Thermionic Emission of Atomic Layer Deposited MoO3/Si UV Photodetectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparing Ultrathin MoO3 Film
2.2. Characterization Methods
3. Results and Discussions
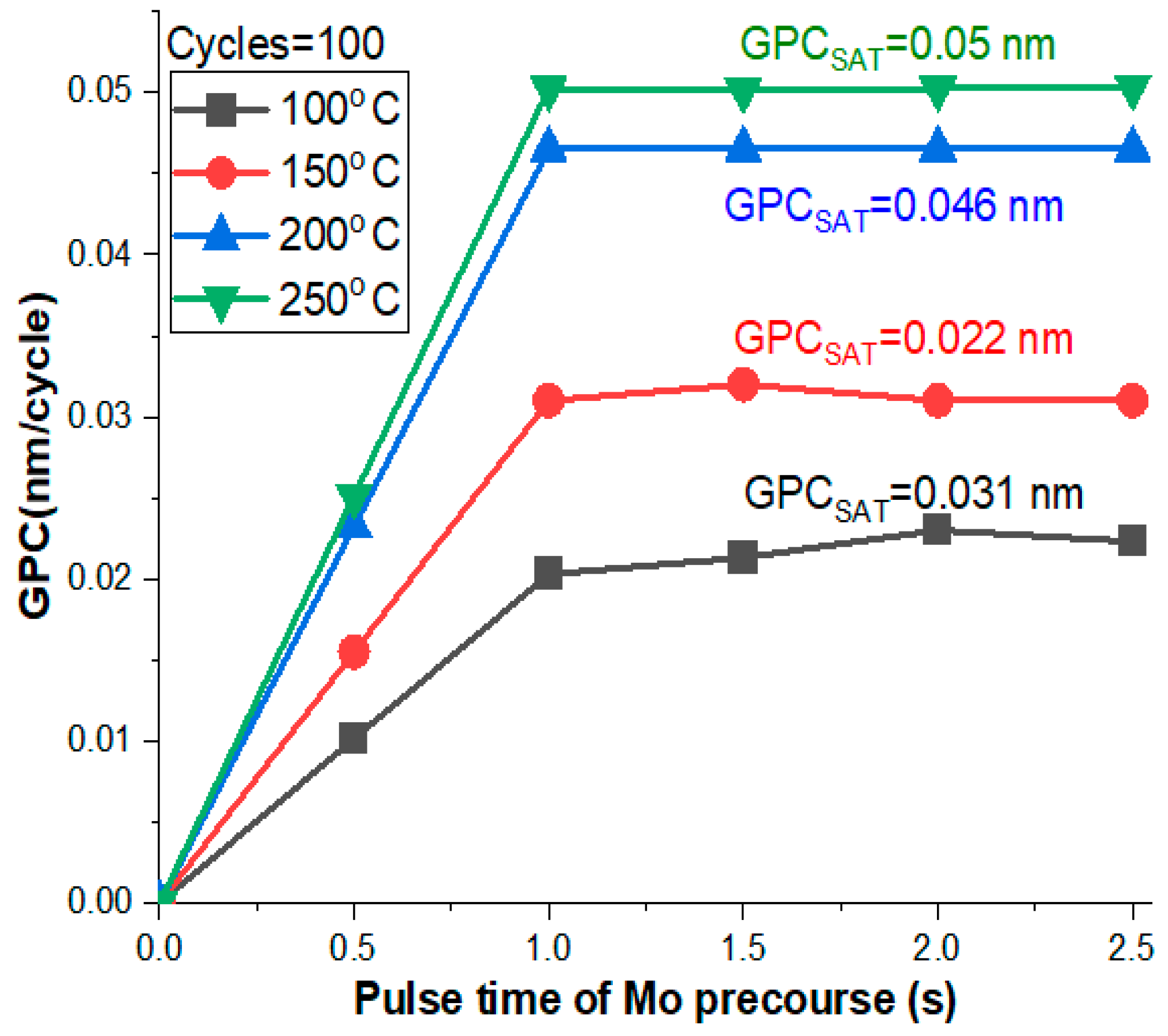
3.1. Growth Per Cycle (GPC)
3.2. Surface Morphology of the Ultrathin Films
3.3. EDS Characterization
3.4. Surface Topography and Roughness
4. Electrical Properties
4.1. Surface Resistance
4.2. Current–Voltage Characteristics
5. Optoelectronic Properties of MoO3/Si under UV
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maroufi, S.; Khayyam Nekouei, R.; Mofarah, S.S.; O’Mullane, A.P.; Yao, Y.; Lim, S.; Cazorla, C.; Sahajwalla, V. Transparent and Flexible Mn1−x−y(CexLay)O2−δ Ultrathin-Film Device for Highly-Stable Pseudocapacitance Application. Adv. Funct. Mater. 2021, 31, 2100880. [Google Scholar] [CrossRef]
- Khan, M.F.; Ahmed, F.; Rehman, S.; Akhtar, I.; Rehman, M.A.; Shinde, P.A.; Khan, K.; Kim, D.K.; Eom, J.; Lipsanen, H.; et al. High Performance Complementary WS2 Devices with Hybrid Gr/Ni Contacts. Nanoscale 2020, 12, 21280–21290. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Q.; Lee, H.G.; Ko, E.K.; Lu, Q.; Noh, T.W. Controllable Thickness Inhomogeneity and Berry Curvature Engineering of Anomalous Hall Effect in SrRuO3 Ultrathin Films. Nano Lett. 2020, 20, 2468–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wang, Q.; Sheng, Y.; Cao, G.; Yang, P.; Shan, Y.; Liao, F.; Muhammad, Z.; Bao, W.; Hu, L.; et al. High-Performance WSe2 Photodetector Based on a Laser-Induced p-n Junction. ACS Appl. Mater. Interfaces 2019, 11, 43330–43336. [Google Scholar] [CrossRef] [PubMed]
- Holler, B.A.; Crowley, K.; Berger, M.H.; Gao, X.P.A. 2D Semiconductor Transistors with Van Der Waals Oxide MoO3 as Integrated High-κ Gate Dielectric. Adv. Electron. Mater. 2020, 6, 2000635. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, W.; Liu, X.; Zhang, L.; Zheng, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum Single Atoms on Tin Oxide Ultrathin Films for Extremely Sensitive Gas Detection. Mater. Horiz. 2020, 7, 1519–1527. [Google Scholar] [CrossRef]
- Juge, R.; Je, S.G.; Chaves, D.D.S.; Buda-Prejbeanu, L.D.; Peña-Garcia, J.; Nath, J.; Miron, I.M.; Rana, K.G.; Aballe, L.; Foerster, M.; et al. Current-Driven Skyrmion Dynamics and Drive-Dependent Skyrmion Hall Effect in an Ultrathin Film. Phys. Rev. Appl. 2019, 12, 044007. [Google Scholar] [CrossRef] [Green Version]
