonsdaleite originating from New Caledonia. Chem. Phys. 2022, 559, 111541. [Google Scholar] [CrossRef]
- Matar, S.F.; Solozhenko, V.L. Ultra-hard rhombohedral carbon by crystal chemistry and ab initio investigations. J. Solid State Chem. 2021, 302, 122354. [Google Scholar] [CrossRef]
- Shiell, T.B.; McCulloch, D.G.; Bradby, J.E.; Haberl, B.; Boehler, R.; McKenzie, D.R. Nanocrystalline hexagonal diamond formed from glassy carbon. Sci. Rep. 2016, 6, 37232. [Google Scholar] [CrossRef]
- Kraus, D.; Ravasio, A.; Gauthier, M.; Gericke, D.O.; Vorberger, J.; Frydrych, S.; Helfrich, J.; Fletcher, L.B.; Schaumann, G.; Nagler, B.; et al. Nanosecond formation of diamond and lonsdaleite by shock compression of graphite. Nat. Commun. 2016, 7, 10970. [Google Scholar] [CrossRef]
- Kulnitskiy, B.; Perezhogin, I.; Dubitskya, G.; Blanka, V. Polytypes and twins in the diamond-lonsdaleite system formed by high-pressure and high-temperature treatment of graphite. Acta Crystallogr. Sect. B 2013, 695, 474–479. [Google Scholar] [CrossRef]
- Murri, M.; Smith, R.L.; McColl, K.; Hart, M.; Alvaro, M.; Jones, A.P.; Németh, P.; Salzmann, C.G.; Corà, F.; Domeneghetti, M.C.; et al. Quantifying hexagonal stacking in diamond. Sci. Rep. 2019, 9, 10334. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L.; Kamb, B. A revised set of values of single-bond radii derived from the observed interatomic distances in metals by correction for bond number and resonance energy. Proc. Natl. Acad. Sci. USA 1986, 83, 3569–3571. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Müller, P. Crystal Structure Refinement: A Crystallographers Guide to SHELXL; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Wolff, A.; Doert, T.; Hunger, J.; Kaiser, M.; Pallmann, J.; Reinhold, R.; Yogendra, S.; Giebeler, L.; Sichelschmidt, J.; Schnelle, W.; et al. Low-Temperature Tailoring of Copper-Deficient Cu3–xP—Electric Properties, Phase Transitions, and Performance in Lithium-Ion Batteries. Chem. Mater. 2018, 30, 7111–7123. [Google Scholar] [CrossRef] [Green Version]
- Pallecchi, I.; Caglieris, F.; Putti, M. Thermoelectric properties of iron-based superconductors and parent compounds. Supercond. Sci. Technol. 2016, 29, 073002. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Tu, J.; Wang, M.; Jiao, S. Cu3P as a novel cathode material for rechargeable aluminum-ion batteries. J. Mater. Chem. A 2019, 7, 8368–8375. [Google Scholar] [CrossRef]




| Compound | Cu2.881(7)As |
|---|---|
| Temperature [K] | 293(2) K |
| Formula weight [g/mol] | 265.54 |
