Cross-Linking, Morphology, and Physico-Mechanical Properties of GTR/SBS Blends: Dicumyl Peroxide vs. Sulfur System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methodology
3. Results and Discussion
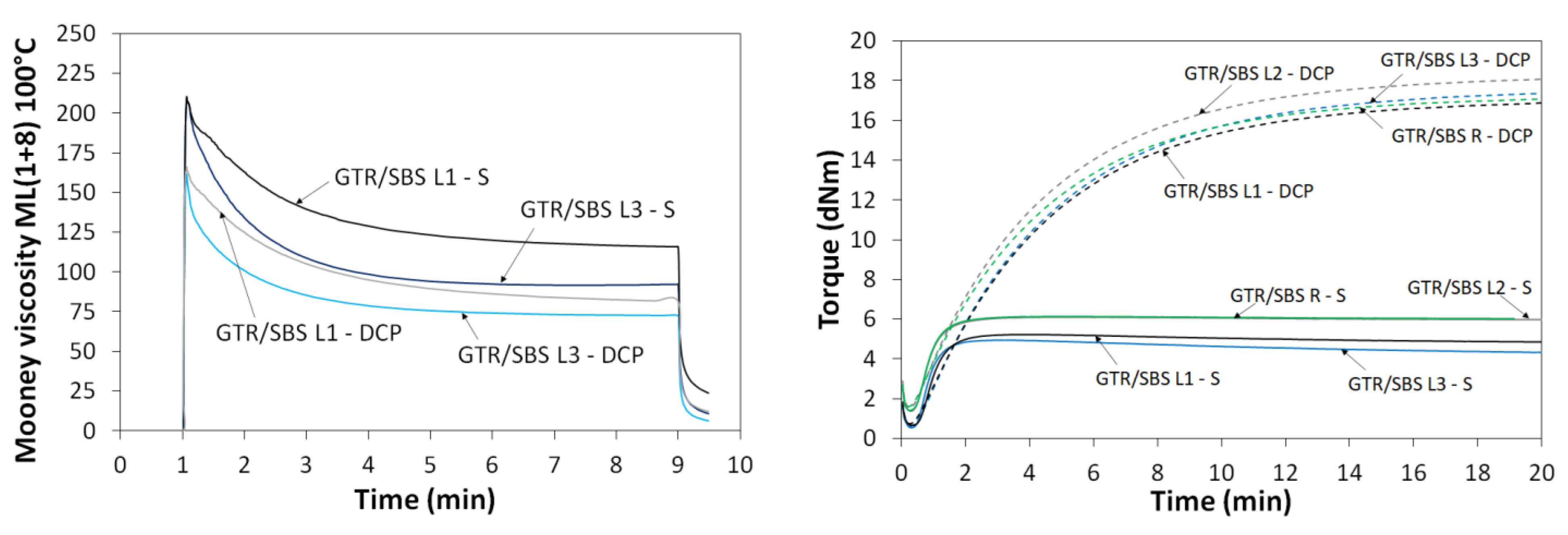
3.1. Mooney Viscosity and Curing Characteristics
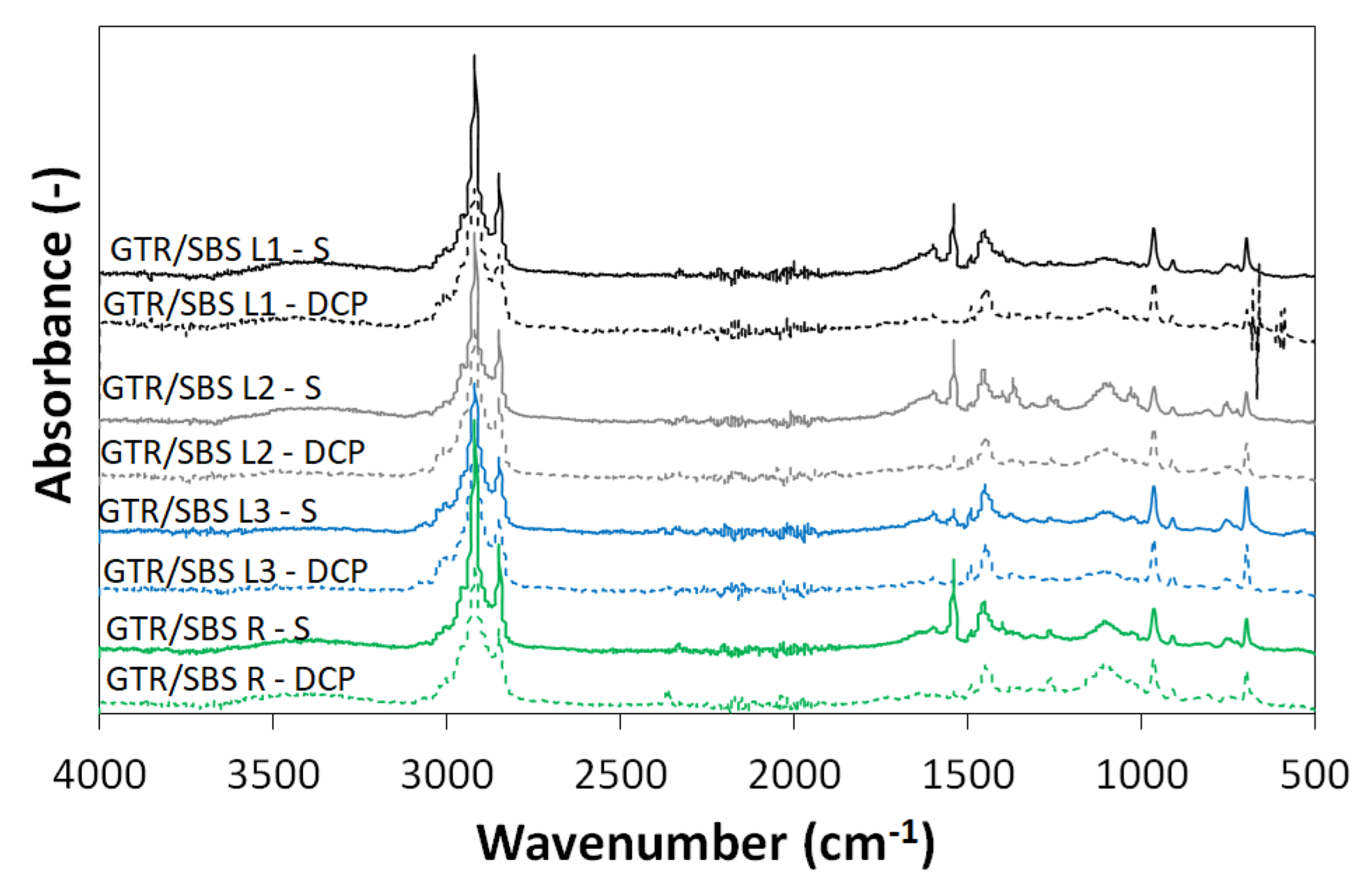
3.2. FTIR Analysis
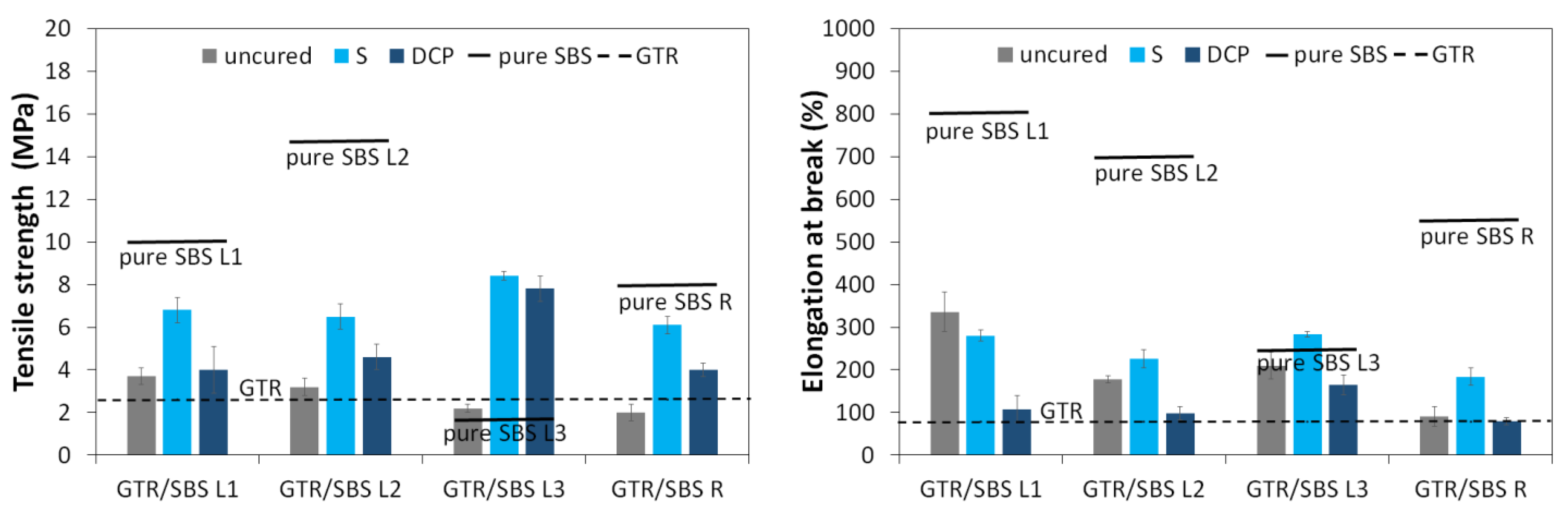
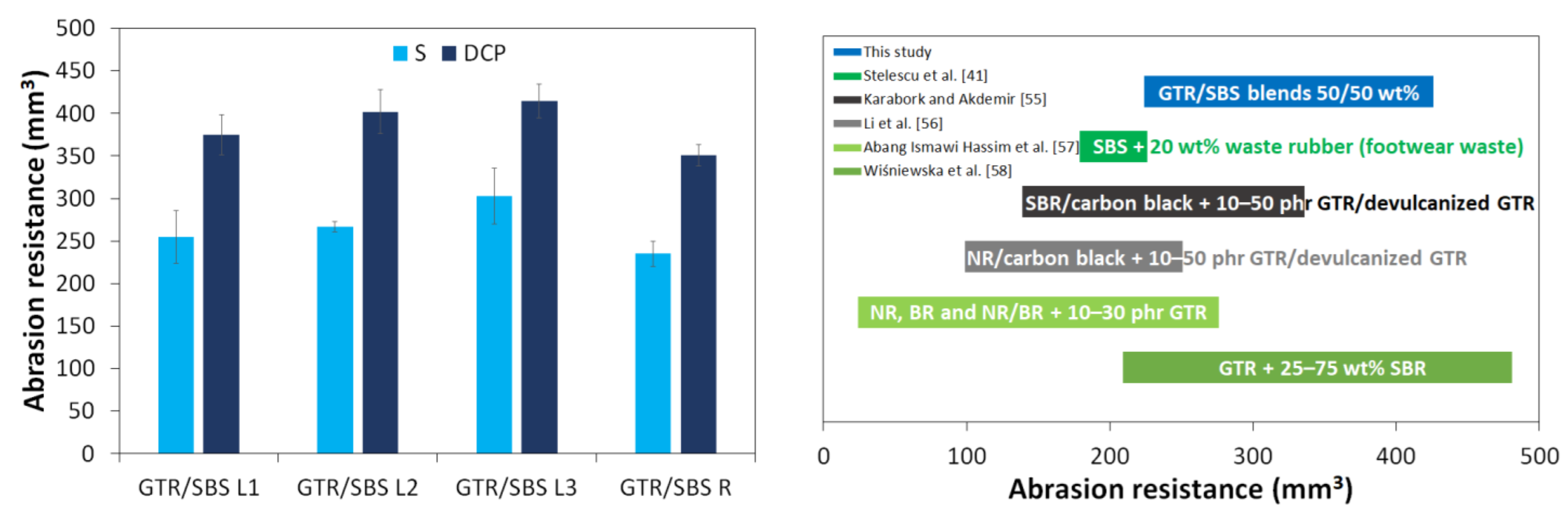
3.3. Physico-Mechanical Properties
| Composition | Processing Method | Mechanical Properties | Observations | Reference | |
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | ||||
| GTR/SBS 50/50 wt% with and without curing system | Internal mixer: 200 °C (8 min) Compression molding: at 170 °C (10 MPa, t90), and cold compression (5 min) for samples without the curing system | 4.1–8.4 MPa (2.0–3.7 MPa without curing) | 80–283% (91–336% without curing) | SBS with low viscosity enhanced processing and tensile properties due to the higher mixing efficiency between GTR and SBS | This study |
| HIPS/EVA/GTR 25/5/70 wt% compatibilized by SBS (up to 18 phr) | Internal mixer: 165 °C (8 min) Compression molding: at 180 °C (15 MPa, 10 min) and cold compression (8 min) | ~6–8 MPa * (3.3 MPa for sample without SBS) | ~115–245% (17.6% for sample without SBS) | SBS had a good compatibilizing effect and improved the tensile properties of the blends studied (the optimal SBS content was 12 phr) | [32] |
| HDPE/GTR 30/70 wt% compatibilized by SBS (up to 15 phr) | Internal mixer: 165 °C (8 min) Compression molding: at 165 °C (15 MPa, 9 min) and cold compression (8 min) | ~12.3–14.8 MPa * (11.8 MPa for sample without SBS) | ~240–260% (185% for sample without SBS) | SBS improved the mechanical properties and elasticity of the blends studied (the optimal SBS content was 12 phr) | [33] |
| SBS + 20 wt% waste rubber (footwear waste) with and without peroxide curing system | Internal mixer: 170 °C (7 min) Compression molding: at 170 °C (300 kN, 6 min) and at 45 °C (300 kN, 10 min) | ~3.4–5.5 MPa * (~4.5 MPa for pure SBS) | ~175–460% * (~580% for pure SBS) | The addition of vulcanized rubber powder (SBR-based) to SBS showed good compatibility between the two polymer phases, which was related to the similar structures of SBS and SBR. Dynamic cross-linking and grafting improved the mechanical properties of the studied materials. The investigated material showed good abrasion resistance. | [41] |
| LLDPE/GTR 34/66 wt% compatibilized by SBS and a DCP-based system (up to 10 wt%) | Kneading mixer: 185 °C (23 min) Compression molding: at 180 °C for 11 min (5 min preheating and 6 min of compression), and at room temperature for 4 min | ~3.0–3.5 MPa * (3.1 MPa * for sample without SBS) | ~50–113% * (43% * for sample without SBS) | Mechanical properties of the studied blends were improved by SBS (the optimal SBS content was 6 wt%) | [53] |
