Effect of Cr on Microstructure and Properties of WVTaTiCrx Refractory High-Entropy Alloy Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
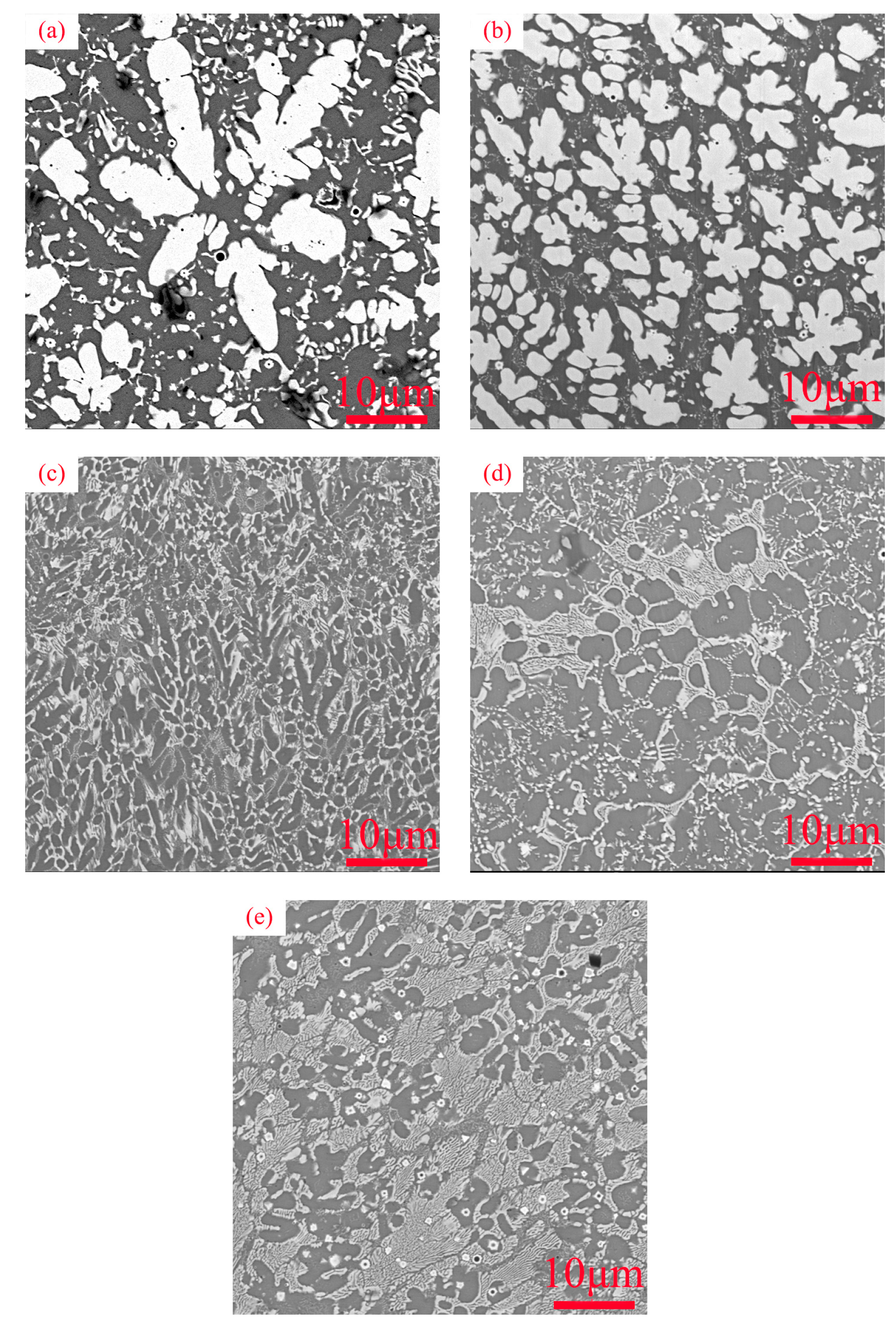
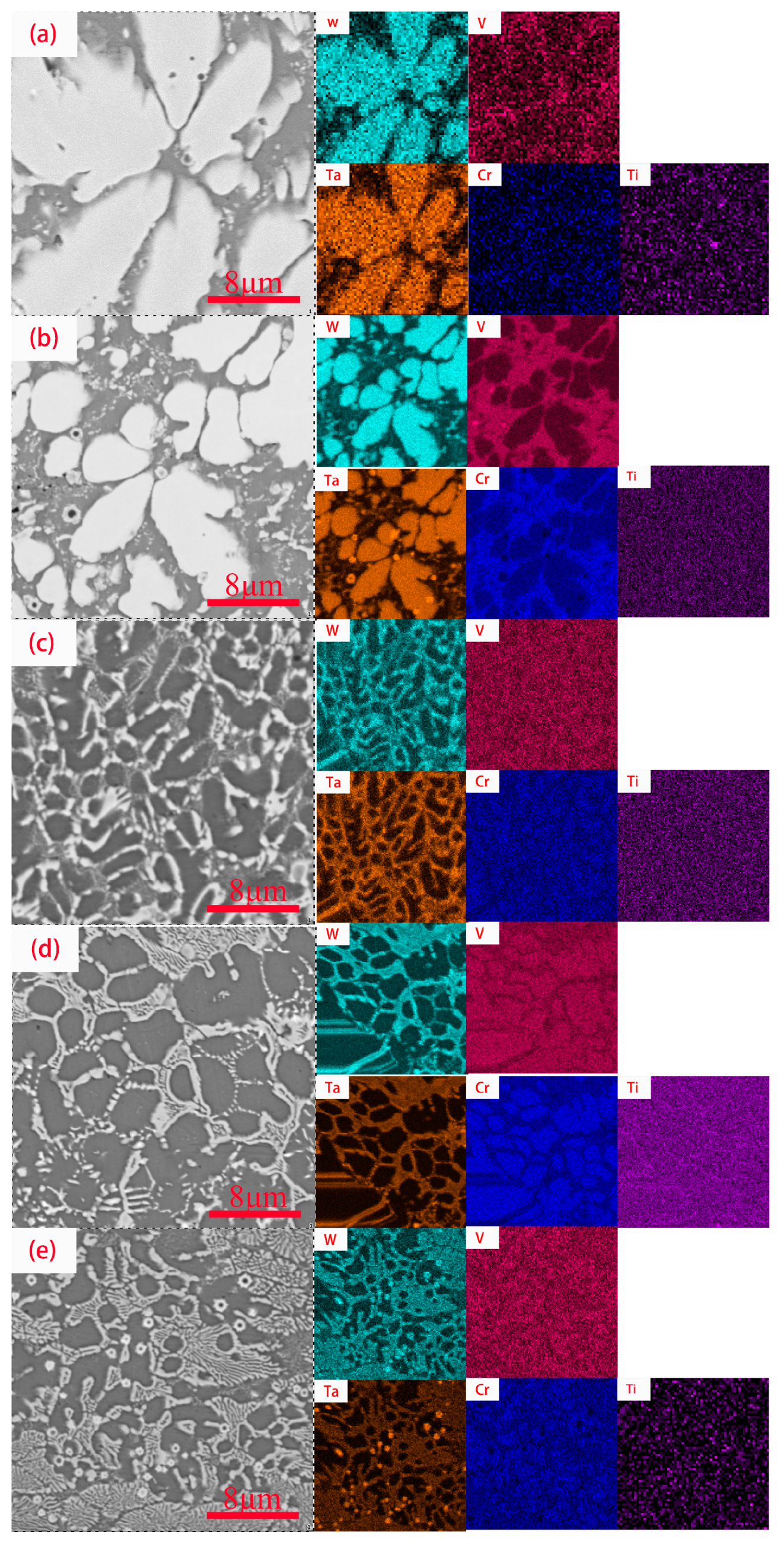
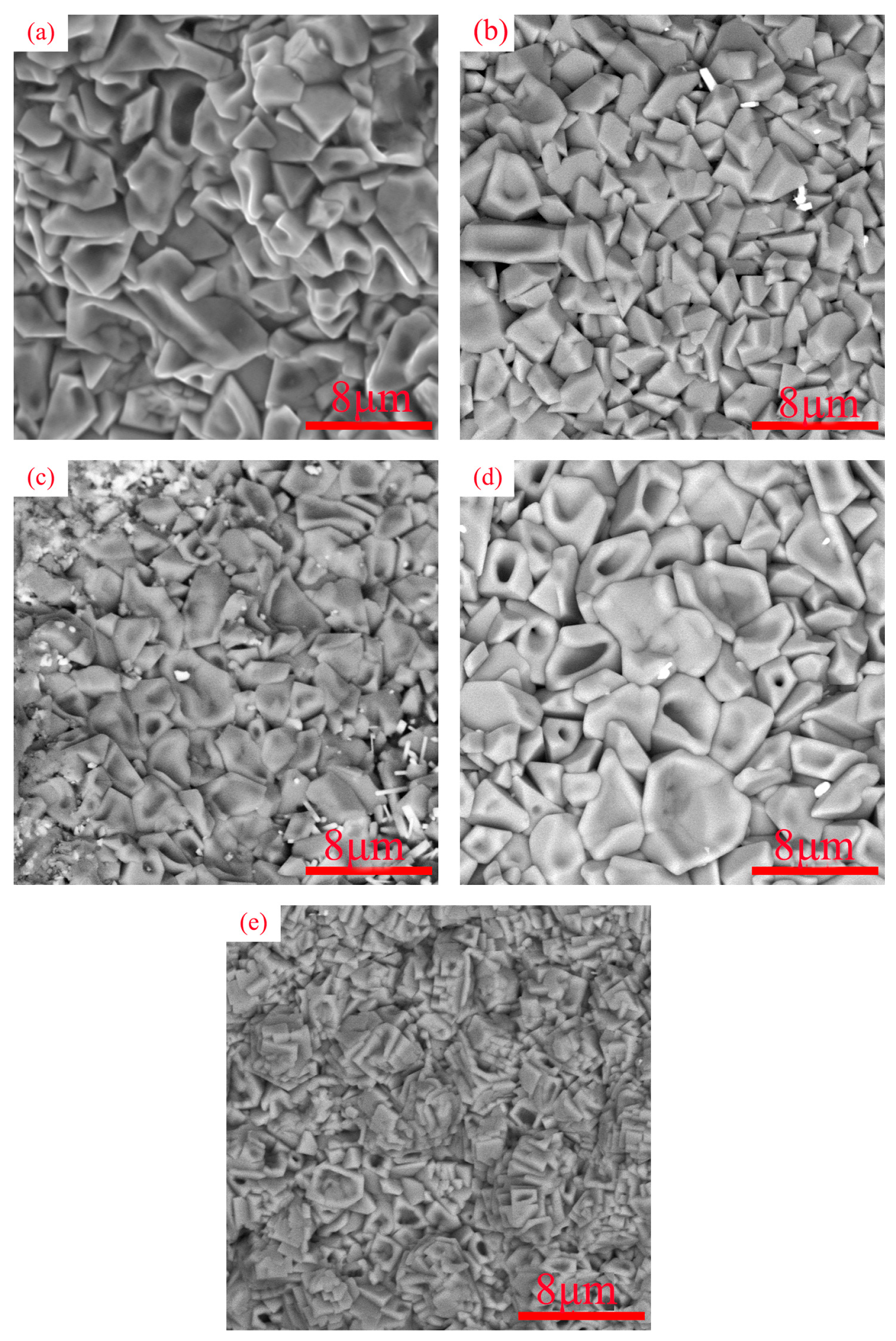
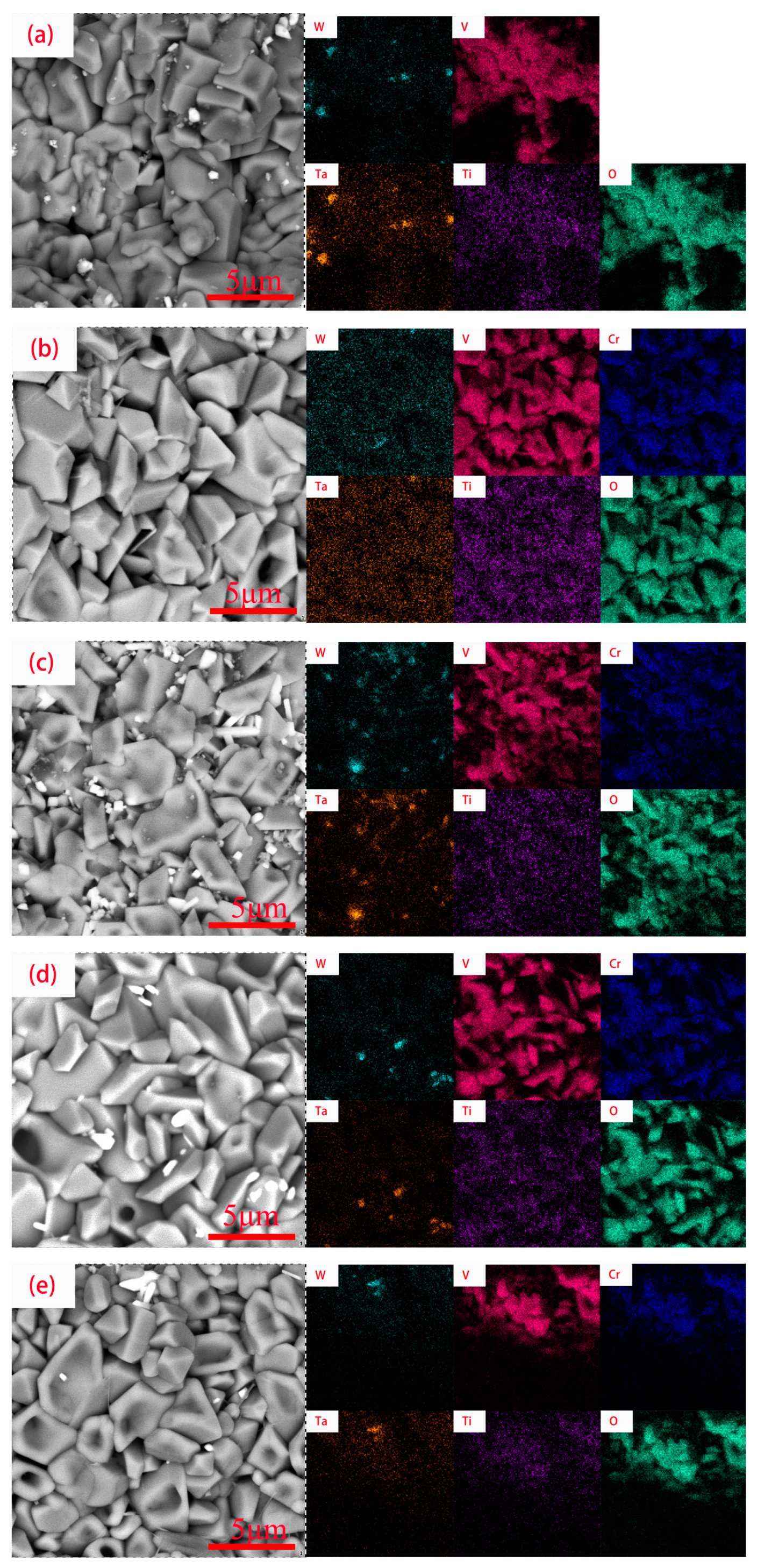
3.1. Microstructure Characterization
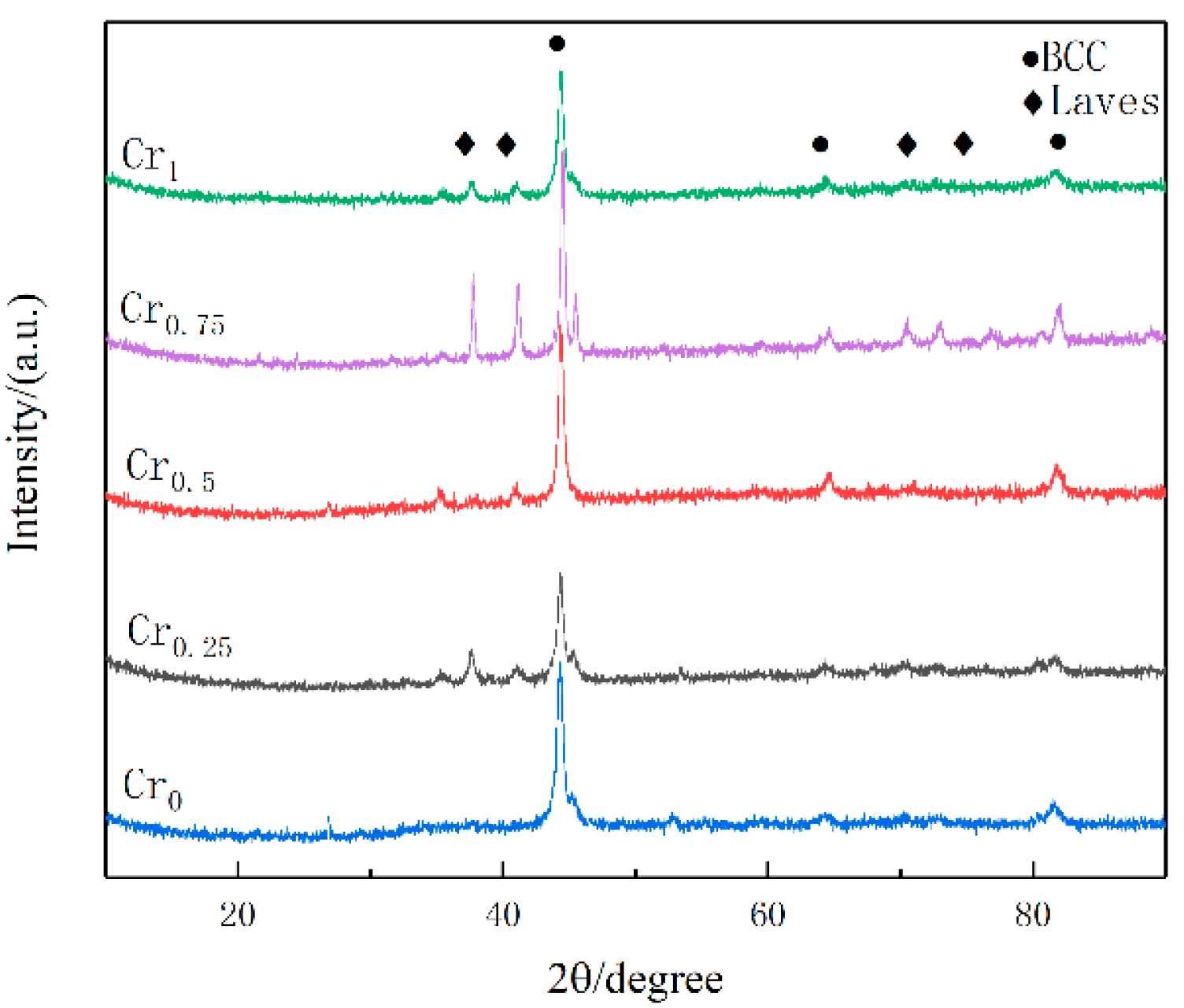
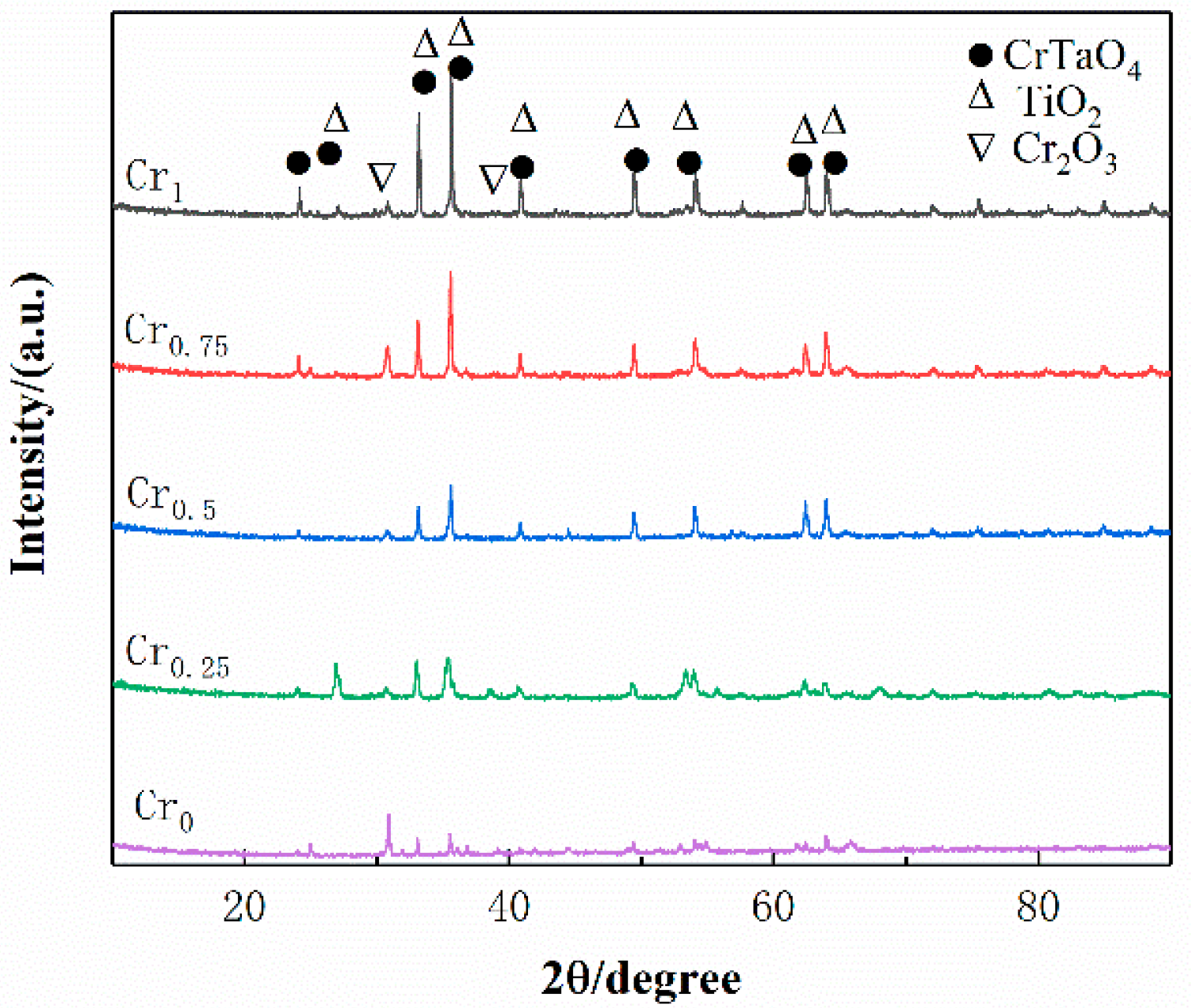
3.2. Phase Analysis
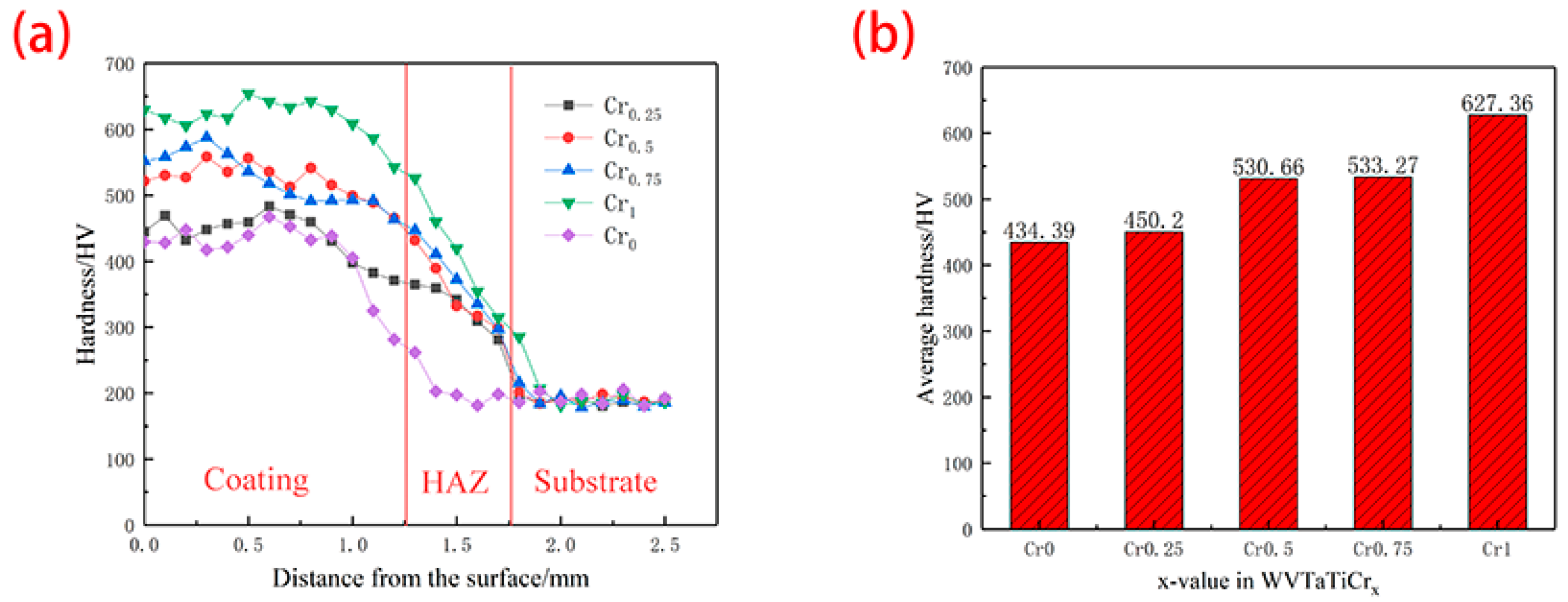
3.3. Hardness
3.4. Oxidation Resistance
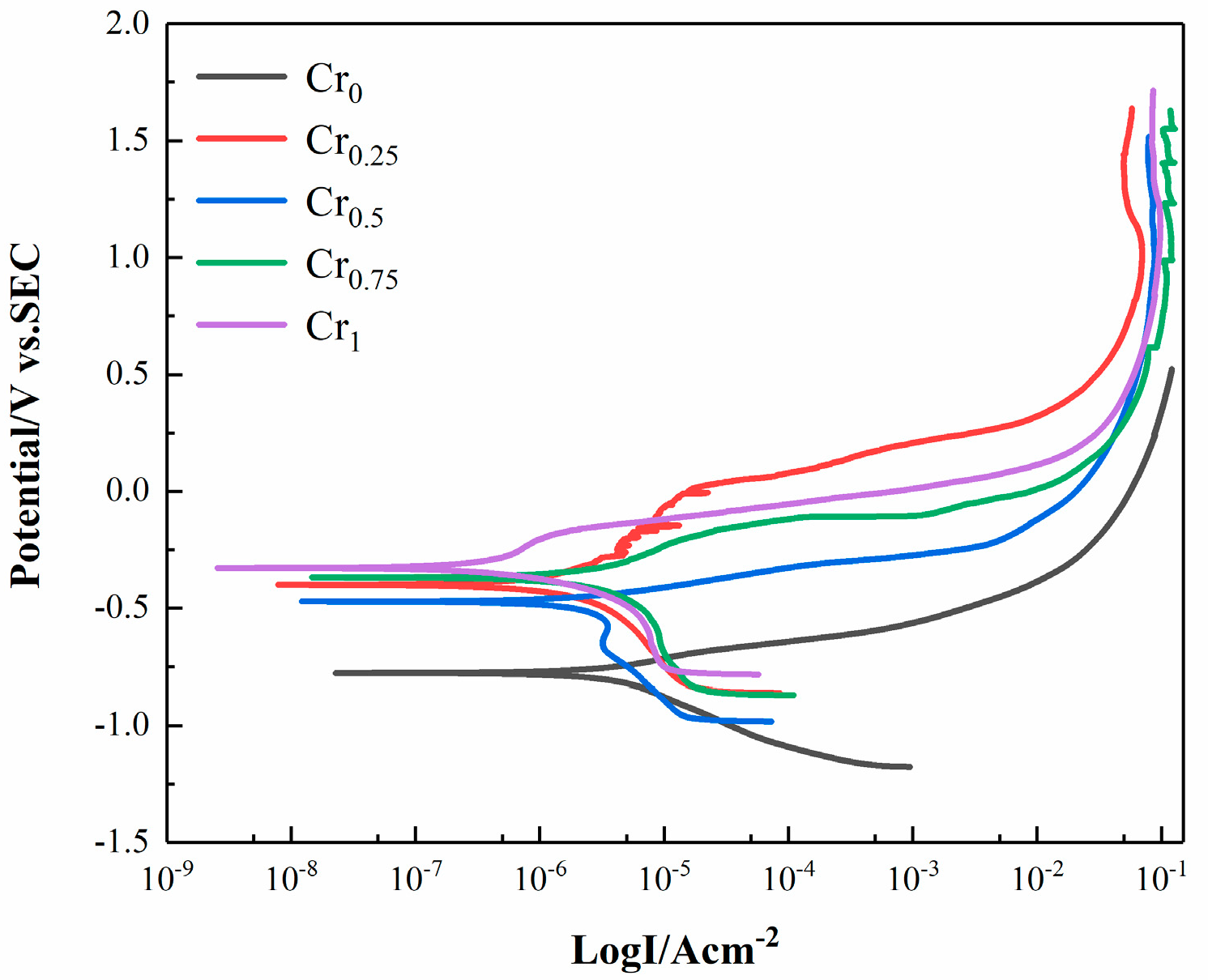
3.5. Corrosion Resistance
4. Conclusions
- (1)
- Laser-cladding-forming figures showed good metallurgical bonding between the coatings and the substrate, a uniform and dense coating structure, and a coating thickness of about 1 mm. The results indicate that laser cladding is feasible for the preparation of RHEA coatings.
- (2)
- The WVTaTi (Cr0) alloy coating consists of the BCC phase, and the addition of Cr promotes the precipitation of the Laves, which is uniformly distributed in the structure. The WVTaTiCr (Cr1) exhibits superior mechanical properties, particularly in terms of its exceptional hardness, high-temperature oxidation resistance, and outstanding corrosion resistance.
- (3)
- The successful preparation of WVTaTiCr alloy coating compensates for the performance shortcomings of some W-based binary alloys, providing a new idea for the development of heat insulation and radiation protection materials for nuclear