| Structural prototype | Cu3P |
| Pearson symbol | hP24-0.71 |
| Crystal system | Hexagonal |
| Space group | P63cm (No. 185) |
| a [Å] | 7.1407(3) |
| c [Å] | 7.3057(6) |
| Unit cell volume [Å3] | 322.61(4) |
| Unit formula per cell, Z | 6 |
| Calculated density, ρ [g/cm3] | 7.97 |
| Absorption coefficient, µ [mm−1] | 43.06 |
| F(000) | 700 |
| Crystal description | Irregular form, metallic luster |
| Theta range [°] | 3.3° ≤ ϑ ≤ 45.2° |
| Index ranges h, k, l | −14 ≤ h ≤ 14 |
| –14 ≤ k ≤ 14 | |
| –14 ≤ l ≤ 14 | |
| Reflections collected | 18668 |
| Absorption correction | Multiscan |
| Refinement method | Full-matrix least-squares on F2 |
| Data/parameter | 991/26 |
| Absolute structure parameter | 0.11(12) |
| Goodness of fit on F2 | 1.08 |
| Final R indices [I > 2σ(I)] | R1 = 0.0349, wR2 = 0.0927 |
| R indices (all data) | R1 = 0.0460, wR2 = 0.0979 |
| Rint/Rsym | 0.0748/0.0255 |
| Largest diff. peak and hole [e–/Å3] | +1.82, –2.27 |
| Atom | Site | Atomic Coordinates | Occ. | Ueq [Å2] | ||
|---|---|---|---|---|---|---|
| x/a | y/b | z/c | ||||
| As | 6c | 0.33416(10) | 0 | 0.0823(4) | 1 | 0.01172(13) |
| Cu1 | 2a | 0 | 0 | 0.00000 * | 1 | 0.0212(3) |
| Cu2 | 6c | 0.7121(2) | 0 | 0.2504(4) | 1 | 0.0239(2) |
| Cu3 | 4b | 1/3 | 2/3 | 0.1550(5) | 1 | 0.0373(5) |
| Cu4 | 6c | 0.36811(19) | 0 | 0.4129(4) | 0.881(7) | 0.0271(4) |
| Atom | Site | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|---|
| As | 6c | 0.01100(17) | 0.0118(2) | 0.0124(2) | 0 | 0.00053(14) | 0.00590(11) |
| Cu1 | 2a | 0.0129(3) | 0.0129(3) | 0.0367(8) | 0 | 0 | 0.00646(15) |
| Cu3 | 6c | 0.0331(4) | 0.0156(4) | 0.0164(4) | 0 | 0.0034(5) | 0.00779(19) |
| Cu2 | 4b | 0.0149(3) | 0.0149(3) | 0.0828(16) | 0 | 0 | 0.00744(16) |
| Cu4 | 6c | 0.0232(4) | 0.0535(10) | 0.0142(5) | 0 | −0.0032(4) | 0.0268(5) |
| Central Atom | Ligands | d [Å] | dobs/ΣrM | Polyhedron |
|---|---|---|---|---|
| As CN = 11 | 1 Cu1 | 2.4605(11) | 0.922 | Pseudo Frank–Kasper polyhedra As@Cu11 |
| 2 Cu2 | 2.4361(8) | 0.913 | ||
| 1 Cu3 | 2.4469(18) | 0.918 | ||
| 2 Cu3 | 2.5541(12) | 0.957 | ||
| 1 Cu3 | 2.9652(16) | 1.111 | ||
| 1 Cu4 | 2.4277(18) | 0.910 | ||
| 1 Cu4 | 2.4601(16) | 0.922 | ||
| 2 Cu4 | 2.8037(10) | 1.051 | ||
| Cu1 CN = 12 | 3 As | 2.4605(10) | 0.922 | Icosahedron Cu@Cu9As3 |
| 3 Cu3 | 2.7483(22) | 1.075 | ||
| 3 Cu3 | 2.7516(21) | 1.076 | ||
| 3 Cu4 | 2.7044(12) | 1.058 | ||
| Cu2 CN = 12 | 3 As | 2.4361(9) | 0.913 | Icosahedron Cu@Cu9As3 |
| 3 Cu3 | 2.6511(12) | 1.037 | ||
| 3 Cu4 | 2.8751(21) | 1.125 | ||
