LCST-UCST Transition Property of a Novel Retarding Swelling and Thermosensitive Particle Gel
Abstract
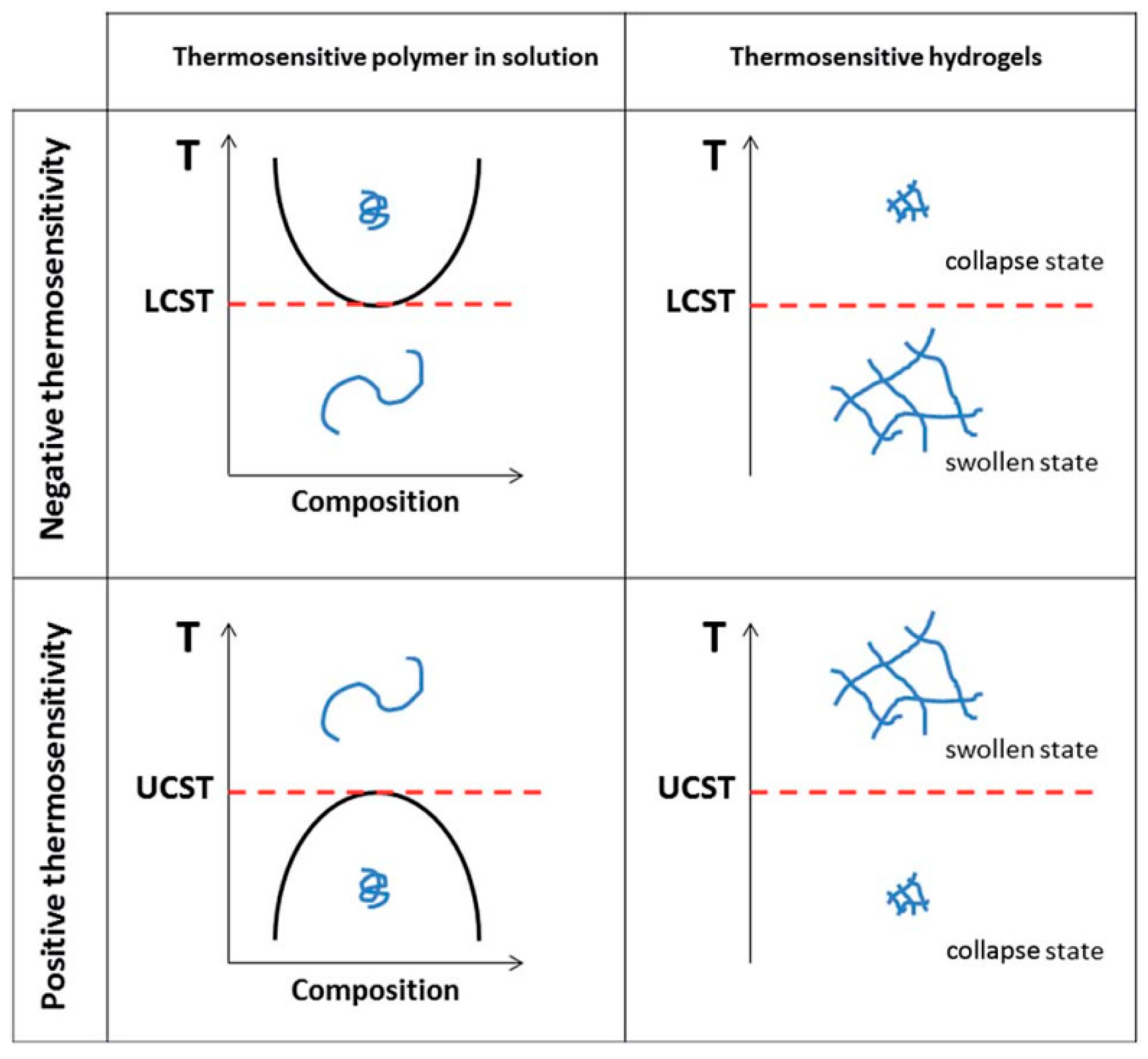
:1. Introduction
2. Experimental Section
2.1. Materials
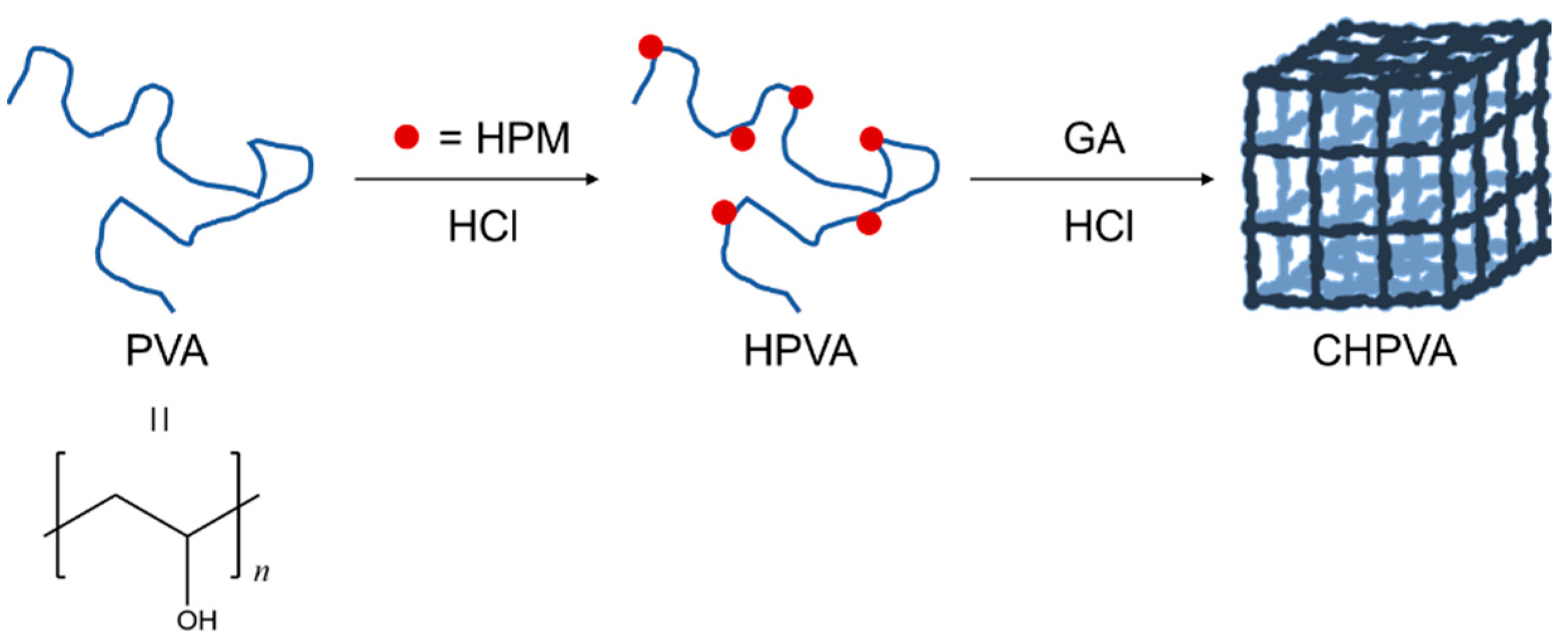