reactors. The improved mechanical and oxidation properties of WVTaTiCr indicate the potential for future use in fusion applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Kumar, R.; Torres, H.; Aydinyan, S.; Antonov, M.; Varga, M.; Hussainova, I.; Rodriguez Ripoll, M. Tribological behavior of Ni-based self-lubricating claddings containing sulfide of nickel, copper, or bismuth at temperatures up to 600 °C. Surf. Coat. Technol. 2023, 456, 129270. [Google Scholar] [CrossRef]
- Hector, T.; Tugce, C.; Jens, H.; Janne, N.; Braham, P.; Manel, R.R. Tribological performance of iron- and nickel-base self-lubricating claddings containing metal sulfides at high temperature. Friction 2022, 10, 2069–2085. [Google Scholar] [CrossRef]
- Couzinié, J.; Dirras, G.; Perrière, L.; Chauveau, T.; Leroy, E.; Champion, Y.; Guillot, I. Microstructure of a near-equimolar refractory high-entropy alloy. Mater. Lett. 2014, 126, 285–287. [Google Scholar] [CrossRef]
- Juan, C.-C.; Tsai, M.-H.; Tsai, C.-W.; Lin, C.-M.; Wang, W.-R.; Yang, C.-C.; Chen, S.-K.; Lin, S.-J.; Yeh, J.-W. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Wu, Y.D.; Cai, Y.H.; Wang, T.; Si, J.J.; Zhu, J.; Wang, Y.D.; Hui, X.D. A Refractory Hf25Nb25Ti25Zr25 High-Entropy Alloy with Excellent Structural Stability and Tensile Properties. Mater. Lett. 2014, 130, 277–280. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Gao, M.C.; Hawk, J.A.; Ma, S.G.; Zhou, H.F.; Zhang, Y. NbTaV-(Ti, W) Refractory High-entropy Alloys: Experiments and Modeling. Mater. Sci. Eng. A 2016, 674, 203–211. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Cai, J.; Ji, S.; Geng, J.; Sun, X.; Li, D. High-strength NbMoTaX refractory high-entropy alloy with low stacking fault energy eutectic phase via laser additive manufacturing. Mater. Des. 2021, 201, 109462. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi-Wen, Z.; Feng-Ge, Z.; Tao, Y.; Chen, X.C. Effect of Powder Particle Size on Microstructure and Mechanical Property of Ni-Based P/M Superalloy Product. J. Iron Steel Res. 2003, 10, 71–74. [Google Scholar]
- Ding, X.; He, J.; Zhong, J.; Wang, X.; Li, Z.; Tian, J.; Dai, P. Effect of Al Addition on Microstructure and Properties of CoCrNi Medium-Entropy Alloy Prepared by Powder Metallurgy. Materials 2022, 15, 9090. [Google Scholar] [CrossRef]
- Thürlová, H.; Průša, F. Influence of the Al Content on the Properties of Mechanically Alloyed CoCrFeNiMnXAl20−X High-Entropy Alloys. Materials 2022, 15, 7899. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.S.; Sourav, A.; Murty, B.; Chelvane, A.; Thangaraju, S. Role of Al and Cr on cyclic oxidation behavior of AlCoCrFeNi2 high entropy alloy. J. Alloys Compd. 2022, 919. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Tan, Z.; He, D.; Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. High temperature oxidation behavior of Al0.6CrFeCoNi and Al0.6CrFeCoNiSi0.3 high entropy alloys. J. Alloys Compd. 2018, 764, 845–852. