Potential Utilization of Ground Eggshells as a Biofiller for Natural Rubber Biocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Pure EGGSHELLS
2.3. Preparation and Characterization of Rubber Compounds
3. Results and Discussion
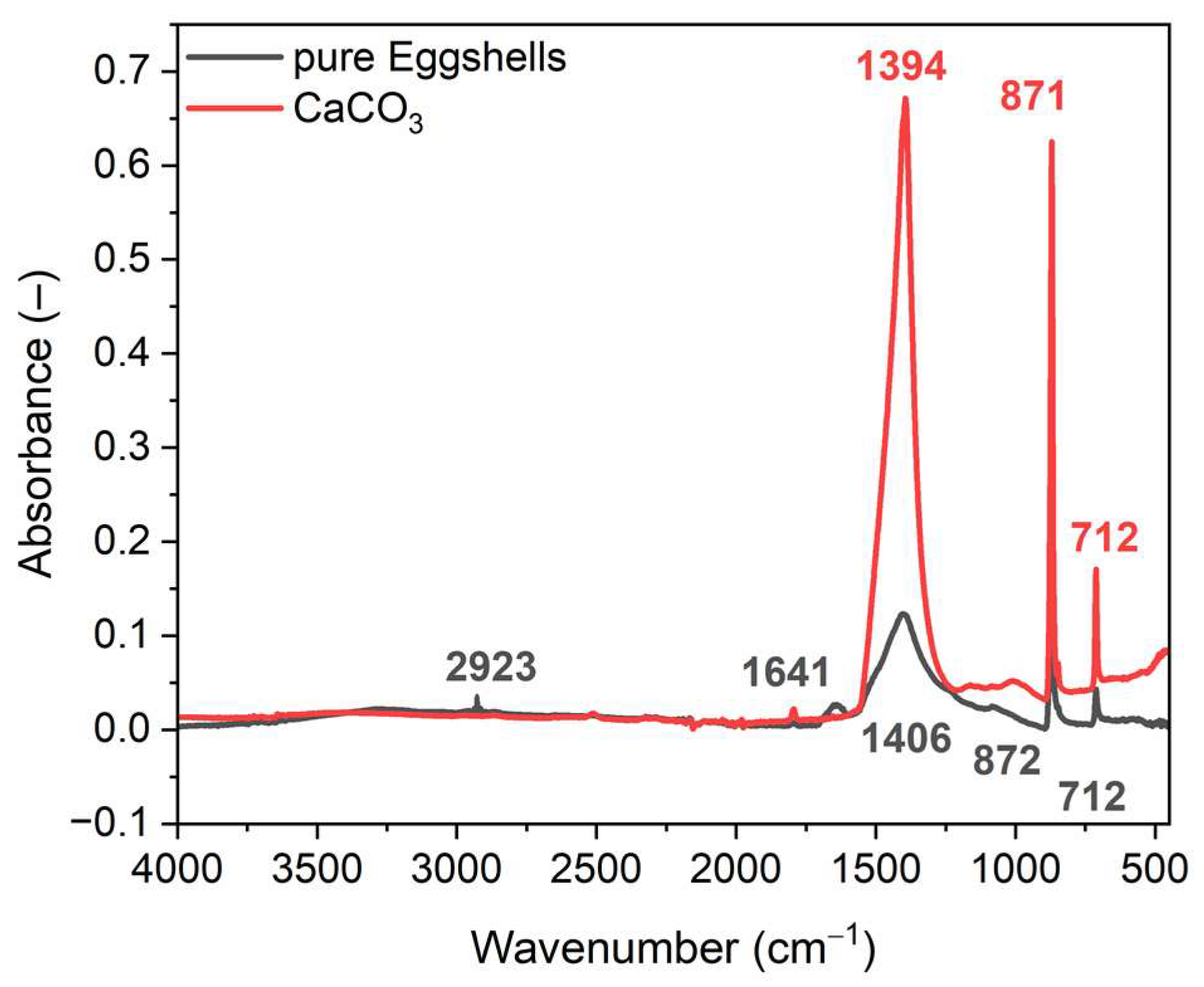
3.1. Characterization of Pure Ground Eggshells
3.2. Cure Characteristics and Crosslink Density of NR Composites Filled with Eggshells
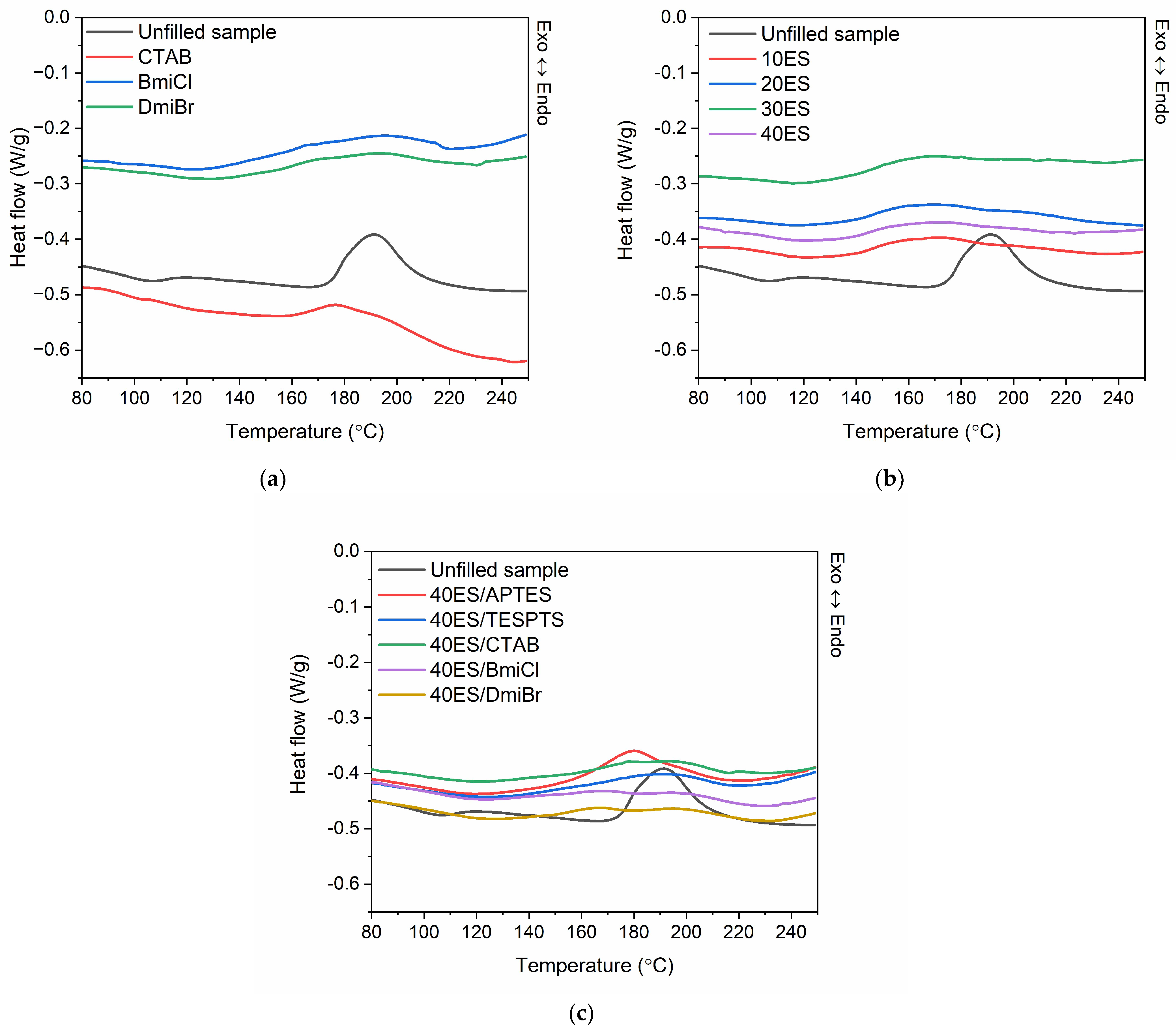
3.3. Differential Scanning Calorimetry (DSC) Analysis of the Crosslinking Process of NR Compounds
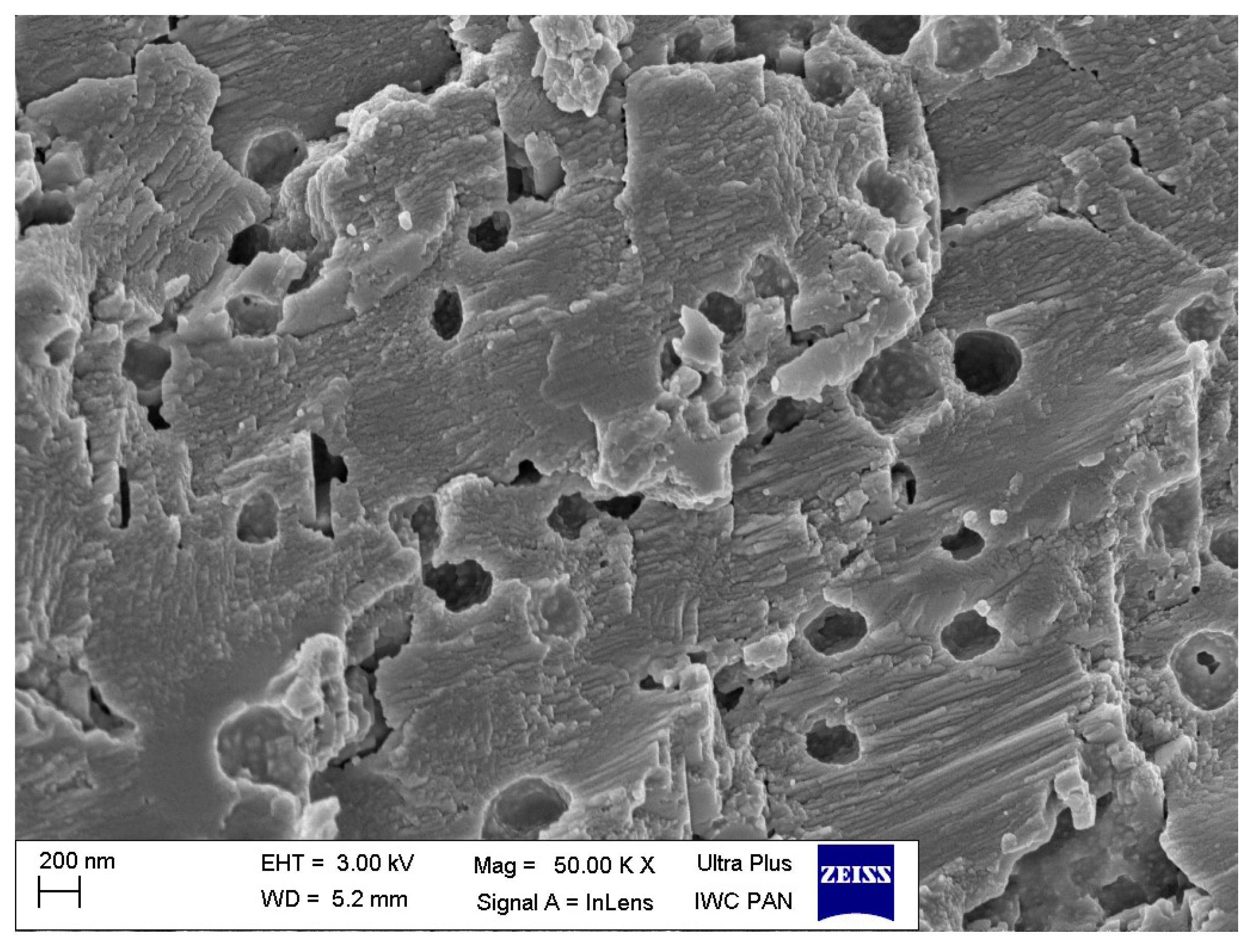
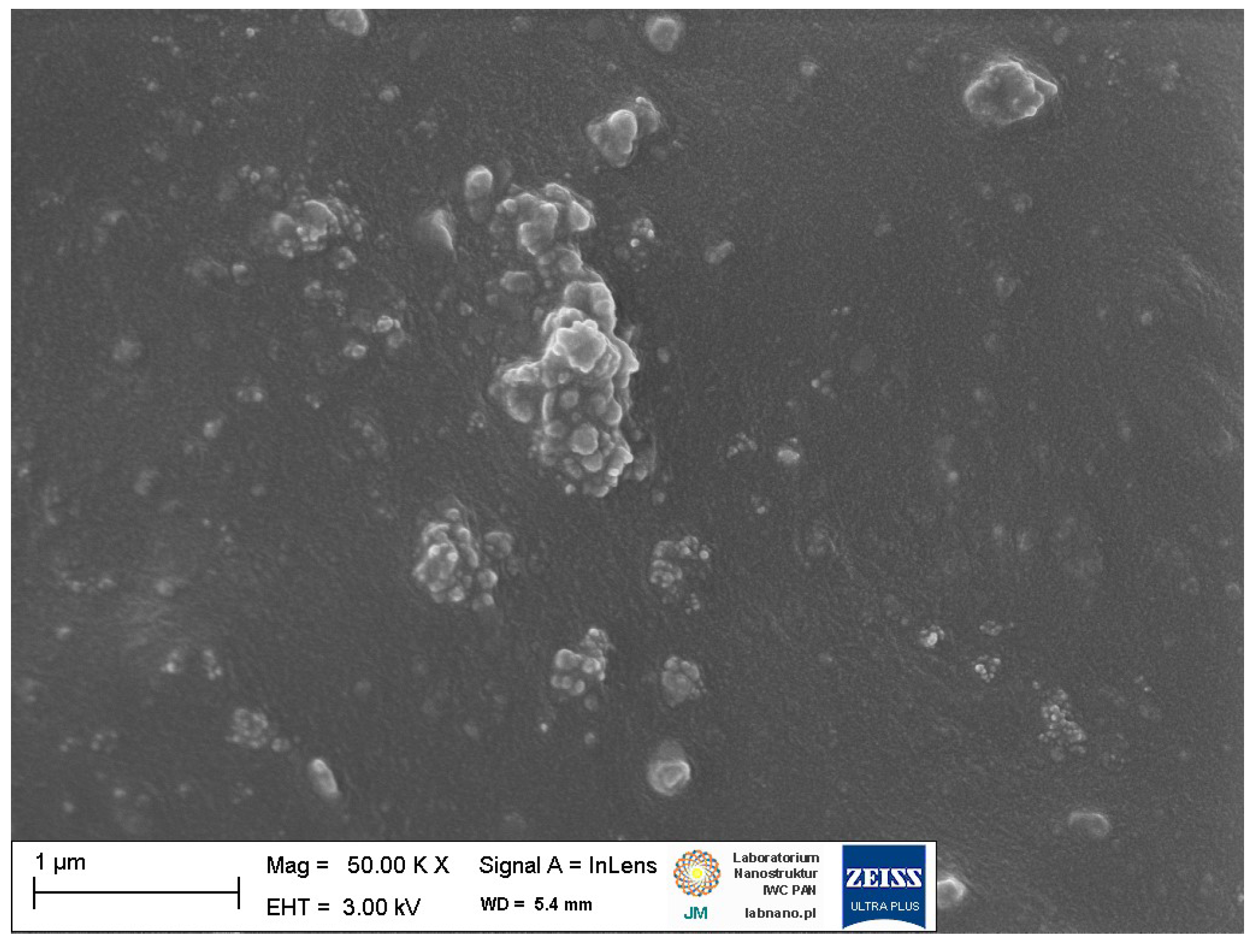
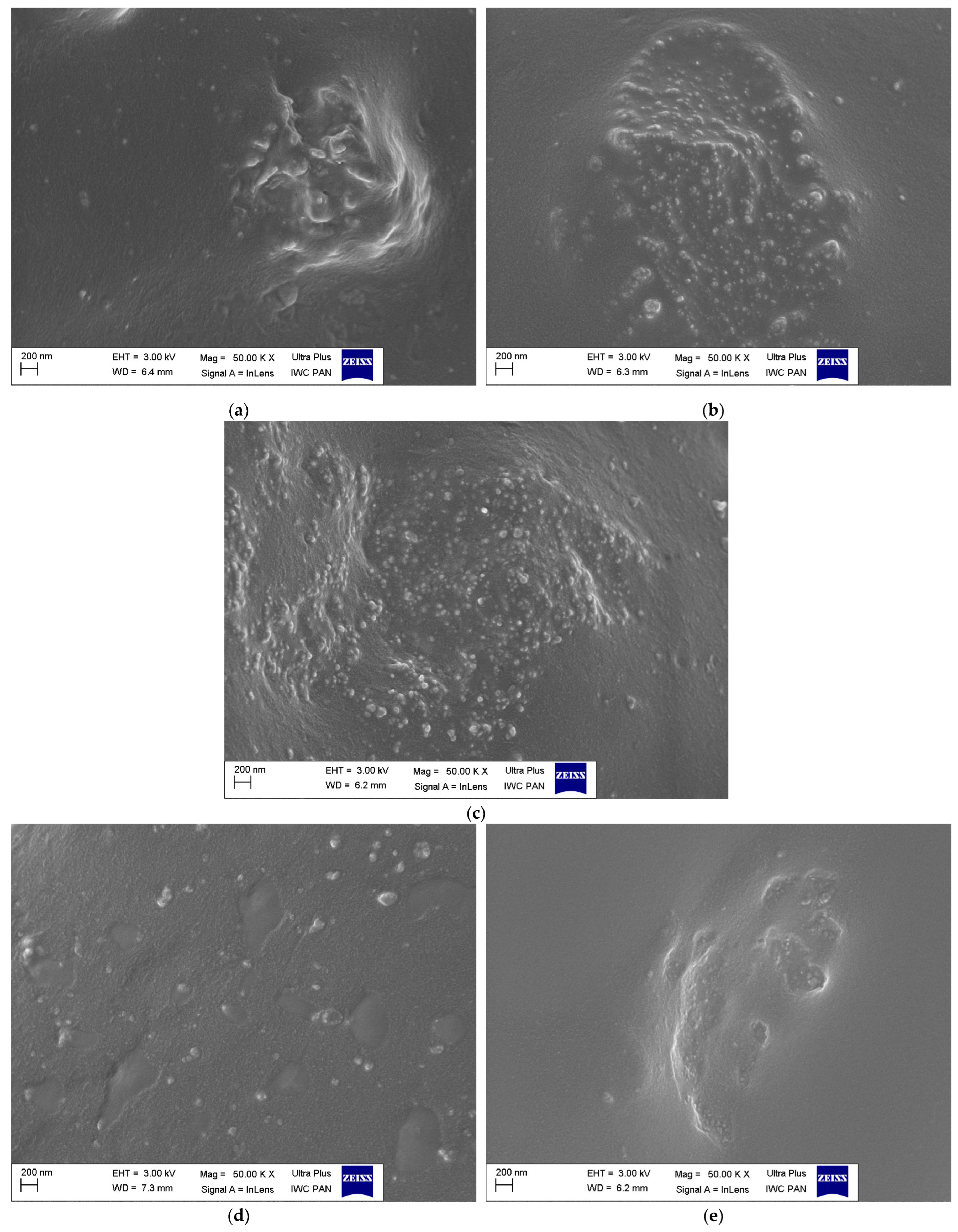
3.4. Dispersion of Ground Eggshells and Curatives in NR Composites
3.5. Tensile Properties and Hardness of NR Composites Filled with Eggshells
3.6. Dynamic Mechanical Properties of NR Composites Filled with Eggshells
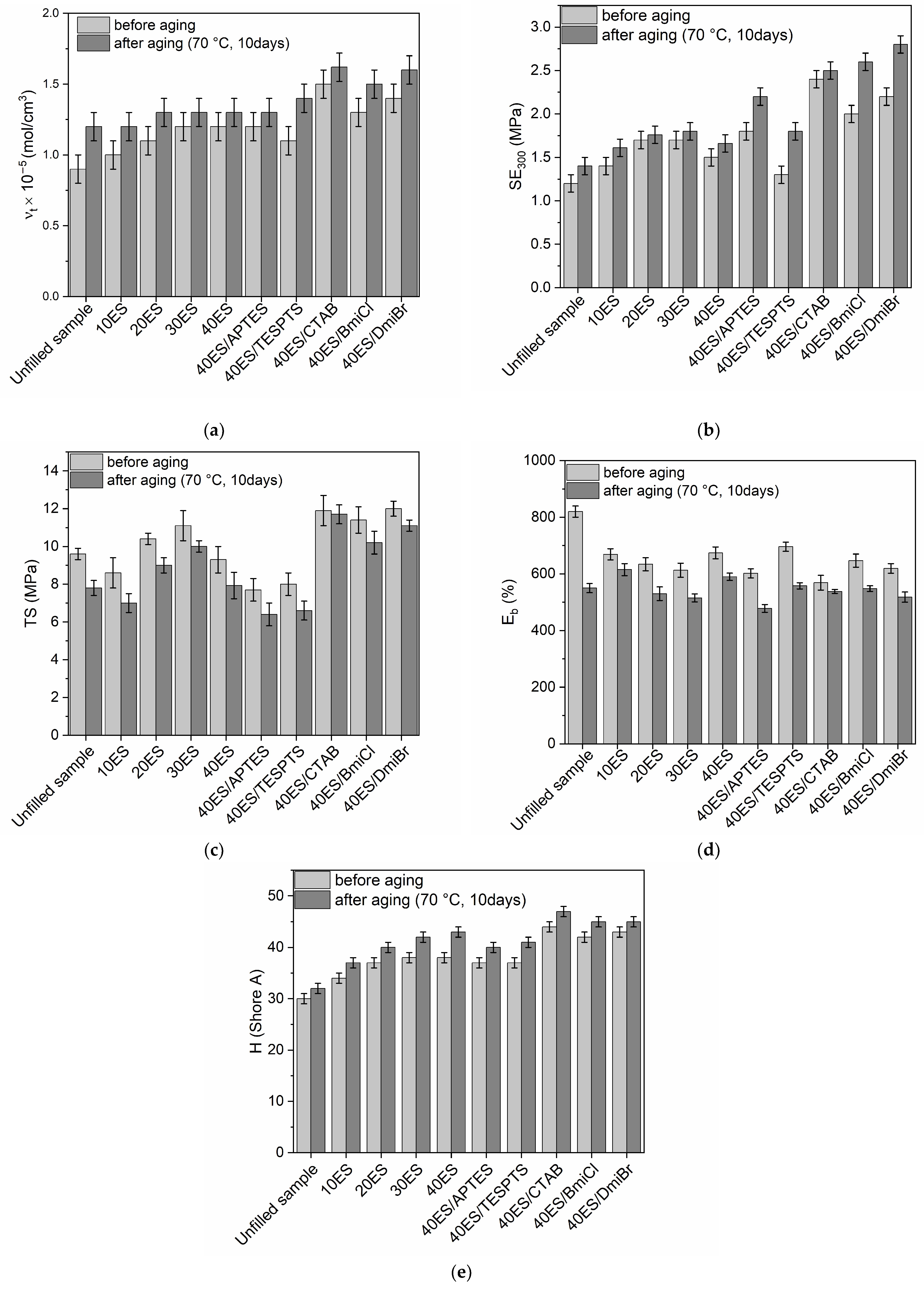
3.7. Thermo-Oxidative Aging Resistance of NR Vulcanizates Filled with Eggshells
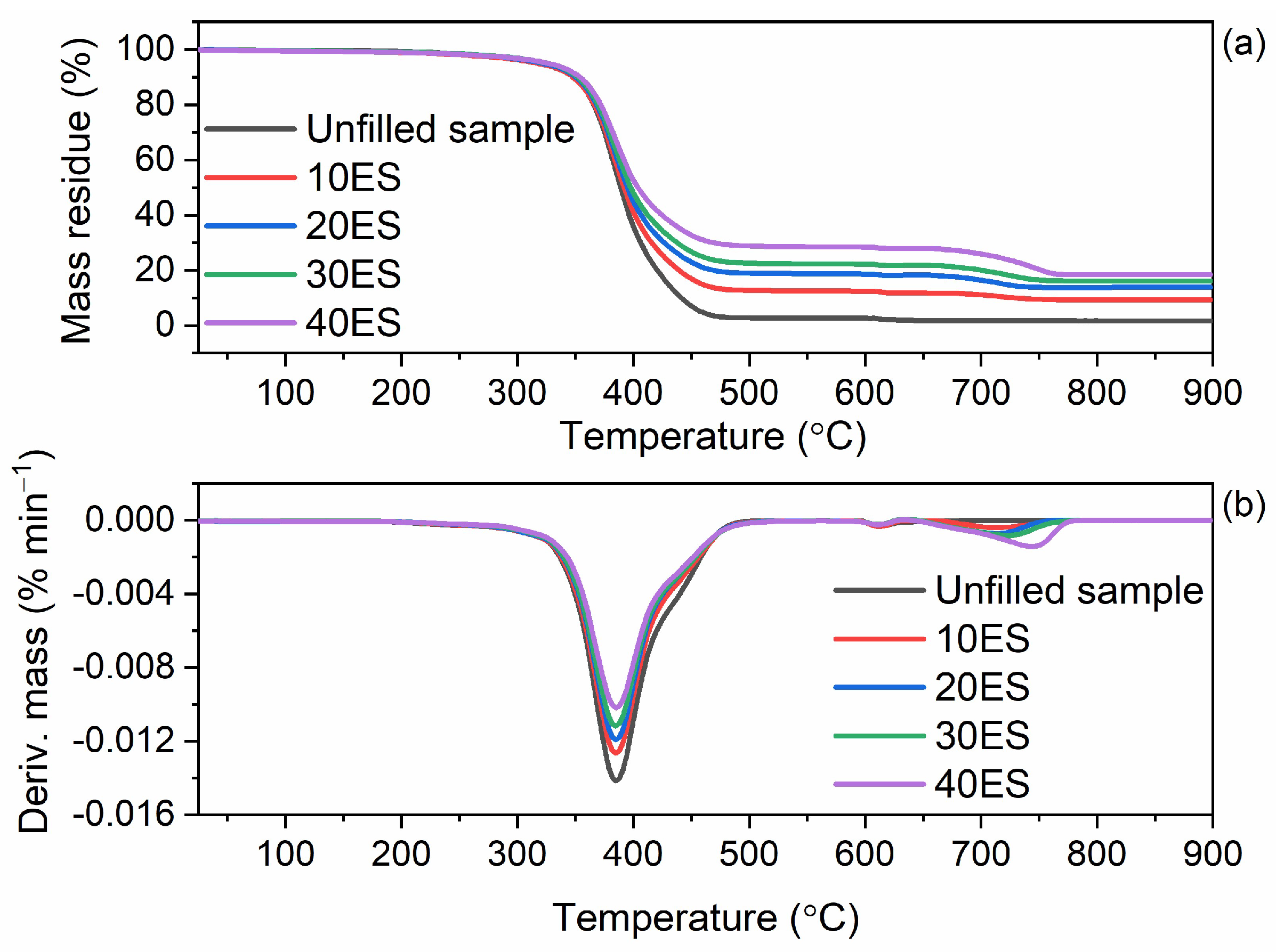
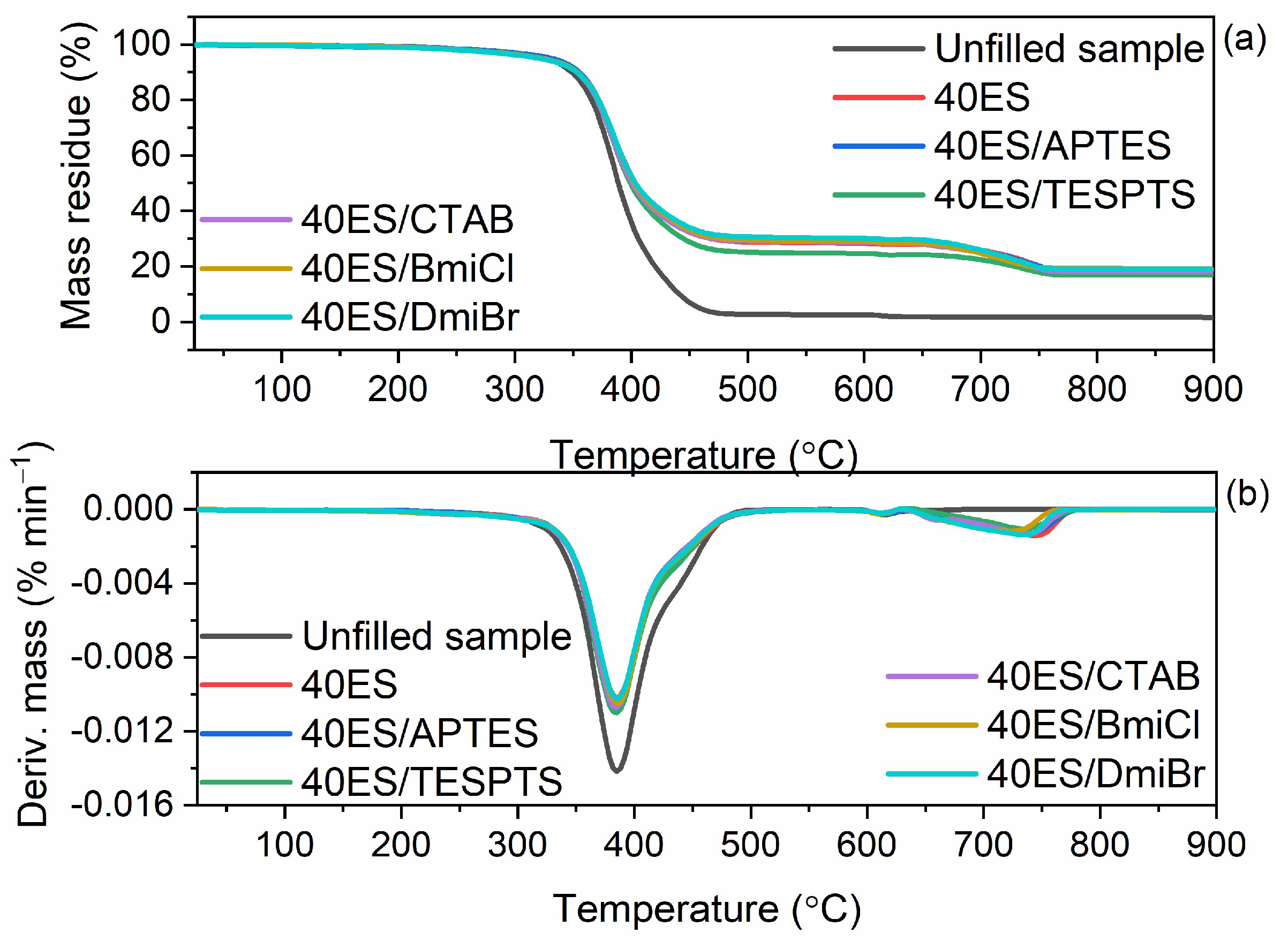
3.8. Thermal Stability of NR Vulcanizates Filled with Eggshells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kamath, S.S.; Chandrappa, R.K. Egg Shell as a Filler in Composite Materials—A Review. J. Mech. Energy Eng. 2020, 4, 335–400. [Google Scholar] [CrossRef]
- Gonzales-Fernandes, M.; Andrade, C.G.B.; Esper, F.J.; Valenzuela-Diaz, F.R.; Wiebeck, H. Improvement of Mechanical Properties in Natural Rubber with Organic Fillers. In Characterization of Minerals, Metals, and Materials 2016; Ikhmayies, S.J., Li, B., Carpenter, J.S., Hwang, J.-Y., Monteiro, S.N., Li, J., Firrao, D., Zhang, M., Peng, Z., Escobedo-Diaz, J.P., et al., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 623–627. [Google Scholar] [CrossRef]
- Mohammed, M.R.; Hadi, A.N. Effect of Egg Shells Powder on Some Mechanical and Physical Properties of Natural Rubber (NR). Iraqi J. Chem. Pet. Eng. 2012, 12, 446–458. [Google Scholar]