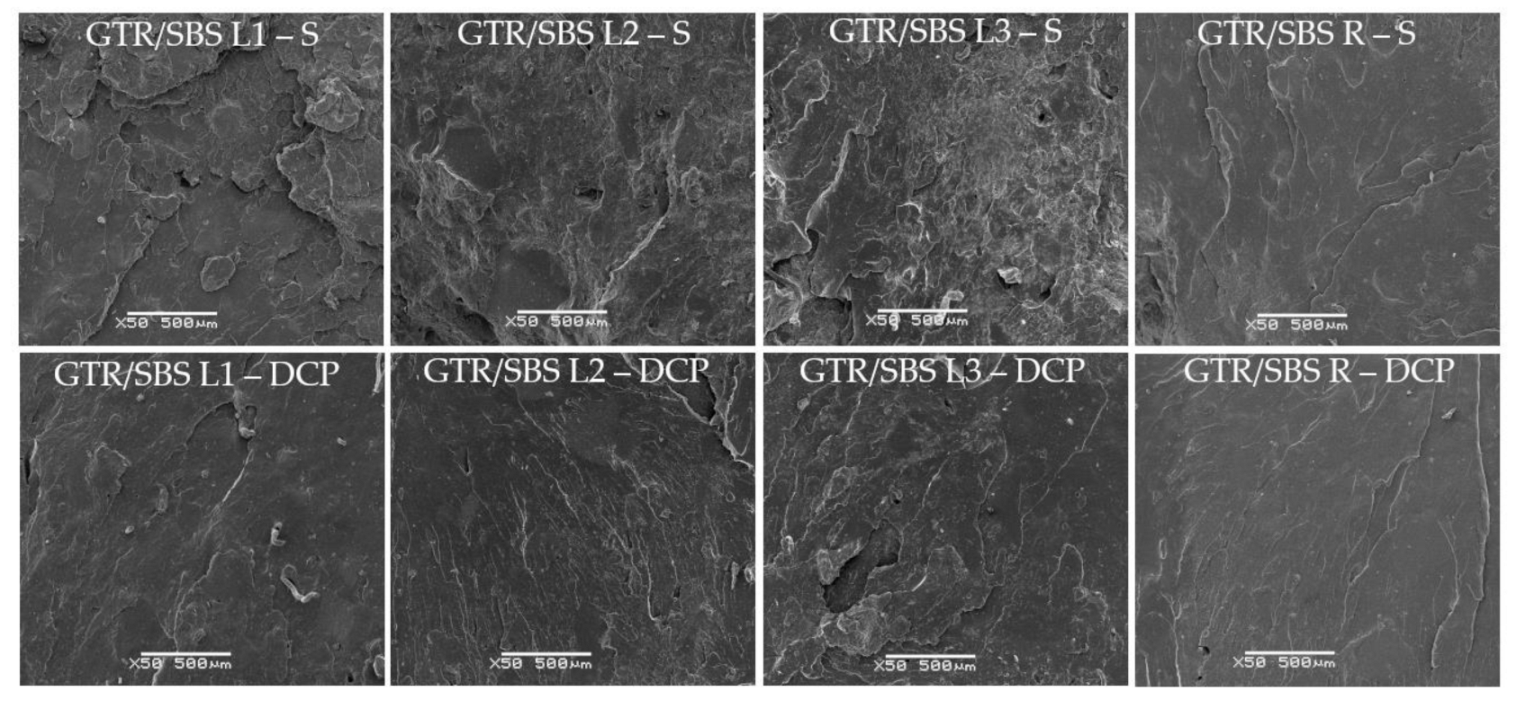
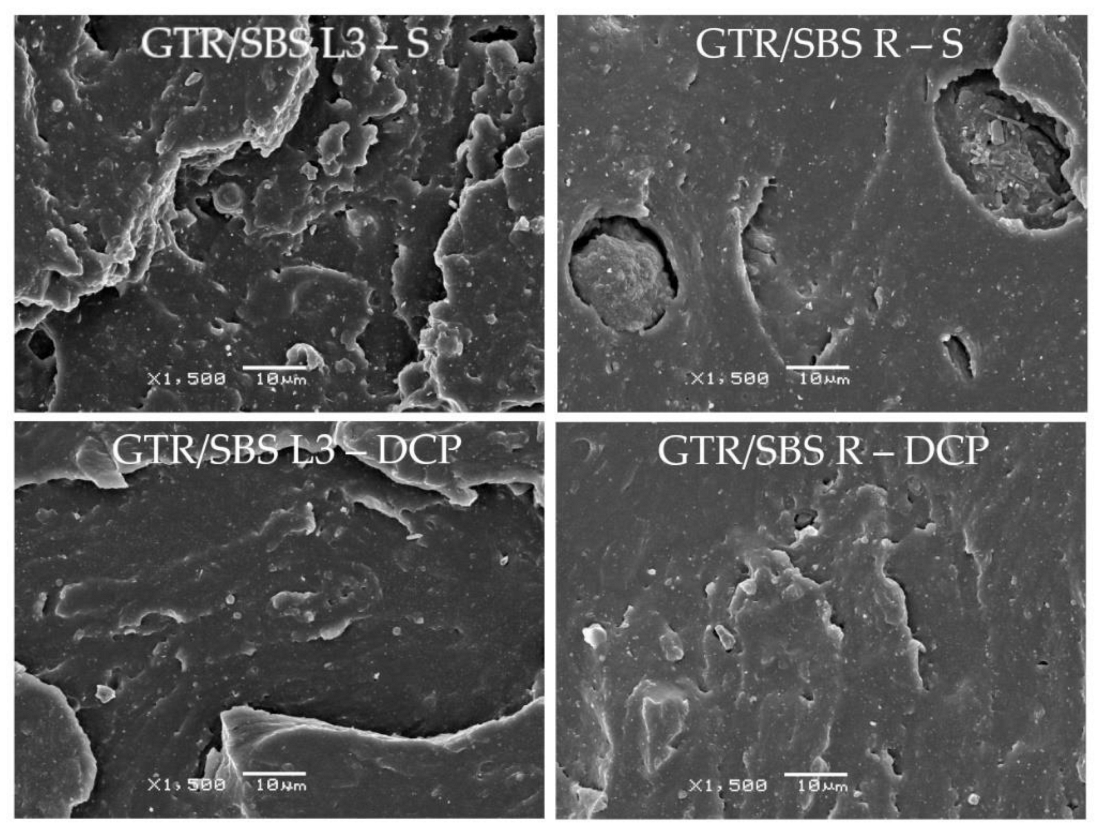
3.4. SEM Analysis
3.5. Thermogravimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czarnecka, B.; Schivinski, B. Do consumers acculturated to global consumer culture buy more impulsively? The moderating role of attitudes towards and beliefs about advertising. J. Glob. Mark. 2019, 32, 219–238. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development, World Bank: Washington, DC, USA, 2018; Available online: https://openknowledge.worldbank.org/handle/10986/30317 (accessed on 31 March 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, D.; Xiao, F. Recent Developments in the application of chemical approaches to rubberized asphalt. Constr. Build. Mater. 2017, 131, 101–113. [Google Scholar] [CrossRef]
- Yoon, S.; Prezzi, M.; Siddiki, N.Z.; Kim, B. Construction of a test embankment using a sand-tire shred mixture as fill material. Waste Manag. 2006, 26, 1033–1044. [Google Scholar] [CrossRef]
- Baričević, A.; Jelčić Rukavina, M.; Pezer, M.; Štirmer, N. Influence of recycled tire polymer fibers on concrete properties. Cem. Concr. Compos. 2018, 91, 29–41. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the use of alternative carbon sources in EAF steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P.; O’Kane, P.; Sahajwalla, V. Reduction of FeO in EAF steelmaking slag by blends of metallurgical coke and end-of-life tyre. Steel Res. Int. 2012, 83, 766–774. [Google Scholar] [CrossRef]
- Conesa, J.A.; Martín-Gullón, I.; Font, R.; Jauhiainen, J. Complete study of the pyrolysis and gasification of scrap tires in a pilot plant reactor. Environ. Sci. Technol. 2004, 38, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Molanorouzi, M.; Mohaved, S.O. Reclaiming waste tire rubber by an irradiation technique. Polym. Degrad. Stab. 2016, 128, 115–125. [Google Scholar] [CrossRef]
- Zedler, Ł.; Klein, M.; Saeb, M.R.; Colom, X.; Cañavate, J.; Formela, K. Synergistic effects of bitumen plasticization and microwave treatment on short-term devulcanization of ground tire rubber. Polymers 2018, 10, 1265. [Google Scholar] [CrossRef]
- Zhang, X.; Saha, P.; Cao, L.; Li, H.; Kim, J. Devulcanization of waste rubber powder using thiobisphenols as novel reclaiming agent. Waste Manag. 2018, 78, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Morera, J.; Verdugo-Manzanares, R.; González, S.; Verdejo, R.; Lopez-Manchado, M.A.; Hernández Santana, M. On the use of mechano-chemically modified ground tire rubber (GTR) as recycled and sustainable filler in styrene-butadiene rubber (SBR) composites. J. Compos. Sci. 2021, 5, 68. [Google Scholar] [CrossRef]