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, N.; Peng, L.L.; Zhao, L.N.; Liu, M. Microstructure and mechanical properties at elevated temperatures of a new Al-containing refractory high-entropy alloy Nb-Mo-Cr-Ti-Al. J. Alloys Compd. 2016, 195, 104001. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Liu, J.X.; Li, F.; Wei, X.; Wu, H.; Zhang, G.J. A high entropy silicide by reactive spark plasma sintering. J. Adv. Ceram. 2019, 8, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Yurchenko, N.; Stepanov, N.; Gridneva, A.; Mishunin, M.; Salishchev, G.; Zherebtsov, S. Effect of Cr and Zr on phase stability of refractory Al-Cr-Nb-Ti-V-Zr high-entropy alloys. J. Alloys Compd. 2018, 757, 403–414. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cropper, A.; Reid, W. Effects of grain size on the microstructure and texture of cold-rolled Ta-2.5W alloy. Int. J. Refract. Met. Hard Mater. 2016, 58, 25–136. [Google Scholar] [CrossRef]
- Arshad, K.; Guo, W.; Wang, J.; Zhao, M.-Y.; Yuan, Y.; Zhang, Y.; Wang, B.; Zhou, Z.-J.; Lu, G.-H. Influence of vanadium precursor powder size on microstructures and properties of W–V alloy. Int. J. Refract. Met. Hard Mater. 2015, 50, 59–64. [Google Scholar] [CrossRef]
- Dai, W.; Liang, S.; Luo, Y.; Yang, Q. Effect of W powders characteristics on the Ti-rich phase and properties of W–10 wt.% Ti alloy. Int. J. Refract. Met. Hard Mater. 2015, 50, 240–246. [Google Scholar] [CrossRef]
- Ma, Y.; Han, Q.-F.; Zhou, Z.-Y.; Liu, Y.-L. First-principles investigation on mechanical behaviors of W–Cr/Ti binary alloys. J. Nucl. Mater. 2016, 468, 105–112. [Google Scholar] [CrossRef]