- Rothon, R.; Paynter, C. Calcium Carbonate Fillers. In Fillers for Polymer Applications; Polymers and Polymeric Composites: A Reference Series; Rothon, R., Ed.; Springer: Cham, Switzerland, 2017; pp. 149–160. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Wu, L.; Younes, M.; Hinckle, M. Biotechnological Applications of Eggshell: Recent Advances. Front. Bioeng. Biotechnol. 2021, 9, 675364. [Google Scholar] [CrossRef] [PubMed]
- Chaithanyasai, A.; Vakchore, P.R.; Umasankar, V. The Micro Structural and Mechanical Property Study of Effects of Egg Shell Particles on The Aluminium 6061. Procedia Eng. 2014, 97, 961–967. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pisitsak, P.; Pechyen, C. Eggshell and Bacterial Cellulose Composite Membrane as Absorbent Material in Active Packaging. Int. J. Polym. Sci. 2016, 2016, 1047606–1047614. [Google Scholar] [CrossRef]
- Opris, H.; Bran, S.; Dinu, C.; Baciut, M.; Prodan, D.A.; Mester, A.; Baciut, G. Clinical Applications of Avian Eggshell-derived Hydroxyapatite. Bosnian J. Basic Med. Sci. 2020, 2, 430–437. [Google Scholar] [CrossRef]
- Trakoolwannachai, V.; Kheolamai, P.; Ummartyotin, S. Development of Hydroxyapatite from Eggshell Waste and a Chitosan-Based Composite: In Vitro Behavior of Human Osteoblast-like Cell (Saos-2) Cultures. Int. J. Biol. Macromol. 2019, 134, 557–564. [Google Scholar] [CrossRef]
- Wu, X.; Stroll, S.I.; Lantigua, D.; Suvarnapathaki, S.; Camci-Unal, G. Eggshell Particle-reinforced Hydrogels for Bone Tissue Engineering: An Orthogonal Approach. Biomater. Sci. 2019, 7, 2675–2685. [Google Scholar] [CrossRef]
- Ingole, V.H.; Vuherer, T.; Maver, U.; Vinchurkar, A.; Ghule, A.V.; Kokol, V. Mechanical Properties and Cytotoxicity of Differently Structured Nanocellulose-Hydroxyapatite Based Composites for Bone Regeneration Application. Nanomaterials 2020, 10, 25. [Google Scholar] [CrossRef]
- Panheleux, M.; Kalin, O.; Gautron, J.; Nys, Y. Features of Eggshell Formation in Guinea Fowl: Kinetics of Shell Deposition, Uterine Protein Secretion and Uterine Histology. Br. Poult. Sci. 2010, 40, 632–643. [Google Scholar] [CrossRef]
