Molecular Understanding of the Interfacial Interaction and Corrosion Resistance between Epoxy Adhesive and Metallic Oxides on Galvanized Steel
Abstract
:1. Introduction
2. Experimental Procedures
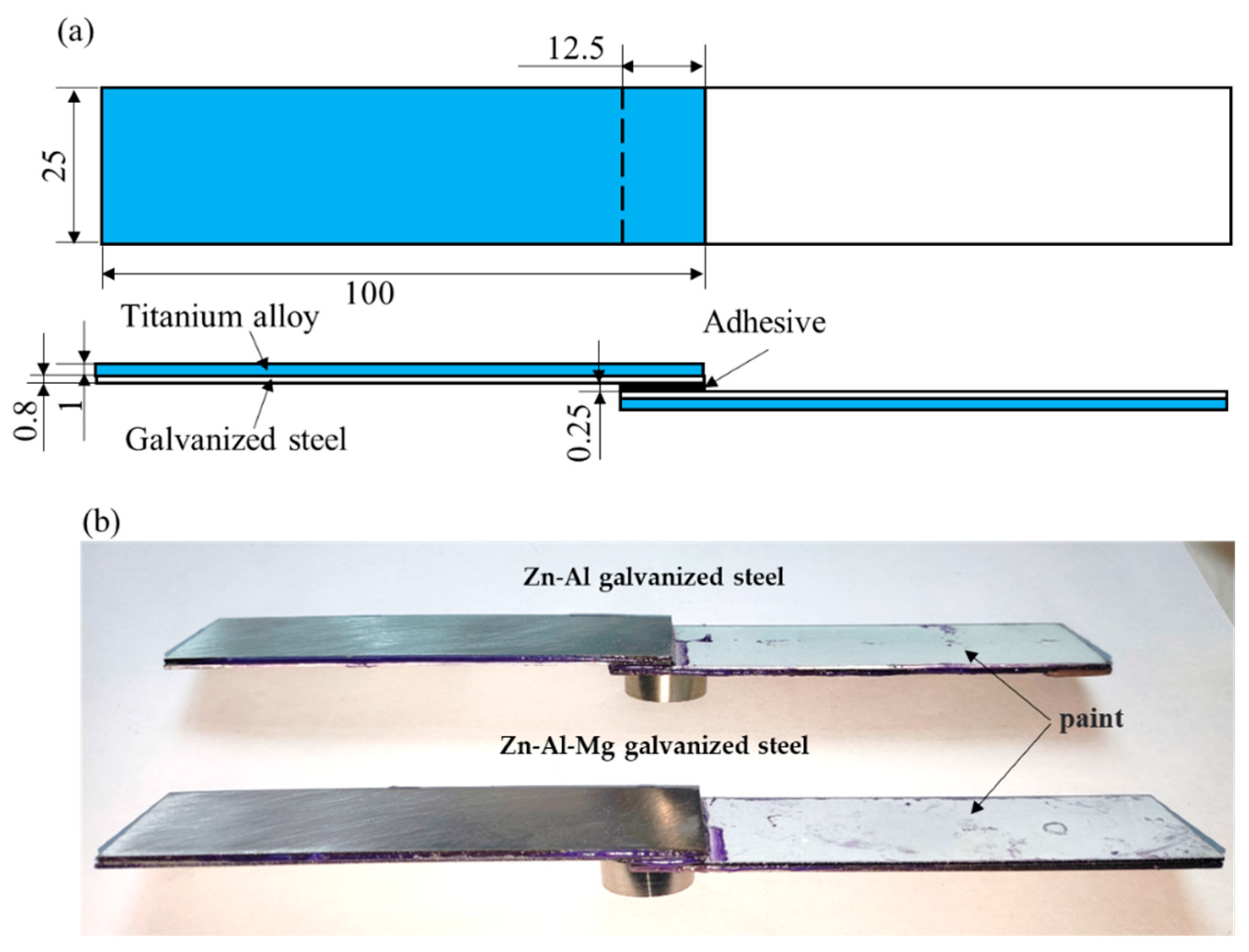
2.1. Materials
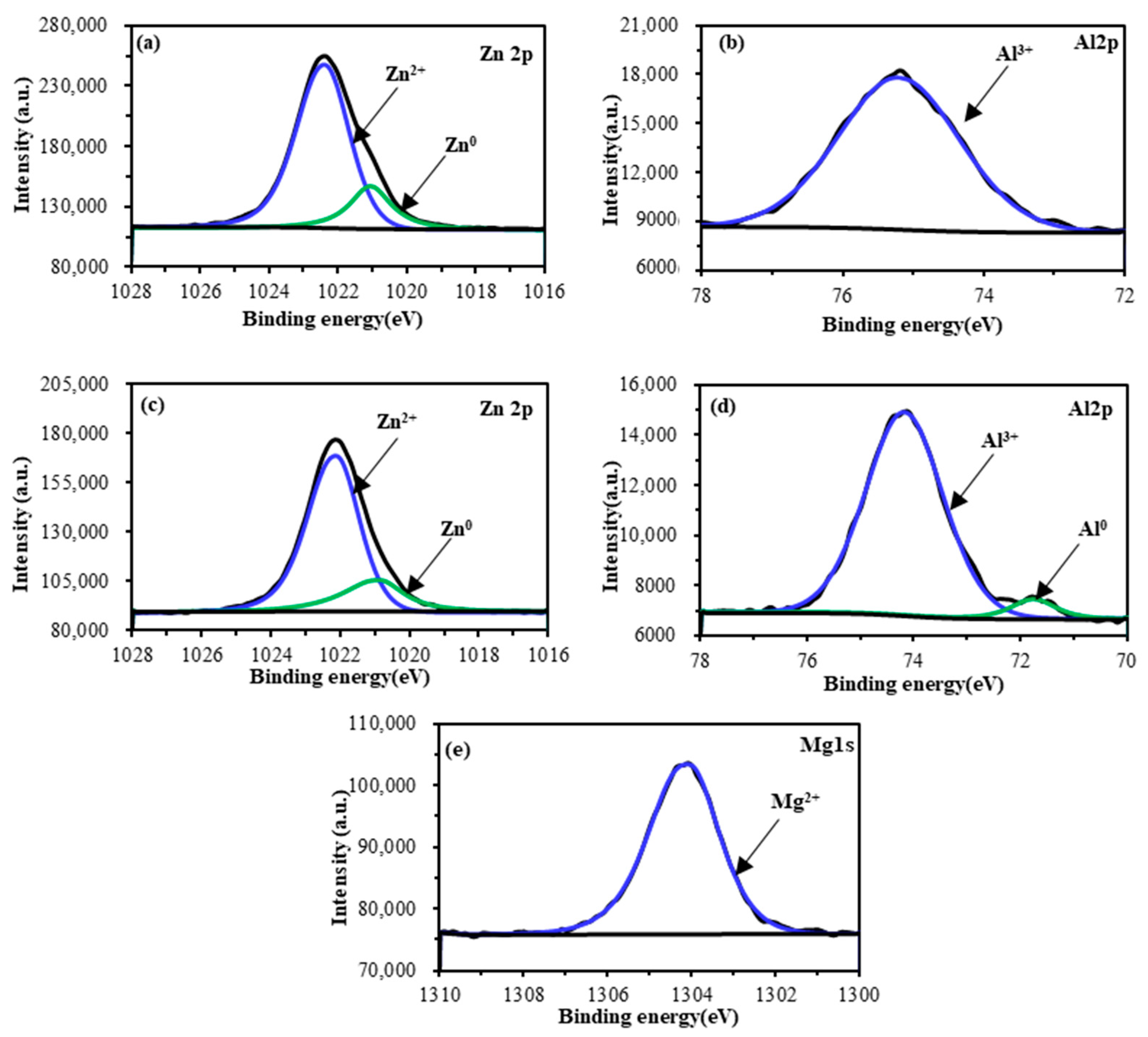
2.2. Characterization
2.3. Water Soak
2.4. Lap-Shear Strength Testing
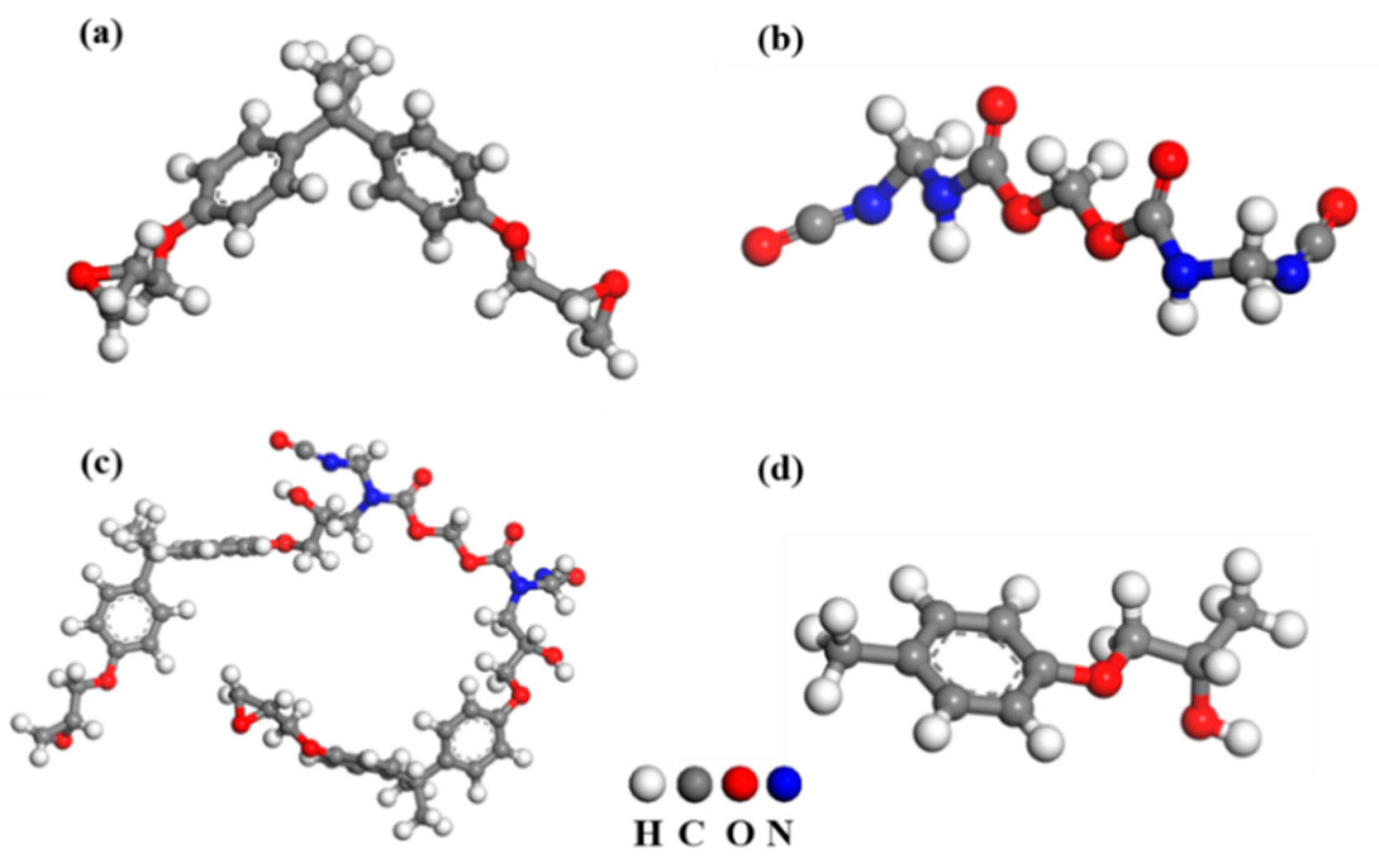
3. Modeling Details
4. Results and Discussion
4.1. Physical and Chemical Properties of Galvanized Steel Surface
4.2. Interfacial Bonding Performance between Epoxy Adhesive and Galvanized Steel
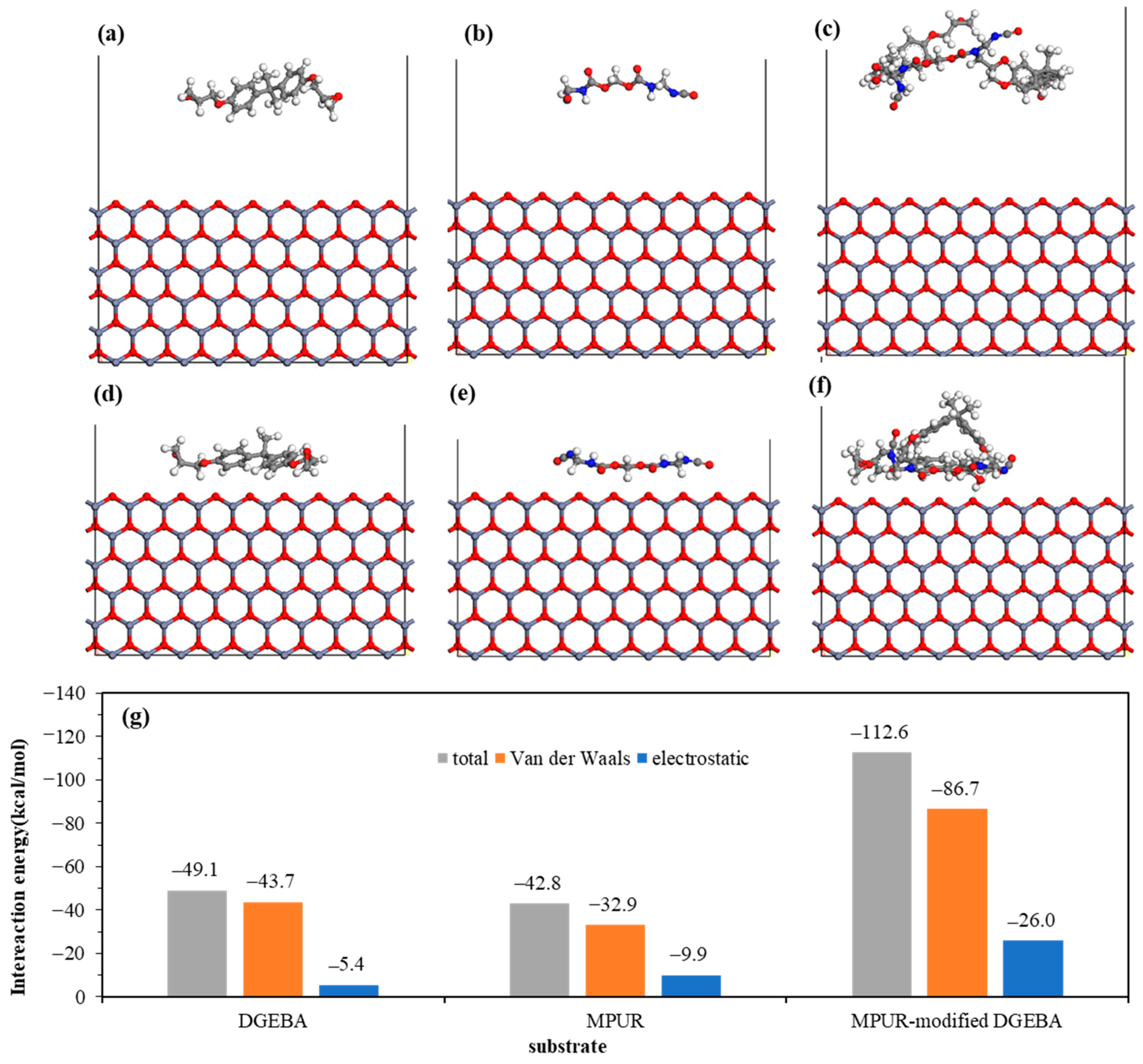
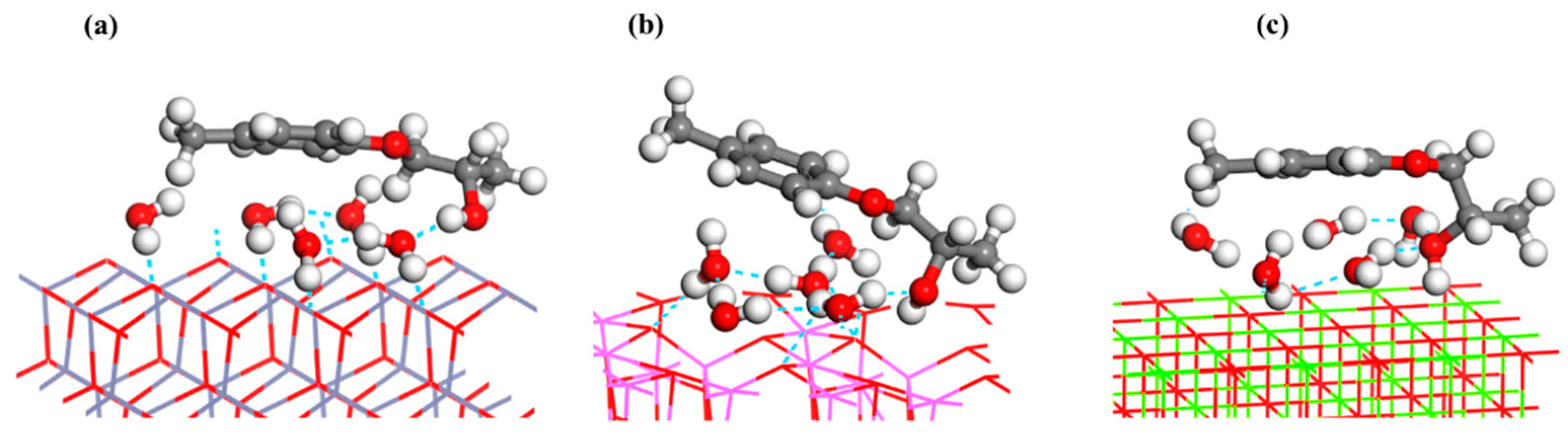
4.3. Molecular Behavior and Adhesion Force at Epoxy Adhesive/Galvanizing Coating Interface
4.4. Corrosion Resistance Mechanism at Epoxy Adhesive/Galvanizing Coating Interface
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yasakau, K.A.; Giner, I.; Vree, C.; Ozcan, O.; Grothe, R.; Oliveira, A.; Grundmeier, G.; Ferreira, M.G.S.; Zheludkevich, M.L. Influence of stripping and cooling atmospheres on surface properties and corrosion of zinc galvanizing coatings. Appl. Surf. Sci. 2016, 389, 144–156. [Google Scholar] [CrossRef]
- Xie, Y.; Du, A.; Zhao, X.; Ma, R.; Fan, Y.; Cao, X. Effect of Mg on Fe–Al interface structure of hot–dip galvanized Zn–Al–Mg alloy coatings. Surf. Coat. Technol. 2018, 337, 313–320. [Google Scholar] [CrossRef]
- Jiang, L.; Volovitch, P.; Wolpers, M.; Ogle, K. Activation and inhibition of Zn–Al and Zn–Al–Mg coatings on steel by nitrate in phosphoric acid solution. Corros. Sci. 2012, 60, 256–264. [Google Scholar] [CrossRef]
- Wan, H.; Min, J.; Zhang, J.; Lin, J.; Sun, C. Effect of adherend deflection on lap-shear tensile strength of laser-treated adhesive-bonded joints. Int. J. Adhes. Adhes. 2020, 97, 102481. [Google Scholar] [CrossRef]