- Rieth, M.; Dudarev, S.L.; De Vicente, S.G.; Aktaa, J.; Ahlgren, T.; Antusch, S.; Armstrong, D.E.J.; Balden, M.; Baluc, N.; Barthe, M.F.; et al. Recent progress in research on tungsten materials for nuclear fusion applications in Europe. J. Nucl. Mater. 2013, 432, 482–500. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Liu, G.; Hu, B.; Wang, J.; Ma, W. Microstructure Stability of V and Ta Microalloyed 12%Cr Reduced Activation Ferrite/Martensite Steel during Long-term Aging at 650 °C. J. Mater. Sci. Technol. 2015, 31, 311–319. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Jin, X.; Liang, Y.; Bi, J.; Li, B. Non-monotonic variation of structural and tensile properties with Cr content in AlCoCrxFeNi2 high entropy alloys. J. Alloys Compd. 2019, 798, 243–248. [Google Scholar] [CrossRef]
- Yan, X.; Guo, H.; Yang, W.; Pang, S.; Wang, Q.; Liu, Y.; Liaw, P.K.; Zhang, T. Al0.3CrxFeCoNi high-entropy alloys with high corrosion resistance and good mechanical properties—ScienceDirect. J. Alloys Compd. 2021, 860, 158436. [Google Scholar] [CrossRef]
- Ben, Q.; Zhang, Y.; Sun, L.; Wang, L.; Wang, Y.; Zhan, X. Wear and Corrosion Resistance of FeCoCrxNiAl High-Entropy Alloy Coatings Fabricated by Laser Cladding on Q345 Welded Joint. Metals 2022, 12, 1428. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.; Miracle, D.B. Low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system: Microstructure and phase analysis. Acta Mater. 