- Sulcis, R.; Vizza, F.; Oberhauser, W.; Ciardelli, F.; Spiniello, R.; Dintcheva, N.T.; Passaglia, E. Recycling ground tire rubber (GTR) scraps as high-impact filler of in situ produced polyketone matrix. Polym. Adv. Technol. 2014, 25, 1060–1068. [Google Scholar] [CrossRef]
- Formela, K.; Korol, J.; Saeb, M.R. Interfacially modified LDPE/GTR composites with non-polar elastomers: From microstructure to macro-behavior. Polym. Test. 2015, 42, 89–98. [Google Scholar] [CrossRef]
- Liu, S.; Peng, Z.; Zhang, Y.; Rodrigue, D.; Wang, S. Compatibilized thermoplastic elastomers based on highly filled polyethylene with ground tire rubber. J. Appl. Polym. Sci. 2022, 139, e52999. [Google Scholar] [CrossRef]
- Kiss, L.; Simon, D.A.; Petrény, R.; Kocsis, D.; Bárány, T.; Mészáros, L. Ground tire rubber filled low-density polyethylene: The effect of particle size. Adv. Ind. Eng. Polym. 2022, 5, 12–17. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Thermoplastic elastomers based on recycled high-density polyethylene/ground tire rubber/ethylene vinyl acetate: Effect of ground tire rubber regeneration on morphological and mechanical properties. J. Thermoplast. Compos. Mater. 2022. [Google Scholar] [CrossRef]
- Garcia, P.S.; de Lima, J.A.; Scuracchio, C.H.; Cruz, S.A. The effect of adding devulcanized rubber on the thermomechanical properties of recycled polypropylene. J. Appl. Polym. Sci. 2021, 138, 50703. [Google Scholar] [CrossRef]
- Kościuszko, A.; Sykutera, D.; Czyżewski, P.; Hoyer, S.; Kroll, L.; Szczupak, B. Processing and mechanical properties of highly filled PP/GTR compounds. Materials 2022, 15, 3799. [Google Scholar] [CrossRef]
- Basso, A.; Zhang, Y.; Linnemann, L.; Hansen, H.N. Study of the distribution of rubber particles in ground tire rubber/polypropylene blends. Mater. Today Proc. 2021, 34, 311–316. [Google Scholar] [CrossRef]
- Stelescu, M.D. Polymer composites based on plasticized PVC and vulcanized nitrile rubber waste powder for irrigation pipes. Int. Sch. Res. Not. 2013, 2013, 726121. [Google Scholar] [CrossRef]
- Tatangelo, V.; Mangili, I.; Caracino, P.; Bestetti, G.; Collina, E.; Anzano, M.; Branduardi, P.; Posteri, R.; Porro, D.; Lasagni, M.; et al. Microbial desulfurization of ground tire rubber (GTR): Characterization of microbial communities and rheological and mechanical properties of GTR and natural rubber composites (GTR/NR). Polym. Degrad. Stab. 2019, 160, 102–109. [Google Scholar] [CrossRef]