2.2. Synthesis of the Copolymer and Hydrogels
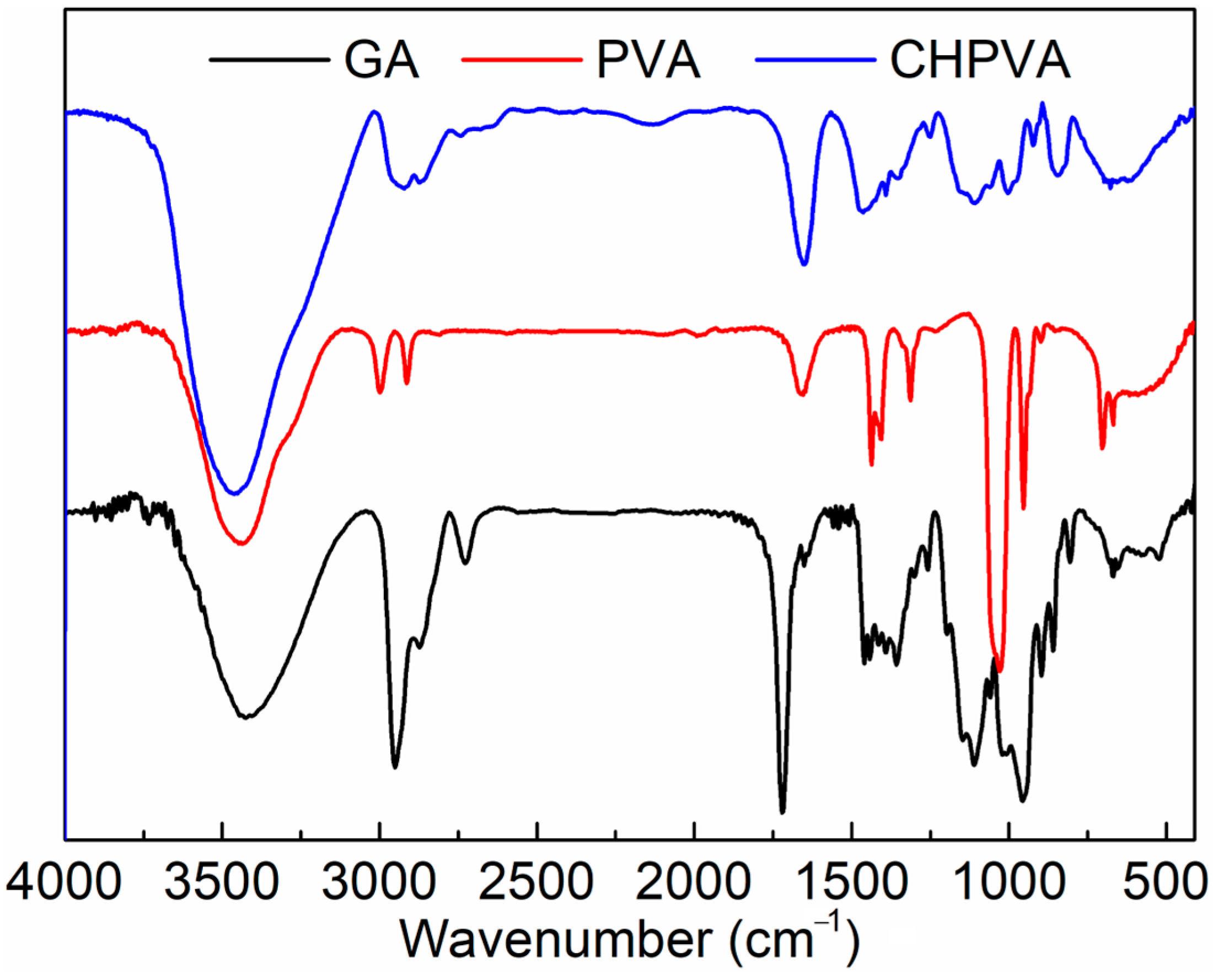
2.3. FTIR and 1H-NMR Measurements
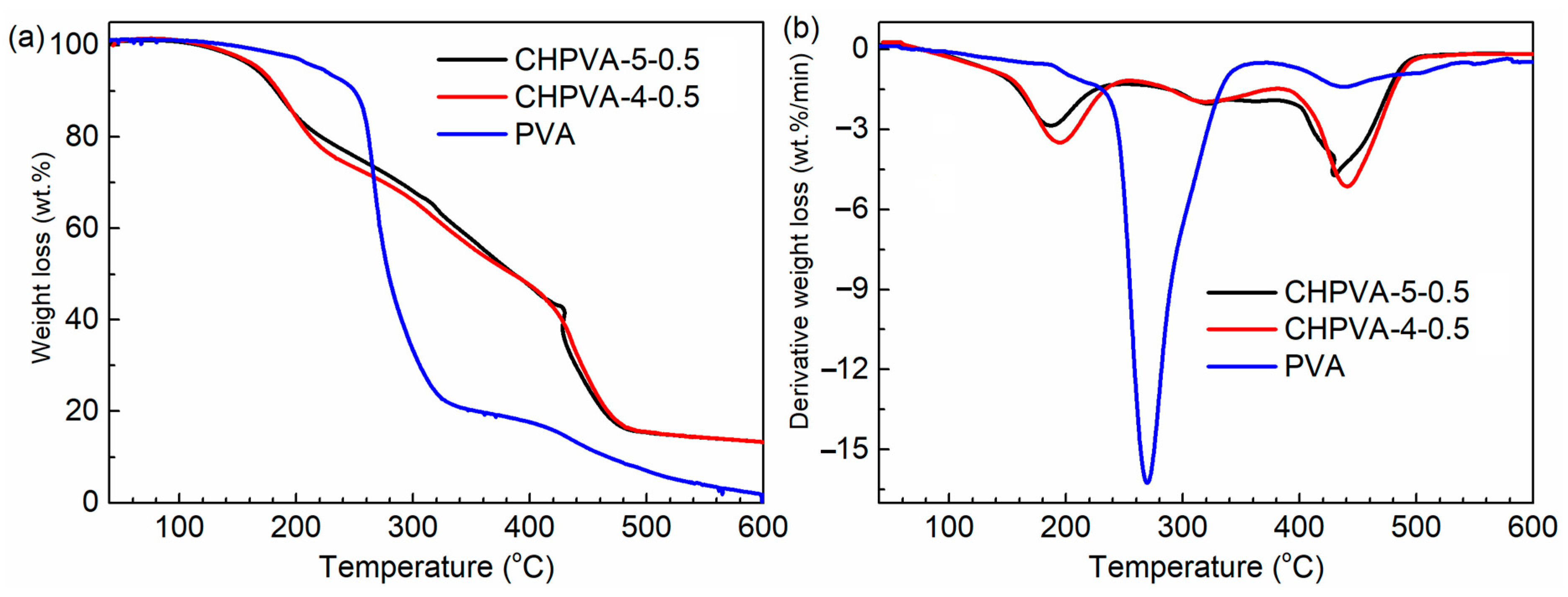
2.4. Thermogravimetric (TG) Analysis
2.5. Scanning Electron Microscope (SEM) Imaging
2.6. Thermosensitive-Transparency