- Wan, H.; Min, J.; Lin, J.; Carlson, B.E.; Maddela, S.; Sun, C. Effect of laser spot overlap ratio on surface characteristics and adhesive bonding strength of an Al alloy processed by nanosecond pulsed laser. J. Manuf. Process. 2021, 62, 555–565. [Google Scholar] [CrossRef]
- Min, J.; Wan, H.; Carlson, B.E.; Lin, J.; Sun, C. Application of laser ablation in adhesive bonding of metallic materials: A review. Opt. Laser Technol. 2020, 128, 106188. [Google Scholar] [CrossRef]
- Ni, J.; Min, J.; Wan, H.; Lin, J.; Wang, S.; Wan, Q. Effect of adhesive type on mechanical properties of galvanized steel/SMC adhesive-bonded joints. Int. J. Adhes. Adhes. 2020, 97, 102482. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Ghaffari, M.; Saeb, M.R.; Ramezanzadeh, B.; De Proft, F.; Terryn, H. A close-up of the effect of iron oxide type on the interfacial interaction between epoxy and carbon steel: Combined molecular dynamics simulations and quantum mechanics. J. Phys. Chem. C 2016, 120, 11014–11026. [Google Scholar] [CrossRef]
- Krüger, T.; Amkreutz, M.; Schiffels, P.; Schneider, B.; Hennemann, O.-D.; Frauenheim, T. Theoretical Study of the Interaction between Selected Adhesives and Oxide Surfaces. J. Phys. Chem. B 2005, 109, 5060–5066. [Google Scholar] [CrossRef]
- Anand, K.; Duguet, T.; Esvan, J.; Lacaze-Dufaure, C. Chemical Interactions at the Al/Poly-Epoxy Interface Rationalized by DFT Calculations and a Comparative XPS Analysis. ACS Appl. Mater. Interfaces 2020, 12, 57649–57665. [Google Scholar] [CrossRef]
- Semoto, T.; Tsuji, Y.; Tanaka, H.; Yoshizawa, K. Role of Edge Oxygen Atoms on the Adhesive Interaction between Carbon Fiber and Epoxy Resin. J. Phys. Chem. C 2013, 117, 24830–24835. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Ramezanzadeh, B. A detailed molecular dynamics simulation and experimental investigation on the interfacial bonding mechanism of an epoxy adhesive on carbon steel sheets decorated with a novel cerium–lanthanum nanofilm. ACS Appl. Mater. Interfaces 2017, 9, 17536–17551. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, F.; Li, H.; Xin, Y.; Yi, Z. Study on the interfacial interactions and adhesion behaviors of various polymer-metal interfaces in nano molding. Polym. Eng. Sci. 2021, 61, 95–106. [Google Scholar] [CrossRef]
- Li, H.; Cai, Z.; Zhou, F.; Liu, D. MD simulation analysis of the anchoring behavior of injection-molded nanopits on polyphenylene sulfide/Cu interface. Compos. Interfaces 2022, 29, 431–446. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.G.; Choe, Y.; Lee, S.G. Mechanism of adhesion of the diglycidyl ether of bisphenol A (DGEBA) to the Fe(100) surface. Compos. Sci. Technol. 2016, 126, 9–16. [Google Scholar] [CrossRef]
- Knaup, J.M.; Köhler, C.; Frauenheim, T.; Blumenau, A.T.; Amkreutz, M.; Schiffels, P.; Schneider, B.; Hennemann, O.-D. Computational Studies on Polymer Adhesion at the Surface of γ-Al2O3. I. The Adsorption of Adhesive Component Molecules from the Gas Phase. J. Phys. Chem. B 2006, 110, 20460–20468. [Google Scholar] [CrossRef]
- Semoto, T.; Tsuji, Y.; Yoshizawa, K. Molecular understanding of the adhesive force between a metal oxide surface and an epoxy resin. J. Phys. Chem. C 2011, 115, 11701–11708. [Google Scholar] [CrossRef]
- Tsurumi, N.; Tsuji, Y.; Masago, N.; Yoshizawa, K. Elucidation of Adhesive Interaction between the Epoxy Molding Compound and Cu Lead Frames. ACS Omega 2021, 6, 34173–34184. [Google Scholar] [CrossRef]
- Duchoslav, J.; Arndt, M.; Steinberger, R.; Keppert, T.; Luckeneder, G.; Stellnberger, K.H.; Hagler, J.; Riener, C.K.; Angeli, G.; Stifter, D. Nanoscopic view on the initial stages of corrosion of hot dip galvanized Zn–Mg–Al coatings. Corros. Sci. 2014, 83, 327–334. [Google Scholar] [CrossRef]