- Karabork, F.; Pehlivan, E.; Akdemir, A. Characterization of styrene butadiene rubber and microwave devulcanized ground tire rubber composites. J. Polym. Eng. 2014, 34, 543–554. [Google Scholar] [CrossRef]
- Simon, D.A.; Pirityi, D.; Tamás-Bényei, P.; Bárány, T. Microwave devulcanization of ground tire rubber and applicability in SBR compounds. J. Appl. Polym. Sci. 2020, 137, 48351. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling waste tires into ground tire rubber (GTR)/rubber compounds: A review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Formela, K. Waste tire rubber-based materials: Processing, performance properties and development strategies. Adv. Ind. Eng. Polym. Res. 2022, 5, 234–247. [Google Scholar] [CrossRef]
- Hejna, A.; Klein, M.; Saeb, M.R.; Formela, K. Towards understanding the role of peroxide initiators on compatibilization efficiency of thermoplastic elastomers highly filled with reclaimed GTR. Polym. Test. 2019, 73, 143–151. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Cañavate, J.; Formela, K. GTR/NBR/silica composites performance properties as a function of curing system: Sulfur versus peroxides. Materials 2021, 14, 5345. [Google Scholar] [CrossRef]
- Radhesh Kumar, C.; Fuhrmann, I.; Karger-Kocsis, J. LDPE-based thermoplastic elastomers containing ground tire rubber with and without dynamic curing. Polym. Degrad. Stab. 2002, 76, 137–144. [Google Scholar] [CrossRef]
- Grigoryeva, O.; Fainleib, A.; Starostenko, O.; Danilenko, I.; Kozak, N.; Dudarenko, G. Ground tire rubber (GTR) reclamation: Virgin rubber/reclaimed GTR (RE)vulcanizates. Rubber Chem. Technol. 2004, 77, 131–146. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Du, F.; Wang, X. Thermoplastic elastomer based on high impact polystyrene/ethylene-vinyl acetate copolymer/waste ground rubber tire powder composites compatibilized by styrene-butadiene-styrene block copolymer. Mater. Chem. Phys. 2012, 136, 1124–1129. [Google Scholar] [CrossRef]
- Wang, L.; Lang, F.; Li, S.; Du, F.; Wang, Z. Thermoplastic elastomers based on high-density polyethylene and waste ground rubber tire composites compatibilized by styrene–butadiene block copolymer. J. Thermoplast. Compos. Mater. 2014, 27, 1479–1492. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Araújo, E.M.; Siqueira, D.D.; de Souza Morais, D.D.; dos Santos Filho, E.A.; Filho, M.V.L. Fook, Incorporation of a recycled rubber compound from the shoe industry in polystyrene: Effect of SBS compatibilizer content. J. Elastomers Plast. 2020, 52, 3–28. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Y.; Wang, Z. Super-hydrophobic and super-oleophilic surface based on high-density polyethylene/waste ground rubber tire powder thermoplastic elastomer. J. Thermoplast. Compos. Mater. 2020, 33, 851–864. [Google Scholar] [CrossRef]