- Gautron, J.; Stapane, L.; Le Roy, N.; Rodriguez-Navarro, A.B.; Hincke, M.T. Avian Eggshell Biomineralization: An Update on Its Structure, Mineralogy and Protein Toolkit. BMC Mol. Cell Biol. 2021, 22, 11–27. [Google Scholar] [CrossRef]
- Ketta, M.; Tumova, E. Eggshell Structure, Measurements, and Quality-Affecting Factors in Laying Hens: A Review. Czech J. Anim. Sci. 2016, 61, 299–301. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian Eggshell Mineralization: Biochemical and Functional Characterization of Matrix Proteins. C. R. Palevol. 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Le Roy, N.; Stapane, L.; Gautron, J.; Hincke, M.T. Evolution of the Avian Eggshell Biomineralization Protein Toolkit: New Insights from Multi-Omics. Front. Genet. 2021, 12, 672433. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, E.I. Comparative Study on the Characteristics of Egg Shells of Some Bird Species. Bull. Chem. Soc. Ethiop. 2009, 23, 159–166. [Google Scholar] [CrossRef]
- Horn, R.; Schlogl, R. Methane Activation by Heterogeneous Catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Maslowski, M.; Rybinski, P.; Strzelec, K. Properties of Chemically Modified (Selected Silanes) Lignocellulosic Filler and Its Application in Natural Rubber Biocomposites. Materials 2020, 13, 4163. [Google Scholar] [CrossRef] [PubMed]
- Miedzianowska, J.; Maslowski, M.; Rybinski, P.; Strzelec, K. Straw/Nano-Additive Hybrids as Functional Fillers for Natural Rubber Biocomposites. Materials 2021, 14, 321. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Tzounis, L.; Pongwisuthiruchte, A.; Potiyaraj, P.E. Effect of Various Surface Treatments on the Performance of Jute Fibers Filled Natural Rubber (NR) Composites. Polymers 2020, 12, 369. [Google Scholar] [CrossRef]
- Sowinska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Effect of SILPs on the Vulcanization and Properties of Ethylene–Propylene–Diene Elastomer. Polymers 2020, 12, 1220. [Google Scholar] [CrossRef]
- Sahu, P.; Gupta, M.K. A Review on the Properties of Natural Fibres and its Bio-composites: Effect of Alkali Treatment. Proc. Inst. Mech. Eng. Part L 2020, 234, 198–217. [Google Scholar] [CrossRef]