- Zheng, R.; Lin, J.-p.; Wang, P.-C.; Wu, Y.-R. Effect of hot-humid exposure on static strength of adhesive-bonded aluminum alloys. Def. Technol. 2015, 11, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Lin, J.; Wang, P.-C.; Zheng, R.; Wu, Q. Effect of long-term neutral salt spray exposure on durability of adhesive-bonded Zr–Ti coated aluminum joint. Int. J. Adhes. Adhes. 2016, 64, 97–108. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, J.; Wang, P.-C.; Zheng, R. Effect of Thermal Exposure on Static and Fatigue Characteristics of Adhesive-bonded Aluminum Alloys. J. Adhesion 2016, 92, 722–738. [Google Scholar] [CrossRef]
- Wang, S.; Min, J.; Lin, J.; Wu, Y. Effect of neutral salt spray (NSS) exposure on the lap-shear strength of adhesive-bonded 5052 aluminum alloy (AA5052) joints. J. Adhes. Sci. Technol. 2019, 33, 549–560. [Google Scholar] [CrossRef]
- Semoto, T.; Tsuji, Y.; Yoshizawa, K. Molecular Understanding of the Adhesive Force between a Metal Oxide Surface and an Epoxy Resin: Effects of Surface Water. Bull. Chem. Soc. Jpn. 2012, 85, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, K.; Murata, H.; Tanaka, H. Density-Functional Tight-Binding Study on the Effects of Interfacial Water in the Adhesion Force between Epoxy Resin and Alumina Surface. Langmuir 2018, 34, 14428–14438. [Google Scholar] [CrossRef]
- Ogata, S.; Takahashi, Y. Moisture-Induced Reduction of Adhesion Strength between Surface Oxidized Al and Epoxy Resin: Dynamics Simulation with Electronic Structure Calculation. J. Phys. Chem. C 2016, 120, 13630–13637. [Google Scholar] [CrossRef]
- Ogata, S.; Uranagase, M. Unveiling the Chemical Reactions Involved in Moisture-Induced Weakening of Adhesion between Aluminum and Epoxy Resin. J. Phys. Chem. C 2018, 122, 17748–17755. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Xu, W. Atomistic molecular modelling of crosslinked epoxy resin. Polymer 2006, 47, 6004–6009. [Google Scholar] [CrossRef]