| 3 Cu4 | 2.9470(21) | 1.153 | ||
| Cu3 CN = 12 | 1 As | 2.4469(18) | 0.918 | Icosahedron Cu@Cu8As4 |
| 2 As | 2.5541(10) | 0.957 | ||
| 1 As | 2.9652(16) | 1.111 | ||
| 1 Cu1 | 2.7483(23) | 1.075 | ||
| 1 Cu1 | 2.7516(23) | 1.076 | ||
| 2 Cu2 | 2.6511(12) | 1.037 | ||
| 2 Cu4 | 2.6722(8) | 1.046 | ||
| 1 Cu4 | 2.5313(15) | 0.990 | ||
| 1 Cu4 | 2.7284(19) | 1.068 | ||
| Cu4 CN = 13 | 1 As | 2.4277(18) | 0.910 | Pseudo Frank–Kasper polyhedra Cu@Cu9As4 |
| 1 As | 2.4601(16) | 0.922 | ||
| 2 As | 2.8037(11) | 1.051 | ||
| 1 Cu1 | 2.7044(15) | 1.058 | ||
| 2 Cu2 | 2.8751(20) | 1.125 | ||
| 2 Cu2 | 2.9740(21) | 1.153 | ||
| 1 Cu3 | 2.5313(15) | 0.990 | ||
| 2 Cu3 | 2.6722(13) | 1.046 | ||
| 1 Cu3 | 2.7284(19) | 1.068 |
| 61/65 | 62/31 | 63 | -c- | --c | |
|---|---|---|---|---|---|
| Total | 218 | 173 | 126 | 5313 | 2881 |
| N (I > 3) | 101 | 89 | 14 | 631 | 1914 |
| 〈I〉 | 6.0 | 7.5 | 0.2 | 0.4 | 16.6 |
| 〈I/σ〉 | 5.5 | 6.5 | 1.1 | 1.3 | 6.6 |
| Compound | Cu2.852(5)As |
|---|---|
| Temperature [K] | 195(2) |
| Formula weight [g/mol] | 256.12 |
| Structural prototype | Own |
| Pearson symbol | hP72 |
| Crystal system | Trigonal |
| Space group | P–3c1 (No. 165) |
| a [Å] | 7.110(2) |
| c [Å] | 21.879(4) |
| Unit cell volume [Å3] | 957.8(6) |
| Unit formula per cell, Z | 18 |
| Calculated density, ρ [g/cm3] | 7.99 |
| Absorption coefficient, µ [mm−1] | 43.21 |
| F(000) | 2083 |
| Crystal description | Irregular form, metallic luster |
| Theta range [°] | 2.8° ≤ ϑ ≤ 36.4° |
| Index ranges h, k, l | −11 ≤ h ≤ 11 |
| –11 ≤ k ≤ 11 | |
| –36 ≤ l ≤ 36 | |
| Reflections collected | 57491 |
| Absorption correction | Multiscan |
| Refinement method | Full-matrix least-squares on F2 |
| Data/parameter | 1568/58 |
| Twin law | −1 0 0 0 −1 0 0 0 −1 |
| BASF | 0.60 |
| Goodness of fit on F2 | 1.17 |
| Final R indices [I > 2σ(I)] | R1 = 0.0481, wR2 = 0.1103 |
| R indices (all data) | R1 = 0.0510, wR2 = 0.1116 |
| Rint/Rsym | 0.0743/0.0210 |
| Largest diff. peak and hole [e–/Å3] | +4.03, –4.11 |
| Atom | Site | Atomic Coordinates | Occ. | Ueq [Å2] | ||
|---|---|---|---|---|---|---|
| x/a | y/b | z/c | ||||
| As1 | 12g | 0.32922(14) | 0.32932(15) | 0.07954(3) | 1 | 0.01017(13) |
| As2 | 6f | 0.34120(17) | 0 | 1/4 | 1 | 0.00890(16) |
| Cu1 | 4d | 1/3 | 2/3 | 0.05029(8) | 1 | 0.0138(3) |
| Cu2 | 4d | 1/3 | 2/3 | 0.39253(9) | 1 | 0.0147(3) |
| Cu3 | 2a | 0 | 0 | 1/4 | 1 | 0.0196(5) |
| Cu4 | 4c | 0 | 0 | 0.11530(10) | 1 | 0.0171(3) |
| Cu5 | 4d | 1/3 | 2/3 | 0.22502(12) | 1 | 0.0232(4) |