- Diskus, M.; Nilsen, O.; Fjellvåg, H.; Diplas, S.; Beato, P.; Harvey, C.; van Schrojenstein Lantman, E.; Weckhuysen, B.M. Combination of Characterization Techniques for Atomic Layer Deposition MoO3 Coatings: From the Amor-phous to the Orthorhombic α-MoO3 Crystalline Phase. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 01A107. [Google Scholar] [CrossRef]
- Abhijith, T.; Kumar, T.V.A.; Reddy, V.S. Organic Bistable Memory Devices Based on MoO3 Nanoparticle Embedded Alq3 Structures. Nanotechnology 2017, 28, 095203. [Google Scholar] [CrossRef]
- Prakash, N.G.; Dhananjaya, M.; Narayana, A.L.; Shaik, D.P.; Rosaiah, P.; Hussain, O.M. High Performance One Dimensional α-MoO3 Nanorods for Supercapacitor Applications. Ceram. Int. 2018, 44, 9967–9975. [Google Scholar] [CrossRef]
- Wang, Z.; Waqas Alam, M.; Lou, Y.; Naka, S.; Okada, H. Enhanced Carrier Injection in Pentacene Thin-Film Transistors by Inserting a MoO3-Doped Pentacene Layer. Appl. Phys. Lett. 2012, 100, 043302. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Ertugrul, S.; Yilmaz, M.; Eker, Y.R. Fast Response of CO2 Room Temperature Gas Sensor Based on Mixed-Valence Phases in Molybdenum and Tungsten Oxide Nanostructured Thin Films. Ceram. Int. 2020, 46, 9839–9853. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Zhang, J.; Luo, Y.; Zhang, C.; Xue, Y.; Wang, G.; Wang, R. Assist More Pt-O Bonds of Pt/MoO3-CNT as a Highly Efficient and Stable Electrocatalyst for Methanol Oxidation and Oxygen Reduction Reaction. J. Alloys Compd. 2021, 873, 159827. [Google Scholar] [CrossRef]
- Alsaif, M.M.Y.A.; Balendhran, S.; Field, M.R.; Latham, K.; Wlodarski, W.; Ou, J.Z.; Kalantar-Zadeh, K. Two Dimensional α-MoO3 Nanoflakes Obtained Using Solvent-Assisted Grinding and Sonication Method: Application for H2 Gas Sensing. Sens. Actuators B Chem. 2014, 192, 196–204. [Google Scholar] [CrossRef]
- Liu, H.; bin Yang, R.; Yang, W.; Jin, Y.; Lee, C.J.J. Atomic Layer Deposition and Post-Growth Thermal Annealing of Ultrathin MoO3 Layers on Silicon Substrates: Formation of Surface Nanostructures. Appl. Surf. Sci. 2018, 439, 583–588. [Google Scholar] [CrossRef]
- Shi, M.L.; Chen, L.; Zhang, T.B.; Xu, J.; Zhu, H.; Sun, Q.Q.; Zhang, D.W. Top-Down Integration of Molybdenum Di-sulfide Transistors with Wafer-Scale Uniformity and Layer Controllability. Small 2017, 13, 1603157. [Google Scholar] [CrossRef] [Green Version]