- Ansarifar, A.; Wang, L.; Ellis, R.J.; Kirtley, S.P.; Riyazuddin, N. Enhancing the Mechanical Properties of Styrene–butadiene Rubber by Optimizing the Chemical Bonding between Silanized Silica Nanofiller and the Rubber. J. Appl. Polym. Sci. 2007, 105, 322–332. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybinski, P. Silane Treatment as an Effective Way of Improving the Reinforcing Activity of Carbon Nanofibers in Nitrile. Materials 2020, 13, 3481. [Google Scholar] [CrossRef]
- Srisuwan, L.; Jarukumjorn, K.; Suppakarn, N. Effect of Silane Treatment Methods on Physical Properties of Rice Husk Flour/Natural Rubber Composites. Adv. Mater. Sci. Eng. 2018, 2018, 458397. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowinska-Baranowska, A. Bromide and Chloride Ionic Liquids Applied to Enhance the Vulcanization and Performance of Natural Rubber Biocomposites Filled with Nanosized Silica. Nanomaterials 2022, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Sowinska, A.; Maciejewska, M.; Grajewska, A. Bis(trifluoromethylsulfonyl)imide Ionic Liquids Applied for Fine-Tuning the Cure Characteristics and Performance of Natural Rubber Composites. Int. J. Mol. Sci. 2021, 22, 3678. [Google Scholar] [CrossRef]
- Sowinska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Task-Specific Ionic Liquids with Lactate Anion Applied to Improve ZnO Dispersibility in the Ethylene-Propylene-Diene Elastomer. Polymers 2021, 13, 774. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.S.; Egodage, S.M.; Walpalage, S. Enhancement of Mechanical Properties of Natural Rubber–Clay Nanocomposites through Incorporation of Silanated Organoclay into Natural Rubber Latex. e-Polymers 2020, 20, 144–153. [Google Scholar] [CrossRef]
- Wulan, P.P.D.K.; Wulandari, H.; Ulwan, S.H.; Purwanto, W.W.; Mulia, K. Modification of Carbon Nanotube’s Dispersion Using Cetyltrimethyl Ammonium Bromide (CTAB) as Cancer Drug Delivery. AIP Conf. Proc. 2018, 1933, 030008. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Yu, X.; Zhang, H.; Yan, S. Study on the Use of CTAB-Treated Illite as an Alternative Filler for Natural Rubber. ACS Omega 2021, 6, 19017–19025. [Google Scholar] [CrossRef]