2.7. Swelling Properties
3. Results and Discussion
3.1. FTIR and 1H-NMR Measurements
3.2. Thermogravimetric Analysis
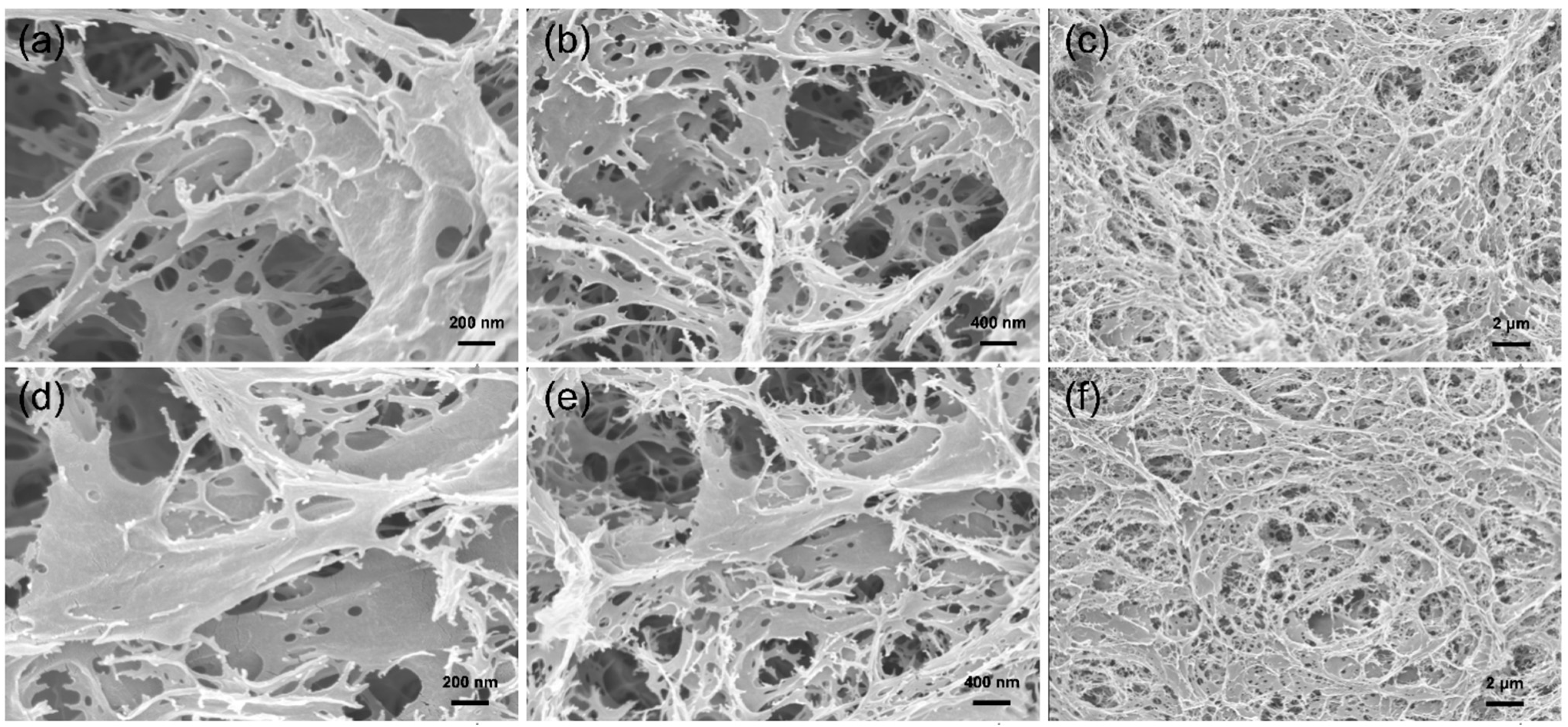
3.3. Micromorphology of CHPVA Hydrogels
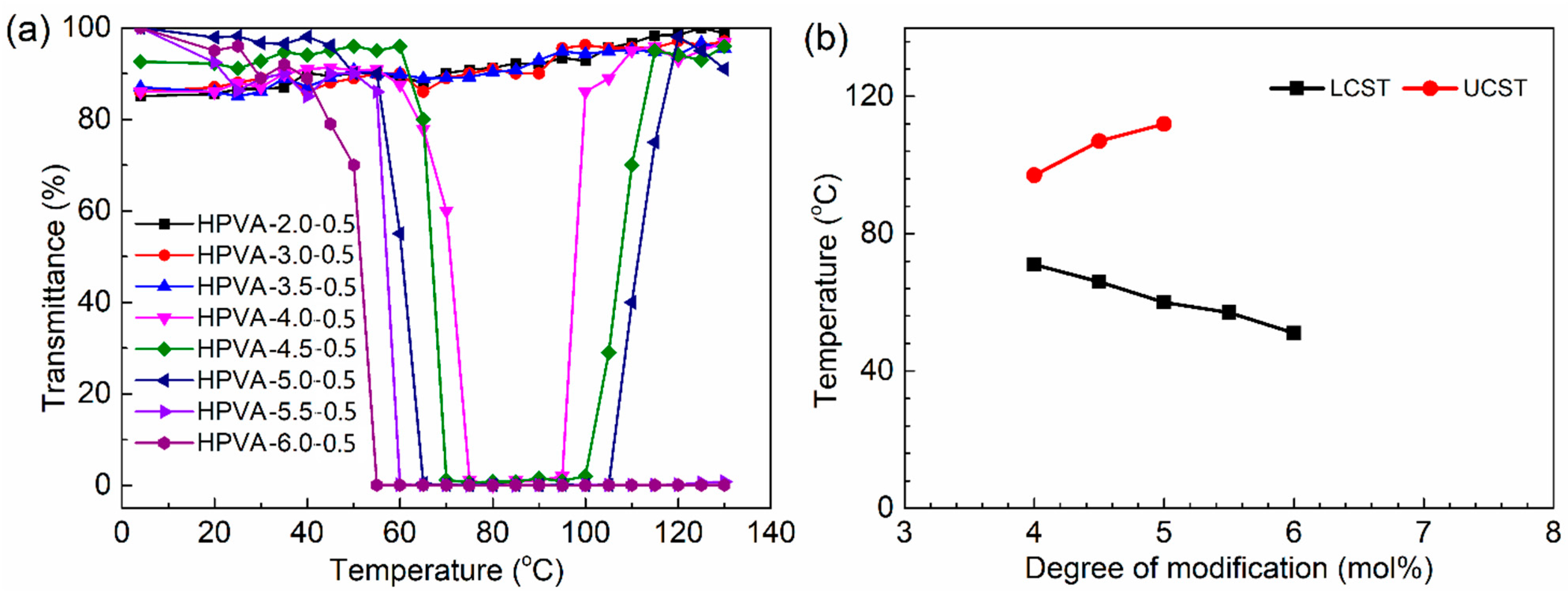