- Martella, C.; Melloni, P.; Cinquanta, E.; Cianci, E.; Alia, M.; Longo, M.; Lamperti, A.; Vangelista, S.; Fanciulli, M.; Molle, A. Engineering the Growth of MoS2 via Atomic Layer Deposition of Molybdenum Oxide Film Precursor. Adv. Electron. Mater. 2016, 2, 1600330. [Google Scholar] [CrossRef]
- Demirtaş, M.; Odacı, C.; Shehu, Y.; Perkgöz, N.K.; Ay, F. Layer and Size Distribution Control of CVD-Grown 2D MoS2 Using ALD-Deposited MoO3 Structures as the Precursor. Mater. Sci. Semicond. Process. 2020, 108, 104880. [Google Scholar] [CrossRef]
- Keller, B.D.; Bertuch, A.; Provine, J.; Sundaram, G.; Ferralis, N.; Grossman, J.C. Process Control of Atomic Layer Deposition Molybdenum Oxide Nucleation and Sulfidation to Large-Area MoS2 Monolayers. Chem. Mater. 2017, 29, 2024–2032. [Google Scholar] [CrossRef]
- Sharma, A.; Mahlouji, R.; Wu, L.; Verheijen, M.A.; Vandalon, V.; Balasubramanyam, S.; Hofmann, J.P.; Erwin Kes-sels, W.M.M.; Bol, A.A. Large Area, Patterned Growth of 2D MoS2 and Lateral MoS2–WS2 Heterostructures for Nano- and Opto-Electronic Applications. Nanotechnology 2020, 31, 255603. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Singh, R. 2D Layered Materials for Ultraviolet Photodetection: A Review. Adv. Opt. Mater. 2021, 9, 2002214. [Google Scholar] [CrossRef]
- Molina-Mendoza, A.J.; Lado, J.L.; Island, J.O.; Angel Niño, M.; Aballe, L.; Foerster, M.; Bruno, F.Y.; López-Moreno, A.; Vaquero-Garzon, L.; van der Zant, H.S.J.; et al. Centimeter-Scale Synthesis of Ultrathin Layered MoO3 by van Der Waals Epitaxy. Chem. Mater. 2016, 28, 4042–4051. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Wang, Z.; Chen, Y.; Zhang, W.; Li, X. Centimeter-Sized 2D α-MoO3 Single Crystal: Growth, Raman Anisotropy, and Optoelectronic Properties. 2D Mater. 2018, 5, 045011. [Google Scholar] [CrossRef]
- Arash, A.; Ahmed, T.; Govind Rajan, A.; Walia, S.; Rahman, F.; Mazumder, A.; Ramanathan, R.; Sriram, S.; Bhaskaran, M.; Mayes, E.; et al. Large-Area Synthesis of 2D MoO3−x for Enhanced Optoelectronic Applications. 2D Mater. 2019, 6, 035031. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, J.; Cao, S.; Gao, H. A Flexible Ultraviolet Photodetector Based on Single Crystalline MoO3 Nanosheets. J. Mater. Chem. C Mater. 2015, 3, 7469–7475. [Google Scholar] [CrossRef]
- Zhong, M.; Zhou, K.; Wei, Z.; Li, Y.; Li, T.; Dong, H.; Jiang, L.; Li, J.; Hu, W. Highly Anisotropic Solar-Blind UV Photodetector Based on Large-Size Two-Dimensional α-MoO3 Atomic Crystals. 2D Mater. 2018, 5, 035033. [Google Scholar] [CrossRef]