- De Almeida Júnior, A.F.; Battistelle, R.A.; Bezerra, B.S.; de Castro, R. Use of scrap tire rubber in place of SBS in modified asphalt as an environmentally correct alternative for Brazil. J. Clean. Prod. 2012, 33, 236–238. [Google Scholar] [CrossRef]
- Navarro, F.J.; Partal, P.; Martȷnez-Boza, F.; Valencia, C.; Gallegos, C. Rheological characteristics of ground tire rubber-modified bitumens. Chem. Eng. J. 2002, 89, 53–61. [Google Scholar] [CrossRef]
- Rasool, R.T.; Song, P.; Wang, S. Thermal analysis on the interactions among asphalt modified with SBS and different degraded tire rubber. Constr. Build. Mater. 2018, 182, 134–143. [Google Scholar] [CrossRef]
- Zhao, M.; Dong, R. Reaction mechanism and rheological properties of waste cooking oil pre-desulfurized crumb tire rubber/SBS composite modified asphalt. Constr. Build. Mater. 2021, 274, 122083. [Google Scholar] [CrossRef]
- Li, H.; Cui, C.; Temitope, A.A.; Feng, Z.; Zhao, G.; Guo, P. Effect of SBS and crumb rubber on asphalt modification: A review of the properties and practical application. J. Traffic Transp. Eng. 2022, 9, 836–863. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Sonmez, M.; Alexandrescu, L.; Nituica, M.; Gurau, D.F.; Georgescu, M. Structure and properties of blends based on vulcanized rubber waste and styrene–butadiene–styrene thermoplastic elastomer. J. Rubber Res. 2022, 25, 421–434. [Google Scholar] [CrossRef]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Vulcanization of natural rubber modified with cashew nut shell liquid and its phosphorylated derivative—A comparative study. Polymer 1998, 39, 4033–4036. [Google Scholar] [CrossRef]
- Khang, T.H.; Ariff, Z.M. Vulcanization kinetics study of natural rubber compounds having different formulation variables. J. Therm. Anal. Calorim. 2012, 109, 1545–1553. [Google Scholar] [CrossRef]
- Lu, K.T.; Chu, Y.C.; Chen, T.C.; Hu, K.H. Investigation of the decomposition reaction and dust explosion characteristics of crystalline dicumyl peroxide. Process Saf. Environ. Prot. 2010, 88, 356–365. [Google Scholar] [CrossRef]
- Przybysz, M.; Hejna, A.; Haponiuk, J.; Formela, K. Structural and thermo-mechanical properties of poly(ε-caprolactone) modified by various peroxide initiators. Polymers 2019, 11, 1101. [Google Scholar] [CrossRef]
- Lu, N.; Shen, M.; Liu, J.; Prakashan, K.; Xin, Z. Effects of posttreatments on the storage stability of reclaimed rubber. Adv. Polym. Technol. 2021, 2021, 6617666. [Google Scholar] [CrossRef]