- Lubura, J.; Kobera, L.; Abbrent, S.; Pavlova, E.; Strachota, B.; Bera, O.; Pavlicevic, J.; Ikonic, B.; Kojic, P.; Strachota, A. Natural Rubber Composites Using Hydrothermally Carbonized Hardwood Waste Biomass as a Partial Reinforcing Filler- Part I: Structure, Morphology, and Rheological Effects during Vulcanization. Polymers 2023, 15, 1176. [Google Scholar] [CrossRef] [PubMed]
- John, M.J.; Thomas, M.G.; Vidhu, H.; Thomas, S. Sustainable Composites Based on Natural Rubber and Biomass Resources. Curr. Appl. Polym. Sci. 2022, 5, 140–150. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Maslowski, M.; Strzelec, K. Improving Performance of Natural Rubber Composites by the Application of Functional Biofiller—Horsetail Modified with Silane Coupling Agents. Cellulose 2023. [Google Scholar] [CrossRef]
- Maslowski, M.; Miedzianowska, J.; Strakowska, A.; Strzelec, K.; Szynkowska, M.I. The Use of Rye, Oat and Triticale Straw as Filler of Natural Rubber Composites. Polym. Bull. 2018, 75, 4607–4626. [Google Scholar] [CrossRef]
- Khaigunha, P.; Wongwuttanasatian, T.; Suksri, A. Effects of Micro-Eggshells Filler on Tracking and Erosion Resistance of Silicone Rubber Composites. Key Eng. Mater. 2020, 846, 37–41. [Google Scholar] [CrossRef]
- ISO 6502-3:2018; Rubber—Measurement of Vulcanization Characteristics Using Curemeters—Part 3: Rotorless Rheometer. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 11357-1:2016; Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 1817:2015; Rubber, Vulcanized or Thermoplastic—Determination of Effect of Liquids. International Organization for Standardization: Geneva, Switzerland, 2017.
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-linked Polymer Networks. II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 868:2003; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 188:2011; Rubber, Vulcanized or Thermoplastic—Accelerated Ageing and Heat Resistance Tests. International Organization for Standardization: Geneva, Switzerland, 2011.