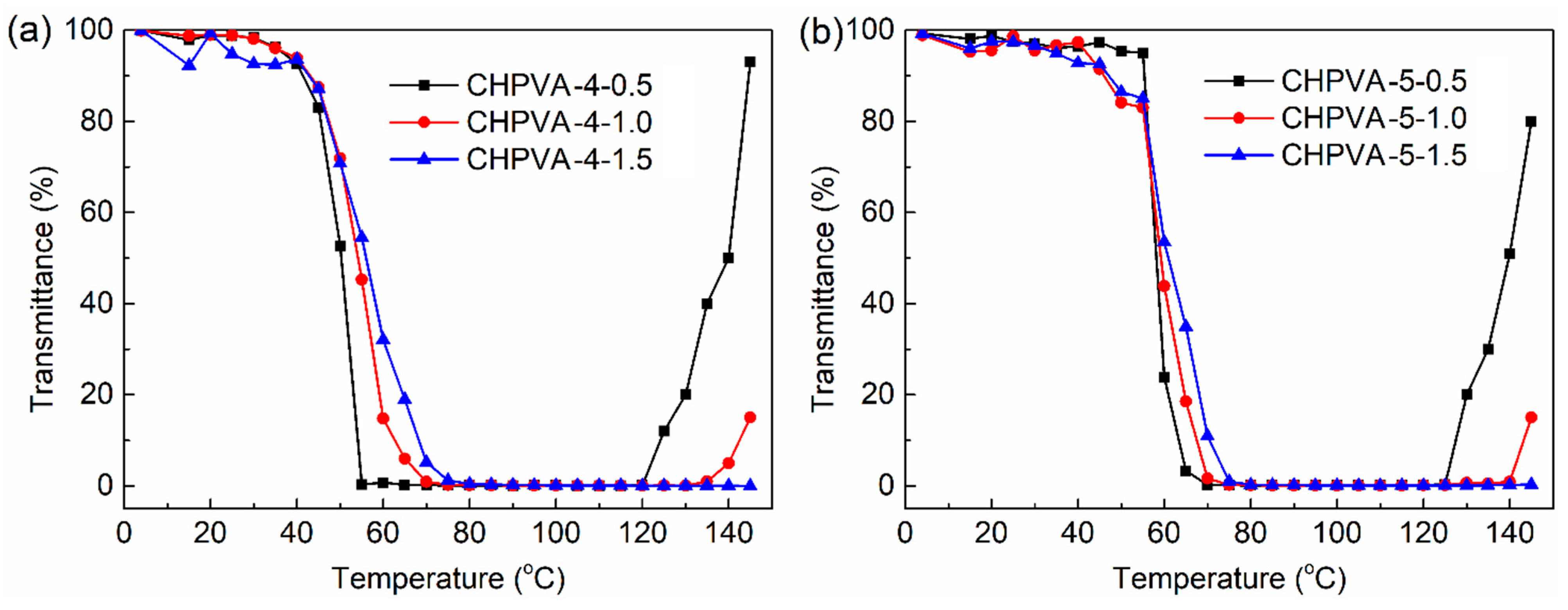
3.4. Thermosensitive-Transparency
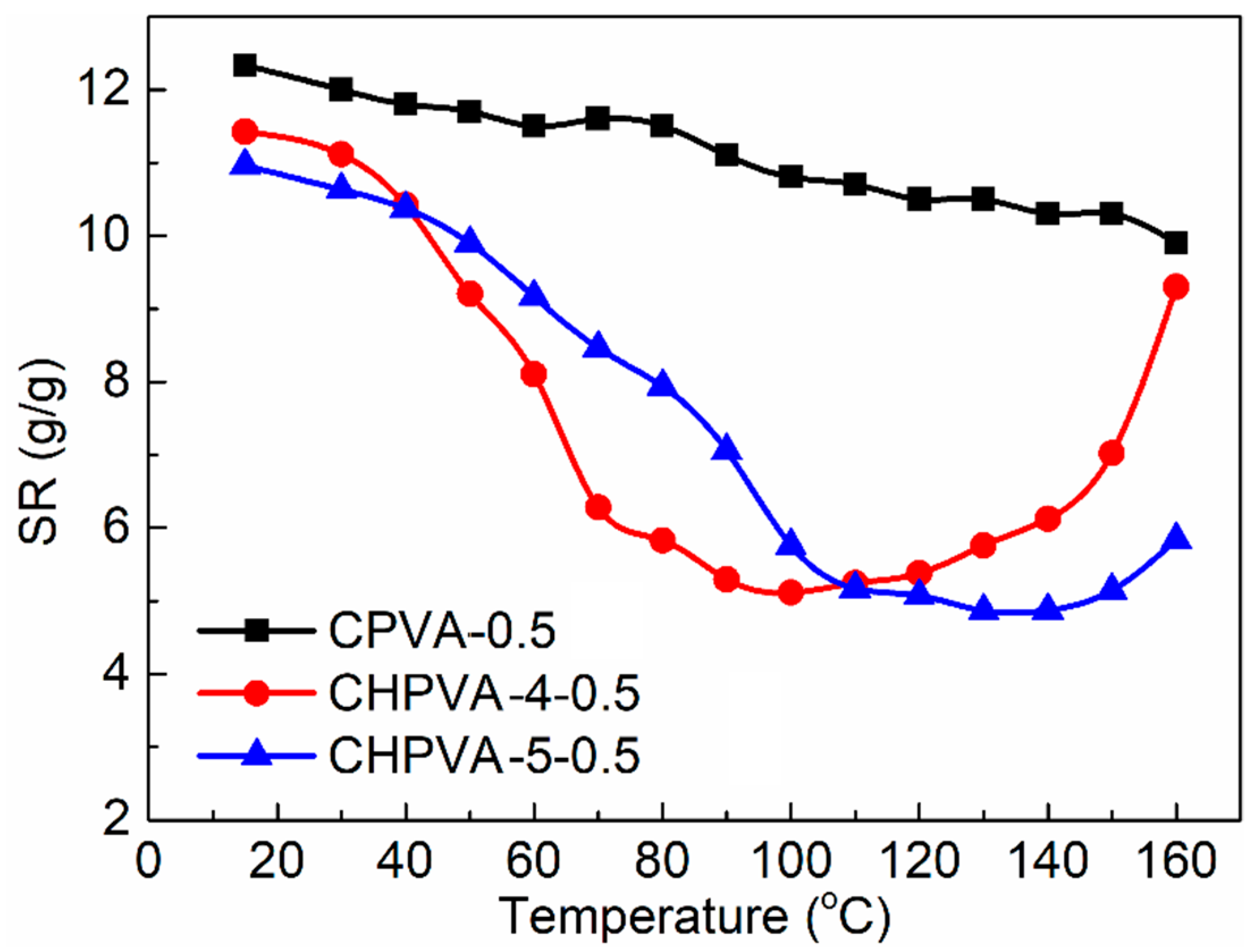
3.5. Swelling Properties
4. Conclusions
- (1)
- A series of CHPVA hydrogels was successfully prepared, of which the structure was confirmed by FTIR and 1H-NMR. The micromorphology of the hydrogel network was presented by SEM imaging.
- (2)
- The measurement of light transmittance with temperature increase shows that the hydrophobic modification of HPVA will reduce the LCST of the polymer solution and increase the UCST. The CHPVA hydrogels with the same crosslinking density present the same tendency. For the CHPVA hydrogels with the same modification degree, both the LCST and UCST increase with the increase in crosslinking density.