- Yao, L. Multi-scale studies of glass transition and uniaxial tensile properties of a commercially available epoxy adhesive using experimental measurements and molecular dynamics simulation. Chin. J. Polym. Sci. 2015, 33, 465–474. [Google Scholar] [CrossRef]
- Amirkhani, F.; Harami, H.R.; Asghari, M. CO2/CH4 mixed gas separation using poly(ether-b-amide)-ZnO nanocomposite membranes: Experimental and molecular dynamics study. Polym. Test. 2020, 86, 106464. [Google Scholar] [CrossRef]
- Liu, W.; Tkatchenko, A.; Scheffler, M. Modeling Adsorption and Reactions of Organic Molecules at Metal Surfaces. Acc. Chem. Res. 2014, 47, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Duchoslav, J.; Steinberger, R.; Arndt, M.; Keppert, T.; Luckeneder, G.; Stellnberger, K.H.; Hagler, J.; Angeli, G.; Riener, C.K.; Stifter, D. Evolution of the surface chemistry of hot dip galvanized Zn–Mg–Al and Zn coatings on steel during short term exposure to sodium chloride containing environments. Corros. Sci. 2015, 91, 311–320. [Google Scholar] [CrossRef]
- Saarimaa, V.; Markkula, A.; Juhanoja, J.; Skrifvars, B.-J. Novel insight to aluminum compounds in the outermost layers of hot dip galvanized steel and how they affect the reactivity of the zinc surface. Surf. Coat. Technol. 2016, 306, 506–511. [Google Scholar] [CrossRef]
- Foster, R.T.; Schroeder, K.J. Adhesive Bonding to Galvanized Steel: I. Lap Shear Strengths and Environmental Durability. J. Adhes. 1987, 24, 259–278. [Google Scholar] [CrossRef]
- Korta, J.; Mlyniec, A.; Uhl, T. Experimental and numerical study on the effect of humidity-temperature cycling on structural multi-material adhesive joints. Compos. Pt. B-Eng. 2015, 79, 621–630. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kallip, S.; Lisenkov, A.; Ferreira, M.G.S.; Zheludkevich, M.L. Initial stages of localized corrosion at cut-edges of adhesively bonded Zn and Zn-Al-Mg galvanized steel. Electrochim. Acta 2016, 211, 126–141. [Google Scholar] [CrossRef]