- Xiang, D.; Han, C.; Zhang, J.; Chen, W. Gap States Assisted MoO3 Nanobelt Photodetector with Wide Spectrum Response. Sci. Rep. 2014, 4, 4891. [Google Scholar] [CrossRef] [Green Version]
- Basyooni, M.A.; Gündoğdu, Y.; Kiliç, H.Ş.; Eker, Y.R. Optical, Optoelectronic, and Third-Order Nonlinear Photonics of Ultrathin Molybdenum Oxide Film Deposited by Atomic Layer Deposition. Phys. Stat. Solidi (A) 2023, 220, 2200689. [Google Scholar] [CrossRef]
- Bertuch, A.; Sundaram, G.; Saly, M.; Moser, D.; Kanjolia, R. Atomic Layer Deposition of Molybdenum Oxide Using Bis(Tert-Butylimido)Bis(Dimethylamido) Molybdenum. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 32, 01A119. [Google Scholar] [CrossRef]
- Vos, M.F.J.; Macco, B.; Thissen, N.F.W.; Bol, A.A.; Kessels, W.M.M. (Erwin) Atomic Layer Deposition of Molybdenum Oxide from (NtBu)2(NMe2)2Mo and O2 Plasma. J. Vac. Sci. Technol. A Vac. Surf. Film. 2015, 34, 01A103. [Google Scholar] [CrossRef] [Green Version]
- Kalanyan, B.; Beams, R.; Katz, M.B.; Davydov, A.v.; Maslar, J.E.; Kanjolia, R.K. MoS2 Thin Films from a (NtBu)2(NMe2)2Mo and 1-Propanethiol Atomic Layer Deposition Process. J. Vac. Sci. Technol. A Vac. Surf. Film. 2018, 37, 010901. [Google Scholar] [CrossRef] [PubMed]
- Diskus, M.; Nilsen, O.; Fjellvåg, H. Growth of Thin Films of Molybdenum Oxide by Atomic Layer Deposition. J. Mater. Chem. 2010, 21, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Chiappim, W.; Fraga, M.A.; Maciel, H.S.; Pessoa, R.S. An Experimental and Theoretical Study of the Impact of the Precursor Pulse Time on the Growth Per Cycle and Crystallinity Quality of TiO2 Thin Films Grown by ALD and PEALD Technique. Front. Mech. Eng. 2020, 6, 80. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Hyde, L.; Hai, Z.; Akbari, M.K.; Kats, E.; Detavernier, C.; Xue, C.; Xu, H. Atomic Layer Deposition-Enabled Single Layer of Tungsten Trioxide across a Large Area. Appl. Mater. Today 2017, 6, 44–53. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.B.; Moon, Y.J.; Kim, S.M.; Jung, H.J.; Seo, M.S.; Lee, K.M.; Kim, S.K.; Lee, S.W. High-Responsivity Deep-Ultraviolet-Selective Photodetectors Using Ultrathin Gallium Oxide Films. ACS Photonics 2017, 4, 2937–2943. [Google Scholar] [CrossRef]