- (3)
- The temperature-increasing swelling experiment shows that the swelling-shrinking-swelling change of CHPVA hydrogels with temperature generate the retarding swelling property of PPGs taking advantage of the temperature gradient and is responsible for the deep migration and efficient water plugging of PPGs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for Enhanced Oil Recovery Technology. Proc. Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Son, H.; Kim, H.; Lee, G.; Kim, J.; Sung, W. Enhanced oil recovery using nanoparticle-stabilized oil/water emulsions. Korean J. Chem. Eng. 2014, 31, 338–342. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Wei, B.; Mao, R.; Tian, Q.; Xiang, H.; Xu, X.; Wood, C. Nanocellulose-Strengthened Particle Gel for Conformance Control in Fractured Tight Formations. Part I: Preparation and Mechanical Stability. Energy Fuels 2020, 34, 5766–5776. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Pu, W.; Li, L.; Bai, B.; Han, Q.; Zhang, Y.; Tang, X. Combining Preformed Particle Gel and Curable Resin-Coated Particles to Control Water Production from High-Temperature and High-Salinity Fractured Producers. SPE J. 2020, 25, 938–950. [Google Scholar] [CrossRef]
- Kumar, A.; Mahto, V.; Sharma, V.P. Reinforced preformed particle gel: Synthesis, characterization and performance evaluation for water shut-off jobs in heterogeneous reservoir. J. Pet. Sci. Eng. 2020, 193, 107408. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, Z.; Gong, H.; Zhu, C.; Sang, Q.; Li, Y.; Dong, M. Synergy of microbial polysaccharides and branched-preformed particle gel on thickening and enhanced oil recovery. Chem. Eng. Sci. 2019, 208, 115138. [Google Scholar] [CrossRef]
- Tang, X.; Liu, Y.; Limin, Y.; Wei, F.; Ma, T.; Ma, H. Laboratory researches on deep fluid diversion agent with high strength and retarding swelling characteristics. Pet. Explor. Dev. 2009, 36, 494–497. [Google Scholar]
- Deng, Y.; Zhou, M. Study on wrapping methods of control initial water-absorbent rate of super absorbent resin. Chem. Eng. Oil Gas 2009, 38, 422–425. [Google Scholar]
- Yu, X.; Pu, W.; Chen, D.; Zhang, J.; Zhou, F.; Zhang, R.; Gu, S. Degradable cross-linked polymeric microsphere for enhanced oil recovery applications. RSC Adv. 2015, 5, 62752–62762. [Google Scholar] [CrossRef]
- Jia, H.; Ren, Q.; Pu, W.; Zhao, J. Swelling Mechanism Investigation of Microgel with Double-Cross-Linking Structures. Energy Fuels 2014, 28, 6735–6744. [Google Scholar] [CrossRef]
- Bai, Y.; Ban, C.; He, S.; Zhao, J.; Zhang, H. Temperature-responsive self-lubricating hydrogel from dynamic Diels-Alder crosslinking for reservoir in-depth profile control. J. Mol. Liq. 2020, 323, 114595. [Google Scholar] [CrossRef]
- Augé, A.; Zhao, Y. What determines the volume transition temperature of UCST acrylamide–acrylonitrile hydrogels? RSC Adv. 2016, 6, 70616–70623. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Lessard, D.G.; Ousalem, M.; Zhu, X.X. Effect of the molecular weight on the lower critical solution temperature of poly(N, N-diethylacrylamide) in aqueous solutions. Can. J. Chem. 2001, 79, 1870–1874. [Google Scholar] [CrossRef]
- Bütün, V.; Armes, S.P.; Billingham, N.C. Synthesis and aqueous solution properties of near-monodisperse tertiary amine methacrylate homopolymers and diblock copolymers. Polymer 2001, 42, 5993–6008. [Google Scholar] [CrossRef]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2020, 31, 2008123. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution: Unexpected Properties from Known Building Blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Huglin, M.B.; Radwan, M.A. Unperturbed dimensions of a zwitterionic polymethacrylate. Polym. Int. 1991, 26, 97–104. [Google Scholar] [CrossRef]
- Schulz, D.N.; Peiffer, D.G.; Agarwal, P.K.; Larabee, J.; Kaladas, J.J.; Soni, L.; Handwerker, B.; Garner, R.T. Phase behaviour and solution properties of sulphobetaine polymers. Polymer 1986, 27, 1734–1742. [Google Scholar] [CrossRef]
- Mary, P.; Bendejacq, D.D.; Labeau, M.-P.; Dupuis, P. Reconciling Low- and High-Salt Solution Behavior of Sulfobetaine Polyzwitterions. J. Phys. Chem. B 2007, 111, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, G.N.; Rowlinson, J.S. The thermodynamic properties of aqueous solutions of polyethylene glycol, polypropylene glycol and dioxane. Trans. Faraday Soc. 1957, 53, 921–931. [Google Scholar] [CrossRef]
- Saeki, S.; Kuwahara, N.; Nakata, M.; Kaneko, M. Upper and lower critical solution temperatures in poly(ethylene glycol) solutions. Polymer 1976, 17, 685–689. [Google Scholar] [CrossRef]
- Bae, Y.C.; Lambert, S.M.; Soane, D.S.; Prausnitz, J.M. Cloud-point curves of polymer solutions from thermooptical measurements. Macromolecules 1991, 24, 4403–4407. [Google Scholar] [CrossRef] [Green Version]