- Legghe, E.; Aragon, E.; Bélec, L.; Margaillan, A.; Melot, D. Correlation between water diffusion and adhesion loss: Study of an epoxy primer on steel. Prog. Org. Coat. 2009, 66, 276–280. [Google Scholar] [CrossRef]
- Cavodeau, F.; Brogly, M.; Bistac, S.; Devanne, T.; Pedrollo, T.; Glasser, F. Hygrothermal aging of an epoxy/dicyandiamide structural adhesive—Influence of water diffusion on the durability of the adhesive/galvanized steel interface. J. Adhes. 2020, 96, 1027–1051. [Google Scholar] [CrossRef]
- Baldan, A. Adhesion phenomena in bonded joints. Int. J. Adhes. Adhes. 2012, 38, 95–116. [Google Scholar] [CrossRef]
- de la Roza, A.O.; DiLabio, G.A. Non-Covalent Interactions in Quantum Chemistry and Physics: Theory and Applications; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tardio, S.; Abel, M.-L.; Carr, R.H.; Watts, J.F. The interfacial interaction between isocyanate and stainless steel. Int. J. Adhes. Adhes. 2019, 88, 1–10. [Google Scholar] [CrossRef]
- Doonan, C.; Riccò, R.; Liang, K.; Bradshaw, D.; Falcaro, P. Metal–Organic Frameworks at the Biointerface: Synthetic Strategies and Applications. Acc. Chem. Res. 2017, 50, 1423–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, P.R.; Roy, S. Is it Possible to Change Wettability of Hydrophilic Surface by Changing Its Roughness? J. Phys. Chem. Lett. 2013, 4, 3692–3697. [Google Scholar] [CrossRef]
- Ashraf, A.; Wu, Y.; Wang, M.C.; Yong, K.; Sun, T.; Jing, Y.; Haasch, R.T.; Aluru, N.R.; Nam, S. Doping-Induced Tunable Wettability and Adhesion of Graphene. Nano Lett. 2016, 16, 4708–4712. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Jo, W.H. Infiltration of Water Molecules into the Oseltamivir-Binding Site of H274Y Neuraminidase Mutant Causes Resistance to Oseltamivir. J. Chem. Inf. Model. 2009, 49, 2735–2741. [Google Scholar] [CrossRef]