- Kruželák, J.; Hložeková, K.; Kvasničáková, A.; Tomanová, K.; Hudec, I. Application of sulfur and peroxide curing systems for cross-Linking of rubber composites filled with calcium lignosulfonate. Polymers 2022, 14, 1921. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the crosslink characteristics and mechanical properties of natural rubber compound via accelerators and reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Colom, X.; Marín-Genesca, M.; Mujal, R.; Formela, K.; Cañavate, J. Structural and physico-mechanical properties of natural rubber/GTR composites devulcanized by microwaves: Influence of GTR source and irradiation time. J. Compos. Mater. 2018, 52, 3099–3108. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Cañavate, J.; Saeb, M.R.T.; Haponiuk, J.; Formela, K. Investigating the impact of curing system on structure-property relationship of natural rubber modified with brewery by-product and ground tire rubber. Polymers 2020, 12, 545. [Google Scholar] [CrossRef]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds—Overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- Zedler, Ł.; Burger, P.; Wang, S.; Formela, K. Ground tire rubber modified by ethylene-vinyl acetate copolymer: Processing, physico-mechanical properties, volatile organic compounds emission and recycling possibility. Materials 2020, 13, 4669. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ding, H.; Wang, X.; Xie, M.; Yu, Z. Blending LLDPE and ground rubber tires. Polym. Plast. Technol. Eng. 2008, 47, 199–202. [Google Scholar] [CrossRef]
- El-Nemr, K.F.; Ali, M.A.; Gad, Y.H. Manifestation of the silicate filler additives and electron beam irradiation on properties of SBR/devulcanized waste tire rubber composites for floor tiles applications. Polym. Compos. 2022, 43, 366–377. [Google Scholar] [CrossRef]
- Karabork, F.; Akdemir, A. Friction and wear behavior of styrene butadiene rubber-based composites reinforced with microwave-devulcanized ground tire rubber. J. Appl. Polym. Sci. 2015, 132, 42419. [Google Scholar] [CrossRef]
- Li, S.; Lamminmäki, J.; Hanhi, K. Effect of ground rubber powder and devulcanizates on the properties of natural rubber compounds. J. Appl. Polym. Sci. 2005, 97, 208–217. [Google Scholar] [CrossRef]
- Abang Ismawi Hassim, D.H.; Abraham, F.; Summerscales, J.; Brown, P. The effect of interface morphology in waste tyre rubber powder filled elastomeric matrices on the tear and abrasion resistance. Express Polym. Lett. 2019, 13, 248–260. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Zedler, Ł.; Formela, K. Processing, performance properties, and storage stability of ground tire rubber modified by dicumyl peroxide and ethylene-vinyl acetate copolymers. Polymers 2021, 13, 4014. [Google Scholar] [CrossRef]