- Dai, T.J.; Liu, Y.C.; Fan, X.D.; Liu, X.Z.; Xie, D.; Li, Y.R. Synthesis of Few-Layer 2H-MoSe2 Thin Films with Wafer-Level Homogeneity for High-Performance Photodetector. Nanophotonics 2018, 7, 1959–1969. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Mane, A.U.; Elam, J.W.; Darling, S.B. Ultrathin Molybdenum Oxide Anode Buffer Layer for Organic Photovoltaic Cells Formed Using Atomic Layer Deposition. Sol. Energy Mater. Sol. Cells 2012, 99, 235–239. [Google Scholar] [CrossRef]
- Guo, Y.; Zou, Y.; Cheng, C.; Wang, L.; Made, R.I.; Goei, R.; Tan, K.W.; Li, S.; Tok, A.I.Y. Noble Metal Alloy Thin Films by Atomic Layer Deposition and Rapid Joule Heating. Sci. Rep. 2022, 12, 2522. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, Y.; Sang, L.; Ma, J.; Shi, H.; Liu, X.; Lu, J.; Zhang, Y. Boosting the Electrochemical Performance of MoO3 Anode for Long-Life Lithium Ion Batteries: Dominated by an Ultrathin TiO2 Passivation Layer. Electrochim. Acta 2018, 269, 241–249. [Google Scholar] [CrossRef]
- Kemell, M.; Färm, E.; Ritala, M.; Leskelä, M. Surface Modification of Thermoplastics by Atomic Layer Deposition of Al2O3 and TiO2 Thin Films. Eur. Polym. J. 2008, 44, 3564–3570. [Google Scholar] [CrossRef]
- Mattinen, M.; King, P.J.; Khriachtchev, L.; Heikkilä, M.J.; Fleming, B.; Rushworth, S.; Mizohata, K.; Meinander, K.; Räisänen, J.; Ritala, M.; et al. Atomic Layer Deposition of Crystalline Molybdenum Oxide Thin Films and Phase Control by Post-Deposition Annealing. Mater. Today Chem. 2018, 9, 17–27. [Google Scholar] [CrossRef]
- Wree, J.L.; Rogalla, D.; Ostendorf, A.; Schierbaum, K.D.; Devi, A. Plasma-Enhanced Atomic Layer Deposition of Molybdenum Oxide Thin Films at Low Temperatures for Hydrogen Gas Sensing. ACS Appl. Mater. Interfaces 2022, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, X.; Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Zhou, X.; Huo, L. Oxygen-Vacancy-Enriched Porous α-MoO3 Nanosheets for Trimethylamine Sensing. ACS Appl. Nano Mater. 2019, 2, 8016–8026. [Google Scholar] [CrossRef]
- Bisht, P.; Kumar, A.; Jensen, I.T.; Ahmad, M.; Belle, B.D.; Mehta, B.R. Enhanced Gas Sensing Response for 2D α-MoO3 Layers: Thickness-Dependent Changes in Defect Concentration, Surface Oxygen Adsorption, and Metal-Metal Oxide Contact. Sens. Actuators B Chem. 2021, 341, 129953. [Google Scholar] [CrossRef]
- Demirezen, S.; Al-Sehemi, A.G.; Yüzer, A.; Ince, M.; Dere, A.; Al-Ghamdi, A.A.; Yakuphanoglu, F. Electrical Characteristics and Photosensing Properties of Al/Symmetrical CuPc/p-Si Photodiodes. J. Mater. Sci. Mater. Electron. 2022, 33, 21011–21021. [Google Scholar] [CrossRef]