- Higuchi, C.; Tanaka, H.; Yoshizawa, K. Molecular understanding of the adhesive interactions between silica surface and epoxy resin: Effects of interfacial water. J. Comput. Chem. 2019, 40, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajat, J.B.; Mišković-Stanković, V.B.; Popić, J.P.; Dražić, D.M. Adhesion characteristics and corrosion stability of epoxy coatings electrodeposited on phosphated hot-dip galvanized steel. Prog. Org. Coat. 2008, 63, 201–208. [Google Scholar] [CrossRef]
- Bajat, J.B.; Mišković-Stanković, V.B.; Bibić, N.; Dražić, D.M. The influence of zinc surface pretreatment on the adhesion of epoxy coating electrodeposited on hot-dip galvanized steel. Prog. Org. Coat. 2007, 58, 323–330. [Google Scholar] [CrossRef]
- Koyanagi, J.; Itano, N.; Yamamoto, M.; Mori, K.; Ishida, Y.; Bazhirov, T. Evaluation of the mechanical properties of carbon fiber/polymer resin interfaces by molecular simulation. Adv. Compos. Mater 2019, 28, 639–652. [Google Scholar] [CrossRef]
- Calvez, P.; Bistac, S.; Brogly, M.; Richard, J.; Verchère, D. Mechanisms of Interfacial Degradation of Epoxy Adhesive/Galvanized Steel Assemblies: Relevance to Durability. J. Adhes. 2012, 88, 145–170. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Al Sagheer, F.; Bumajdad, A. Aramid-Zirconia Nanocomposite Coating With Excellent Corrosion Protection of Stainless Steel in Saline Media. Front. Chem. 2020, 8, 391. [Google Scholar] [CrossRef]
- Olarte, J.; Martinez de Ilarduya, J.; Zulueta, E.; Ferret, R.; Garcia-Ortega, J.; Lopez-Guede, J.M. Online Identification of VLRA Battery Model Parameters Using Electrochemical Impedance Spectroscopy. Batteries 2022, 8, 238. [Google Scholar] [CrossRef]


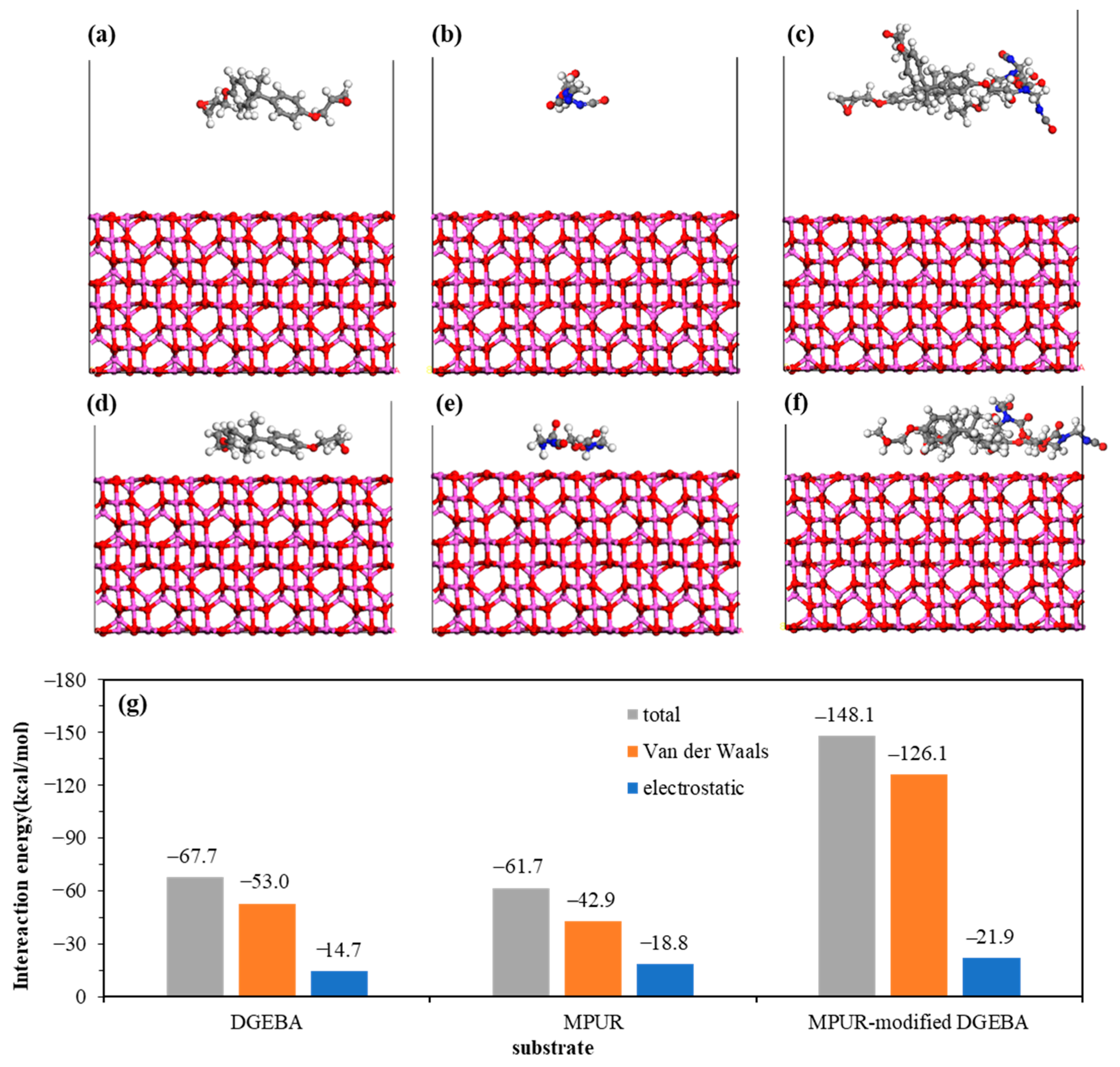