2013, 61, 1545–1557. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Powder Metallurgy Processing of a WxTaTiVCr High-Entropy Alloy and Its Derivative Alloys for Fusion Material Applications. Sci. Rep. 2017, 7, 1926. [Google Scholar] [CrossRef] [Green Version]
- Moshtaghi, M.; Safyari, M.; Mori, G. Hydrogen absorption rate and hydrogen diffusion in a ferritic steel coated with a micro- or nanostructured ZnNi coating. Electrochem. Commun. 2022, 134, 107169. [Google Scholar] [CrossRef]
- Erario, M.d.l.Á.; Croce, E.; Moviglia Brandolino, M.T.; Moviglia, G.; Grangeat, A.M. A review on laser cladding of high-entropy alloys, their recent trends and potential applications. J. Manuf. Process. 2021, 68, 225–273. [Google Scholar]
- Liu, H.; Gao, Q.; Dai, J.; Chen, P.; Gao, W.; Hao, J.; Yang, H. Microstructure and high-temperature wear behavior of CoCrFeNiWx high-entropy alloy coatings fabricated by laser cladding. Tribol. Int. 2022, 172, 107574. [Google Scholar] [CrossRef]
- Zhou, J.; Kong, D. Friction–Wear performances and oxidation behaviors of Ti3AlC2 reinforced Co–based alloy coatings by laser cladding. Surf. Coat. Technol. 2021, 408, 126816. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Liu, C.-G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Cheng, Y.-H.; Chen, Y.-X.; Liang, X.-B. Composition design and preparation process of refractory high-entropy alloys: A review. Int. J. Refract. Met. Hard Mater. 2022, 105, 105836. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Miracle, D.B.; Woodward, C.F. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloys Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Gao, X.; Chen, R.; Liu, T.; Fang, H.; Wang, L.; Su, Y. High deformation ability induced by phase transformation through adjusting Cr content in Co-Fe-Ni-Cr high entropy alloys. J. Alloys Compd. 2022, 895, 162564. [Google Scholar] [CrossRef]
- Holcomb, G.R.; Tylczak, J.H.; Carney, C.S. Oxidation of CoCrFeMnNi High Entropy Alloys. Jom 2015, 67, 2326–2339. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Yu, X.; Tang, D. Role of Cr in the High-Temperature Oxidation Behavior of CrxMnFeNi High-Entropy Alloys at 800 °C in Air. Soc. Sci. Electron. Publ. 2022, 15, 110211. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Q.; Li, X.; Dai, P. The effect of N on the oxidation resistance of CoCrFeMnNi high entropy alloy. Met. Heat Treat. 2017, 42, 7. (In Chinese) [Google Scholar] [CrossRef]


| Element | W | V | Ta | Ti | Cr |
|---|---|---|---|---|---|
| Atomic radius (Å) | 1.37 | 1.32 | 1.43 | 1.46 | 1.25 |
| Melting point (°C) | 3407 | 1890 | 2996 | 1660 | 1857 |
| Alloys | Identification | W | V | Ta | Ti | Cr |
|---|---|---|---|---|---|---|
| WVTaTi | Cr0 | 39.65 | 10.99 | 39.03 | 10.33 | |
| WVTaTiCr0.25 | Cr0.25 | 38.57 | 10.69 | 37.97 | 10.12 | 2.74 |
| WVTaTiCr0.5 | Cr0.5 | 37.55 | 10.40 | 36.96 | 9.78 | 5.31 |
| WVTaTiCr0.75 | Cr0.75 | 36.58 | 10.14 | 36 | 9.52 | 7.76 |