| Cu6 | 12g | 0.06180(2) | 0.39440(2) | 0.13869(5) | 1 | 0.0218(2) |
| Cu7 | 12g | 0.34900(2) | 0.30257(16) | 0.19163(5) | 1 | 0.01458(19) |
| Cu8 | 12g | 0.34710(2) | 0.03300(2) | 0.02891(5) | 0.778(5) | 0.0135(3) |
| Atom | Site | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|---|
| As1 | 12g | 0.0087(3) | 0.0092(3) | 0.0126(3) | −0.0027(2) | 0.0003(2) | 0.0045(3) |
| As2 | 6f | 0.0110(4) | 0.0083(3) | 0.0083(3) | −0.00024(18) | −0.0005(4) | 0.0055(2) |
| Cu1 | 4d | 0.0112(4) | 0.0112(4) | 0.0189(7) | 0.000 | 0.000 | 0.0056(2) |
| Cu2 | 4d | 0.0114(4) | 0.0114(4) | 0.0213(7) | 0.000 | 0.000 | 0.0057(2) |
| Cu3 | 2a | 0.0071(5) | 0.0071(5) | 0.0446(15) | 0.000 | 0.000 | 0.0036(3) |
| Cu4 | 4c | 0.0122(4) | 0.0122(4) | 0.0269(8) | 0.000 | 0.000 | 0.0061(2) |
| Cu5 | 4d | 0.0102(4) | 0.0102(4) | 0.0494(12) | 0.000 | 0.000 | 0.0051(2) |
| Cu6 | 12g | 0.0358(6) | 0.0331(6) | 0.0145(4) | 0.0085(4) | 0.0106(4) | 0.0307(5) |
| Cu7 | 12g | 0.0175(4) | 0.0100(4) | 0.0150(3) | 0.0044(3) | 0.0003(4) | 0.0059(4) |
| Cu8 | 12g | 0.0116(6) | 0.0247(7) | 0.0082(4) | 0.0035(5) | 0.0027(4) | 0.0121(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mödlinger, M.; Provino, A.; Solokha, P.; Caglieris, F.; Ceccardi, M.; Macciò, D.; Pani, M.; Bernini, C.; Cavallo, D.; Ciccioli, A.; et al. Cu3As: Uncommon Crystallographic Features, Low-Temperature Phase Transitions, Thermodynamic and Physical Properties. Materials 2023, 16, 2501. https://doi.org/10.3390/ma16062501
Mödlinger M, Provino A, Solokha P, Caglieris F, Ceccardi M, Macciò D, Pani M, Bernini C, Cavallo D, Ciccioli A, et al. Cu3As: Uncommon Crystallographic Features, Low-Temperature Phase Transitions, Thermodynamic and Physical Properties. Materials. 2023; 16(6):2501. https://doi.org/10.3390/ma16062501
Chicago/Turabian StyleMödlinger, Marianne, Alessia Provino, Pavlo Solokha, Federico Caglieris, Michele Ceccardi, Daniele Macciò, Marcella Pani, Cristina Bernini, Dario Cavallo, Andrea Ciccioli, and et al. 2023. "Cu3As: Uncommon Crystallographic Features, Low-Temperature Phase Transitions, Thermodynamic and Physical Properties" Materials 16, no. 6: 2501. https://doi.org/10.3390/ma16062501
APA StyleMödlinger, M., Provino, A., Solokha, P., Caglieris, F., Ceccardi, M., Macciò, D., Pani, M., Bernini, C., Cavallo, D., Ciccioli, A., & Manfrinetti, P. (2023). Cu3As: Uncommon Crystallographic Features, Low-Temperature Phase Transitions, Thermodynamic and Physical Properties. Materials, 16(6), 2501. https://doi.org/10.3390/ma16062501