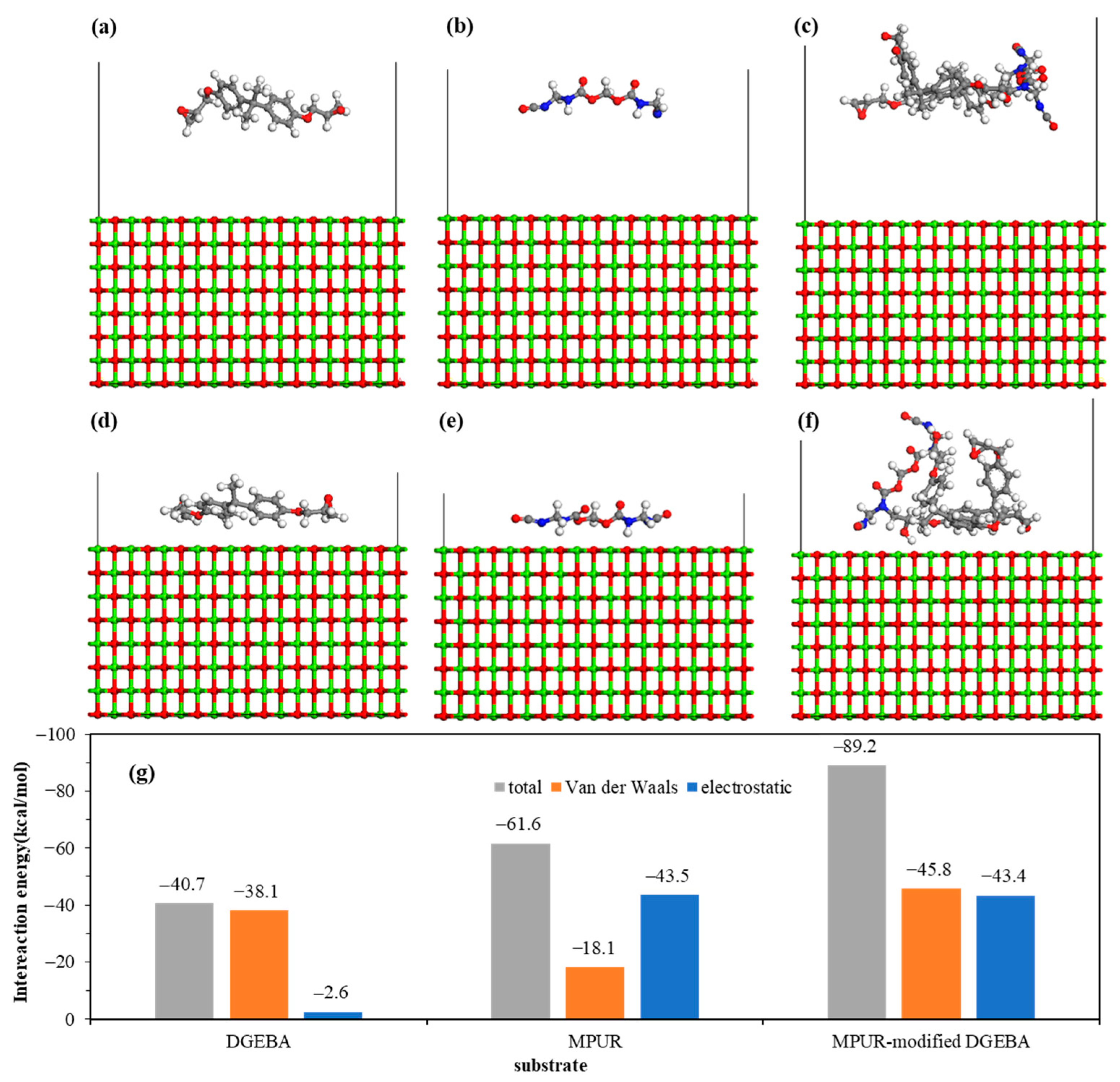
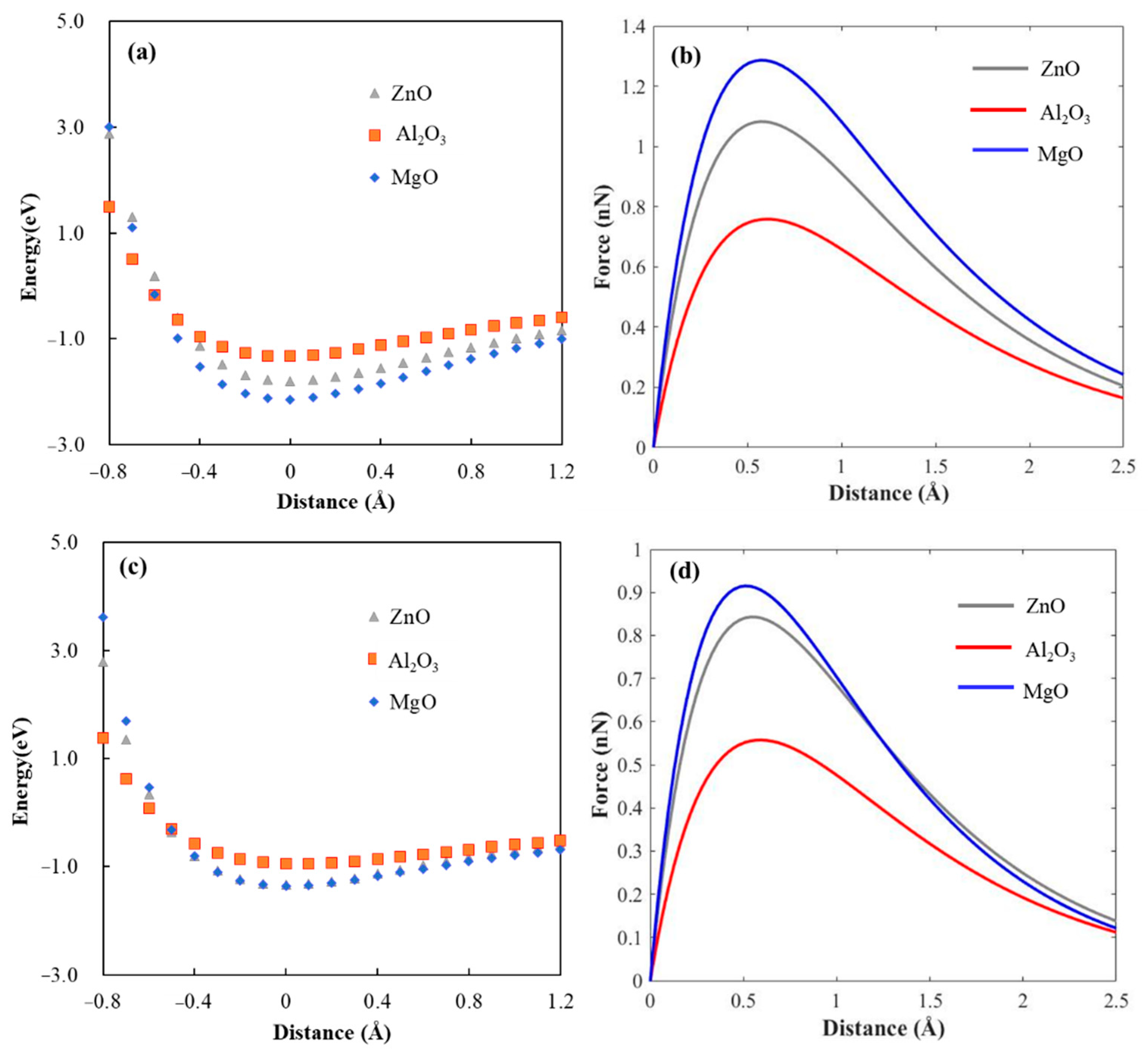

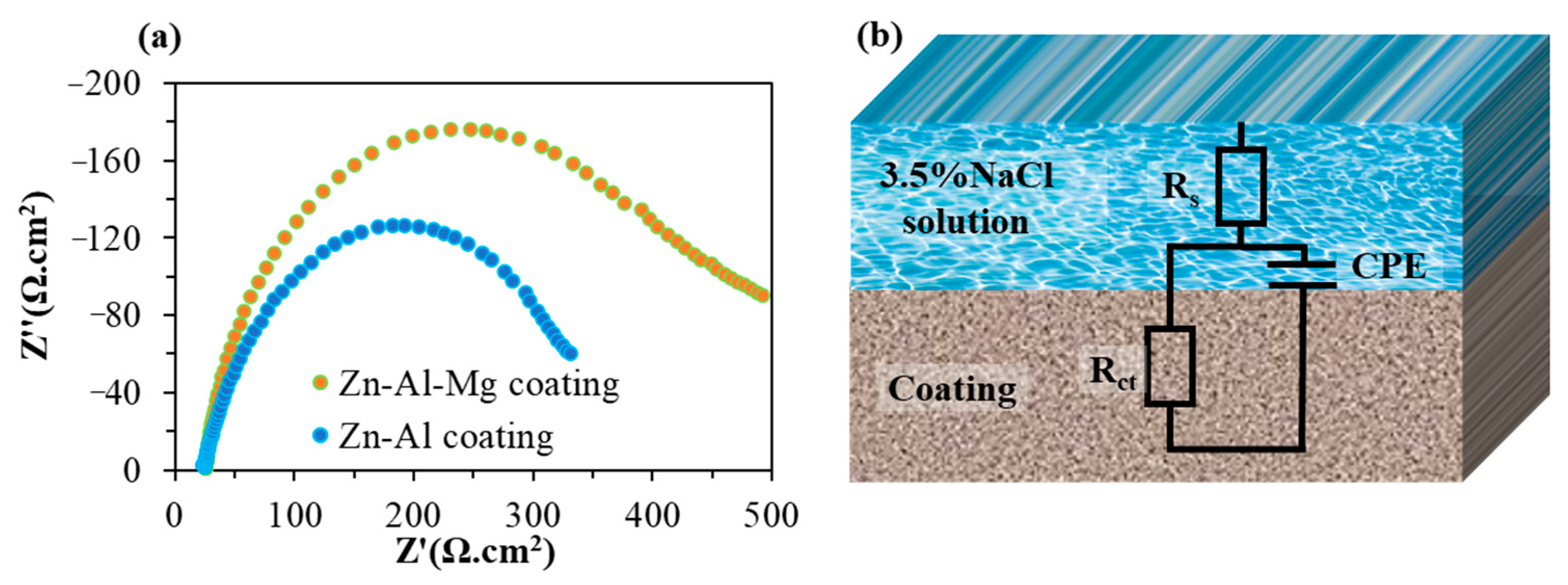
| Element | Atomic % | |
|---|---|---|
| Zn–Al | Zn–Al–Mg | |
| Zn | 13.10 | 8.37 |
| Al | 9.00 | 7.41 |
| Mg | 0.05 | 2.16 |
| Fe | 0.69 | 0.36 |
| O | 37.47 | 42.27 |
| C | 39.70 | 39.43 |
| Model | Oxides in Coating | E0/eV | /Å−1 | Fmax/nN | Smax/GPa in This Work | Smax/GPa in Reference |
|---|---|---|---|---|---|---|
| dry | ZnO | 1.80 | 0.8331 | 1.08 | 0.96 | 0.62 [17], 0.61 [17], 1.55 [18], 2.18 [18], 1.546 [25] |
| Al2O3 | 1.33 | 0.8748 | 0.76 | 0.68 | ||
| MgO | 2.14 | 0.8324 | 1.28 | 1.14 | ||
| water | ZnO | 1.34 | 0.7929 | 0.84 | 0.75 | 0.51 [24], 0.50 [24], 1.38 [46] |
| Al2O3 | 0.95 | 0.8512 | 0.56 | 0.50 | ||
| MgO | 1.36 | 0.7413 | 0.91 | 0.81 |
| Sample | Rs (Ω·cm2) | Rct (Ω·cm2) | CPE-T (s-n/Ω·cm2) | CPE-P | χ2 |
|---|---|---|---|---|---|
| Zn–Al coating | 23.65 | 342.10 | 4.66 × 10−5 | 0.79 | 0.0007 |
| Zn–Al–Mg coating | 24.83 | 461.10 | 3.24 × 10−5 | 0.85 | 0.0048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhao, Y.; Wan, H.; Lin, J.; Min, J. Molecular Understanding of the Interfacial Interaction and Corrosion Resistance between Epoxy Adhesive and Metallic Oxides on Galvanized Steel. Materials 2023, 16, 3061. https://doi.org/10.3390/ma16083061
Li S, Zhao Y, Wan H, Lin J, Min J. Molecular Understanding of the Interfacial Interaction and Corrosion Resistance between Epoxy Adhesive and Metallic Oxides on Galvanized Steel. Materials. 2023; 16(8):3061. https://doi.org/10.3390/ma16083061
Chicago/Turabian StyleLi, Shuangshuang, Yanliang Zhao, Hailang Wan, Jianping Lin, and Junying Min. 2023. "Molecular Understanding of the Interfacial Interaction and Corrosion Resistance between Epoxy Adhesive and Metallic Oxides on Galvanized Steel" Materials 16, no. 8: 3061. https://doi.org/10.3390/ma16083061