- Dormidontova, E.E. Role of Competitive PEO−Water and Water−Water Hydrogen Bonding in Aqueous Solution PEO Behavior. Macromolecules 2002, 35, 987–1001. [Google Scholar] [CrossRef]
- Dormidontova, E.E. Influence of End Groups on Phase Behavior and Properties of PEO in Aqueous Solutions. Macromolecules 2004, 37, 7747–7761. [Google Scholar] [CrossRef]
- Van Durme, K.; Van Assche, G.; Nies, E.; Van Mele, B. Phase Transformations in Aqueous Low Molar Mass Poly(vinyl methyl ether) Solutions: Theoretical Prediction and Experimental Validation of the Peculiar Solvent Melting Line, Bimodal LCST, and (Adjacent) UCST Miscibility Gaps. J. Phys. Chem. B 2007, 111, 1288–1295. [Google Scholar] [CrossRef]
- Van Assche, G.; Van Mele, B.; Li, T.; Nies, E. Adjacent UCST Phase Behavior in Aqueous Solutions of Poly(vinyl methyl ether): Detection of a Narrow Low Temperature UCST in the Lower Concentration Range. Macromolecules 2011, 44, 993–998. [Google Scholar] [CrossRef]
- Wu, G.; Chen, S.; Zhan, Q.; Wang, Y. Well-Defined Amphiphilic Biodegradable Comb-Like Graft Copolymers: Their Unique Architecture-Determined LCST and UCST Thermoresponsivity. Macromolecules 2011, 44, 999–1008. [Google Scholar] [CrossRef]
- Christova, D.; Ivanova, S.; Ivanova, G. Water-soluble temperature-responsive poly(vinyl alcohol-co-vinyl acetal)s. Polym. Bul. 2003, 50, 367–372. [Google Scholar] [CrossRef]
- Shiomi, T.; Imai, K.; Watanabe, C.; Miya, M. Thermodynamic and conformational properties of partially butyralized poly(vinyl alcohol) in aqueous solution. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 1305–1312. [Google Scholar] [CrossRef]
- Longenecker, R.; Mu, T.; Hanna, M.; Burke, N.A.D.; Stöver, H.D.H. Thermally Responsive 2-Hydroxyethyl Methacrylate Polymers: Soluble–Insoluble and Soluble–Insoluble–Soluble Transitions. Macromolecules 2011, 44, 8962–8971. [Google Scholar] [CrossRef]
- Buscall, R.; Corner, T. The phase-separation behaviour of aqueous solutions of polyacrylic acid and its partial sodium salts in the presence of sodium chloride. Eur. Polym. J. 1982, 18, 967–974. [Google Scholar] [CrossRef]
- Flory, P.J.; Osterheld, J.E. Intrinsic Viscosities of Polyelectrolytes. Poly-(acrylic Acid). J. Phys. Chem. 1954, 58, 653–661. [Google Scholar] [CrossRef]
- Bokias, G.; Staikos, G.; Iliopoulos, I. Solution properties and phase behaviour of copolymers of acrylic acid with N-isopropylacrylamide: The importance of the intrachain hydrogen bonding. Polymer 2000, 41, 7399–7405. [Google Scholar] [CrossRef]
- Fang, J.; Bian, F.; Shen, W. A study on solution properties of poly(N,N-diethylacrylamide-co-acrylic acid). J. Appl. Polym. Sci. 2008, 110, 3373–3378. [Google Scholar] [CrossRef]
- Ning, J.; Kubota, K.; Li, G.; Haraguchi, K. Characteristics of zwitterionic sulfobetaine acrylamide polymer and the hydrogels prepared by free-radical polymerization and effects of physical and chemical crosslinks on the UCST. React. Funct. Polym. 2013, 73, 969–978. [Google Scholar] [CrossRef]
- Toncheva, V.D.; Ivanova, S.D.; Velichkova, R.S. Modified poly(vinyl acetals). Eur. Polym. J. 1992, 28, 191–198. [Google Scholar] [CrossRef]

| Sample | [HPM]/[PVA] | Degree of Hydrophobic Modification (%) | [GA]/[PVA] |
|---|---|---|---|
| CPVA-0.5 | 0 | 0 | 0.5% |
| CHPVA-2-0.5 | 2% | 1.98% | 0.5% |
| CHPVA-3-0.5 | 3% | 3.12% | 0.5% |
| CHPVA-4-0.5 | 4% | 4.05% | 0.5% |
| CHPVA-4-1.0 | 4% | 4.05% | 1.0% |
| CHPVA-4-1.5 | 4% | 4.05% | 1.5% |
| CHPVA-5-0.5 | 5% | 5.23% | 0.5% |
| CHPVA-5-1.0 | 5% | 5.23% | 1.0% |
| CHPVA-5-1.5 | 5% | 5.23% | 1.5% |
| CHPVA-6-0.5 | 6% | 6.16% | 0.5% |
| Ion | Concentration (mg/L) |
|---|---|
| Na+ | 7.16 × 104 |
| Ca2+ | 1.13 × 104 |
| Mg2+ | 1.16 × 103 |
| Cl− | 13.37 × 104 |
| Br− | 179.78 |
| SO42− | 149.952 |
| HCO3− | 33.84 |
| I− | 9.89 |
| Total | 21.81 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Guo, J.; Kang, C. LCST-UCST Transition Property of a Novel Retarding Swelling and Thermosensitive Particle Gel. Materials 2023, 16, 2761. https://doi.org/10.3390/ma16072761
Li L, Guo J, Kang C. LCST-UCST Transition Property of a Novel Retarding Swelling and Thermosensitive Particle Gel. Materials. 2023; 16(7):2761. https://doi.org/10.3390/ma16072761
Chicago/Turabian StyleLi, Liang, Jixiang Guo, and Chuanhong Kang. 2023. "LCST-UCST Transition Property of a Novel Retarding Swelling and Thermosensitive Particle Gel" Materials 16, no. 7: 2761. https://doi.org/10.3390/ma16072761
APA StyleLi, L., Guo, J., & Kang, C. (2023). LCST-UCST Transition Property of a Novel Retarding Swelling and Thermosensitive Particle Gel. Materials, 16(7), 2761. https://doi.org/10.3390/ma16072761