- Szadkowski, B.; Kuśmierek, M.; Sliwka-Kaszynska, M.; Marzec, A. Structure and Stability Characterization of Natural Lake Pigments Made from Plant Extracts and Their Potential Application in Polymer Composites for Packaging Materials. Materials 2022, 15, 4608. [Google Scholar] [CrossRef]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1,3-Diamino-2-Hydroxypropane-N,N,N0,N0-tetraacetic Acid Stabilized Amorphous Calcium Carbonate: Nucleation, Transformation and Crystal Growth. Cryst. Eng. Comm. 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Li, H.-Y.; Tan, Y.-Q.; Zhang, L.; Zhang, Y.-X.; Song, Y.-H.; Ye, Y.; Xia, M.-S. Bio-filler from Waste Shellfish Shell: Preparation, Characterization, and its Effect on the Mechanical Properties on Polypropylene Composites. J. Hazard. Mater. 2012, 217, 256–262. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczynski, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and Applicational Evaluation of the Rigid Polyurethane Foam Composites with Egg Shell Waste. Polym. Degrad. Stabil. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of Chicken Eggshell on the Flame-Retardant and Smoke Suppression Properties of an Epoxy-Based Traditional APP-PER-MEL System. Polym. Compos. 2018, 40, 2712–2723. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a Biocomposite Based on Green Polyethylene Biopolymer and Eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.H.S.; Maddila, S.N.; Jonnalagadda, S.B. One-Pot Green Synthesis of Novel 5,10-Dihydro-1H-Pyrazolo[1,2-b]Phthalazine Derivatives with Eco-Friendly Biodegradable Eggshell Powder as Efficacious Catalyst. Res. Chem. Intermed. 2020, 46, 3067–3083. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Dash, S.; Kamruddin, M.; Aijkumar, P.K.; Tyagi, A.K.; Raj, B. Nanocrystalline and Metastable Phase Formation in Vacuum Thermal Decomposition of Calcium Carbonate. Thermochim. Acta. 2000, 363, 129–135. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Koga, N. Thermal Decomposition of Biomineralized Calcium Carbonate: Correlation between the Thermal Behavior and Structural Characteristics of Avian Eggshell. ACS Sust. Chem. Eng. 2018, 6, 5283–5295. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Luque, A.; Rodriguez-Navarro, A.B.; Ortega-Huertas, M. Thermal Decomposition of Calcite: Mechanisms of Formation and Textural Evolution of CaO Nanocrystals. Am. Min. 2009, 94, 578–593. [Google Scholar] [CrossRef]
- Skorczewska, K.; Lewandowski, K.; Szewczykowski, P.; Wilczewski, S.; Szulc, J.; Stopa, P.; Nowakowska, P. Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites. Polymers 2022, 14, 4372. [Google Scholar] [CrossRef]
- Datta, R.N.; Ingham, F.A.A. Rubber Additives—Compounding Ingredients. In Rubber Technologist’s Handbook, 1st ed.; De, S.K., White, J.K., Eds.; Rapra Technology Limited: Shawbury, UK, 2001; pp. 167–181. [Google Scholar]
- Naik, S.D.; Doraiswamy, L.K. Phase transfer catalysis: Chemistry and engineering. AIChE J. 1998, 44, 612–646. [Google Scholar] [CrossRef]
- Gu, Y.; Li, G. Ionic Liquids-Based Catalysis with Solids: State of the Art. Adv. Synth. Catal. 2009, 351, 817–847. [Google Scholar] [CrossRef]
- Przybyszewska, M.; Zaborski, M. Effect of Ionic Liquids and Surfactants on Zinc Oxide Nanoparticle Activity in Crosslinking of Acrylonitrile Butadiene Elastomer. J. Appl. Polym. Sci. 2010, 116, 155–164. [Google Scholar] [CrossRef]
- Goudarzi, T.; Spring, D.W.; Paulino, G.H.; Lopez-Pamies, O. Filled elastomers: A theory of Filler Reinforcement Based on Hydrodynamic and Interphasial Effects. J. Mech. Phys. Solids 2015, 80, 37–67. [Google Scholar] [CrossRef]
- Khimi, S.R.; Pickering, K.L. A New Method to Predict Optimum Cure Time of Rubber Compound Using Dynamic Mechanical Analysis. J. Appl. Polym. Sci. 2014, 131, 4008. [Google Scholar] [CrossRef]
- Maslowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Crop Residues as an Alternative to Vulcanizates with Common Fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Roshin, R.; Sreelekshmi, R.V.; Menon, A.R.R. Cetyltrimethyl Ammonium Bromide Modified Kaolin as a Reinforcing Filler for Natural Rubber. J. Polym. Environm. 2018, 26, 39–47. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowinska, A. Influence of Fillers and Ionic Liquids on the Crosslinking and Performance of Natural Rubber Biocomposites. Polymers 2021, 13, 1656. [Google Scholar] [CrossRef]
- Sowinska-Baranowska, A.; Maciejewska, M. The Potential Application of Starch and Walnut Shells as Biofillers for Natural Rubber (NR) Composites. Int. J. Mol. Sci. 2022, 23, 7968. [Google Scholar] [CrossRef]
- Misman, M.A.; Rashid, A.; Yahya, S.R. Modification and Application of Starch in Natural Rubber Latex Composites. Rubber Chem. Technol. 2018, 91, 184–204. [Google Scholar] [CrossRef]
- Yin, P.; Dong, X.; Zhou, W.; Zha, D.; Xu, J.; Guo, B.; Li, P. A Novel Method to Produce Sustainable Biocomposites Based on Thermoplastic Corn-Starch Reinforced by Polyvinyl Alcohol Fibers. RSC Adv. 2020, 10, 23632–23643. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and Physical Modifications of Starch for Renewable Polymeric Materials. Mater. Today Sustain. 2020, 7–8, 100028–100106. [Google Scholar] [CrossRef]
- Kocetkovs, V.; Radenkovs, V.; Juhnevica-Radenkova, K.; Jakovlevs, D.; Muizniece-Brasava, S. The Impact of Eggshell Thickness on the Qualitative Characteristics of Stored Eggs Produced by Three Breeds of Laying Hens of the Cage and Cage-Free Housed Systems. Appl. Sci. 2022, 12, 11539. [Google Scholar] [CrossRef]