- Sonnier, R.; Leroy, E.; Clerc, L.; Bergeret, A.; Lopez-Cuesta, J.-M.; Bretelle, A.-S.; Ienny, P. Compatibilizing thermoplastic/ground tyre rubber powder blends: Efficiency and limit. Polym. Test. 2008, 27, 901–907. [Google Scholar] [CrossRef]
- Nadal Gisbert, P.A.; Crespo Amorós, J.E.; López Martínez, J.; Macias Garcia, A. Study of thermal degradation kinetics of elastomeric powder (ground tire rubber). Polym. Plast. Technol. Eng. 2007, 47, 36–39. [Google Scholar] [CrossRef]
- Garcia, P.S.; de Sousa, F.D.B.; de Lima, J.A.; Cruz, S.A.; Scuracchio, C.H. Devulcanization of ground tire rubber: Physical and chemical changes after different microwave exposure times. Express Polym. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Cui, H.; Yang, J.; Liu, Z. Thermogravimetric analysis of two Chinese used tires. Thermochim. Acta 1999, 333, 173–175. [Google Scholar] [CrossRef]
- Knappe, S.; Urso, C. Applications of thermal analysis in the rubber industry. Thermochim. Acta 1993, 227, 35–42. [Google Scholar] [CrossRef]

| Item * | Method | SBS Copolymer | |||
|---|---|---|---|---|---|
| SBS L1 (SBS L7322) | SBS L2 (SBS L7342) | SBS L3 (SBS L7417) | SBS R (SBS R7382) | ||
| Content of bound styrene (wt%) | Producer’s internal procedure | 27.5–30.5 | 28.5–31.5 | 36.0–38.0 | 28.5–31.5 |
| Melt flow index at 190 °C/5 kg (g/10 min) | Producer’s internal procedure | 3.0–9.0 | - | 16.0–25.0 | - |
| Tensile strength (MPa) | ASTM D 412 | ≥10.0 | ≥14.7 | ≥1.7 | ≥8 |
| Modulus at 300% (MPa) | ASTM D 412 | ≥2.0 | ≥2.0 | - | ≥2.0 |
| Elongation at break (%) | ASTM D 412 | ≥800 | ≥700 | ≥250 | ≥550 |
| Hardness (Shore A) | ASTM D 2240 | 69–81 | 77–83 | 80–92 | 77–87 |
| Volatile matter content (wt%) | ASTM D 5668 | ≤0.5 | |||
| Ash content (wt%): (a) Calcium stearate or zinc stearate (b) Silica | ASTM D 5667 | ≤0.3 ≤1.2 | |||
| Properties | Sample Code | |||||||
|---|---|---|---|---|---|---|---|---|
| GTR/SBS L1 | GTR/SBS L2 | GTR/SBS L3 | GTR/SBS R | |||||
| S | DCP | S | DCP | S | DCP | S | DCP | |
| Mooney viscosity ML (1+4) 100 °C | 127.4 | 94.7 | - | - | 98.3 | 78.9 | - | - |
| Mooney viscosity ML (1+8) 100 °C | 116.5 | 82.5 | - | - | 91.8 | 73.0 | - | - |
| Minimum torque (dNm) | 0.7 | 0.7 | 1.4 | 1.6 | 0.5 | 0.6 | 1.4 | 1.6 |
| Maximum torque (dNm) | 5.2 | 16.9 | 6.2 | 17.1 | 4.9 | 17.4 | 6.1 | 18.1 |
| Extent of cure (dNm) | 4.6 | 16.2 | 4.7 | 15.5 | 4.4 | 16.8 | 4.7 | 16.5 |
| Scorch time (min) | 0.6 | 0.5 | 0.7 | 0.4 | 0.7 | 0.4 | 0.6 | 0.5 |
| Optimal cure time (min) | 1.7 | 9.7 | 1.6 | 9.6 | 1.4 | 9.9 | 1.6 | 9.5 |