- Sze, S.M.; Ng, K.K. Physics of Semiconductor Devices; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780470068328. [Google Scholar]
- Rhoderick, E.H.; Williams, R.H. Metal-Semiconductor Contacts; Clarendon Press: Oxford, UK; Oxford University Press: Oxford, UK, 1988; ISBN 0198593368. [Google Scholar]
- Dimitriadis, C.A.; Logothetidis, S.; Alexandrou, I. Schottky Barrier Contacts of Titanium Nitride on N-type Silicon. Appl. Phys. Lett. 1998, 66, 502. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, Z.; Su, M.; Liu, P.; Mai, W.; Xie, W. Self-Powered, High-Speed and Visible-Near Infrared Response of MoO3–x/n-Si Heterojunction Photodetector with Enhanced Performance by Interfacial Engineering. ACS Appl. Mater. Interfaces 2015, 7, 25981–25990. [Google Scholar] [CrossRef]
- Tataroglu, A.; Ocaya, R.; Dere, A.; Dayan, O.; Serbetci, Z.; Al-Sehemi, A.G.; Soylu, M.; Al-Ghamdi, A.A.; Yaku-phanoglu, F. Ruthenium(II) Complex Based Photodiode for Organic Electronic Applications. J. Electron. Mater. 2018, 47, 828–833. [Google Scholar] [CrossRef]
- Cheung, S.K.; Cheung, N.W. Extraction of Schottky Diode Parameters from Forward Current-Voltage Characteristics. Appl. Phys. Lett. 1986, 49, 85–87. [Google Scholar] [CrossRef]
- Norde, H. A Modified Forward I-V Plot for Schottky Diodes with High Series Resistance. J. Appl. Phys. 1979, 50, 5052–5053. [Google Scholar] [CrossRef]
- Ashery, A.; Gaballah, A.E.H.; Ahmed, E.M. Negative Series Resistance and Photo-Response Properties of Au/PPY-MWCNTs Composite/TiO2/Al2O3/n-Si/Al Photodiode. Mater. Res. Express 2022, 9, 016301. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Shaban, M.; Ramazan Eker, Y.; Yilmaz, M. Efficient MoWO3/VO2/MoS2/Si UV Schottky Photodetectors; MoS2 Optimization and Monoclinic VO2 Surface Modifications. Sci. Rep. 2020, 10, 15926. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.E.; Buyukharman, M.; Basyooni, M.A.; Efe Görmez, A.; Sezgin, A.; Ramazan Eker, Y.; Yılmaz, M. Phase Modulation of MoO2–MoO3 Nanostructured Thin Films through W-Doping; Utilizing UV Photodetection and Gas Sensing Applications. Selcuk. Univ. J. Sci. Fac. 2022, 48, 34–45. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Tihtih, M.; Orsini, A.; Salvatori, S.; Alfryyan, N.; Ramazan Eker, Y.; Attia, G.F.; Yılmaz, M.; Sule, A.; et al. Nanostructured MoS2 and WS2 Photoresponses under Gas Stimuli. Nanomaterials 2022, 12, 3585. [Google Scholar] [CrossRef]
