- Dennis, J.E.; Xiao, S.-Q.; Agarwal, M.; Fink, D.J.; Heuer, A.H.; Caplan, A.I. Microstructure of Matrix and Mineral Components of Eggshells from White Leghorn Chickens (Gallus Gallus). J. Morphol. 1996, 228, 287–306. [Google Scholar] [CrossRef]
- Damaziak, K.; Marzec, A. Analysis of Ultrastructure and Microstructure of Blackbird (Turdus Merula) and Song Thrush (Turdus Philomelos) Eggshell by Scanning Electron Microscopy and X-ray Computed Microtomography Nature Reports. Sci. Rep. 2022, 12, 11875. [Google Scholar] [CrossRef] [PubMed]
- Deepalekshmi, P.; Sadasivuni, K.K.; Yves, G.; Qipeng, G.; Sabu, T. Carbon Nanotube Based Elastomer Composites—An Approach Towards Multifunctional Materials. J. Mater. Chem. C. 2014, 40, 8446–8485. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, S.; He, H.; Lu, Y.; Shi, J.; Liu, J.; Zhu, H. Ultra-small SiO2 Nanoparticles Highly Dispersed on Non-covalent Functionalized Reduced Graphene Oxide Nanoplatelets for High-perfomance Elastomer Applications. Compos. Sci. Technol. 2020, 198, 108297. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaborski, M. Ionic Liquids Applied to Improve the Dispersion of Coagent Particles in an Elastomer. J. Compos. 2013, 2013, 286534. [Google Scholar] [CrossRef]
- Haddad, B.; Mittal, J.; Paolone, A.; Villemin, D.; Debdab, M.; Mimanne, G.; Habibi, A.; Hamidi, Z.; Boumediene, M.; Belarbi, E.-h. Synthesis and Characterization of Egg Shell (ES) and Egg Shell with Membrane (ESM) Modified by Ionic Liquids. Chem. Data. Coll. 2021, 33, 100717. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Fritzsche, J.; Klüppel, M.; Heinrich, G. Coupling Activity of Ionic Liquids between Diene Elastomers and Multi-Walled Carbon Nanotubes. Carbon 2009, 47, 3313–3321. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, P.; Wang, Z.; Chen, L.; Xu, H.; Chen, S. Magnesium Hydroxide Modified by 1-N-Tetradecyl-3-Carboxymethyl Imidazolium Chloride and its Effects on the Properties of LLDPE. Polym. Eng. Sci. 2011, 51, 1519–1524. [Google Scholar] [CrossRef]
- Rader, C.P. Vulcanization of Rubber—A. Sulfur and Non-Peroxides. In Basic Elastomer Technology, 1st ed.; Baranwal, K.C., Stephens, H.L., Eds.; Rubber Division: Akron, OH, USA, 2001; pp. 165–191. [Google Scholar]
- Akiba, M.; Hashim, A.S. Vulcanization and Crosslinking in Elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Maslowski, M.; Miedzianowska, J.; Czylkowska, A.; Strzelec, K. Horsetail (Equisetum Arvense) as a Functional Filler for Natural Rubber Biocomposites. Materials 2020, 12, 2526. [Google Scholar] [CrossRef]
- Moreno, R.M.B.; Ferreira, M.; Goncalves, P.; Capparelli, M.L.H. Technological Properties of Latex and Natural Rubber of Hevea Brasiliensis Clones. Sci. Agric. 2005, 62, 112–126. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Xie, W.-Y.; He, F.-M.; Zhu, D.; Liu, S.; Zhang, L.; Liao, S. The role of non-rubber components acting as endogenous antioxidants on thermal-oxidative aging behavior of natural rubber. Polym. Test. 2022, 111, 107614. [Google Scholar] [CrossRef]
- Choi, S.S. Influence of Thermal Aging in Change of Crosslink Density and Deformation of Natural Rubber Vulcanizates. Bull. Korean Chem. Soc. 2000, 21, 628–634. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cho, M.; Nam, J.-D.; Lee, Y. Effect of ZnO Particle Sizes on Thermal Aging Behavior of Natural Rubber Vulcanizates. Polym. Degrad. Stab. 2018, 148, 50–55. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, J.C.; Lee, S.G.; Joo, Y.L. Influence of the Cure Systems on Long Time Thermal Aging Behaviors of NR Composites. Macromol. Res. 2008, 16, 561–566. [Google Scholar] [CrossRef]
- Kruzelak, J.; Hudec, I.; Dosoudil, R. Influence of Thermo-oxidative and Ozone Ageing on the Properties of Elastomeric Magnetic Composites. Polym. Degrad. Stab. 2012, 97, 921–928. [Google Scholar] [CrossRef]
- Coran, A.Y. Chemistry of the Vulcanization and Protection of Elastomers: A Review of the Achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Masek, A.; Cichosz, S.; Piotrowska, M. Biocomposites of Epoxidized Natural Rubber/Poly(lactic acid) Modified with Natural Fillers (Part I). Int. J. Mol. Sci. 2021, 22, 3150. [Google Scholar] [CrossRef]
- Rasid, Z.A. The Thermal Stability Property of Bio-composites: A Review. In InCIEC 2014: Proceedings of the International Civil and Infrastructure Engineering Conference 2014, Kota Kinabalu, Malaysia, 28 September–1 October 2014; Hassan, R., Yusoff, M., Alisibramulisi, A., Amin, N.M., Ismail, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 667–680. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Massoumi, I.; Hassan, A. Comparison of Polylactic Acid/kenaf and Polylactic Acid/rise Husk Composites: The Influence of the Natural Fibers on the Mechanical, Thermal and Biodegradability Properties. J. Polym. Environ. 2010, 18, 422–429. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, S. Biodegradable Polymers/bamboo Fiber Biocomposite with Bio-based Coupling Agent. Compos. Part A 2006, 37, 80–89. [Google Scholar] [CrossRef]
- Mohanty, S.; Verma, S.K.; Nayak, S.K. Dynamic Mechanical and Thermal Properties of MAPE Treated Jute/HDPE Composites. Compos. Sci. Technol. 2005, 2, 538–547. [Google Scholar] [CrossRef]
- Phumnok, E.; Khongprom, P.; Ratanawilai, S. Preparation of Natural Rubber Composites with High Silica Contents Using a Wet Mixing Process. ACS Omega 2022, 7, 8364–8376. [Google Scholar] [CrossRef] [PubMed]
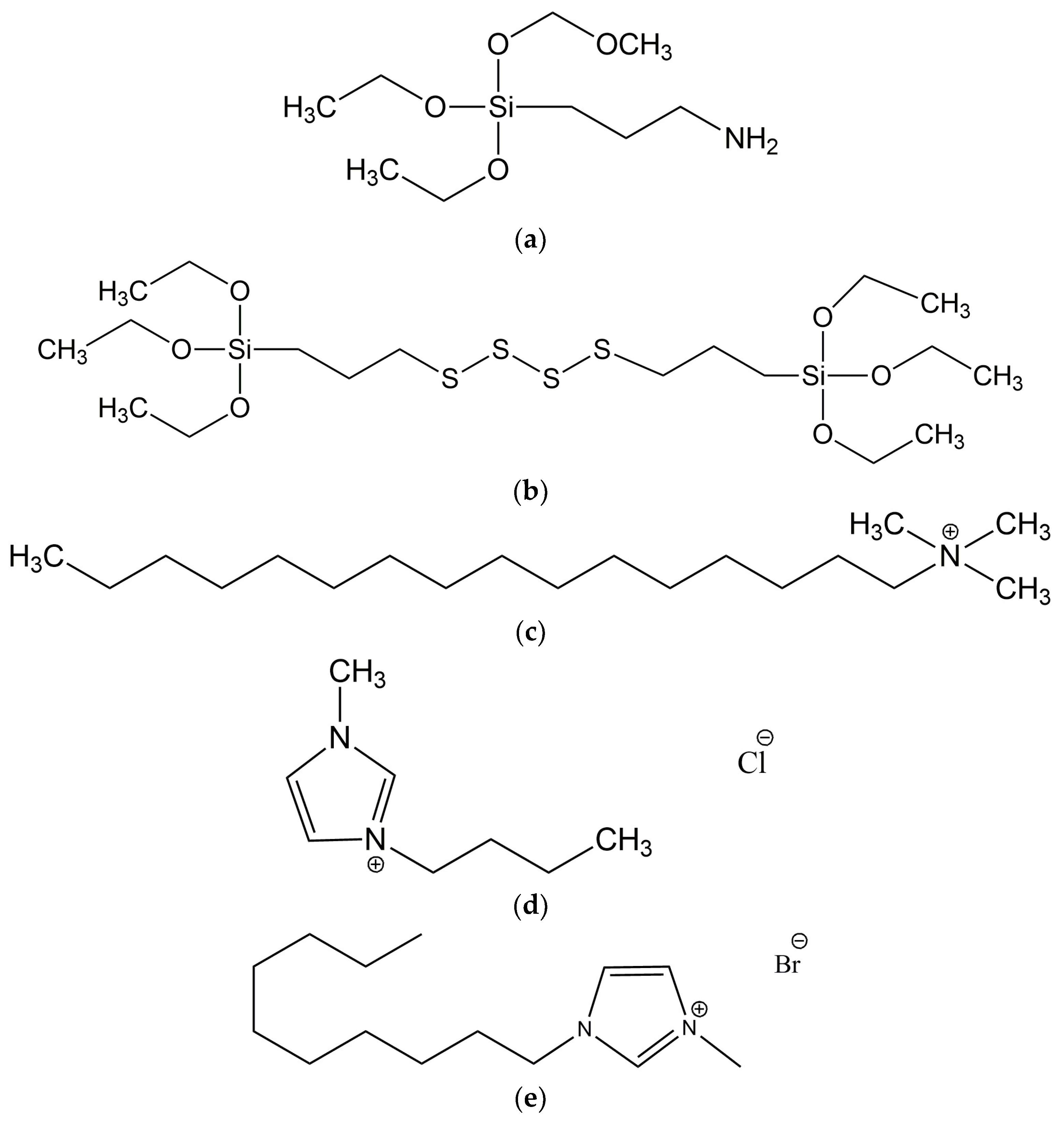



| Compound | Unfilled Sample | CTAB | BmiCl | DmiBr |