| Cure rate index (min−1) | 90.9 | 10.9 | 111.1 | 10.9 | 142.9 | 10.5 | 100.0 | 11.1 |
| Sample Code | Hardness (Shore A) | Density (g/cm3) | Swelling Degree (%) | Sol Fraction (%) | |
|---|---|---|---|---|---|
| GTR * | - | 57 ± 1 | 1.149 ± 0.007 | 169 ± 4 | 10.5 ± 0.3 |
| GTR/SBS L1 | S | 66 ± 1 | 1.075 ± 0.008 | 318 ± 4 | 9.7 ± 0.2 |
| DCP | 67 ± 1 | 1.043 ± 0.001 | 201 ± 2 | 7.1 ± 0.1 | |
| GTR/SBS L2 | S | 68 ± 2 | 1.072 ± 0.002 | 277 ± 6 | 9.4 ± 0.2 |
| DCP | 73 ± 2 | 1.042 ± 0.001 | 186 ± 4 | 7.1 ± 0.2 | |
| GTR/SBS L3 | S | 78 ± 2 | 1.086 ± 0.004 | 368 ± 10 | 12.2 ± 1.1 |
| DCP | 76 ± 2 | 1.044 ± 0.003 | 228 ± 2 | 9.2 ± 0.1 | |
| GTR/SBS R | S | 67 ± 2 | 1.074 ± 0.008 | 248 ± 4 | 9.6 ± 0.1 |
| DCP | 70 ± 2 | 1.043 ± 0.006 | 205 ± 3 | 9.0 ± 0.3 | |
| Sample Code | Decomposition Temperature (°C) | Residue Mass at 800 °C | ||||
|---|---|---|---|---|---|---|
| T−2% | T−5% | T−10% | T−50% | |||
| GTR | - | 230.9 | 290.0 | 342.4 | 445.3 | 36.9 |
| SBS L1 | - | 365.7 | 384.2 | 404.2 | 451.5 | 0.7 |
| GTR/SBS L1 | S | 263.4 | 330.4 | 368.6 | 448.1 | 20.6 |
| DCP | 267.8 | 338.2 | 373.7 | 453.5 | 17.9 | |
| SBS L2 | - | 362.2 | 380.0 | 399.0 | 451.9 | 0.4 |
| GTR/SBS L2 | S | 264.2 | 330.9 | 370.0 | 449.3 | 19.9 |
| DCP | 264.3 | 336.9 | 373.7 | 453.1 | 18.0 | |
| SBS L3 | - | 353.4 | 376.7 | 395.3 | 454.0 | 0.2 |
| GTR/SBS L3 | S | 262.7 | 330.7 | 370.2 | 449.6 | 19.8 |
| DCP | 255.2 | 332.8 | 371.7 | 452.6 | 18.9 | |
| SBS R | - | 363.4 | 381.2 | 399.7 | 452.5 | 0.8 |
| GTR/SBS R | S | 259.3 | 329.0 | 369.1 | 448.3 | 20.3 |
| DCP | 269.6 | 340.6 | 376.6 | 454.3 | 18.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodak, A.; Susik, A.; Kowalkowska-Zedler, D.; Zedler, Ł.; Formela, K. Cross-Linking, Morphology, and Physico-Mechanical Properties of GTR/SBS Blends: Dicumyl Peroxide vs. Sulfur System. Materials 2023, 16, 2807. https://doi.org/10.3390/ma16072807
Rodak A, Susik A, Kowalkowska-Zedler D, Zedler Ł, Formela K. Cross-Linking, Morphology, and Physico-Mechanical Properties of GTR/SBS Blends: Dicumyl Peroxide vs. Sulfur System. Materials. 2023; 16(7):2807. https://doi.org/10.3390/ma16072807
Chicago/Turabian StyleRodak, Agata, Agnieszka Susik, Daria Kowalkowska-Zedler, Łukasz Zedler, and Krzysztof Formela. 2023. "Cross-Linking, Morphology, and Physico-Mechanical Properties of GTR/SBS Blends: Dicumyl Peroxide vs. Sulfur System" Materials 16, no. 7: 2807. https://doi.org/10.3390/ma16072807
APA StyleRodak, A., Susik, A., Kowalkowska-Zedler, D., Zedler, Ł., & Formela, K. (2023). Cross-Linking, Morphology, and Physico-Mechanical Properties of GTR/SBS Blends: Dicumyl Peroxide vs. Sulfur System. Materials, 16(7), 2807. https://doi.org/10.3390/ma16072807