| Samples | Rs (Ohm/sq) | Type | Ns (cm−2) |
|---|---|---|---|
| S1 | 6.6 × 106 | N | 2.0 × 109 |
| S2 | 9.3 × 106 | N | 1.1 × 109 |
| S3 | 1.1 × 105 | N | 7.6 × 1010 |
| S4 | 1.3 × 106 | N | 4.1 × 109 |
| Under Dark | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | (TE) | Cheung (H) | dV/dlnI | Nord (F) | |||||||
| n | n | n | n | ||||||||
| S1-100 °C | 13.5 | 0.67 | 2.98 × 108 | 13.6 | 0.73 | 3.30 × 108 | 16.4 | 4.45 × 108 | 13.5 | 0.66 | 1.26 × 107 |
| S2-150 °C | 13.7 | 0.68 | 7.46 × 107 | 13.1 | 0.64 | 7.70 × 107 | 11.9 | 4.17 × 107 | 13.7 | 0.67 | 7.84 × 106 |
| S1-200 °C | 13.3 | 0.58 | 1.81 × 107 | 15.3 | 0.71 | 1.30 × 107 | 14.2 | 1.89 × 107 | 13.6 | 0.60 | 3.5 × 105 |
| S1-250 °C | 13.6 | 0.66 | 1.28 × 107 | 13.4 | 0.63 | 9.15 × 106 | 13.4 | 2.00 × 107 | 13.7 | 0.65 | 4.67 × 106 |
| Under UV Illumination | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | (TE) | Cheung (H) | dV/dlnI | Nord (F) | |||||||
| n | n | n | n | ||||||||
| S1-100 °C | 11.9 | 0.71 | 2.8 × 106 | 12.1 | 0.70 | 1.47 × 106 | 12.6 | 1.38 × 106 | 11.7 | 0.67 | 6.75 × 107 |
| S2-150 °C | 16.8 | 0.60 | 3.51 × 106 | 14.8 | 0.70 | 1.34 × 106 | 14.8 | 1.1 × 106 | 14.8 | 0.64 | 1.1 × 106 |
| S1-200 °C | 12.8 | 0.58 | 1.81 × 105 | 12.8 | 0.63 | 1.85 × 105 | 15.8 | 6.0 × 105 | 15.9 | 0.62 | 6.0 × 105 |
| S1-250 °C | 15.7 | 0.68 | 1.23 × 106 | 13.6 | 0.65 | 6.5 × 105 | 13.6 | 13.6 × 105 | 13.4 | 0.64 | 3.6 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basyooni, M.A.; Gaballah, A.E.H.; Tihtih, M.; Derkaoui, I.; Zaki, S.E.; Eker, Y.R.; Ateş, Ş. Thermionic Emission of Atomic Layer Deposited MoO3/Si UV Photodetectors. Materials 2023, 16, 2766. https://doi.org/10.3390/ma16072766
Basyooni MA, Gaballah AEH, Tihtih M, Derkaoui I, Zaki SE, Eker YR, Ateş Ş. Thermionic Emission of Atomic Layer Deposited MoO3/Si UV Photodetectors. Materials. 2023; 16(7):2766. https://doi.org/10.3390/ma16072766
Chicago/Turabian StyleBasyooni, Mohamed A., A. E. H. Gaballah, Mohammed Tihtih, Issam Derkaoui, Shrouk E. Zaki, Yasin Ramazan Eker, and Şule Ateş. 2023. "Thermionic Emission of Atomic Layer Deposited MoO3/Si UV Photodetectors" Materials 16, no. 7: 2766. https://doi.org/10.3390/ma16072766
APA StyleBasyooni, M. A., Gaballah, A. E. H., Tihtih, M., Derkaoui, I., Zaki, S. E., Eker, Y. R., & Ateş, Ş. (2023). Thermionic Emission of Atomic Layer Deposited MoO3/Si UV Photodetectors. Materials, 16(7), 2766. https://doi.org/10.3390/ma16072766