|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 |
| ZnO | 5 | 5 | 5 | 5 |
| St.A. | 1 | 1 | 1 | 1 |
| Sulfur | 2 | 2 | 2 | 2 |
| MBT | 2 | 2 | 2 | 2 |
| CTAB | - | 2 | - | - |
| BmiCl | - | - | 2 | - |
| DmiBr | - | - | - | 2 |
| Compound | 10ES | 20ES | 30ES | 40ES | 40ES /APTES | 40ES /TESPTS | 40ES/ CTAB | 40ES/ BmiCl | 40ES /DmiBr |
|---|---|---|---|---|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| ZnO | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| St.A. | 1 | 1 | 1 | 1 | - | - | - | - | - |
| Sulfur | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| MBT | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| ES | 10 | 20 | 30 | 40 | 40 | 40 | 40 | 40 | 40 |
| APTES | - | - | - | - | 2 | - | - | - | - |
| TESPTS | - | - | - | - | - | 2 | - | - | - |
| CTAB | - | - | - | - | - | - | 2 | - | - |
| BmiCl | - | - | - | - | - | - | - | 2 | - |
| DmiBr | - | - | - | - | - | - | - | - | 2 |
| Biofiller | T5% (°C) | TDTG (°C) | ∆m25–650 °C (%) | ∆m650–1100 °C (%) | Residue at 1100 °C (%) |
|---|---|---|---|---|---|
| Pure eggshells | 403 | 836 | 6.3 | 42.3 | 51.4 |
| CaCO3 | 667 | 829 | 3.9 | 38.9 | 57.2 |
| NR Composite | Smin (dNm) | ∆S (dNm) | t02 (min) | t90 (min) | νt × 10−5 (mol/cm3) |
|---|---|---|---|---|---|
| Unfilled sample | 0.4 | 5.1 | 1 | 2 | 0.9 |
| CTAB | 0.4 | 7.4 | 1 | 2 | 1.6 |
| BmiCl | 0.4 | 6.9 | 1 | 2 | 1.4 |
| DmiBr | 0.4 | 7.1 | 1 | 2 | 1.4 |
| 10ES | 0.4 | 5.9 | 1 | 2 | 1.0 |
| 20ES | 0.6 | 6.8 | 1 | 2 | 1.1 |
| 30ES | 0.6 | 7.2 | 1 | 2 | 1.2 |
| 40ES | 0.4 | 7.4 | 1 | 2 | 1.2 |
| 40ES/APTES | 0.6 | 7.5 | 1 | 2 | 1.2 |
| 40ES/TESPTS | 0.3 | 6.7 | 1 | 3 | 1.1 |
| 40ES/CTAB | 0.3 | 9.2 | 1 | 3 | 1.5 |
| 40ES/BmiCl | 0.3 | 8.6 | 1 | 2 | 1.3 |
| 4ES/DmiBr | 0.2 | 9.5 | 1 | 2 | 1.4 |
| NR Composite | Tvul (°C) | −∆Hvul (J/g) |
|---|---|---|
| Unfilled sample | 174–210 | 13.8 |
| CTAB | 145–218 | 10.9 |
| BmiCl | 138–218 | 10.1 |
| DmiBr | 138–220 | 9.9 |
| 10ES | 136–213 | 9.2 |
| 20ES | 137–229 | 11.6 |
| 30ES | 138–208 | 10.6 |
| 40ES | 138–208 | 7.6 |
| 40ES/APTES | 153–204 | 11.6 |
| 40ES/TESPTS | 158–216 | 7.6 |
| 40ES/CTAB | 140–214 | 7.0 |
| 40ES/BmiCl | 135–221 | 7.0 |
| 40ES/DmiBr | 140–222 | 7.3 |
| NR Vulcanizate | SE300 (MPa) | TS (MPa) | Eb (%) | H (Shore A) |
|---|---|---|---|---|
| Unfilled sample | 1.2 ± 0.1 | 9.6 ± 0.3 | 820 ± 20 | 31 ± 1 |
| 10ES | 1.4 ± 0.1 | 8.6 ± 0.8 | 669 ± 20 | 34 ± 1 |
| 20ES | 1.7 ± 0.1 | 10.4 ± 0.3 | 634 ± 25 | 37 ± 1 |
| 30ES | 1.7 ± 0.2 | 11.1 ± 0.8 | 613 ± 23 | 38 ± 1 |
| 40ES | 1.5 ± 0.1 | 9.3 ± 0.7 | 674 ± 21 | 38 ± 1 |
| 40ES/APTES | 1.8 ± 0.1 | 7.7 ± 0.6 | 602 ± 16 | 37 ± 1 |
| 40ES/TESPTS | 1.3 ± 0.1 | 8.0 ± 0.6 | 696 ± 16 | 37 ± 1 |
| 40ES/CTAB | 2.4 ± 0.1 | 11.9 ± 0.8 | 569 ± 26 | 44 ± 1 |
| 40ES/BmiCl | 2.0 ± 0.1 | 11.4 ± 0.7 | 627 ± 23 | 42 ± 1 |
| 40ES/DmiBr | 2.2 ± 0.1 | 12.0 ± 0.4 | 619 ± 17 | 43 ± 1 |
| NR Vulcanizate | Tg (°C) | tan δTg (−) | tan δ25 °C (−) | tan δ60 °C (−) |
|---|---|---|---|---|
| Unfilled sample | −68 ± 1 | 2.7± 0.1 | 0.06 ± 0.02 | 0.05 ± 0.01 |
| CTAB | −69 ± 1 | 2.3 ± 0.1 | 0.05 ± 0.02 | 0.04 ± 0.01 |
| BmiCl | −69 ± 1 | 2.6 ± 0.1 | 0.05 ± 0.02 | 0.04 ± 0.01 |
| DmiBr | −69 ± 1 | 2.6 ± 0.1 | 0.05 ± 0.02 | 0.04 ± 0.01 |
| 10ES | −69 ± 1 | 2.6 ± 0.1 | 0.05 ± 0.02 | 0.03 ± 0.01 |
| 20ES | −69 ± 1 | 2.6 ± 0.1 | 0.05 ± 0.02 | 0.03 ± 0.01 |
| 30ES | −67 ± 1 | 2.4 ± 0.1 | 0.05 ± 0.02 | 0.03 ± 0.01 |
| 40ES | −69 ± 1 | 2.4 ± 0.1 | 0.05 ± 0.02 | 0.04 ± 0.01 |
| 40ES/APTES | −69 ± 1 | 2.5 ± 0.1 | 0.06 ± 0.02 | 0.04 ± 0.01 |
| 40ES/TESPTS | −69 ± 1 | 2.6 ± 0.1 | 0.06 ± 0.02 | 0.03 ± 0.01 |
| 40ES/CTAB | −69 ± 1 | 2.3 ± 0.1 | 0.04 ± 0.02 | 0.03 ± 0.01 |
| 40ES/BmiCl | −70 ± 1 | 2.6 ± 0.1 | 0.03 ± 0.02 | 0.03 ± 0.01 |
| 40ES/DmiBr | −69 ± 1 | 2.6 ± 0.1 | 0.03 ± 0.02 | 0.03 ± 0.01 |
| NR Composite | Af (−) |
|---|---|
| Unfilled sample | 0.5 ± 0.1 |
| 10ES | 0.7 ± 0.1 |
| 20ES | 0.7 ± 0.1 |
| 30ES | 0.7 ± 0.1 |
| 40ES | 0.7 ± 0.1 |
| 40ES/APTES | 0.7 ± 0.1 |
| 40ES/TESPTS | 0.7 ± 0.1 |
| 40ES/CTAB | 0.9 ± 0.1 |
| 40ES/BmiCl | 0.8 ± 0.1 |
| 40ES/DmiBr | 0.8 ± 0.1 |
| NR Vulcanizate | T5% (°C) | TDTG (°C) | ∆m25–600 °C (%) | ∆m600–900 °C (%) | Residue at 900 °C %) |
|---|---|---|---|---|---|
| Unfilled sample | 322 | 398 | 97.1 | 0.9 | 1.9 |
| 10ES | 319 | 396 | 87.2 | 3.0 | 9.8 |
| 20ES | 320 | 395 | 81.2 | 4.6 | 14.2 |
| 30ES | 324 | 395 | 77.6 | 5.8 | 16.6 |
| 40ES | 328 | 396 | 71.3 | 9.7 | 19.0 |
| 40ES/APTES | 331 | 395 | 70.9 | 10.0 | 19.1 |
| 40ES/TESPTS | 322 | 395 | 72.3 | 10.6 | 17.1 |
| 40ES/CTAB | 325 | 395 | 71.1 | 10.3 | 18.6 |
| 40ES/BmiCl | 325 | 397 | 70.7 | 10.0 | 19.3 |
| 40ES/DmiBr | 324 | 396 | 69.7 | 10.9 | 19.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowińska-Baranowska, A.; Maciejewska, M. Potential Utilization of Ground Eggshells as a Biofiller for Natural Rubber Biocomposites. Materials 2023, 16, 2988. https://doi.org/10.3390/ma16082988
Sowińska-Baranowska A, Maciejewska M. Potential Utilization of Ground Eggshells as a Biofiller for Natural Rubber Biocomposites. Materials. 2023; 16(8):2988. https://doi.org/10.3390/ma16082988
Chicago/Turabian StyleSowińska-Baranowska, Anna, and Magdalena Maciejewska. 2023. "Potential Utilization of Ground Eggshells as a Biofiller for Natural Rubber Biocomposites" Materials 16, no. 8: 2988. https://doi.org/10.3390/ma16082988
APA StyleSowińska-Baranowska, A., & Maciejewska, M. (2023). Potential Utilization of Ground Eggshells as a Biofiller for Natural Rubber Biocomposites. Materials, 16(8), 2988. https://doi.org/10.3390/ma16082988