| WVTaTiCr1 | Cr1 | 35.66 | 9.88 | 35.09 | 9.28 | 10.08 |
| Alloys | Cr0 | Cr0.25 | Cr0.5 | Cr0.75 | Cr1 |
|---|---|---|---|---|---|
| Δm/s | 10.41 | 8.56 | 7.98 | 6.86 | 5.12 |
| Δm/(s·t) | 0.21 | 0.17 | 0.16 | 0.14 | 0.10 |
| Oxide | Cr2O3 | TiO2 | CrTaO4 | Ta2O5 |
|---|---|---|---|---|
| ΔG (KJ/mol O2) | −538 | −713 | −568 | −598 |
| Alloys | Ecorr (V) | Icorr (uA/cm2) | V (mm/a) |
|---|---|---|---|
| Cr0 | −0.7783 | 6.466 × 10−6 | 2.959 |
| Cr0.25 | −0.4005 | 6.848 × 10−6 | 2.545 |
| Cr0.5 | −0.4663 | 1.059 × 10−6 | 0.394 |
| Cr0.75 | −0.3692 | 1.972 × 10−5 | 7.329 |
| Cr1 | −0.3198 | 4.337 × 10−7 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Sun, Z.; Li, C.; Wang, Z. Effect of Cr on Microstructure and Properties of WVTaTiCrx Refractory High-Entropy Alloy Laser Cladding. Materials 2023, 16, 3060. https://doi.org/10.3390/ma16083060
Xu Z, Sun Z, Li C, Wang Z. Effect of Cr on Microstructure and Properties of WVTaTiCrx Refractory High-Entropy Alloy Laser Cladding. Materials. 2023; 16(8):3060. https://doi.org/10.3390/ma16083060
Chicago/Turabian StyleXu, Zhaomin, Zhiping Sun, Cheng Li, and Zhiming Wang. 2023. "Effect of Cr on Microstructure and Properties of WVTaTiCrx Refractory High-Entropy Alloy Laser Cladding" Materials 16, no. 8: 3060. https://doi.org/10.3390/ma16083060
APA StyleXu, Z., Sun, Z., Li, C., & Wang, Z. (2023). Effect of Cr on Microstructure and Properties of WVTaTiCrx Refractory High-Entropy Alloy Laser Cladding. Materials, 16(8), 3060. https://doi.org/10.3390/ma16083060






